| Research Article | ||
Open Vet. J.. 2025; 15(9): 4635-4649 Open Veterinary Journal, (2025), Vol. 15(9): 4635-4649 Research Article Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley ratsBilal Ahmad1, Farhat Jabeen1, Mudassir Hassan2, Salma Ikram3, Maria Manan4, Muhammad Haseeb5 and Muhammad Kashif Zahoor1*1Department of Zoology, Government College University, Faisalabad, Pakistan 2Department of Zoology, Baba Guru Nanak University, Nankana Sahib, Pakistan 3Department of Physics, Government College University, Faisalabad, Pakistan 4Department of Pharmacology, Government College University, Faisalabad, Pakistan 5Department of Veterinary Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan *Corresponding Author: Muhammad Kashif Zahoor. Department of Zoology, Government College University, Faisalabad, Pakistan. Email: kashif.zahoor [at] gcuf.edu.pk Submitted: 11/05/2025 Revised: 24/07/2025 Accepted: 12/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
AbstractBackground: Carbon nanoparticles (CNPs) are extremely small particles mainly composed of carbon atoms, typically ranging from 1 to 100 nm in size. Although CNPs have promising applications in various fields, they can damage cell membranes, cause toxicity, and potentially induce mutations that may lead to cancer. Aim: This study aimed to investigate the dose-dependent effects of CNPs on the liver in rats. Methods: Twenty-five male Sprague–Dawley rats (weight, 100–130 g) were acclimated and randomly divided into five groups. Groups I, II, and III received intraperitoneal injections of CNPs at doses of 19.5, 58.5, and 97.5 mg/kg of body weight for 28 consecutive days, respectively. The saline control group received 0.5 ml of normal saline. Liver samples were collected for biochemical and histological analyses. Results: Rats exposed to higher CNP doses showed significant weight loss. Serum alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and bilirubin levels were markedly modulated, especially in the high-dose group. Lipid metabolism was also disrupted. Histological studies revealed hepatocellular degeneration, inflammatory infiltration, and sinusoidal dilation at higher CNP concentrations. Conclusion: The results revealed that exposure to CNPs, particularly at higher doses, may lead to liver toxicity and pose health risks. As the use of CNPs becomes more widespread, understanding their biological effects is essential to ensure their safe use for the reasonable development of nanotechnology. Keywords: Carbon nanoparticles (CNPs), Toxicity assessment, Hepatotoxicity, Histological analysis, Biochemical markers. IntroductionIn recent decades, nanotechnology has emerged as a transformative force, revolutionizing various scientific and industrial fields, including medicine, materials science, electronics, and environmental engineering. Among the many types of engineered nanomaterials, carbon-based nanoparticles have garnered significant attention owing to their unique physical, chemical, and biological properties, such as high surface area-to-volume ratio, chemical stability, and multifunctional capabilities (Principi et al., 2016; Rawat et al., 2017; Fang et al., 2019;Lee and Jun 2019; Vorobyova et al., 2022; ALRashdi et al., 2023; Rathore et al., 2023). These characteristics make carbon nanoparticles (CNPs) valuable for a wide array of applications, including drug delivery, biosensing, imaging, cancer therapy, and even consumer products such as textiles, cosmetics, and food packaging (Strojny et al., 2015; Wang et al., 2018; Shah et al., 2021; Vilas-Boas and Vinken, 2021; Selvakumar et al., 2023; Tan et al., 2023; Hannan et al., 2024; Sharma et al., 2021, 2024). Despite their actantial benefits, the increasing use of CNPs has raised growing concerns about their possible adverse health effects, particularly due to their ability to interact with biological systems in ways that differ significantly from larger-sized materials (Patil et al., 2016; Huang et al., 2018; Madannejad et al., 2019; Xiang et al., 2020; Witkowska et al., 2022; Dien et al., 2024). The liver, a central organ for metabolism and detoxification, is particularly vulnerable to nanoparticle accumulation and associated toxicity (Kermanizadeh et al., 2015; Yang et al., 2016; Kumar et al., 2017; Sun et al., 2018; Chen et al., 2019a, 2019b; Zhang et al., 2019b, 2022; Rana, 2020; Singh et al., 2020b; Yu et al., 2020; Kim et al., 2024a). Nanoparticles can enter the human body via inhalation, ingestion, or dermal exposure, and a large fraction is sequestered by the liver once in systemic circulation. The reticuloendothelial system of this organ, particularly Kupffer cells and sinusoidal endothelial cells, plays a primary role in nanoparticle uptake, potentially leading to structural and functional disruptions (Hou et al., 2017a, 2017b; Adedara et al., 2018; Kubes and Jenne, 2018; Abu Gazia and El-Magd, 2019). Oxidative stress is one of the primary mechanisms underlying nanoparticle-induced toxicity (Xu et al., 2016, 2024; Yang et al., 2016). Excessive generation of reactive oxygen species (ROS) coupled with an imbalance in antioxidant defenses can initiate lipid peroxidation, DNA damage, protein oxidation, and ultimately cell death (Kermanizadeh et al., 2015; Francis and Devasena, 2018; Zhang et al., 2018; Zhao et al., 2019; Chrishtop et al., 2021; He et al., 2022; Guo et al., 2022; Aschner et al., 2023; Azizah et al., 2024). Prolonged or repeated exposure to CNPs can result in significant oxidative damage in sensitive organs such as the liver, kidney, and brain (Angoth et al., 2017; Elshater et al., 2018; Saeedi et al., 2019; Cornu et al., 2020; Awogbindin et al., 2021; Al Mutairi et al., 2023; Herrera-Rodríguez et al., 2023; Kamt et al., 2023; Das et al., 2024). Furthermore, the method of nanoparticle synthesis can influence their toxicity. Although chemical methods allow for large-scale production, they may introduce toxic residues on the particle surface. Green synthesis methods using biological agents such as bacteria, fungi, and plant extracts offer an environmentally friendly alternative with reduced toxicity risks (Ohkawa et al., 1979; Albini et al., 2015; Pourali et al., 2017; Fang et al., 2018; De Menezes et al., 2019; Madannejad et al., 2019; Vorobyova et al., 2022; Florek et al., 2023; Nathawat et al., 2023; Dien et al., 2024). However, understanding how the physical properties of CNPs, such as size, surface charge, and agglomeration, translate into biological responses remains a key challenge in nanotoxicology (Sedlak and Lindsay, 1968; Linet al., 2018; Galassi et al., 2020; Guzmán-Mendoza et al., 2020; Dien et al., 2024; Gao et al., 2024). Given the critical role of the liver in maintaining physiological homeostasis through functions such as xenobiotic metabolism, nutrient storage, and biosynthesis, assessing the potential hepatotoxic effects of CNPs is essential (Helmy Abdou et al., 2019). Previous studies have shown that hepatocytes and other liver cells are susceptible to nanoparticle-induced injury, which may manifest as inflammation, necrosis, or apoptosis, depending on the dose and duration of exposure (Helmy Abdou et al., 2019; Hassan et al., 2020; Mohammadi et al., 2020; Rana, 2020; Modrzynska et al., 2021; Sun et al., 2023; Dien et al., 2024; He et al., 2024). This study aimed to investigate the hepatotoxic effects of CNPs in male Sprague–Dawley rats. By evaluating biochemical markers of liver function alongside histological changes in hepatic tissue, this study seeks to elucidate CNP-induced dose-dependent toxicological responses. The findings of this study will contribute to a better understanding of nanoparticle–liver interactions and support the development of safer nanomaterials for biomedical and industrial applications. Materials and MethodsProduction of CNPsCNPs were synthesized following the method described by Sun et al. (2014). Briefly, 72 g of sugar was dissolved in 100 ml of deionized water and stirred continuously using a magnetic stirrer at 50°C for 70 minutes to ensure homogenous mixing. The solution was then transferred into a Teflon-lined hydrothermal autoclave and heated in a muffle furnace at 200°C for 8 hours. The resulting solid was filtered, washed with ethanol, and neutralized to a pH of approximately 7.68 upon cooling to room temperature. The material was dried for 28 hours, ground into a fine powder using a mortar and pestle for 2 hours, and further heated at 180°C for 2 hours to complete the synthesis. The final CNP product was stored in Eppendorf tubes for subsequent use. Characterization of CNPsStructural and morphological characterization of the synthesized CNPs was conducted following the protocol of Kermanizadeh et al. (2015). X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and scanning electron microscopy (SEM) were employed. Animal modelsA total of 25 male Sprague–Dawley rats (100–130 g) were procured and acclimatized at the animal facility of Government College University, Faisalabad. The rats were maintained under standard laboratory conditions, temperature 24.0°C ± 2.5°C, relative humidity 50%–70%, and a 12-hour light/dark cycle. The rats had free access to standard feed and water (Li et al., 2016a; Shah et al., 2017; ALRashdi et al., 2023; Hannan et al., 2024). Table 1. Grouping of male Sprague–Dawley rats and their treatment.
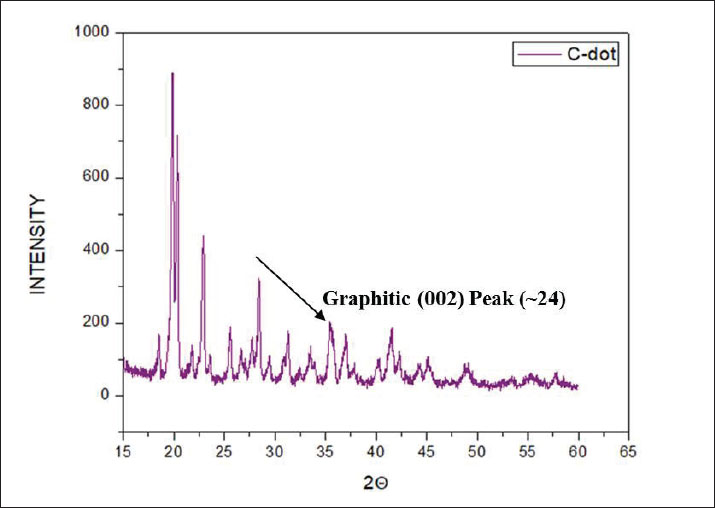
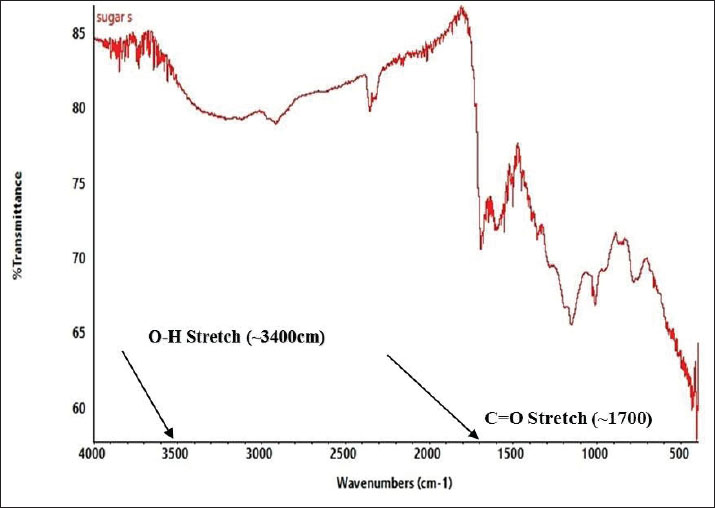
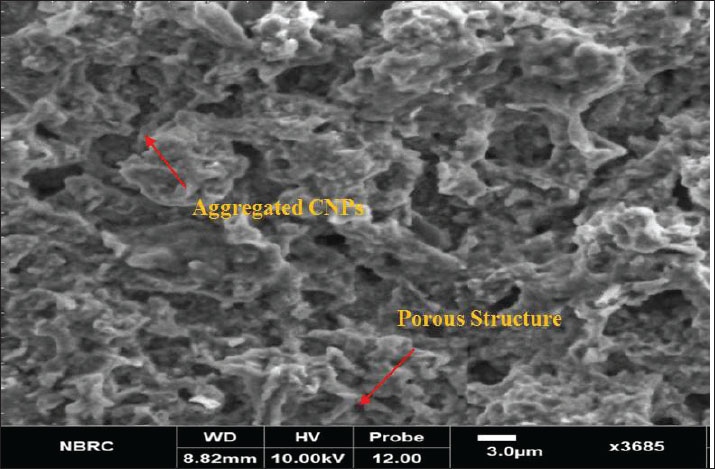
Preparation of the CNP exposure mediumCNPs (99% purity) were dispersed in deionized water to prepare a stock solution at a concentration of 90 mg/ml. The suspension was placed in sterile 50-ml Falcon tubes and sonicated at 65°C for 60 minutes to ensure uniform dispersion before administration (Li et al., 2016b). Experimental designFollowing the acclimatization period, the rats were divided into five equal groups (n=5/group). Groups I, II, and III received intravenous doses of CNPs at concentrations of 19.5, 58.5, and 97.5 mg/kg BW, respectively. Group IV (Control) was provided only with standard feed and water. Group V (Saline) was administered 0.5 ml of normal saline (Table 1). CNP treatment was administered daily for 28 consecutive days. The rats were mercifully euthanized at the end of the trial (Li et al., 2016ab). Body weightThe body weights of all rats were recorded weekly throughout the 28-day experiment using an electronic balance. Rats with comparable initial body weight were selected to minimize variability. Weight gain or loss trends were plotted to assess any CNP-induced changes (Kermanizadeh et al., 2015; Saber et al., 2015; Reyes et al., 2022). Preparation of the blood and tissue samplesAfter 28 days of exposure, the animals were fasted for 24 hours and sedated using chloroform. Blood samples were collected from the jugular vein using ethylenediaminetetraacetic acid-coated 3-ml tubes and stored at 4ºC. The livers of the rats were then dissected and preserved for further histological and biochemical analyses (Kermanizadeh et al., 2015). Parameters of liver functionSerum was separated and analyzed using an automated clinical chemistry analyzer to evaluate liver function by measuring alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) levels (Li et al., 2016a, 2016b). Serum biochemistryApproximately 1.5 ml of blood was collected into non-anticoagulant tubes for serum analysis. Key biochemical parameters, including bilirubin, ALT, AST, and albumin-to-globulin (A/G) ratio, were assessed using a Microlab 300 chemical analyzer (ELITech Group, USA) (Li et al., 2016a, 2016b). Histological analysisLiver samples from each group were processed histologically to identify structural alterations due to CNP exposure using a protocol adapted from Li et al. (2016a, 2016b). Liver tissues were excised, rinsed in saline, and fixed for 48 hours in 10% neutral buffered formalin for 48 hours. Fixed tissues were dehydrated using graded ethanol: 80% ethanol for 1 hour, then 90% ethanol for 2 hours, placed overnight, then 100% ethanol for 2 hours (repeated twice). Dehydrated tissues were cleared in xylene (1–2 hours) and embedded in paraffin wax. Sections (3–4 µm thick) were cut using a microtome (Histo-Line MR-2258, Italy) and mounted on glass slides. After deparaffinization and rehydration, tissues were stained with hematoxylin and eosin (H&E). The prepared slides were examined using a fluorescence microscope (Nikon DS-Fi2) under 10x, 20x, and 40x magnification to observe histological changes. Statistical analysisData were statistically analyzed using Minitab 17 software. A one-way analysis of variance was employed to assess significant differences among groups, with a threshold of p < 0.05. Tukey’s post hoc test was used for pairwise comparisons. Ethical approvalAll animal procedures were approved by the Institutional Animal Ethics Committee of Government College University, Faisalabad (Ref. No. 416-A; dated 27-06-2024). ResultsCharacterization of CNPsX-ray diffraction analysis of CNPsX-ray diffraction analysis of the synthesized CNPs revealed a notable peak at 2θ=19.88°, indicating a mixed phase of crystalline and amorphous carbon. The broad signal around 20° indicates disordered carbon structures, while the absence of sharp peaks at higher angles indicates a largely nanocrystalline morphology, likely comprising both turbostratic and graphitic domains (Fig. 1). FTIR analysis of CNPsThe FTIR spectrum displayed characteristic peaks corresponding to the functional groups on the surface. A broad band between 3,200 and 3,500 cm−¹ indicates –OH or –NH stretching, while a peak near 1,600–1,700 cm−¹ corresponds to C=O stretching. Signals below 1,500 cm−¹, within the fingerprint region, suggest the presence of C-O and C-N bonds (Fig. 2). These functional groups influence the biological activity and stability of the particles. SEM analysis of CNPsSEM imaging revealed that the CNPs possessed an irregular, porous surface and exhibited agglomeration, likely due to van der Waals forces. Imaging parameters included a magnification of 3,685 × 10.00 kV accelerating voltage, and 3.0 µm scale bar (Fig. 3). The observed morphology shows a high surface area, enhancing their actantial for biological interactions.
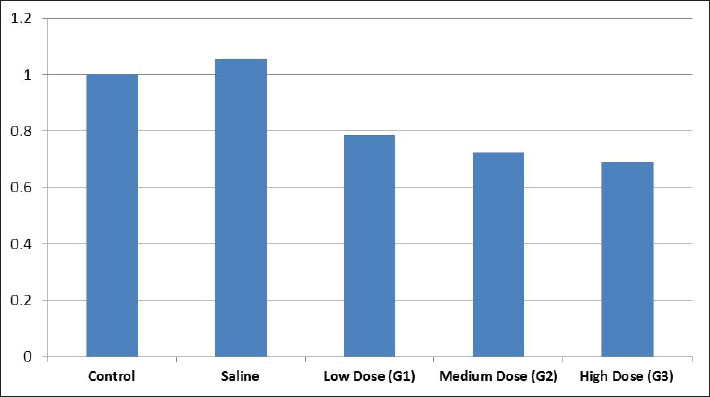
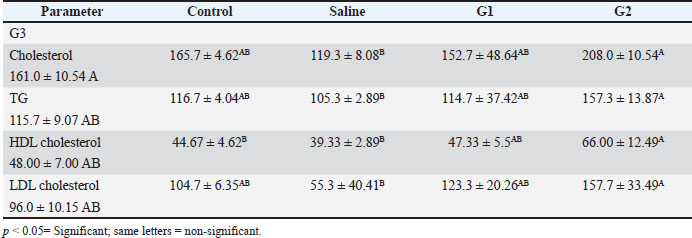
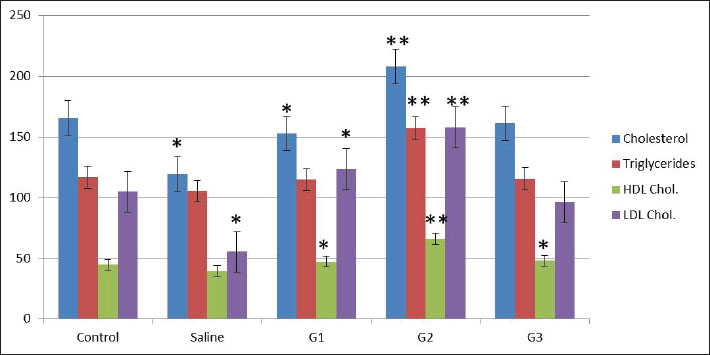
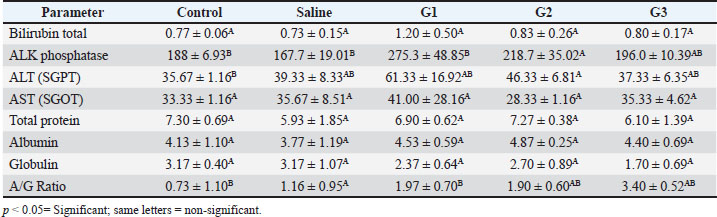
Fig. 1. X-ray diffraction pattern of synthesized CNPs. General observations of the experimentsAll rats initially showed normal behavior and consistent intake of food. However, rats treated with higher CNP doses (58.5 and 97.5 mg/kg BW) developed reduced activity, appetite loss, and weight decline over time. The highest dose group exhibited the most severe toxicity symptoms, including behavioral changes and visible health deterioration. At the end of the study, only the control and low-dose groups maintained normal physiological conditions. Evaluation of changes in body weightBody weight was tracked over 28 days. Rats treated with 58.5 and 97.5 mg/kg doses showed significant weight loss compared with controls (p < 0.05), showing dose-dependent systemic toxicity. No notable differences in weight were observed between the control and saline groups. The results of ANOVA confirmed significant intergroup variation (p=0.002, F=9.78), warranting further post hoc analysis (data not shown). The normalized liver-to-body weight ratio showed that liver weight was dose-dependently decreased (Fig. 4). Changes in lipid profile in ratsLipid profile analysis revealed that Group 2 (58.5 mg/kg BW) showed significant increases in total cholesterol, triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels compared with the saline group (p < 0.05), indicating disrupted lipid metabolism (Table 2 and Fig. 5). Elevated HDL and LDL levels in G2 show actantial cardiovascular and metabolic effects. Groups G1 and G3 exhibited moderate to non-significant changes. Group 3 showed the same cholesterol level as the control and low TG, LDL, and HDL levels, possibly due to an unknown genetic or enzymatic feedback mechanism that has been triggered and possibly initiates detoxification metabolism. CNP exposure and rat liver functionTable 3 shows that ALK phosphatase levels were significantly higher in Group 2 (58.5 mg/kg BW) compared to the control and saline groups (p < 0.05), indicating possible liver enzyme stress. The ALT and AST levels remained consistent across all groups, indicating minimal liver injury. The total protein content decreased in all treatment groups. Albumin levels increased in all treatments, whereas globulin levels showed no significant changes. The A/G ratio varied between the groups, indicating actantial shifts in the protein balance.
Fig. 2. FTIR spectrum of CNPs. Histological analysisHistological examination of liver tissue after 28 days of CNP exposure revealed dose-dependent structural changes indicative of hepatic toxicity. Liver sections from untreated rats showed normal architecture (Fig. 6), with radially arranged hepatocytes around the central vein. No signs of inflammation, necrosis, or fibrosis were observed. The liver tissue of Group 1 male Sprague–Dawley rats exposed to a low dose of CNPs (19.5 mg/kg body weight) exhibited mild sinusoidal congestion and slight inflammatory infiltration. Hepatocytes remained largely normal with no evidence of necrosis or fibrosis, which show minimal hepatic impact. Group 2 showed moderate structural changes, including sinusoidal enlargement and hepatocyte vacuolization, after exposure to a medium dose (58.5 mg/kg body weight) of CNPs. Mild congestion and early immune cell infiltration indicated the onset of inflammation. Group 3 rats exposed to a high dose (97.5 mg/kg body weight) of CNPs exhibited severe histological alterations, including pronounced sinusoidal dilation, hepatocyte vacuolation, inflammatory infiltration, and signs of cellular degeneration, suggesting significant hepatotoxicity. Liver histology of male Sprague–Dawley rats in the normal saline group, serving as the control group, which received 0.5 ml of normal saline; remained normal, similar to the control group, with preserved architecture and no pathological changes (Fig. 6). DiscussionThe results demonstrate that male Sprague–Dawley rats develop liver toxicity as a result of intravenous CNP exposure at certain dosage levels. Laboratory data, markers of oxidative stress, and histological tests established that nanoparticles tend to damage liver tissue, especially because it functions as a key metabolic and detoxification organ (Wu and Tang, 2018; Khashan et al., 2020). Synthetic CNPs possess structural and chemical properties that play an essential role in studying their biological activities and toxicity actantial (Kim et al., 2015; Kurantowicz et al., 2015; Kumar et al., 2017; Lee and Jun, 2019; Kumar Babele et al., 2021; Kuznetsov et al., 2022; Dien et al., 2024). An XRD test was performed to evaluate the resulting carbon material to be crystalline carbon, along with amorphous materials. The XRD peak located at 2θ=19.88° demonstrates a mixture of turbostratic and graphitic carbon structures that might affect the biological system penetration based on previous research findings on similar CNPs (Liu et al., 2018; Yu et al., 2020). The functional bonds O-H, C=O, and C-O/C-N became visible through FTIR spectroscopy analysis. Patel Liu et al. (2019) and Singh et al. (2025) recognized functional groups on nanoparticles as actantial contributors to oxidative stress mechanisms and cellular interactions, leading to cytotoxic effects. The rough and porous appearance shown by SEM analysis combined with aggregate tendencies indicates an actantial improved cellular uptake that might result in hepatotoxicity, as described in Wang et al. (2017). Carbon-based nanoparticles have received criticism from past studies about their cytotoxic properties; thus, thorough safety assessments must precede biomedical applications (Dvir et al., 2020; Zhang et al., 2022; Kim et al., 2024b).
Fig. 3. Scanning electron microscopy (SEM) analysis of CNPs.
Fig. 4. Ratio of liver weight to body weight. Table 2. The toxic effects of varying concentrations of CNPs on liver function markers in male Sprague–Dawley rats following a 28-day intraperitoneal exposure, expressed as mean (±SD).
Fig. 5. Graph of the lipid profile. The current findings from rats that received higher doses of CNPs indicate that systemic toxicity might exist. A substantial decline in the movements of rats became apparent along with a decrease in appetite and a major weight reduction when rats were given CNPs at doses of 19.5, 58.5, and 97.5 mg/kg, respectively, but normal expressions remained constant for both the control and saline groups. Consistent with previous research, nanoparticle contact has been found to induce physiological pressure along with metabolic system modifications (Nakamura and Watano, 2018; Singh et al., 2020a; Zhang et al., 2021a–d; Nasim et al., 2024). Table 3. Effects of CNP exposure on rat liver function and protein profile.
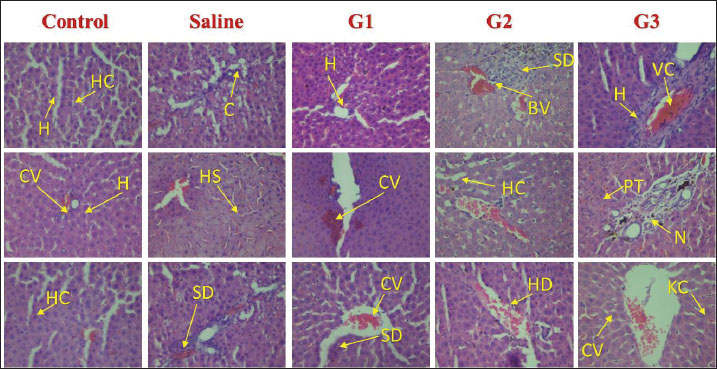
Fig. 6. Photomicrographs of liver tissue from male Sprague–Dawley rats administered with different doses of CNPs captured at 40x magnification. Histological examination of H&E-stained liver tissues revealed a progressive pattern of hepatic damage across the experimental groups. The control group exhibited normal liver architecture with intact central veins (CV) and hepatic cords (HC). The saline group remained largely normal, with only mild SD. Early signs of liver injury appear in Group 1 (G1), including mild congestion (C) and hepatocyte (H) swelling. Group 2 (G2) exhibited moderate damage, with evident vascular congestion (VC), HD, and inflammatory infiltration. The most severe changes are seen in Group 3 (G3), where advanced liver injury is indicated by marked necrosis (N), cytoplasmic vacuolation (CV), hemorrhage (H), and disorganized hepatic structure. These findings show that the experimental compound likely induces a dose-dependent hepatotoxic effect. HC, Hepatic Cords; H, Hepatocytes; C, Congestion; BV, Blood Vessel Congestion; SD, Sinusoidal Dilation; HS, Hepatic Sinusoids; HD, Hepatocellular degeneration; VC, Vascular Congestion; PT, Portal triad; CV, Cytoplasmic Vacuolation; N, Necrosis; KC, Kupffer Cells. Body weight alterations are one of the primary markers of systemic toxicity. Rats that received higher CNP doses lost substantial amounts of weight because the accumulation of nanoparticles within their bodies affected their metabolic processes. CNP exposure led to major changes in body weight, serum biochemical data, and oxidative stress readings, suggesting that liver dysfunction might persist and affect overall wellness (Zhang et al., 2019a; Singh et al., 2020a). It has also been established through rodent nanoparticle research that weight loss occurs due to oxidative stress and inflammation, which disrupts both nutrient absorption and energy metabolism (Chen et al., 2019a; Singh et al., 2020ab). In the current study, both liver and body weight were found to be decreased. Weight reduction in the low-, medium-, and high-dose groups implies that CNP contact harms regular metabolic operations by potentially destructive hepatic means. Weight loss appears due to liver dysfunction because this organ leads protein synthesis and energy regulation, similar to impairments that result in weight loss (Ali et al., 2018). In the present study, studies on liver function biomarkers showed that Group 3 had the same cholesterol level as the control and low TG, LDL, and HDL levels. A more plausible explanation could be that an unknown genetic or enzymatic feedback mechanism has been triggered, which could initiate metabolism detoxification. However, the liver weight showed a significant decrease, revealing a high level of toxicity, which is supported by images. The altered levels of ALT, AST, ALP, and bilirubin, along with the A/G ratio, demonstrated the hepatotoxicity of CNP exposure. The release of ALT and AST enzymes in the bloodstream indicates liver cell damage because these enzymes come from damaged cells (Aebi, 1974; Adedara et al., 2018; Ahmadi et al., 2017a, 2017b; Garcia-Garcia et al., 2021). Elevated ALP activity indicates hepatobiliary stress, which possibly arises from damaged bile ducts (Kumari et al., 2018). The research findings support previous observations about nanoparticles, which demonstrate elevated bilirubin levels mainly seen in the high-dose group because of nanoparticle-induced oxidative stress and liver functional impairment (Abdelhalim and Jarrar, 2016). The researchers observed no substantial variations in total bilirubin levels during the study period, indicating that CNP exposure affects liver function but does not cause major disruptions to bilirubin metabolic processes (Kim et al., 2024a). Research findings demonstrating alterations in lipid metabolism strongly demonstrate that workplace exposure to CNP causes liver toxicity. The statistical analysis using ANOVA revealed substantial modifications in LDL cholesterol (p=0.012) and triglyceride (p=0.012) levels, together with HDL cholesterol (p=0.008) levels, indicating aberrant lipid homeostasis. Previous research has demonstrated how nanoparticle exposure leads to disturbances in lipid profiles, which may occur through oxidative stress pathways (Chen et al., 2019b; Liu et al., 2020; Zhang et al., 2021a–c). The findings of increased ALP levels (p=0.009) support the mechanism that CNPs disrupt hepatic lipid metabolism according to studies showing how nanoparticles cause hepatic stress and biliary dysfunction (Sun et al., 2018). The absence of significant changes in total protein and globulin levels (p > 0.05) demonstrates that CNP exposure causes minimal impairment of protein synthesis or metabolism according to results reported by Chen et al. (2014). The statistically significant change in A/G ratio (p=0.020) implies that CNPs might affect the relationship between albumin and globulin, as experts have associated this pattern with oxidative stress changes in protein distribution changes in prior research (Zhou et al., 2020). The multiple hepatotoxic effects of CNPs occur through oxidative stress, inflammatory mechanisms, and cellular interactions. Scientific evidence demonstrates that nanoparticles trigger the production of ROS, which generates lipid peroxidation alongside protein damage and mitochondrial impairment, leading to cellular death (Xia et al., 2009). The presence of nanoparticles within liver tissues results in signaling pathway activation, which subsequently causes hepatocellular damage and liver dysfunction (Sharma et al., 2021). The present results establish that extended CNP exposure damages liver function via oxidative and inflammatory responses. Under a microscope, research on liver tissues demonstrated the harmful reactions that stem from CNP exposure. All dosage levels in the high-dose groups provoked cellular degeneration of hepatocytes with vacuolation, while inflammatory infiltration appeared alongside sinusoidal dilation. The examined tissue alterations match the results of Rajput et al. (2018) when documenting nanoparticle-related hepatic damage. Inflammatory cells that enter the tissue indicate that CNP accumulation initiates an immune reaction that potentially leads to prolonged liver harm (Li et al., 2017). Researchers investigating carbon-based nanomaterials demonstrated dose-dependent hepatic tissue damage because excessive doses caused hepatocyte vacuolation, sinusoidal congestion, and inflammation (Zhang et al., 2019b; Li et al., 2021). Our findings are consistent with those reported by Kumar et al. (2022), who showed that immune cells enter the liver and cause inflammation because of nanoparticle exposure. The findings from this research establish that CNP exposure causes hepatotoxicity that increases in severity at different dosage levels based on measured liver function biomarker changes, elevated oxidative stress, and distinctive tissue alterations in the liver. The observations of liver toxicity require extensive safety evaluations of CNPs to assess both short- and long-term risks to hepatic health. The next phase of research needs to clarify how CNPs generate their toxic effects by investigating structural changes in oxidative stress and inflammatory signaling pathways and mitochondrial dysfunction at the molecular level. Studying these pathways would enable scientists to develop possible mitigation solutions (Dien et al., 2024). According to Kim et al. (2024a), nanoparticle modifications applied to surfaces represent a promising method for both reducing particle reactivity and increasing biocompatibility. Supplementation with antioxidants has demonstrated actantial as a way to protect the liver from nanoparticle-induced damage through oxidative stress mechanisms. Illuminating the safety aspect of carbon-based nanoparticles is essential because their use continues to expand across numerous industries. ConclusionIt was concluded that male Sprague–Dawley rats exposed to intravenous CNPs developed liver toxicity according to the dose. The liver is highly sensitive to nanoparticles because nanoparticle exposure leads to major alterations in liver function markers, oxidative stress indicators, lipid metabolism, and tissue structure disruption. Structural analysis of CNPs validated their composition through crystalline phases and amorphous features along with surface functional groups and porous structure because these factors determine biological reactions and actantial toxicity levels. Weight loss, behavioral changes, and biochemical alterations were observed in rats who received elevated doses of CNPs, indicating that CNPs have systemic effects. The microscopic examination showed harm to liver cells together with inflammation and tissue destruction, matching the toxicological effects caused by oxidative stress and inflammatory pathways. Interestingly, the lipid metabolic profile also showed that a high dose could have possibly triggered the detoxification mechanism. However, when histological alterations and liver weight to body weight ratio are taken together, it becomes clear that nanoparticles cause toxicity to the liver. Thus, the safety of carbon-based nanoparticles has become more crucial since their industrial applications continue to increase. New research should focus on understanding CNP-induced liver damage at the molecular level, along with creating new strategies to decrease their toxicity through surface alteration or antioxidant investigation. The industrial and biomedical use of CNPs necessitates responsible management practices to lower the potential health dangers. AcknowledgmentsThe facilities and support provided by the Department of Zoology, Government College University Faisalabad; the Department of Pharmacology, Government College University Faisalabad; and the Department of Physics, Government College University Faisalabad are highly acknowledged. Conflict of interestThe authors declare that there is no conflict of interest. FundingNone. Authors’ contributionsFJ designed and supervised the study. BA performed the experiments. SI helped in nanoparticle preparation and SEM. MKZ, MM, and MH helped in rearing and experimentation. MKZ and MH helped with data analyses and during the write-up. All authors have read and approved the final version of the manuscript. Moreover, all authors declare that they have no competing interests. Data availabilityAll data were provided in the manuscript. ReferencesAbdelhalim, M.A.K. and Jarrar, B.M. 2016. Hepatotoxicity induced by gold nanoparticles in rats: a histological and biochemical study. Int. J. Nanomed. 11, 453–465. Abu Gazia, M. and El-Magd, M.A. 2019. Effect of pristine and functionalized multiwalled carbon nanotubes on rat renal cortex. Acta Histochem. 121(2), 207–217. Adedara, I.A., Anao, O.O., Forcados, G.E., Awogbindin, I.O., Agbowo, A., Ola-Davies, O.E., Patlolla, A.K., Tchounwou, P.B. and Farombi, E.O. 2018. Low doses of multi-walled carbon nanotubes elicit hepatotoxicity in rats with markers of oxidative stress and pro-inflammatory cytokine induction. Biochem. Biophys. Res. Commun. 503(4), 3167–3173. Aebi, H. 1974. Catalase. Methods of enzymatic analysis. Elsevier, Vol. 2, pp: 673–684; doi:10.1016/B978-0-12-091302-2.50032-3 Ahmadi, H., Ramezani, M., Yazdian-Robati, R., Behnam, B., Razavi Azarkhiavi, K., Hashem Nia, A., Mokhtarzadeh, A., Matbou Riahi, M., Razavi, B.M. and Abnous, K. 2017a. Acute toxicity of functionalized single wall carbon nanotubes: a biochemical, histopathologic and proteomics approach. Chemico-Biol. Interact. 275, 196–209. Ahmadi, M., Elmongy, H., Madrakian, T. and Abdel-Rehim, M. 2017b. Nanomaterials as sorbents for sample preparation in bioanalysis: a review. Anal. Chim. Acta 958, 1–21. Al Mutairi, M.A., Bin Saeedan, N.M., Alnabati, K.K., Alotaibi, A., Al-Mayouf, A.M., Ali, R. and Alowaifeer, A.M. 2023. Characterization of engineered titanium dioxide nanoparticles in selected food. Food Addit. Contamin. 16(3), 266–273. Ali, M., Smith, J., Khan, R., Williams, D. and Brown, P. 2018. Metabolic disturbances and weight loss in rodents exposed to nanoparticles. Toxicol. Rep. 5, 123–132. Albini, A., Pagani, A., Pulze, L., Bruno, A., Principi, E., Congiu, T. and Noonan, D.M. 2015. Environmental impact of multi-wall carbon nanotubes in a novel model of exposure: systemic distribution, macrophage accumulation, and amyloid deposition. Int. J. Nanomed. 10, 6133–6145. ALRashdi, B.M., Germoush, M.O., Sani, S.S., Ayub, I., Bashir, W., Hussain, B., Mazhar, M., Ali, S., Zahid, Z., Ayesha, S. and Rafique, A. 2023. Biosynthesis of Salvia hispanica based silver nanoparticles and evaluation of their antibacterial activity in-vitro and rat model. Pak. Vet. J. 43(2), 283–289. Angoth, B., Lingabathula, H. and Yellu, N.R. 2017. Assessment of extrapulmonary toxicity induced by carbon nanomaterials following intra-tracheal instillation in rats. Asian J. Pharm. Clin. Res. 10(5), 82–86. Aschner, M., Skalny, A., Santamaria, A., Buha-Dordevic, A., Tizabi, Y., Jiang, Y. and Tinkov, A.A. 2023. From mechanisms to implications: understanding the molecular neurotoxicity of titanium dioxide nanoparticles. Front. Biosci. Landmark. 28(9), 1–21. Awogbindin, I.O., Maduako, I.C., Adedara, I.A., Owumi, S.E., Ajeleti, A.O., Owoeye, O., Patlolla, A.K., Tchounwou, P.B. and Farombi, E.O. 2021. Kolaviron ameliorates hepatic and renal dysfunction associated with multiwalled carbon nanotubes in rats. Environ. Toxicol. 36(1), 67–76. Azizah, R.N., Verheyen, G.R., Shkedy, Z. and Van Miert, S. 2024. Overview of in vitro-in vivo extrapolation approaches for the risk assessment of nanomaterial toxicity. NanoImpact 35, 100524; doi:10.1016/j.ni.100524 Chen, N., Wang, H., Huang, Q., Li, J., Yan, J., He, D., Fan, C. and Song, H. 2014. Long-term effects of nanoparticles on nutrition and metabolism. Small 10(18), 3603–3611. Chen, L., Zhang, H., Wang, J., Li, Y., Zhao, X. and Liu, M. 2019a. Hepatic metabolism and nanoparticle interactions: an in vivo study. J. Nanomed. Res. 11(4), 302–315. Chen, R., Zhang, L., Wang, Y., Zhao, L., Chen, X. and Xu, Z. 2019b. Effects of NPs on lipid metabolism and cardiovascular health. Toxicol. Rep. 6, 132–141. Chrishtop, V.V., Prilepskii, A.Y., Nikonorova, V.G. and Mironov, V.A. 2021. Nanosafety vs. nanotoxicology: adequate animal models for testing in vivo toxicity of nanoparticles. Toxicology 462, 152952. Cornu, R., Béduneau, A. and Martin, H. 2020. Influence of nanoparticles on liver tissue and hepatic functions: a review. Toxicology 430, 152344. Das, S.K., Sen, K., Ghosh, B., Ghosh, N., Sinha, K. and Sil, P.C. 2024. Molecular mechanism of nanomaterials induced liver injury: a review. World J. Hepatol. 16(4), 566–600. De Menezes, B.R.C., Rodrigues, K.F., da Silva Fonseca, B.C., Ribas, R.G., do Amaral Montanheiro, T.L. and Thim, G.P. 2019. Recent advances in the use of carbon nanotubes as smart biomaterials. J. Mater. Chem. B 7(9), 1343–1360. Dien, E.E.E., Mansour, Y.A., Ahmed, Y.H., Galal, M.K., Rashad, M.M., Mahmoud, M.Y. and Hussein, S. 2024. Effect of multi-walled carbon nanotubes on kidney of male albino rats with the actantial ameliorative effect of alpha lipoic acid. J. Adv. Vet. Res. 14, 96–102. Dvir, T., Timko, B.P., Kohane, D.S. and Langer, R. 2020. Nanotechnological strategies for engineering complex tissues. In: Nano-enabled medical applications, 1st ed. Jenny Stanford Publishing, pp: 351–382. Elshater, A.E.A., Haridy, M.A., Salman, M.M., Fayyad, A.S. and Hammad, S. 2018. Fullerene C60 nanoparticles ameliorated cyclophosphamide-induced acute hepatotoxicity in rats. Biomed. Pharmacol. 97, 53–59. Fang, H., Cui, Y., Wang, Z. and Wang, S. 2018. Toxicological assessment of multi-walled carbon nanotubes combined with nonylphenol in male mice. PLoS One 13(7), e0200238. Fang, R., Chen, K., Yin, L., Sun, Z., Li, F. and Cheng, H.M. 2019. The regulating role of carbon nanotubes and graphene in lithium-ion and lithium–sulfur batteries. Adv. Mater. 31(9), 1800863. Florek, E., Witkowska, M., Szukalska, M., Richter, M., Trzeciak, T., Miechowicz, I. and Giersig, M. 2023. Oxidative stress in long-term exposure to multi-walled carbon nanotubes in male rats. Antioxidants 12, 464. Francis, A.P. and Devasena, T. 2018. Toxicity of carbon nanotubes: a review. Toxicol. Ind. Health 34(3), 200–210. Galassi, T.V., Antman-Passig, M., Yaari, Z., Jessurun, J., Schwartz, R.E. and Heller, D.A. 2020. Long-term in vivo biocompatibility of single-walled carbon nanotubes. PLoS One 15(5), e0226791. Gao, C., Wang, M., Zheng, Y., Zhang, L., He, J., Liu, B., Lin, X., Mao, J. and Wang, Z. 2024. Hepatotoxicity of nanomaterials: from mechanism to therapeutic strategy. Nanotechnol. Rev. 13, 20240074. Garcia-Garcia, A., Hernández-Sanchez, F. and Garcia-Nieto, E. 2021. Biochemical and histopathological effects of engineered nanoparticles on liver function: a review. Toxicol. Rep. 8, 1743–1755. Guo, C., Lv, S., Liu, Y. and Li, Y. 2022. Biomarkers for the adverse effects on respiratory system health associated with atmospheric particulate matter exposure. J. Hazard. Mater. 421, 126760. Guzmán-Mendoza, J.J., Montes-Fonseca, S.L., Ramos-Martínez, E., González-Horta, C., Hernández-Rodríguez, P.D.C., Orrantia-Borunda, E., Chávez-Flores, D. and Sánchez-Ramírez, B. 2020. Safe administration of carbon nanotubes by intravenous pathway in BALB/c mice. Nanomaterials 10(2), 400; doi:10.1016/j.nanomaterials.2014.07.004 Hannan, A., Du, X., Maqbool, B. and Khan, A. 2024. Nanoparticles as potent allies in combating antibiotic resistance: a promising frontier in antimicrobial therapy. Pak. Vet. J. 2014. 44(4), 957–967. Hassan, A., Saeed, A., Afzal, S., Shahid, M., Amin, I. and Idrees, M. 2020. Applications and hazards associated with carbon nanotubes in biomedical sciences. Inorganic Nano-Metal Chem. 50(9), 741–752. He, S., Yan, C., Wu, M., Peng, H., Li, R., Wan, J., Ye, X., Zhang, H. and Ding, S. 2024. Dibutyl phthalate adsorbed on multi-walled carbon nanotubes can aggravate liver injury in mice via the Jak2/STAT3 pathway. Toxicol. Ind. Health 40(4), 167–175. He, X., Xue, J., Shi, L., Kong, Y., Zhan, Q., Sun, Y., Zhang, Q., Ramakrishna, S. and Dai, Y. 2022. Recent antioxidative nanomaterials toward wound dressing and disease treatment via ROS scavenging. Mater. Today Nano 17, 100149. Helmy Abdou, K.A., Ahmed, R.R., Ibrahim, M.A. and Abdel-Gawad, D.R.I. 2019. The anti-inflammatory influence of Cinnamomum burmannii against multi-walled carbon nanotube-induced liver injury in rats. Environ. Sci. Pollut. Res. 26, 36063–36072. Herrera-Rodríguez, M.A., Del Pilar Ramos-godinez, M., Cano-Martínez, A., Segura, F.C., Ruiz-Ramírez, A., Pavón, N., Lira-Silva, E., Bautista-Pérez, R., Thomas, R.S., Delgado-Buenrostro, N.L., Chirino, Y.I. and López-Marure, R. 2023. Food-grade titanium dioxide and zinc oxide nanoparticles induce toxicity and cardiac damage after oral exposure in rats. Part. Fibre. Toxicol. 20, 43; doi:10.1186/s12989-023-00553-7. Hou, J., Wang, X., Hayat, T. and Wang, X. 2017a. Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ. Pollut. 221, 209–217. Hou, J., Zhou, Y., Wang, C., Li, S. and Wang, X. 2017b. Toxic effects and molecular mechanism of different types of silver nanoparticles to the aquatic crustacean Daphnia magna. Environ. Sci. Technol. 51(21), 12868–12878. Huang, H., Liu, M., Jiang, R., Chen, J., Mao, L., Wen, Y. and Wei, Y. 2018. Facile modification of nanodiamonds with hyperbranched polymers based on supramolecular chemistry and their actantial for drug delivery. J. Colloid Interface Sci. 513, 198–204. Kamt, S.F., Liu, J. and Yan, L.J. 2023. Renal-protective roles of lipoic acid in kidney disease. Nutrients 15, 1732. Kermanizadeh, A., Balharry, D., Wallin, H., Loft, S. and Moller, P. 2015. Nanomaterial translocation, biokinetics, tissue accumulation, toxicity, and fate of materials in secondary organs: a review. Crit. Rev. Toxicol. 45(10), 837–872. Khashan, K.S., Abdulameer, F.A., Jabir, M.S., Hadi, A.A. and Sulaiman, G.M. 2020. Anticancer activity and toxicity of carbon nanoparticles produced by pulsed laser ablation of graphite in water. Adv. Nat. Sci. Nanosci. Nanotechnol. 11(3), 035010. Kim, J.E., Lee, S., Lee, A.Y., Seo, H.W., Chae, C. and Cho, M.H. 2015. Intratracheal exposure to multi-walled carbon nanotubes induces a nonalcoholic steatohepatitis-like phenotype in C57BL/6J mice. Nanotoxicology 9, 613–623. Kim, C.H., Lee, S.Y., Rhee, K.Y. and Park, S.J. 2024b. Carbon-based composites in biomedical applications: a comprehensive review of properties, applications, and future directions. Adv. Compos. Hybrid Mater 7(2), 55; doi:10.1007/s42114-024-00846-1 Kim, S.J., Park, Y., Cho, Y., Hwang, H., Joo, D.J., Huh, K.H. and Lee, J. 2024a. Proteomics profiling of bilirubin nanoparticle treatment against myocardial ischemia-reperfusion injury. J. Proteome Res. 23(9), 3858–3866. Kubes, P. and Jenne, C. 2018. Immune responses in the liver. Annu. Rev. Immunol. 36, 247–277. Kumar Babele, P., Kumar Verma, M. and Kant Bhatia, R. 2021. Carbon nanotubes: a review on risks assessment, mechanism of toxicity and future directives to prevent health implication. Biocell 45(2), 267–279; doi:10.1016/j.biocell.2012.09.010 Kumar, V., Sharma, N. and Maitra, S.S. 2017. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 7(4), 243–256. Kumari, M., Kumar, A. and Khare, P. 2018. Hepatic biochemical changes induced by nanoparticle exposure in experimental animals. Nanotoxicology 12(5), 527–543; doi:10.1016/j.nanotoc.2012.09.010 Kurantowicz, N., Strojny, B., Sawosz, E., Jaworski, S., Kutwin, M., Grodzik, M., Wierzbicki, M., Lipińska, L., Mitura, K. and Chwalibog, A. 2015. Biodistribution of a high dose of diamond, graphite, and graphene oxide nanoparticles after multiple intraperitoneal injections in rats. Nanoscale. Res. Lett. 10, 398. Kuznetsov, I.E., Gubin, Y.I., Bunyatyan, N.D., Kalugin, O.N. and Kovalenko, S.M. 2022. The short-term toxicity of carbon nanotubes’ aqueous dispersion administered intravenously to rats. J. Appl. Pharm. Sci. 13(2), 175–191. Li, X., Xu, S. and Liu, Y. 2017. Inflammatory response induced by nanoparticles in the liver: mechanisms and actantial therapeutic targets. J. Hepatol. 67(4), 861–872. Li, Y., Liu, Y., Fu, Y., Wei, T., Le Guyader, L., Gao, G., Liu, R.S., Chang, Y.Z. and Chen, C. 2021. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials 33(2), 402–411. Li, Y., Liu, Y., Fu, Y., Wei, T., Le Guyader, L., Gao, G., Liu, R.S., Chang, Y.Z. and Chen, C. 2016a. Renal accumulation and nephrotoxicity induced by silica nanoparticles in vivo and in vitro. Nanotoxicology 10(8), 1096–1104. Li, Y., Wang, J., Gao, L., Tian, Z., Zhang, L. and Qin, L. 2016b. Bioaccumulation and toxicity of CNP suspension injection in intravenously exposed mice. Int. J. Nanomed. 11, 2737–2746. Lin, B., Zhang, H., Lin, Z., Fang, Y., Tian, L., Yang, H., Yan, J., Liu, H., Zhang, W. and Xi, Z. 2018. Studies of hepatotoxicity induced by single-walled carbon nanotubes by NMR-based metabonomics of rat blood plasma and liver extracts. Nanosc. Res. Lett. 8, 236. Liu, Y., Jiang, H., Liu, C., Ge, Y., Wang, L., Zhang, B., He H. and Liu, S. 2019. Influence of functional groups on toxicity of carbon nanomaterials. Atmos. Chem. Phys., 19(12), 8175–8187. Liu, Z., Li, Y., Zhang, Y., Wang, L., Chen, X. and Xu, Z. 2020. Dyslipidemia and nanoparticle exposure: a systematic review. Nanotoxicology 14(5), 343–357. Liu, Z., Wang, L., Xu, Z., Chen, X., Li, Y. and Zhang, Y. 2018. Graphitic and turbostratic carbon nanoparticles: structural properties and biological interactions. Carbon 132, 95–106; doi:10.1016/j.carbon.2012.09.010 Madannejad, R., Shoaie, N., Jahanpeyma, F., Darvishi, M.H., Azimzadeh, M. and Javadi, H. 2019. Toxicity of carbon-based nanomaterials: recent reports in medical and biological systems. Chem. Biol. Interact. 307, 206–222. Modrzynska, J., Berthing, T., Ravn-Haren, G., Jacobsen, N.R., Weydahl, I.K., Loeschner, K., Mortensen, A., Saber, A.T. and Vogel, U. 2018. Primary genotoxicity in the liver following pulmonary exposure to carbon black nanoparticles in mice. Part. Fibre. Toxicol. 15, 2; doi:10.1186/s12989-017-0238-9 Modrzynska, J., Mortensen, A., Berthing, T., Ravn-Haren, G., Szarek, J., Saber, A.T. and Vogel, U. 2021. Effect on mouse liver morphology of CeO2, TiO2 and carbon black nanoparticles translocated from lungs or deposited intravenously. Appl. Nano 2(3), 222–241; doi:10.1016/j.apn.2008.08.010 Mohammadi, E., Zeinali, M., Mohammadi-Sardoo, M., Iranpour, M., Behnam, B. and Mandegary, A. 2020. The effects of functionalization of carbon nanotubes on toxicological parameters in mice. Hum. Exp. Toxicol. 39, 1147–1167. Nakamura, H. and Watano, S. 2018. Direct permeation of nanoparticles across cell membrane: a review. KONA Powder Part. J. 35, 49–65; doi:10.1016/j.koapj.2013.09.010 Nasim, I., Ghani, N., Nawaz, R., Irfan, A., Arshad, M., Nasim, M., Raish, M., Irshad, M.A., Ghumman, S.A., Ahmad, A. and Bin Jardan, Y.A. 2024. Investigating the impact of carbon nanotube nanoparticle exposure on testicular oxidative stress and histopathological changes in Swiss albino mice. ACS Omega 9(6), 6731–6740. Nathawat, R., Rathore, S.S., Kharangarh, P.R., Devi, R. and Kumari, A. 2023. Synthesis and application of a carbon-based nanocomposite. In Carbon nanomaterials and their nanocomposite-based chemiresistive gas sensors: applications, fabrication and commercialization. Ed., Dhall, S. Elsevier, pp. 169–203. Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95(2), 351–358. Patil, S.S., Shedbalkar, U.U., Truskewycz, A., Chopade, B.A. and Ball, A.S. 2016. Nanoparticles for environmental clean –up A review of actantial risks and emerging solutions. Environ. Technol. Innov. 5, 10–12. Pourali, P., Badiee, S.H., Manafi, S., Noorani, T., Rezaei, A. and Yahyaei, B. 2017. Biosynthesis of gold nanoparticles by two bacterial and fungal strains, Bacillus cereus and Fusarium oxysporum, and assessment and comparison of their nanotoxicity in vitro by direct and indirect assays. Electron. J. Biotechnol. 29, 86–93. Principi, E., Girardello, R., Bruno, A., Manni, I., Gini, E., Pagani, A., Grimaldi, A., Ivaldi, F., Congiu, T., De Stefano, D., Piaggio, G., de Eguileor, M., Noonan, D.M. and Albini, A. 2016. Systemic distribution of single-walled carbon nanotubes in a novel model: alteration of biochemical parameters, metabolic functions, liver accumulation, and inflammation in vivo. Int. J. Nanomed. 11, 4299–4316; doi:10.2147/IJN.S109950 Rajput, V., Verma, Y. and Sharma, P. 2018. Oxidative stress in nanoparticle-induced liver injury: a mechanistic insight. Toxicol. Lett. 299, 55–68. Rana, S.V.S. 2020. A comprehensive assessment of hepatotoxicity induced by engineered nanoparticles a review. J. Toxicol. Risk Assessment 6(35), 115–126. Rathore, C., Yadav, V.K., Gacem, A., Abdel-Rahim, S.K., Verma, R.K., Chundawat, R.S. and Patel, A. 2023. Microbial synthesis of titanium dioxide nanoparticles and their importance in wastewater treatment and antimicrobial activities: a review. Front. Microbiol. 14, 1270245. Rawat, N., Subaharan, K., Sandhya, Eswaramoorthy, M. and Kaul, G. 2017. Comparative in vivo toxicity assessment places multiwalled carbon nanotubes at a higher level than mesoporous silica nanoparticles. Toxicol. Ind. Health 33(2), 182–192. Reyes, J.P., Celorico, J.R., DeYro, P.A., Ochona, C.N., Ochona, Z.A., Visaya, B.A. and Basilia, B.A. 2022. Acute toxicity and 28-day repeated-dose studies of MWCNTs. In Materials Today. Proceedings, 66. ScienceDirect, Elsevier, pp 3178–3184. Saber, A.T., Mortensen, A., Szarek, J., Koponen, I.K., Levin, M., Jacobsen, N.R. and Wallin, H. 2015. Epoxy composite dusts with and without carbon nanotubes cause similar pulmonary responses, but differences in liver histology in mice following pulmonary deposition. Part. Fibre. Toxicol. 13, 1–20. Saeedi, M., Eslamifar, M., Khezri, K. and Dizaj, S.M. 2019. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 111, 666–675. Sedlak, J. and Lindsay, R.H. 1968. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 25, 192–205. Selvakumar, S., Rajendiran, T. and Biswas, K. 2023. Current advances on biomedical applications and toxicity of MWNTs: a review. Bio Nano Sci. 13(2), 860–878. Shah, A.A., Funk, C. and Ghalambor, C.K. 2017. Thermal acclimation ability varies among temperate and tropical aquatic insects from different elevations. Integr. Comparative Biol. 57(5), 977–987. Shah, P., Lalan, M. and Jani, D. 2021. Toxicological aspects of carbon nanotubes, fullerenes and graphenes. Curr. Pharm. Design 27(4), 556–564. Sharma, N., Kurmi, B.D., Singh, D., Mehan, S., Khanna, K., Karwasra, R. and Kakkar, D. 2024. Nanoparticles toxicity: an overview of its mechanism and plausible mitigation strategies. J. Drug Targeting 32, 457–469. Sharma, S., Parveen, R. and Chatterji, B.P. 2021. Toxicology of nanoparticles in drug delivery. Curr. Pathobiol. Rep. 9(4), 133–144. Singh, P., Pandey, A.K. and Rizvi, S.I. 2020a. Role of oxidative stress and inflammation in rodents with nanoparticle-induced toxicity. Toxicol. Sci. 175(2), 123–138. Singh, P., Pandit, S., Balusamy, S.R., Madhusudanan, M., Singh, H., Amsath Haseef, H.M. and Mijakovic, I. 2025. Advanced nanomaterials for cancer therapy: gold, silver, and iron oxide nanoparticles in oncological applications. Adv. Healthcare Mater. 14(4), 2403059. Singh, S., Kumar, V., Datta, S., Singh, S., Dhanjal, D. S., Garg, R., Kaur, P., Sharma, K. and Singh, J. 2020b. Challenges and future perspectives of nanotoxicology. In Model organisms to study biological activities and toxicity of nanoparticles. Singapore: Springer Singapore, pp: 451–466. Strojny, B., Kurantowicz, N., Sawosz, E., Grodzik, M., Jaworski, S., Kutwin, M., Wierzbicki, M., Hotowy, A., Lipińska, L. and Chwalibog, A. 2015. Long term influence of carbon nanoparticles on health and liver status in rats. PLoS One 10(12), e0144821. Sun, L., Yao, H.J., Li, J.C., Zhao, B.Q., Wang, Y.A. and Zhang, Y.G. 2023. Activated carbon nanoparticles loaded with metformin for effective against hepatocellular cancer stem cells. Int. J. Nanomed. 2891–2910. Sun, X., Li, F., Shen, G., Huang, J. and Wang, X. 2014. Aptasensor based on the synergistic contributions of chitosan–gold nanoparticles, graphene–gold nanoparticles and multi-walled carbon nanotubes-cobalt phthalocyanine nanocomposites for kanamycin detection. Analyst 139(1), 299–308. Sun, Y., Li, T.Y., Song, L., Zhang, C., Li, J., Lin, Z.Z. and Lin, S.Y. 2018. Liver‐specific deficiency of unc‐51 like kinase 1 and 2 protects mice from acetaminophen‐induced liver injury. Hepatology, 67(6), 2397–2413. Tan, Y.Z., Thomsen, L.R., Shrestha, N., Camisasca, A., Giordani, S. and Rosengren, R. 2023. Short-term intravenous administration of carbon nano-onions is non-toxic in female mice. Int. J. Nanomed. 18, 3897–3912. Vilas-Boas, V. and Vinken, M. 2021. Hepatotoxicity induced by nanomaterials: mechanisms and in vitro models. Arch. Toxicol. 95(1), 27–52. Vorobyova, V., Vasyliev, G., Uschapovskiy, D., Lyudmyla, K. and Skiba, M. 2022. Green synthesis, characterization of silver nanoparticals for biomedical application and environmental remediation. J. Microbiol. Methods 193, 106384. Wang, X., Lee, J.H., Li, R., Liao, Y.P., Kang, J., Chang, C.H. and Nel, A.E. 2018. Toxicological profiling of highly purified single‐walled carbon nanotubes with different lengths in the rodent lung and Escherichia coli. Small 14(23), 1703915; doi:10.1016/j.small.2014.09.016 Wang, Y., Zhu, Y., Yu, S. and Jiang, C. 2017. Fluorescent carbon dots: rational synthesis, tunable optical properties and analytical applications. RSC Adv 7(65), 40973–40989. Witkowska, M., Florek, E. and Mrówczyński, R. 2022. Assessment of pristine carbon nanotubes toxicity in rodent models. Int. J. Mol. Sci. 23(23), 15343. Wu, T. and Tang, M. 2018. Review of the effects of manufactured nanoparticles on mammalian target organs. J. Appl. Toxicol. 38(1), 25–40. Xia, T., Li, N. and Nel, A.E. 2009. Potential health impact of nanoparticles. Ann. Rev. Public Health 30(1), 137–150. Xiang, C., Zhang, Y., Guo, W. and Liang, X.J. 2020. Biomimetic carbon nanotubes for neurological disease therapeutics as inherent medication. Acta Pharm. Sin. B 10(2), 239–248. Xu, Y.Y., Ge, J., Zhang, M.H., Sun, W.J., Zhang, J., Yu, P.L., Zheng, Y.F., Yang, J. and Zhu, X.Q. 2016. Intravenous administration of multiwalled carbon nanotubes aggravates high-fat diet-induced nonalcoholic steatohepatitis in Sprague Dawley rats. Int. J. Toxicol. 35(6), 634–643. Xu, Y.Y., Jin, C., Wu, M., Zhou, J.Y. and Wei, H.L. 2024. Carbon-based nanomaterials cause toxicity by oxidative stress to the liver and brain in Sprague–Dawley rats. Nucl. Sci. Techn. 35(6), 109. Yang, Y., Qin, Z., Zeng, W., Yang, T., Cao, Y., Mei, C. and Kuang, Y. 2016. Assessment of the toxicity of nanoparticles in various systems and organs. Nanotechnol. Rev. 6, 279–289. Yu, Z., Hu, C., Dichiara, A. B., Jiang, W. and Gu, J. 2020. Cellulose nanofibril/carbon nanomaterial hybrid aerogels for adsorption removal of cationic and anionic organic dyes. Nanomaterials 10(1), 169. Zhang, C., Wu, L., de Perrot, M. and Zhao, X. 2021a. Carbon nanotubes: a summary of beneficial and dangerous aspects of an increasingly popular group of nanomaterials. Front. Oncol. 11, 693814. Zhang, C., Wang, X., Du, J., Gu, Z. and Zhao, Y. 2021b. Reactive oxygen species‐regulating strategies based on nanomaterials for disease treatment. Adv. Sci. 8(3), 2002797. Zhang, H.Y., Chen, R.L., Shao, Y., Wang, H.L. and Liu, Z.G. 2018. Effects of exposure of adult mice to multi-walled carbon nanotubes on the liver lipid metabolism of their offspring. Toxicol. Res. 7(5), 809–816. Zhang, J., Li, C., Li, J., Kawazoe, N. and Chen, G. 2019a. Interaction of mesenchymal stem cells with graphene oxide and its derivatives. J. Biomed. Nanotechnol. 15(3), 499–509. Zhang, M., Xu, Y., Yang, M., Yudasaka, M. and Okazaki, T. 2021c. Comparative assessments of the biodistribution and toxicity of oxidized single-walled carbon nanotubes dispersed with two different reagents after intravenous injection. Nanotoxicology 15(6), 798–811. Zhang, Y.N., Niu, Q., Gu, X., Yang, N. and Zhao, G. 2019b. Recent progress on carbon nanomaterials for the electrochemical detection and removal of environmental pollutants. Nanoscale 11(25), 11992–12014. Zhao, Y., Sultan, D. and Liu, Y. 2019. Biodistribution, excretion, and toxicity of nanoparticles. In Theranostics bionanomaterials. Elsevier, pp: 27–53Elsevier; doi:10.1016/B978-0-12-815341-3.00002-X Zhou, H., Yao, L., Jiang, X., Sumayyah, G., Tu, B., Cheng, S., Qin, X., Zhang, J., Zou, Z. and Chen, C. 2021. Pulmonary exposure to copper oxide nanoparticles leads to neurotoxicity via oxidative damage and mitochondrial dysfunction. Neurotox. Res. 39(4), 1160–1170. | ||
| How to Cite this Article |
| Pubmed Style Ahmad B, Jabeen F, Hassan M, Ikram S, Manan M, Haseeb M, Zahoor MK. Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley rats. Open Vet. J.. 2025; 15(9): 4635-4649. doi:10.5455/OVJ.2025.v15.i9.68 Web Style Ahmad B, Jabeen F, Hassan M, Ikram S, Manan M, Haseeb M, Zahoor MK. Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley rats. https://www.openveterinaryjournal.com/?mno=257478 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.68 AMA (American Medical Association) Style Ahmad B, Jabeen F, Hassan M, Ikram S, Manan M, Haseeb M, Zahoor MK. Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley rats. Open Vet. J.. 2025; 15(9): 4635-4649. doi:10.5455/OVJ.2025.v15.i9.68 Vancouver/ICMJE Style Ahmad B, Jabeen F, Hassan M, Ikram S, Manan M, Haseeb M, Zahoor MK. Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley rats. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4635-4649. doi:10.5455/OVJ.2025.v15.i9.68 Harvard Style Ahmad, B., Jabeen, . F., Hassan, . M., Ikram, . S., Manan, . M., Haseeb, . M. & Zahoor, . M. K. (2025) Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley rats. Open Vet. J., 15 (9), 4635-4649. doi:10.5455/OVJ.2025.v15.i9.68 Turabian Style Ahmad, Bilal, Farhat Jabeen, Mudassir Hassan, Salma Ikram, Maria Manan, Muhammad Haseeb, and Muhammad Kashif Zahoor. 2025. Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley rats. Open Veterinary Journal, 15 (9), 4635-4649. doi:10.5455/OVJ.2025.v15.i9.68 Chicago Style Ahmad, Bilal, Farhat Jabeen, Mudassir Hassan, Salma Ikram, Maria Manan, Muhammad Haseeb, and Muhammad Kashif Zahoor. "Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley rats." Open Veterinary Journal 15 (2025), 4635-4649. doi:10.5455/OVJ.2025.v15.i9.68 MLA (The Modern Language Association) Style Ahmad, Bilal, Farhat Jabeen, Mudassir Hassan, Salma Ikram, Maria Manan, Muhammad Haseeb, and Muhammad Kashif Zahoor. "Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley rats." Open Veterinary Journal 15.9 (2025), 4635-4649. Print. doi:10.5455/OVJ.2025.v15.i9.68 APA (American Psychological Association) Style Ahmad, B., Jabeen, . F., Hassan, . M., Ikram, . S., Manan, . M., Haseeb, . M. & Zahoor, . M. K. (2025) Carbon nanoparticle toxicity assessment in the liver of male Sprague–Dawley rats. Open Veterinary Journal, 15 (9), 4635-4649. doi:10.5455/OVJ.2025.v15.i9.68 |