| Research Article | ||
Open Vet. J.. 2025; 15(9): 4726-4734 Open Veterinary Journal, (2025), Vol. 15(9): 4726-4734 Research Article The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewesSarmad Talib Abdulazeez and Sarmad Abdulrazzq Abbood Alsaadi*Department of Animal Production, Faculty of Agriculture, University of Kirkuk, Kirkuk, Iraq *Corresponding Author: Sarmad Abbood Alsaadi, Department of Animal Production, Faculty of Agriculture, University of Kirkuk, Kirkuk, Iraq. Email: dr.sarmadalsaadi [at] uokirkuk.edu.iq Submitted: 22/05/2025 Revised: 27/07/2025 Accepted: 10/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
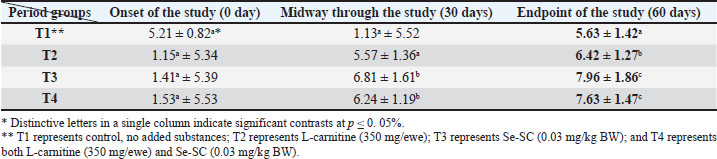
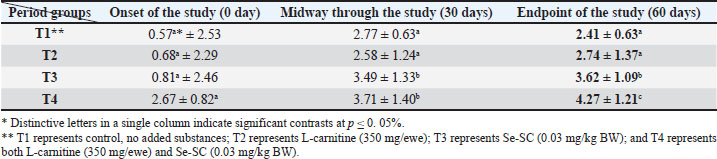
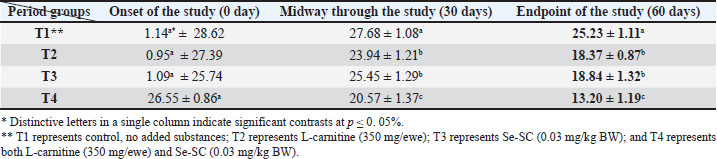
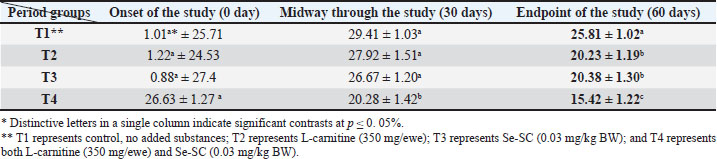
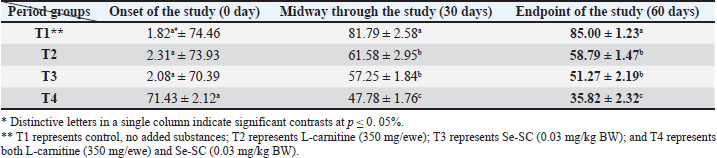
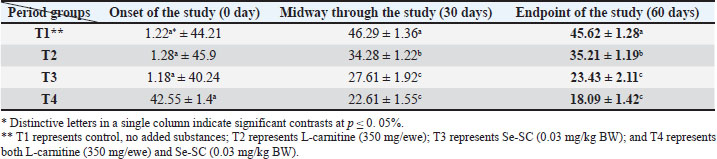
AbstractBackground: This study was conducted to investigate the biochemical impact of acetyl-L-carnitine (350 mg/ewe) administration with or without selenium-fortified Saccharomyces cerevisiae (0.03 mg/kg body weight) on 24 mature ewes for 60 days. Aim: This study aimed to investigate the biophysiological changes in some biochemical traits by adopting acetyl-L-carnitine with or without selenium-fortified S. cerevisiae and their combination. Methods: This study investigated the effect of acetyl-L-carnitine (350 mg/ewe) with or without selenium-fortified S. cerevisiae (0.03 mg/kg body weight) on 24 mature ewes for 60 days. Results: This study investigated the biochemical impact of acetyl-l-carnitine and selenium-fortified S. cerevisiae (Se-SC) on the metabolic and liver health of Awassi ewes over 60 days. A total of 24 mature ewes were randomly assigned to four treatment groups: a control group (no additives), a group receiving L-carnitine (350 mg/ewe), a group receiving selenium-fortified S. cerevisiae (0.03 mg/kg BW), and a combined treatment group (L-carnitine and Se-SC). The study focused on key biochemical parameters, including blood glucose, total protein, albumin, liver enzymes (AST and ALT), cholesterol, and triglycerides. The combined treatment (T4) significantly improved metabolic and liver health, reducing blood glucose, cholesterol, triglycerides, and liver enzymes, while enhancing total protein and albumin levels. Individual treatments also showed beneficial effects, with Se-SC enhancing protein metabolism and L-carnitine optimizing lipid and glucose levels. These findings suggest that the synergistic effects of acetyl-L-carnitine and Se-SC may offer a promising strategy for improving metabolic regulation and liver function in ruminants, particularly in livestock management practices aiming for improved health and productivity. Conclusion: Both acetyl-L-carnitine and selenium-fortified S. cerevisiae improved metabolic and liver health in ewes, with the combined treatment being the most effective. Keywords: Acetyl-L-carnitine, Saccharomyces cerevisiae, Selenium, Biochemical, Sheep. IntroductionL-carnitine is essentially synthesized within the kidneys and liver from lysine and methionine amino acids, with the assistance of fundamental cofactors such as vitamin C, press, niacin (B3), and vitamin B6 in most creatures (Al-Mafarji and Alsaadi, 2023). L-carnitine is crucial in fat oxidation and converts it to energy (Vecchio et al., 2021). The organic impact of carnitine is its involvement in the oxidation of long-chain fatty acids (beta) after being translocated over the inner membrane of mitochondria and to the intermembrane space of mitochondria, where the oxidation process takes place and vitality is produced (Pirmadah et al., 2020). Subsequently, it produces vitality as ATP, which the body uses to perform its crucial capacities (Gracious et al., 2022). Carnitine acts as an antioxidant, which reduces the harm from free radicals and improves well-being (Sawicka et al., 2020). Scientists have turned to the arrangement of nourishment complements, a combination of two or more components that have a crucial organic impact on cultivated creatures, such as selenium-improved yeast (Zhang et al., 2023). Selenium (Se) is an essential trace element with important physiological functions, essentially due to its incorporation into selenoproteins such as glutathione peroxidases (GPx), thioredoxin reductases (TrxR), and iodothyronine deiodinases (DIO) (Zhang et al., 2023). GPx proteins catalyze the diminishment of hydrogen peroxide and lipid hydroperoxides, thereby moderating oxidative harm to cellular components (Barchielli et al., 2022). TrxR maintains intracellular redox adjustment by directing thioredoxin, which is crucial for DNA repair and apoptosis (Hosnedlova et al., 2017). DIO proteins tweak the activation/deactivation of thyroid hormones, affecting metabolic rate and vitality consumption (Xu et al., 2022). Selenium-fortified Saccharomyces cerevisiae (Se-SC). This shape is productively ingested and consolidated into selenoproteins, improving the antioxidant capacity and metabolic control of ruminants (Cui et al., 2021). The synergistic combination of Se-SC and acetyl-L-carnitine may intensify these benefits, as L-carnitine promotes and bolsters mitochondrial work and fatty acid oxidation (Ringseis et al., 2012). Yeast is a single-celled, oval-shaped fungal kingdom (Li et al., 2024). Yeast has become one of the most important things used in microbiological nutrition in the field of breeding around the world, as yeast works to improve the metabolism coming from the feed, since every livestock feed formulation includes precise fiber content, which are digested through microbial digestion; hence, the use of yeasts lies in improving digestion (Ringseis et al., 2012). Then, they can produce countless numbers inside the rumen where microbial digestion occurs (Cui et al., 2021). As revealed in ruminants, Se-fortified yeast enhances selenoprotein activity and synergizes with L-carnitine to mitigate oxidative stress and improve hepatic function. The biological nutrition that relies on yeast significantly regulates the ecological balance in the intestines of animals and enhances digestion and absorption, strengthening immunity, and improving animal production (Gong et al., 2023). The hypothesis posits that combined supplementation will yield superior outcomes compared with individual treatments, leveraging the complementary roles of acetyl-L-carnitine in energy metabolism and Se-yeast in oxidative stress mitigation. Materials and MethodsStudy placeThis experiment was conducted at the creature field of the Office of Creature Production at the Faculty of Agriculture at Kirkuk University. The duration of the experiment was 60 days. Ewes are accommodated at semi-enclosed, uniformly sized sheds with dimensions of 5.5 × 3.5 m, provided with food managers that measure about 250 × 35 × 15 cm3 and a 50-l capacity for tap water to each ewe. AnimalsTwenty-four mature Awassi ewes with a normal age of years and a weight of 55 kg were used in this study. Animals were housed and fed collectively in semi-open barns. Experimental design and treatmentsEwes were separated into four experimental groups, each including six sheep of homogeneous weights. The treated groups were then haphazardly distributed among each other. The 1st control group of ewes were administered orally with tap water as it was. The 2nd group was administered with 350 mg L-carnitine/ewe. The 3rd group was administered 0.03 mg Se-fortified S. cerevisiae/kg BW. The 4th group was administered a mixture of 350 mg L-Carnitine/ewe and 0.03 mg Se-fortified S. cerevisiae/kg BW. Blood samplesEspecially at the onset, mid, and endpoint of the think about, blood samples for hematological measurements are taken from the jugular vein using a non-essential 5 ml size sterilized syringe. Each blood sample was placed in anticoagulant-devoid test tubes and centrifuged under standard ambient conditions for approximately 15 minutes. Serum samples were kept at the collection point and centrifuged for 15 minutes at a rotational speed of 3,500 cpm. At that point, the samples were divided into the smallest tests and scattered in sterile test tubes. Biochemical parameters were measured in the serum samples using standardized methods: Blood Glucose: Determined spectrophotometrically using the glucose oxidase-peroxidase (GOD-POD) method. Total Protein and Albumin: Quantified using the Biuret and BCG dye-binding methods. Liver enzymes (AST and ALT): Measured via kinetic assays based on the recommendations of the International Federation of Clinical Chemistry. Lipid Profile (Cholesterol and Triglycerides): Enzymatically analyzed using cholesterol oxidase-peroxidase (CHOD-PAP) and glycerol-3-phosphate oxidase (GPO-PAP) strategies. The following measures were executed to guarantee the expositor’s precision and unwavering quality: Calibration: All tests were calibrated using certified reference materials (CRMs) and standard bends arranged from known concentrations of analytes. Inside Controls: Commercially accessible control sera (typical and neurotic ranges) were run nearby tests to confirm the accuracy of the measurements. Connect- and Intra-Assay Changeability: The coefficients of variation (CV) were maintained below 5% for all parameters. Instrument Upkeep: Normal support and execution checks were conducted on spectrophotometers and computerized analyzers. Dazzle Reproduces: A subset of tests was analyzed in copy to confirm reproducibility. These protocols adhered to the International Council for Harmonization (ICH) guidelines and were performed in an accredited laboratory setting. Statistical testData were analyzed using a one-way completely randomized design (CRD). A one-way analysis of variance was conducted to determine the significance of differentiation between groups, followed by Duncan’s multiple range test for post hoc analysis (Duncan, 1955). Normality assumptions were tested according to the Shapiro–Wilk test, and the homogeneity of variances was evaluated using Levene’s test. A Bonferroni correction was applied to adjust for multiple comparisons to control for type I errors. A p-value ≤ 0.05 was considered statistically significant (SAS, 2012). Ethical approvalNone of the experimental animals were harmed or slaughtered and all the standard breeding supplies and Means were provided to ensure the welfare of the animal throughout the study period. ResultsBlood glucoseAt the initiation of the study, there were no significant differences between the groups (p > 0.05). At the end of the study, T4 (combined treatment) showed a significant reduction in blood glucose (46.08 ± 1.26 mg/dl, p=0.032) compared with that in the control group (T1), and both T2 (L-Carnitine) and T3 (Se-SC) showed significant reductions (p ≤ 0.05) compared with that in T1. At the end of the study, T4 exhibited the most pronounced reduction (46.08 ± 1.26 mg/dl, p=0.032), followed by T2 (60.37 ± 1.16 mg/dl, p=0.001) and T3 (55.92 ± 1.37 mg/dl, p=0.004) compared to the control group (76.61 ± 1.02 mg/dl). Total proteinNo considerable differences were noted between the groups (p > 0.05) at the initiation of the study. At the end of the study, T4 (7.63 ± 1.47 g/dl, p=0.005) showed the highest increase in total protein, which was significantly higher (p ≤ 0.05) than that in T1 (5.63 ± 1.42 g/dl), with no significant change observed in T2. At the end of the study, T4 (7.63 ± 1.47 g/dl, p=0.005) exhibited a significant increase (p ≤ 0.05) compared with T1 (5.63 ± 1.42 g/dl) and T2 (6.42 ± 1.27 g/dl, p=0.02). Serum albuminNo significant differences were found between the groups (p > 0.05) at the initiation of the study. At the end of the study, T3 (Se-SC) displayed a significant increase (p ≤ 0.05) in albumin (3.49 ± 1.33 g/dl, p=0.03) compared with T1, and T4 exhibited a higher increase (p ≤ 0.05) (3.71 ± 1.40 g/dl, p=0.02). At the end of the study, T4 (4.27 ± 1.21 g/dl, p=0.002) showed the greatest increase (p ≤ 0.05) in albumin, followed by T3 (3.62 ± 1.09 g/dl, p=0.02) and T2 (2.74 ± 1.37 g/dl, p=0.03) compared to T1 (2.41 ± 0.63 g/dl). Aspartate aminotransferaseNo significant differences were found between the groups (p > 0.05) at the initiation of the study. At the end of the study, T4 (13.20 ± 1.19 U/l, p=0.001) showed the greatest reduction in AST levels compared to T1 (25.23 ± 1.11 U/l), with T2 (18.37 ± 0.87 U/l, p=0.004) and T3 (18.84 ± 1.32 U/l, p=0.003) also showing significant reductions compared to T1. At the end of the study, T4 exhibited the most pronounced reduction in AST (13.20 ± 1.19 U/l, p=0.001), followed by T2 (18.37 ± 0.87 U/l, p=0.004) and T3 (18.84 ± 1.32 U/l, p=0.003) compared to T1 (25.23 ± 1.11 U/l). Alanine aminotransferaseNo significant differences were found at the initiation of the study (p > 0.05). At the midpoint of the study, T2 (20.23 ± 1.19 U/l, p=0.01) significantly reduced ALT levels compared with T1 (25.81 ± 1.02 U/l). At the end of the study, T4 (15.42 ± 1.22 U/l, p=0.002) exhibited the most significant reduction in ALT, followed by T2 (20.23 ± 1.19 U/l, p=0.01) and T3 (20.38 ± 1.30 U/l, p=0.02) compared with T1 (25.81 ± 1.02 U/l). CholesterolNo significant differences were found between the groups (p > 0.05) at the initiation of the study. At the end of the study, T4 (35.82 ± 2.32 mg/dl, p=0.001) showed the greatest reduction in cholesterol compared with T1 (85.00 ± 1.23 mg/dl), and both T2 (58.79 ± 1.47 mg/dl, p=0.004) and T3 (51.27 ± 2.19 mg/dl, p=0.003) showed significant reductions. At the end of the study, T4 (35.82 ± 2.32 mg/dl, p=0.001) exhibited the most pronounced reduction, followed by T2 (58.79 ± 1.47 mg/dl, p=0.004) and T3 (51.27 ± 2.19 mg/dl, p=0.003) compared to T1 (85.00 ± 1.23 mg/dl). TriglyceridesNo significant differences were observed at the onset of the study (p > 0.05). At the end of the study, T4 (18.09 ± 1.42 mg/dl, p=0.001) showed the most significant reduction in TGs compared with T1 (45.62 ± 1.28 mg/dl), with T2 (35.21 ± 1.19 mg/dl, p=0.002) and T3 (23.43 ± 2.11 mg/dl, p=0.003) also exhibiting significant reductions. At the end of the study, T4 (18.09 ± 1.42 mg/dl, p=0.001) exhibited the greatest reduction in TGs, followed by T3 (23.43 ± 2.11 mg/dl, p=0.003) and T2 (35.21 ± 1.19 mg/dl, p=0.002) compared with T1 (45.62 ± 1.28 mg/dl). DiscussionThe results of the study in Table 1 agree with the results of a study by Helal et al. (2018), who pointed out that L-carnitine plays a significant role in regulating glucose levels through various physiological and biochemical mechanisms, which explains the reduction in blood glucose levels in T2 and T4 Carnitine-treated groups. It primarily facilitates the transport of long-chain fatty acids to the mitochondria, which sustains oxidation to produce energy (Radhi et al., 2024). This vital process helps shift the body’s metabolism from glucose utilization to fat oxidation, which can lower blood glucose levels (Martín et al., 2022). One of the key components is the direction of quality expression related to the LD system. L-carnitine supplementation upregulates mitochondrial fatty acid beta-oxidation, whereas it downregulates cholesterol acids and triglyceride amalgamation in muscle and liver tissues (Seemann et al., 2024). This move not only improves fat utilization but also diminishes lipid aggregation, contributing to better glucose control. Table 1. L-carnitine impact with/without Se-SC in blood glucose (mg/dl) (Mean ± SE).
Additionally, L-carnitine has antioxidant properties that protect cells from oxidative stress, which is often linked to insulin resistance and elevated blood glucose levels (Ribas et al., 2014). L-carnitine may improve resistance to infection and help manage blood glucose levels by supporting mitochondrial capacities and diminishing oxidative harm (Luo et al., 2022). The findings concurred with Mousaie (2021), who expressed that Se-enriched S. cerevisiae, through and through, reduced blood glucose levels in Awassi ewes, particularly when combined with acetyl-L-carnitine. The diminished blood glucose can be attributed to the antioxidant properties of Se, its potential to form insulin sensitivity, and its agreeable vitality with L-carnitine in updating the lipids destructive absorption framework (Gao et al., 2024). These findings suggest that Se-enriched yeast is a valuable supplement for improving ruminant glucose absorption, especially when combined with other metabolic enhancers, such as L-carnitine. Based on the data in Table 2, the advancement in blood protein levels, which is consistent with the comes about of MartÃn et al. (2022), may be due to the capacity of L-carnitine to improve protein amalgamation and diminish protein degradation, particularly in skeletal muscle, which might lead to muscle development and by and large protein levels within the blood (Montesano et al., 2015), while decreasing plasma smelling salt levels, demonstrating progressed nitrogen utilization and protein digestion system (Salihi and Alsaadi, 2025). In addition to the increment within the serum protein of ewes that ate Se-fortified yeast, Hosnedlova et al. (2017) demonstrated that Se-fortified yeast may help secure proteins from oxidative harm, possibly affecting overall protein levels. Se supplementation may improve supplement retention and utilization, including basic amino acids, which are the construction blocks of proteins (Huang et al., 2023). Saccharomyces cerevisiae can impact rumen maturation by tweaking microbial action. This can indirectly impact protein synthesis and degradation within the rumen (Odunfa et al., 2024). Additionally, the probiotic properties of S. cerevisiae can improve gut health and nutrient absorption, further supporting protein synthesis (Anjum et al., 2018). As for the results of Table 3, represented by a significant increase in blood albumin values for carnitine treatment, these results agreed with the conclusions of Zhang et al. (2025) by comparing the single effect of L-carnitine or Se-fortified yeast or by combining them is not much variant, both of them have the potential to positively influence blood albumin levels in ruminants through their effects on energy metabolism, antioxidant function, and protein synthesis (Keller et al., 2011; Gunun et al., 2022). The concurrent organization of these supplements may have a synergistic impact, upgrading albumin combination, and contributing to the overall metabolic well-being of ruminant species (Lee et al., 2014). Regarding the impact of L-carnitine on hepatic chemical movement, observational investigations suggest that L-carnitine may have an advantageous impact on the decrease in ALT and AST levels, as shown in Tables 4 and 5. These results are consistent with those of Shaalan et al. (2015). Türker et al. (2011) found that the administration of both L-carnitine and Se-enriched yeast alone or combined led to a reduction in the level of ALT and AST activity and improved the functional state of the liver. The defensive effects of L-carnitine are likely attributable to its capacity to upgrade mitochondrial function and promote lipid digestion (Alsaadi et al., 2023a). L-carnitine has been shown to improve mitochondrial function and enhance fatty acid metabolism, thereby significantly reducing fat accumulation within the liver, a condition known as hepatic steatosis (Abu-El-Zahab et al., 2019). This collection is regularly connected with expanded levels of hepatic chemicals. In addition, it constricts oxidative stress and irritation, which are basic contributors to the pathogenesis of hepatocellular damage and the ensuing elevation of AST and ALT levels (Safar and Alsaadi, 2025). Conversely, a Se-enriched yeast has been shown to impact hepatic chemical action by increasing the usefulness of antioxidant proteins, thus protecting hepatocytes from oxidative damage (Amir Shareef et al., 2022; Xu et al., 2022). This study may have resulted in diminished concentrations of liver chemicals within the circulation system, subsequently indicating that liver function was upgraded through the help of metabolic forms that relieve fat aggregation and improve overall hepatic function (Alsaadi et al., 2025). Se-enriched S. cerevisiae regularly illustrates a synergistic impact when combined with other cancer prevention agents, such as L-carnitine, in this manner contributing to upgraded hepatoprotection and a diminishment in chemical levels (Alhasaniah, 2023). The current study showed a significant decrease in both cholesterol and triglyceride levels in all treated groups, whether it was carried out either with L-carnitine alone, with Se-enriched S. cerevisiae yeast, or both, as shown in Tables 6 and 7. The results of our study are consistent with the findings of Liu et al. (2019), who suggested that L-carnitine supplementation may be beneficial in reducing the levels of cholesterol and triglycerides, particularly among individuals with high cholesterol levels or metabolic disorders (Pooyandjoo et al., 2016). The current consideration is anticipated to result from the compound’s capacity to inhibit fatty corrosive absorption, leading to a decrease in lipid aggregation. In addition, explore outlines that L-carnitine has the potential to advance the levels of high-density lipoprotein (HDL) cholesterol, which is usually known as the “good” cholesterol (Qana et al., 2023). L-carnitine can potentially diminish triglyceride levels, especially in people diagnosed with hypertriglyceridemia or metabolic disorders (Xu et al., 2017). This is likely inferable to its work in enabling greasy acids, which, along these lines, diminishes the accessibility of free fatty acids for TG union (Alsaadi et al., 2023b). L-carnitine has the potential to combat corpulence, which may lead to a reduction in TG and cholesterol levels (Carlson et al., 2012). This effect is likely mediated through lipolysis inhibition and the subsequent decrease in free fatty acid discharge. The antioxidant properties of this compound may contribute to the control of oxidative stress, a condition associated with dyslipidaemia (Barchielli et al., 2022). The results of the study bunches that those treated with Se-enriched S. cerevisiae yeast also came in symmetric with the conclusions of a study by Aremmt et al. (2019), who observed that the inclusion of Se-enriched S. cerevisiae in the apportion recorded a noteworthy variation in the levels of cholesterol and triglycerides. In addition, it was consistent with Faccenda et al. (2020), who ascertained a significant reduction in cholesterol and triglyceride levels compared with a control group by administering 15 g-SC/day to the cows fed diets. The decreased lipid level may be because Se-SC plays an important role as an antioxidant, reduces free radicals, and thus can reduce harmful fats (triglycerides, LDL, and VLDL). Se is recognized for its antioxidant properties and association with completely different metabolic processes. Moreover, S. cerevisiae, a yeast species, has been suggested to influence intestinal prosperity and lipid absorption (Amir Shareef et al., 2022). The synergistic impacts of these two components on blood cholesterol and triglyceride levels have been inspected (Omar and Alsaadi, 2022). Se-enriched yeast can lead to a decrease in cholesterol concentrations as well as low-density lipoprotein cholesterol, as illustrated in a few studies (Ponce de León et al., 2002). The antioxidant properties of Se have the potential to moderate oxidative stress, which is related to the oxidation of low-density lipoprotein (LDL) cholesterol, a noteworthy contributor to the pathogenesis of atherosclerosis (Alsaadi et al., 2023c). In addition, the yeast component has the potential to overhaul the digestive tract microbiota, which may, in turn, affect the cholesterol absorption framework (Wang et al., 2010). Se-enriched yeast has been shown to reduce TG concentrations. Se plays a crucial role in the control of the lipid assimilation framework, and insufficiency in this component has been associated with dyslipidemia (Yoshinaga et al., 2018). This supplement can potentially extend the value of chemicals included within the lipid absorption framework, particularly glutathione peroxidase, by making strides in Se status. This protein plays an important role in oxidative stress and improving health state (Alsaadi and Abass, 2020). Table 2. L-carnitine impact with/without Se-SC protein (g/dl) (Mean ± SE).
Table 3. L-carnitine impact in albumin with/without Se-SC (g/dl) (Mean ± SE).
Table 4. L-carnitine impact in AST with/without Se-SC (IU/l) (Mean ± SE).
Table 5. L-carnitine impact in ALT with/without Se-SC (IU/l) (Mean ± SE).
Table 6. L-carnitine impact with/without Se-SC in serum cholesterol (mg/dl) (Mean ± SE).
Table 7. L-carnitine impact with/without Se-SC in serum triglycerides (mg/dl) (Mean ± SE).
ConclusionThe current study investigated the effects of acetyl-L-carnitine (350 mg/ewe), either as an independent administration or in conjunction with Se-fortified S. cerevisiae (0.03 mg/kg BW), which showed an important effect on obvious biochemical traits in making ewes over a term of 60 days. The study results led to a significant reduction in blood glucose levels, an increase in protein, cholesterol, and triglyceride levels, and a decrease in liver enzyme activity, particularly in AST and ALT. Other than that, there was an increase in the blood albumin concentrations. The disclosures of this think almost emphasize the potential of acetyl-l-carnitine and Se-fortified S. cerevisiae to motivate productive biophysical alterations, illustrating their effectiveness in updating metabolic and hepatic prosperity in ewes. Future examinations may examine the long-term impacts of these mediations and examine their vital abilities in other animal populations. AcknowledgmentsThe authors extend their thanks and gratitude to all persons and staff of all institutions who helped accomplish this work. Conflict of interestThe authors declare no conflict of interest. FundingNone. Authors contributionsBoth authors contributed equally to this manuscript. Both authors have read and approved the final version of the manuscript. Data availabilityAll data are provided in the revised manuscript. ReferencesAbu-El-Zahab, H.S.H., Hamza, R.Z., Montaser, M.M., El-Mahdi, M.M. and Al-Harthi, W.A. 2019. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice. Ecotoxicol. Environ. Saf. 173, 419–428. Alhasaniah, A.H. 2023. L-carnitine: nutrition, pathology, and health benefits. Saudi J. Biol. Sci. 30(2), 103555; doi:10.1016/j.sjbs.2022.103555 Al-Mafarji, D.A.T. and Alsaadi, S.A.A. 2023. The physiological effect of different types of water used to thermally stressed ewes during the summer season. IOP Conf. Ser. Earth Environ. Sci. 1262(7), 72061. Alsaadi, S.A. 2023. Biophysiological impacts of some herbs extracts as antioxidants, an advanced contemporary. Kirkuk Univ. J. Agricult. Sci. 14(2), 145–161; doi:10.58928/KU23.14214 Alsaadi, S.A., Abdulazeez, S.T. and Baker, A.G. 2023. The biophysiological impact of aqueous extract of turamic with or without folic acid in awassi ewes, comparative study. IOP Conf. Ser. Earth Environ. Sci. 1252(1), 12147. Alsaadi, S.A.R., Abdulazeez, S.T. and Noaman, H.A. 2025. The bio-physiological impact of different concentrations of ginger aqueous extract in Awassi Ewes. Egypt. J. Vet. Sci. 56, 1099–1104; doi:10.21608/ejvs.2024.279035.1965 Alsaadi, S.A.A. and Abass, K.S. 2020. Benincasa hispida is an antioxidant of possible physiological importance: a comparative review. Plant Arch. 20(2), 2833–2838. Amir Shareef, M., Al-Rawi, F.T., Kareem, J.A.M., Thabit Jassim Alrawi, S., Omar, A.A. and Muneeb Alrawi, H. 2023. Effect of Saccharomyces cerevisiae Fortified with selenium on the hematological and biochemical some biochemical traits of local Iraqi Goat. Arch. Razi Inst. 78(4), 1213–1216; doi:10.32592/ARI.2023.78.4.1213 Anjum, M.I., Javaid, S., Ansar, M.S. and Ghaffar, A. 2018. Effects of yeast (Saccharomyces cerevisiae) supplementation on intake, digestibility, rumen fermentation and milk yield in Nili-Ravi buffaloes. Iran. J. Vet. Res. 19(2), 96–100. Aremmt, M.K., Mohammed, T.R. and Alrawi, S.T. 2019. Effect of yeast (Saccharomyces cerevisiae) supported by Selenium and zinc on the lipid profile of local sheep males. Al-Anbar J. Vet. Sci. 12(1), 89–96; doi:10.3796/AJAS.2019.12.1.10 Barchielli, G., Capperucci, A. and Tanini, D. 2022. The role of selenium in pathologies: an updated review. Antioxidants 11(2), 251; doi:10.3390/antiox11020251 Carlson, D.B., Mcfadden, J.W., D’Angelo, A., Woodworth, J.C. and Drackley, J.K. 2007. Dietary l-carnitine affects periparturient nutrient metabolism and lactation in multiparous cows. J. Dairy Sci. 90(7), 3422–3441. Cui, X., Wang, Z., Tan, Y., Chang, S., Zheng, H., Wang, H., Yan, T., Guru, T. and Hou, F. 2021. Selenium yeast dietary supplement affects rumen bacterial population dynamics and fermentation parameters in alpine meadows. Front. Microbiol. 12, 663945; doi:10.3389/fmicb.2021.663945 Duncan, D. 1955. Multiple ranges and multiple F-test. Biometrics 11, 24. Faccenda, A., Zambom, M.A., De Avila, A.S., Schneider, C.R., Werle, C.H., Anschau, F.A., Almeida, A.R.E., Lange, M.J. and Dos Santos, G.T. 2020. Performance and milk composition of Holstein cows fed with dried malt bagasse and selenium-enriched Saccharomyces cerevisiae. Livestock Sci. 238, 104081; doi:10.1016/j.livsci.2020.104081 Gao, S., Qiu, H., Chen, F., Yang, G., Hou, L., Dong, J. and Dong, W. 2024. Effects of high-dose selenium-enriched Saccharomyces cerevisiae on growth performance, antioxidant status, tissue fat content and selenium concentration, and selenoenzyme mRNA expression in chicks. Poultry Sci. 103(12), 104312; doi:10.1016/j.psj.2024.104312 Gong, A., Liu, W., Lin, Y., Huang, L. and Xie, Z. 2023. Adaptive laboratory evolution reveals the selenium efflux process to improve selenium tolerance mediated by the membrane sulfite pump in Saccharomyces cerevisiae. Microbiol. Spectr. 11(3), 132623; doi:10.1128/spectrum.01326-23 Gunun, N., Sanjun, I., Kaewpila, C., Foiklang, S., Cherdthong, A., Wanapat, M., Polyorach, S., Khota, W., Kimprasit, T., Kesorn, P., Milintawisamai, N. and Gunun, P. 2022. Effect of dietary supplementation of hydrolyzed yeast on growth performance, digestibility, rumen fermentation, and hematology in growing beef cattle. Anim. Open Access J. 12(18), 2473. Helal, M.A.Y., Hefnawy, A.G., Abokora, S.Y. and Koptan, A.S. 2018. Evaluation of L-carnitine in experimentally the treatment of experimentally induced hypomagnesemia in sheep. Benha Vet. Med. J. 35(2), 31–43. Hosnedlova, B., Kepinska, M., Skalickova, S., Fernandez, C., Ruttkay-Nedecky, B., Malevu, T.D., Sochor, J., Baron, M., Melcova, M., Zidkova, J. and Kizek, R. 2017. A summary of new findings on the biological effects of selenium in selected animal species—a critical review. Int. J. Mol. Sci. 18(10), 2209. Huang, Q., Wang, S., Yang, X., Han, X., Liu, Y., Khan, N.A. and Tan, Z. 2023. Effects of organic and inorganic selenium on selenium bioavailability, growth performance, antioxidant status, and meat quality of local beef cattle in China. Front. Vet. Sci. 10, 1171751; doi:10.3389/fvets.2023.1171751 Keller, J., Ringseis, R., Priebe, S., Guthke, R., Kluge, H. and Eder, K. 2011. Effect of L-Carnitine on the hepatic transcript profile in piglets as an animal model. Nutr. Metab. 8, 76; doi:10.1186/1743-7075-8-76 Lee, B.J., Lin, J.S., Lin, Y.C. and Lin, P.T. 2014. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: a randomized, placebo-controlled trial. Nutr. J. 13, 79; doi:10.1186/1475-2891-13-79 Li, X., Shi, L., Song, P., Cai, W., Luo, X. and Zhao, B. 2024. Certification of new selenium-enriched yeast and supplement reference materials for selenomethionine using two independent measurement strategies. Molecules 29(1), 235; doi:10.3390/molecules29010235 Liu, G., Ding, Y., Chen, Y. and Yang, Y. 2020. Effect of energy intake and L-carnitine on fattening performance, carcass traits, meat quality, blood metabolites, and gene expression of lamb. Small Ruminant Res. 183, 106025. Luo, W., Chen, P., Zhang, X., Zhang, Y., Zhang, S., Sun, K., He, F., Li, L., Zhang, N., Xiong, Y., Guo, Z., Du, Z. and Wen, A. 2022. Effect of adding L-carnitine to high-fat/low-protein diets of common carp (Cyprinus carpio) and the mechanism of fat and protein metabolism regulation. Aquac. Nutr. 2022, 3768368. Martín, A., Giráldez, F.J., Cremonesi, P., Castiglioni, B., Biscarini, F., Ceciliani, F., Santos, N. and Andrés, S. 2022. Dietary administration of L-carnitine during the fattening period of early feed restricted lambs modifies ruminal fermentation but does not improve feed efficiency. Front. Physiol. 13, 840065; doi:10.3389/fphys.2022.840065 Mohamed, H.E. and Ibrahim, H.N. 2024. Impact of Saccharomyces enriched with Selenium on growth performance and metabolic status of sheep exposed to heat stress. Egypt. J. Anim. Health 4(2), 1–21. Montesano, A., Senesi, P., Luzi, L., Benedini, S. and Terruzzi, I. 2015. Potential therapeutic role of L-Carnitine in skeletal muscle oxidative stress and atrophy conditions. Oxid. Med. Cell Longev. 2015, 646171; doi:10.1155/2015/646171 Mousaie, A. 2021. Dietary supranutritional supplementation of selenium-enriched yeast improves feed efficiency and blood antioxidant status of growing lambs reared under warm environmental conditions. Trop. Anim. health Prod. 53(1), 138; doi:10.1007/s11250-021-02588-4 Odunfa, O.A., Dhungana, A., Huang, Z., Yoon, I. and Jiang, Y. 2024. Effects of a liquid and dry Saccharomyces cerevisiae fermentation product feeding program on ruminal fermentation, total tract digestibility, and plasma metabolome of Holstein steers receiving a grain-based diet. J. Anim. Sci. 102, 223. Oh, H., Park, C.H. and Jun, D.W. 2022. Impact of L-carnitine supplementation on liver enzyme normalization in patients with chronic liver disease: a meta-analysis of randomized trials. J. Pers. Med. 12(7), 1053; doi:10.3390/jpm12071053 Omar, E. and Alsaadi, S. 2022. Comparative effects of aqueous extract of local basil seeds, vitamin C, and Selenium as Anti-Heat Stress in some hematological traits at Iraqi Awassi Sheep. Kirkuk Univ. J. Agricult. Sci. 13(3), 109–122; doi:10.58928/ku22.13309 Pirmadah, F., Ramezani-Jolfaie, N., Mohammadi, M., Talenezhad, N., Clark, C.C.T. and Salehi-Abargouei, A. 2020. Does l-carnitine supplementation affect serum levels of enzymes mainly produced by liver? A systematic review and meta-analysis of randomized controlled clinical trials. Eur. J. Nutr. 59(5), 1767–1783. Ponce De Leon, C.A., Bayon, M.M., Paquin, C. and Caruso, J.A. 2002. Selenium incorporation into Saccharomyces cerevisiae cells: a study of different incorporation methods. J. Appl. Microbiol. 92(4), 602–610. Pooyandjoo, M., Nouhi, M., Shab-Bidar, S., Djafarian, K. and Olyaeemanesh, A. 2016. Effect of L-Carnitine on weight loss, body composition, and lipid profile: a systematic review and meta-analysis of randomized controlled trials. J. Res. Med. Sci. 21, 62. Qana, H.A., Mohammed, A.M., Samira, H.A. and Sarmad, A.A. 2023. The requirements of chickens for nutritional compounds for growth: an advanced nutritional perspective. Kirkuk Univ. J. Agric. Sci. 14(3), 17–33. Radhi, D.D., Al-Aidi, R.H.A. and Khalaf, A.A. 2024. an impact of intravenous injection of L-carnitine on the sexual characteristics of aged sheep. J. Anim. Health Prod. 12(1), 258–262. Ribas, G.S., Vargas, C.R. and Wajner, M. 2014. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 533(2), 469–476. Ringseis, R., Keller, J. and Eder, K. 2012. Role of Carnitine in regulating glucose homeostasis and insulin sensitivity: evidence from in vivo and in vitro studies with Carnitine supplementation and Carnitine deficiency. Eur. J. Nutr. 51(1), 1–18; doi:10.1007/s00394-011-0284-2 Safar, T.S. and Alsaadi, S.A.R. 2025. Physiological impact of an aqueous solution of Spirulina algae with or without folic acid on local awassi ewes within medium and late pregnancy stages and indicators of growth in newborn lambs. Egypt. J. Vet. Sci. 56(7), 1541–1545; doi:10.21608/ejvs.2024.284770.2029 Salihi, A. and Alsaadi, S. 2025. Effect of Spirulina algae powder and folic acid on some physiological parameters during the early stages of pregnancy local ewe’s pregnancy in the summer. Egypt. J. Vet. Sci. 56(7), 1507–1512; doi:10.21608/ejvs.2024.283832.2019 SAS/STAT 2012. Users guide for personal computers. Release. 9:12. Cary, NC: SAS Institute, Inc. Sawicka, A.K., Renzi, G. and Olek, R.A. 2020. The bright and the dark sides of L-carnitine supplementation: a systematic review. J. Int. Soc. Sports Nutr. 17(1), 49; doi:10.1186/s12970-020-00377-2 Seemann, L., Frahm, J., Kersten, S., Bühler, S., Meyer, U., Visscher, C., Huber, K. and Dänicke, S. 2024. Dietary L-carnitine supplementation modifies blood parameters of mid-lactating dairy cows during standardized lipopolysaccharide-induced inflammation. Front. Immunol. 15, 1390137. Shaalan, S., El-wakkad, A.S.E. and Saleh, H. 2015. Protective effect of L-carnitine and baker yeast saccharomyces cerevisiae against hepatic toxicity induced by valproate as antiepileptic drug in rats. Int. J. Pharm. Pharm. Sci. 7(5), 89–95. Türker, Y., Nazıroğlu, M., Gümral, N., Çelik, O., Saygın, M., Çömlekçi, S. and Flores-Arce, M. 2011. Selenium and L-carnitine reduce oxidative stress in the heart of rat induced by 2.45-GHz radiation from wireless devices. Biol. Trace Element Res. 143(3), 1640–1650; doi:10.1007/s12011-011-8994-0 Vecchio, M., Chiaramonte, R., Testa, G. and Pavone, V. 2021. Clinical effects of L-carnitine supplementation on physical performance in healthy subjects, the key to success in rehabilitation: a systematic review and meta-analysis from the rehabilitation point of view. J. Funct. Morphol. Kinesiol. 6(4), 93; doi:10.3390/jfmk6040093 Wang, Z., Zhang, L. and Tan, T. 2010. High cell density fermentation of Saccharomyces cerevisiae GS2 for selenium-enriched yeast production. Korean J. Chem. Eng. 27, 1836–1840; doi:10.1007/s11814-010-0300-x Xu, L., Lu, Y., Wang, N. and Feng, Y. 2022. Role and mechanisms of selenium supplementation on fatty liver-associated disorder. Antioxidants 11(5), 922; doi:10.3390/antiox11050922 Xu, Y., Jiang, W., Chen, G., Zhu, W., Ding, W., Ge, Z., Tan, Y., Ma, T. and Cui, G. 2017. L-Carnitine treatment of insulin resistance: a systematic review and meta-analysis. Adv. Clin. Exp. Med. 26(2), 333–338. Yoshinaga, M., How, S., Blanco, D., Murdoch, I.S., Grudny, M., Powers, S.L., Molina, N., Rosen, B.P. and Welch, A.Z. 2018. Directed evolution of Saccharomyces cerevisiae for increased selenium accumulation. Microorganisms 6(3), 81. Zhang, F., Li, X. and Wei, Y. 2023. Selenium and selenoproteins in health. Biomolecules 13(5), 799; doi:10.3390/biom13050799 Zhang, Z., Zhu, L., Zhang, H., Yu, D., Yin, Z. and Zhan, X. 2025. Comparative study on the effects of selenium-enriched yeasts with different selenomethionine contents on gut microbiota and metabolites. Int. J. Mol. Sci. 26(7), 3315; doi:10.3390/ijms26073315 | ||
| How to Cite this Article |
| Pubmed Style Abdulazeez ST, Alsaadi SAA. The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewes. Open Vet. J.. 2025; 15(9): 4726-4734. doi:10.5455/OVJ.2025.v15.i9.77 Web Style Abdulazeez ST, Alsaadi SAA. The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewes. https://www.openveterinaryjournal.com/?mno=256860 [Access: January 26, 2026]. doi:10.5455/OVJ.2025.v15.i9.77 AMA (American Medical Association) Style Abdulazeez ST, Alsaadi SAA. The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewes. Open Vet. J.. 2025; 15(9): 4726-4734. doi:10.5455/OVJ.2025.v15.i9.77 Vancouver/ICMJE Style Abdulazeez ST, Alsaadi SAA. The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewes. Open Vet. J.. (2025), [cited January 26, 2026]; 15(9): 4726-4734. doi:10.5455/OVJ.2025.v15.i9.77 Harvard Style Abdulazeez, S. T. & Alsaadi, . S. A. A. (2025) The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewes. Open Vet. J., 15 (9), 4726-4734. doi:10.5455/OVJ.2025.v15.i9.77 Turabian Style Abdulazeez, Sarmad Talib, and Sarmad Abdulrazzq Abbood Alsaadi. 2025. The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewes. Open Veterinary Journal, 15 (9), 4726-4734. doi:10.5455/OVJ.2025.v15.i9.77 Chicago Style Abdulazeez, Sarmad Talib, and Sarmad Abdulrazzq Abbood Alsaadi. "The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewes." Open Veterinary Journal 15 (2025), 4726-4734. doi:10.5455/OVJ.2025.v15.i9.77 MLA (The Modern Language Association) Style Abdulazeez, Sarmad Talib, and Sarmad Abdulrazzq Abbood Alsaadi. "The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewes." Open Veterinary Journal 15.9 (2025), 4726-4734. Print. doi:10.5455/OVJ.2025.v15.i9.77 APA (American Psychological Association) Style Abdulazeez, S. T. & Alsaadi, . S. A. A. (2025) The vital impact of acetyl-L-carnitine and selenium-fortified Saccharomyces cerevisiae on biochemical parameters in Awassi ewes. Open Veterinary Journal, 15 (9), 4726-4734. doi:10.5455/OVJ.2025.v15.i9.77 |