| Research Article | ||
Open Vet. J.. 2025; 15(9): 4418-4431 Open Veterinary Journal, (2025), Vol. 15(9): 4418-4431 Research Article Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpioHayder A.H. Al-Hasson1,2,3,4*, Khalidah S. Al-Niaeem4 and Monia ElBour21Faculty of Mathematical, Physical and Natural Sciences of Tunis, University of Tunis EL Manar, Tunis, Tunisia 2LRINSTM1605, National Institute of Marine Sciences and Technologies, University of Tunis – Carthage, Tunis, Tunisia 3Basrah Education Directorate, Basrah, Iraq 4Department of Fisheries and Marine Resources, College of Agriculture, University of Basrah, Basrah, Iraq *Corresponding Author: Hayder A.H. Al-Hasson. Basrah Education Directorate, Basrah, Iraq. Email: haydera77 [at] gmail.com Submitted: 06/05/2025 Revised: 21/07/2025 Accepted: 08/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
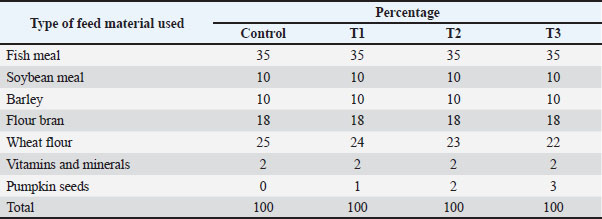
AbstractBackground: Common carp (Cyprinus carpio) is a nutritionally valuable species with high demand in local and regional markets. Aim: This study evaluated the effects of dietary pumpkin seed (Cucurbita pepo) supplementation on the health status and immune response of farmed C. carpio challenged with Vibrio parahaemolyticus. Methods: Pumpkin seeds were selected for their rich content of essential nutrients and potential immunostimulatory properties. Seventy-two healthy fish (average weight 85.16 ± 0.10 g) were randomly assigned to four dietary groups receiving 0%, 1%, 2%, or 3% pumpkin seed powder for 12 weeks. Fish were fed to near satiation twice daily. After 56 days, a 3-hour crowding stress test (40 kg/m³) followed by 24 hours of recovery was performed. Key parameters assessed included growth performance, feed efficiency, hematological and biochemical markers, serum lysozyme activity, body composition, and immune-antioxidant response. Following this, 12 fish per group were intraperitoneally injected with V. parahaemolyticus, and mortality was recorded. Results: Fish fed pumpkin seed-supplemented diets, particularly at the 2% level, showed significant improvements in growth, immune markers, feed utilization, and survival rate post-challenge compared to the control group. Conclusion: These findings suggest that pumpkin seed supplementation enhances immune function and disease resistance in C. carpio, supporting its use as a functional ingredient in aquafeed formulations. Keywords: Medicinal herbs, Pumpkin seed, Cucurbita pepo, Cyprinus carpio, Vibrio parahaemolyticus. IntroductionInfectious diseases significantly reduce aquaculture productivity by impairing fish growth, increasing mortality rates, and lowering feed efficiency. Outbreaks often result in sudden, large-scale losses, leading to decreased harvest volumes and supply chain disruptions. These diseases can account for up to 20%–30% of total production costs in affected systems due to direct losses, treatment expenses, and the need for increased biosecurity measures. According to Hwihy et al. (2025), disease outbreaks remain the primary barrier to the sustainable expansion of the aquaculture sector, particularly in intensive farming operations. Despite the role of aquaculture in enhancing sustainable food security, fish farming faces major challenges, primarily disease outbreaks (Oftebro, 2025). With aquaculture now supplying more fish for human consumption than wild capture fisheries, and aquafeeds accounting for 60%–80% of total production costs in intensive systems (FAO, 2024), sustainable feed solutions are increasingly important. Recent advances in aquafeeds strategies aim to reduce the dependency on fishmeal by replacing it with more sustainable, plant-based ingredients (Fernandez-Maestú et al., 2025). Multiple Vibrio species—such as V. parahaemolyticus, V. alginolyticus, V. harveyi, V. owensii, and V. campbellii—have been identified as common pathogens in farmed aquatic species (Nor-Amalina et al., 2017). Al-Shammari (2024) reported the isolation of V. parahaemolyticus and other Vibrio strains from Cyprinus carpio in Basra, while Abdelaziz et al. (2017) identified similar pathogens in species like Dusky Grouper (Epinephelus marginatus), Egyptian Sole (Solea aegyptiaca), and Striped Mullet (Mugil cephalus). Optimizing feed formulations in recirculating or hydroponic systems can significantly improve productivity and cost efficiency (Hassan et al., 2021). Natural feed additives with medicinal properties have attracted interest owing to their safety, lack of drug resistance, and absence of harmful residues (Dadras et al., 2023). These additives are typically biodegradable, non-toxic, and consumer-friendly (Abdel-Razek et al., 2019). To address health challenges in aquaculture, researchers have explored alternative bio-based treatments and immunoprophylactic approaches, including phototherapy, nanotechnology-based solutions, probiotics, prebiotics, synbiotics, bacteriophage therapy, vaccines, quorum-sensing inhibitors, antimicrobial peptides, biosurfactants, bacteriocins, stem cell applications, and targeted diagnostic-based treatments (Elgendy et al., 2024). In recent years, functional feed additives have been applied to improve the health and growth of various aquaculture species (Wannavijit et al., 2024). However, for common carp—a widely cultured, omnivorous, agastric species prone to distal intestinal enteritis (Rašković et al., 2024)—data on safe and effective bio-supplementation remains limited. Pumpkin seeds (Cucurbita pepo), members of the Cucurbitaceae family, are cultivated globally and include the major species C. pepo, C. maxima, and C. moschata (Velasco et al., 2017). These plants produce seeds rich in bioactive compounds, including sterols, unsaturated fatty acids, tocopherols, selenium, carotenoids, magnesium, cucurbitina, phytosterols, amino acids, β-tocopherol, squalene, phytoestrogens, and phenolic compounds (Keskin Çavdar, 2019). Pumpkin seeds are considered important functional ingredients in both human and animal diets due to their high nutritional value and antioxidant properties (Lee et al., 2003). Medicinal herbs, such as Origanum majorana (Kadhim, 2022), Tinospora cordifolia (Al-Turaihi, 2023), and pumpkin seeds, have immunomodulatory potential and are being explored as natural immunoadjuvants in vaccine strategies (Monsang et al., 2025). Similarly, Karatas et al. (2025) reported that the specific growth rate (SGR), weight gain (WG), and feed conversion ratio (FCR) of common carp treated with pumpkin seed powder improved with increasing dietary pumpkin seed extract levels. They also noticed markedly elevated biomarkers, such as total protein, trypsin, amylase, lipase, superoxide dismutase (SOD), catalase (CAT), and lysozyme (LYZ). In this study, pumpkin seeds were tested as a dietary supplement for C. carpio, owing to their appetite-stimulating effects and antibacterial activity. Materials and MethodsThe experimental diets were prepared using finely ground pumpkin seed powder, which was stored until needed. Four homogeneous diets were developed to include 0% (control), 1%, 2%, and 3% pumpkin seed powder (Table 1). All ingredients were carefully ground and mixed with 100 ml of water to form a uniform blend. The mixture was then pelleted using a laboratory mincer following the method outlined by Abdel-Tawwab and Abbass (2017). The resulting diets were dried at 40 °C until they reached a constant weight and subsequently stored in plastic bags for future use. Fish rearing and feedingHealthy C. carpio samples were obtained from the Fish Farming Unit at the Aquaculture Center, College of Agriculture, University of Basrah. The fish were transported to the aquaculture laboratory in the Department of Fish and Marine Resources using aerated plastic bags containing water from their original pond. Upon arrival, the fish were visually examined to ensure the absence of external disease symptoms. To reduce the risk of pathogen transmission, the fish were disinfected using a 3% saline solution for 5 minutes, following the protocol of Herwig et al. (1979). Following disinfection, the fish were randomly allocated to plastic tanks (30 × 40 × 60 cm³) for a 14-days acclimation period. During acclimation, fish were fed a laboratory-prepared control diet at 2% of body weight, twice daily. For the 56-day experimental trial, 72 healthy fish with an average initial weight of 95.39 ± 2.86 g were randomly assigned to 12 tanks. Each tank contained six fish, with three replicate tanks per dietary treatment. Fish were fed experimental diets twice daily at a feeding rate of 3%–5% of body weight, adjusted according to biomass throughout the trial period. Physical and chemical properties of the water samplesThe water quality was monitored regularly to ensure optimal conditions for fish health throughout the experimental period. The temperature was maintained between 25°C and 30°C (Hepher, 1988), the dissolved oxygen level between 5-7.9 mg/l (Szlaminska and Przybyl, 1986), and the pH concentration should not exceed 8.6 (Swain et al., 2020). Common carp can tolerate a salinity level of about 5 g/l (Peteri, 2009). Weekly water samples were collected from each aquarium to evaluate key physicochemical parameters. On-site pH, salinity, and dissolved oxygen measurements were conducted using a HOJILA AZ86031 5-in-1 Water Quality Meter (USA). Un-ionized ammonia (NH3) concentrations were determined using a Multi-Parameter Ion Analyzer (HANNA Instruments, RI, USA). Additionally, nitrate and nitrite levels were measured weekly. The water temperature was recorded daily using thermometers mounted on each aquarium. Growth performance and survival of fishAt the conclusion of the feeding trial, the fish in each aquarium were collected, counted, and weighed. The growth performance and feed efficiency metrics were determined using the following formulas: • WG (g): W2−W1, where W1 and W2 represent the initial and final weights, respectively. • SGR (%/day):=100 (Ln W2 Ln W1)/T, where T is the duration of the experiment in days. • Feed Intake (g feed/fish): Total feed consumed by all fish in an aquarium divided by the number of fish in that aquarium. • FCR: feed intake/fish WG. • Fish survival rate (%):100×(Final fish count/Initial fish count). Table 1. Components of the feed used in this study.
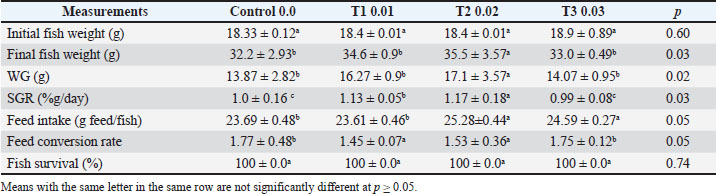
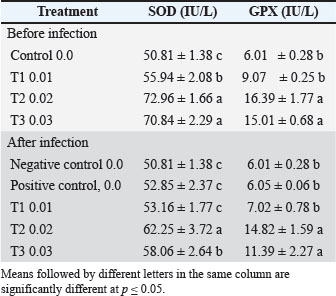
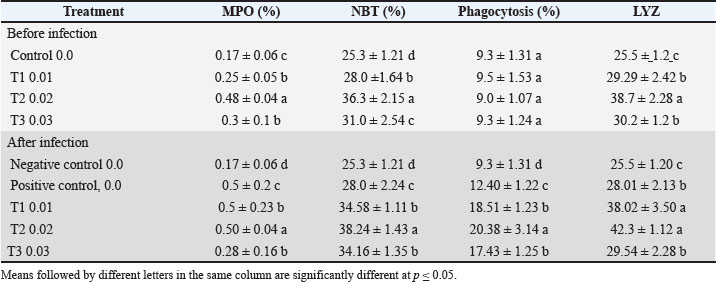
Antioxidant enzyme assaysAt the end of the 56-day feeding trial, fish were fasted for 24 hours before sampling to reduce metabolic variability. Three fish were randomly selected from each tank and anesthetized using 30 mg/l buffered tricaine methane sulfonate (MS-222). Blood samples were collected from the caudal vein using heparinized syringes. The samples were immediately centrifuged at 5,000 × g for 15 minutes at room temperature. The resulting plasma was separated and stored at −20°C for later biochemical and antioxidant analyses. Plasma antioxidant enzyme activities were measured using commercial diagnostic reagent kits (MyBioSource Inc., San Diego, CA) according to the manufacturer’s protocols. The levels of malondialdehyde (MDA), an indicator of lipid peroxidation, were determined spectrophotometrically at 532 nm using the thiobarbituric acid reactive substances method described by Ohkawa et al. (1979). Enzymatic antioxidant activities of SOD, CAT, and glutathione peroxidase (GPX) were quantified using spectrophotometric methods based on established protocols: McCord and Fridovich (1969) for SOD, Aebi (1984) for CAT, and Paglia and Valentine (1967) for GPX. Immunity assaysLeukocyte superoxide anion production was assessed using the Nitroblue tetrazolium (NBT) reduction assay, following the method of Rook et al. (1985). All reagents were obtained from Sigma-Aldrich (St. Louis, MO). LYZ activity was determined using a turbidimetric assay, as described by Caruso et al. (2002), which measures the rate of M. lysodermaticus cell wall lysis. Myeloperoxidase (MPO) activity in the serum was evaluated according to the protocol established by Quade and Roth (1994). Bacteria challengeThe pathogenic bacterium V. parahaemolyticus was obtained from the Department of Fish and Marine Resources, College of Agriculture, Basrah University, Iraq. The cells were cultured on nutrient agar at 30°C under light exposure for 24 hours. At the end of the feeding trial, the fish from each treatment group were pooled and randomly divided into two subgroups. Each subgroup contained 10 fish housed in duplicate 30-l tanks. The first subgroup was exposed to V. parahaemolyticus via a sublethal dose following the method described by Schäperclaus (1992). Each fish received a 0.1-ml intraperitoneal injection of a 24-hours broth culture of virulent V. parahaemolyticus (5 × 105 CFU/ml). The second subgroup, which served as the control, was injected with 0.1 ml of saline solution. All fish were monitored for 10 days while being fed with their respective diets. During this period, infected fish were observed for signs of morbidity and mortality. The bacteria were re-isolated from moribund or recently deceased fish and identified using the API 20E system for Gram-negative bacteria. At the end of the bacterial challenge, plasma samples were collected from three randomly selected live fish per tank, and the previously measured parameters were re-evaluated. The relative percent survival (RPS) was calculated 10 days post-challenge using the method outlined by Amend (1981) as follows: RPS=100 × [1 (% mortality in treated fish/% mortality in control fish)]. Statistical analysisBefore the statistical analysis, the normality of the data distribution was assessed using the Kolmogorov–Smirnov test. Bartlett’s test was used to evaluate the homogeneity of variances among the different treatment groups. Subsequently, the data were analyzed using one-way ANOVA to assess the effects of pumpkin seeds. For antioxidant and immune response data before and after bacterial infection, a two-way analysis of variance was conducted to examine the effects of both pumpkin seeds and bacterial infection as two independent factors. Mean differences were evaluated at a 5% significance level using Duncan’s test for post hoc analysis. All statistical analyses were performed using the Statistical Package for the Social Sciences version 20 (SPSS, Richmond, VA), following the procedures outlined by Dytham (2011). Ethical approvalThis report adheres to all ethical guidelines for fish care and handling as outlined by the 2005 Canadian Council on Animal Care (CCAC) 2005. ResultsPhysical and chemical properties of the water samplesThe water quality parameters were regularly monitored. The water temperature, salinity, dissolved oxygen, pH, nitrate, nitrite, and ammonia. 25.01°C ± 0.32°C, 1.68 ± 0.04 ppt, 7.90 ± 0.3 mg/l, 7.68 ± 0.13, 0.27 ± 0.02, 0.14 ± 0.01, and 0.03 ± 0.01, respectively. Fish growthDiets supplemented with pumpkin seeds were observed to improve fish growth and feed efficiency. Intestinal histological assessment of intestinal tissues showed improvement in intestinal health after 70 days of feeding. All treated groups, particularly T2, exhibited preserved epithelial structure, uniform intestinal villi, increased crypt depth, a higher number of goblet cells, reduced villi fusion, and less degeneration and necrosis compared with other treatments. Fish fed a diet containing 2% pumpkin seeds exhibited higher final weight, WG, and SGR compared with the control group, indicating significantly enhanced performance (p < 0.05) (Table 2 and Fig. 1). Antioxidants’ responsePlasmatic SOD and GPX activity were notably influenced by the inclusion of pumpkin seed supplements in the diet. Additionally, both SOD and GPX activities were significantly impacted by the combined effects of dietary pumpkin seed supplementation and bacterial infection (p < 0.05) (Table 3 and Figs. 2 and 3). Immunity responseThe study demonstrated that pumpkin seed supplementation and bacterial infection significantly influenced the levels of (MPO), NBT, phagocytosis, and LYZ (p > 0.05) (Table 4 and Fig. 4). Post-feeding analysis revealed that all indicators showed marked improvements compared to pre-feeding levels, with all activities further elevated following bacterial infection. Dietary inclusion of pumpkin seeds dose-dependently enhanced fish resistance to V. parahaemolyticus, with no mortality observed in infected fish during the experimental period. Table 4 illustrates the LYZ activity in C. carpio fed with diets containing pumpkin seeds, revealing a significant increase (p ≤ 0.05) compared with the control group. The highest activity (38.7 ± 2.28) was recorded in fish fed 2% pumpkin seeds, followed by (30.2 ± 1.2) in those receiving 3%, with significant differences (p ≤ 0.05) between these groups. Phagocytosis activity in C. carpio initially showed a significant decrease (p ≤ 0.05) in pumpkin-seed-fed groups compared to the control before the bacterial challenge. However, following the challenge, the highest phagocytosis activity (20.38 ± 3.14) was observed in fish fed with 2% pumpkin seeds, while the lowest activity (9.3 ± 1.31) was noted in the negative control group. Bacteria challengeIn this study, no mortality was observed among the experimentally infected fish with V. parahaemolyticus experimentally at a semi-lethal dose of 6.9 × CFU/ ml 710 for 14 days in the control group and the other groups throughout the experimental period. The results of the current study showed that during laboratory infection, the clinical behavior of fish is represented by abnormal movement, an unbalanced condition, and a decrease in the movement of pectoral fins, which are signs of approaching the state of death of common carp fish. External pathological signs of experimentally infected common carp were recorded, represented by the presence of hemorrhagic foci on the skin. DiscussionThis study revealed that feeding C. carpio with a diet supplemented with pumpkin seeds positively impacted their growth in a dose-dependent manner. The highest growth rates were observed in fish fed a 2% pumpkin seed diet, which consumed greater quantities of feed. This effect could be attributed to the enhanced palatability of the diet, which is likely due to the high oleic and linoleic acid content of pumpkin seeds. Pumpkin seeds are rich in proteins and essential fatty acids, such as omega-3 and omega-6, which promote the health of fish skin and fins (Martins et al., 2023). Additionally, the dietary fiber in pumpkin seeds promotes better digestive health, facilitating improved nutrient absorption and overall growth and development in fish. Table 2. Growth performance and feed use of C. carpio fed with diets supplemented with various amounts of pumpkin seeds for 56 days.
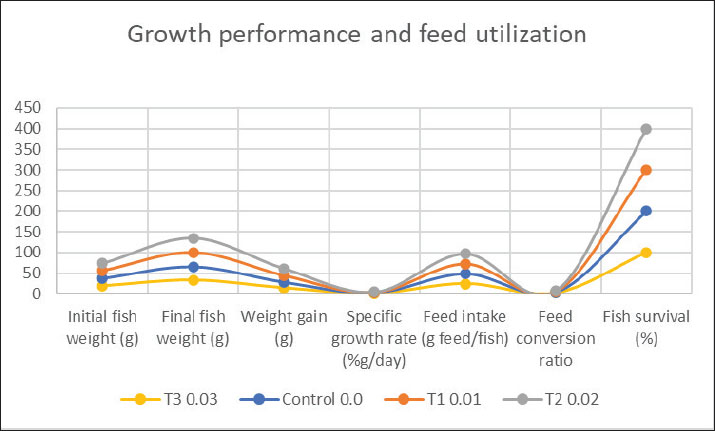
Fig. 1. Growth performance and feed use of C. carpio fed diets supplemented with various amounts of pumpkin seeds for 56 days. Table 3. Effect of pumpkin seeds on serum antioxidant activity.
Previous pharmacological research highlights the hepatoprotective, antioxidant, antimicrobial, and anti-inflammatory properties of P. sativa (Perez-Gutierrez, 2016). Moreover, pumpkin seeds are rich in carbohydrates, proteins, crude fiber, crude oil, and unsaturated fatty acids, particularly linoleic and oleic acids, and are a significant source of antioxidants (Abd-Elnoor, 2019). Pumpkin seeds also had high protein, oil, and oleic and linoleic acids but low dietary fiber content (Öztürk and Turhan, 2020). Fish growthThis study demonstrated that pumpkin seeds had the most pronounced positive impact on intestinal structure, significantly improving all three measured parameters: villus length, villus width, and muscle thickness. Pumpkin seed supplementation was found to enhance the growth performance of C. carpio compared with the control group. The initial weights of the carp were similar across all groups, with the control group at 18.33 g and the T3 group at 18.9 g. The final weights revealed that the fish fed with pumpkin seeds (T1, T2, and T3) grew more than those in the control group, with T2 achieving the highest final weight of 35.5 g. This aligns with the findings of Kadhim (2022), who reported that diets enriched with plant-based ingredients led to significant weight increases in carp, emphasizing the nutritional benefits of such diets. This is consistent with the findings of Ibrahim et al. (2024), who documented significant enhancements in final body weight and total WG in Nile tilapia when moringa was included in their diet. This also agrees with the findings of Karatas et al. (2025), who reported that the SGR, WG, and FCR of common carp treated with pumpkin seed powder were significantly higher.
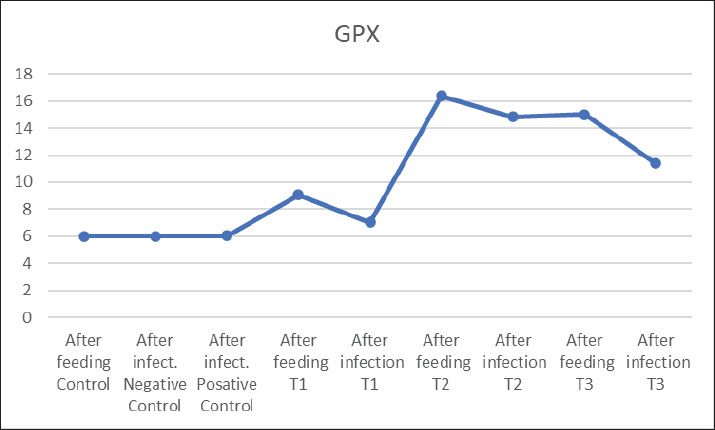
Fig. 2. GPX levels in the blood of common carp after 56 days of feeding on different proportions of pumpkin seeds and after 14 days of experimental infection with V. parahaemolyticus.
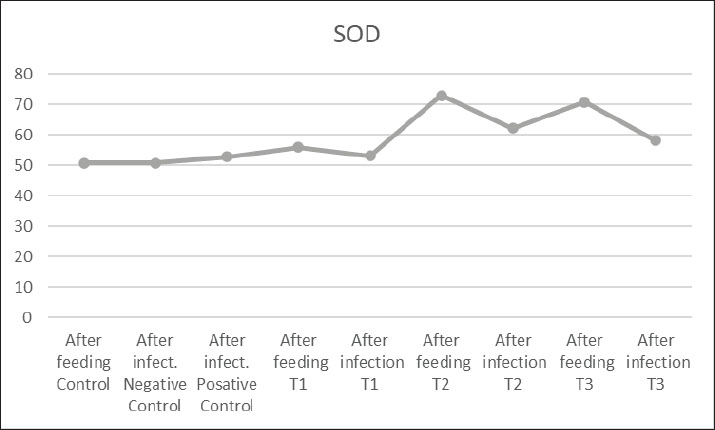
Fig. 3. SOD in the blood of common carp after 56 days of feeding on different proportions of pumpkin seeds and after 14 days of experimental infection with V. parahaemolyticus. Table 4. Immune parameters of the common carp.
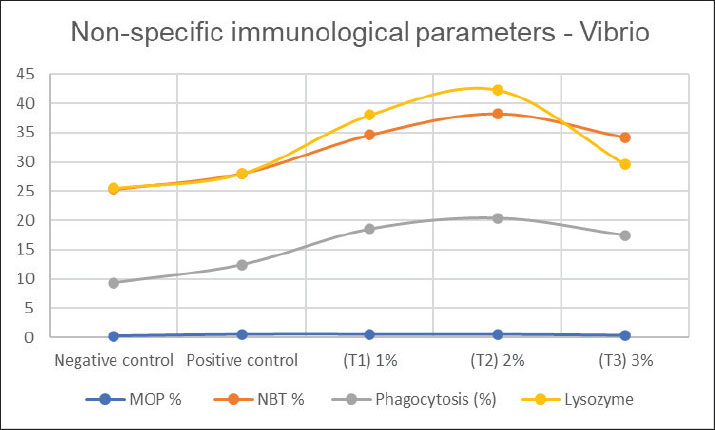
Fig. 4. Nonspecific immunological parameters in the blood of common carp after 56 days of feeding on different proportions of pumpkin seeds and after 14 days of experimental infection with V. parahaemolyticus. WG followed a similar pattern, with T2 demonstrating the best performance (17.1 g), while the control group showed the lowest gain (13.87 g). This finding is supported by research by Kadhim (2022), who indicated that fish receiving diets with higher levels of plant proteins exhibited improved WG compared with those on standard diets. The results indicate that the inclusion of pumpkin seeds positively impacts carp growth. The SGR results indicated that T2 (1.17% g/day) and T1 (1.13% g/day) had the highest growth rates, whereas the control group lagged at 1.0% g/day. These results are consistent with those of Seden et al. (2009), who noted that the SGRs of fish could significantly improve with the addition of plant-based protein sources to their diets. The improvement in SGR highlights the efficiency of pumpkin seeds as a nutrient source for growth promotion. Feed intake varied slightly, with T2 having the highest intake at 25.28 g feed/fish, indicating that the pumpkin seed-enriched diets were more attractive to carp. This aligns with the research by Diler et al. (2017), who found that the palatability of plant-based feeds significantly increased consumption in various fish species. The reason is attributed to the distinctive aromatic flavor of Origanum vulgare, which leads to increased feed intake. It also contains many active compounds that stimulate the secretion of digestive enzymes, thereby increasing food consumption and nutrient absorption. The findings indicate that incorporating pumpkin seeds not only improves growth metrics but also enhances feed consumption. The FCR was lowest in T1 (1.45), indicating the highest efficiency in converting feed into WG. T2 showed a slightly higher FCR (1.53), whereas the control group demonstrated the least efficient conversion at 1.77. This pattern aligns with the results of Mohammadi et al. (2020), who found that feeding Nile tilapia a diet containing 0.5% moringa with O. vulgare resulted in optimal WG, SGR, and FCR. The observed effects were attributed to the growth-promoting properties of active compounds, such as thymol and carvacrol, combined with the aromatic flavor that enhances feed palatability, stimulates digestive enzyme activity, and supports the metabolism of proteins, fats, and carbohydrates—qualities (Tiyaprasertkul et al., 2025). Improved FCR in the pumpkin seed-fed groups indicates enhanced nutrient use. Notably, all groups reported a survival rate of 100%, suggesting that the pumpkin seed-based diets did not adversely affect fish health. This is crucial because high survival rates are a key indicator of the effectiveness of aquaculture feeds (Oke et al. 2017). The safety and health of the fish in the study underscore the viability of using pumpkin seed-based diets in aquaculture. In summary, these findings indicate that incorporating pumpkin seeds into carp diets significantly enhances growth performance, as demonstrated by increased final weights, WGs, SGRs, and improved FCRs because “Pumpkin is an important source of carotenoids, a variety of amino acids, vitamins and minerals, useful fibers, so it has a high therapeutic and health care function with great nutritional and technological potential” (Ceclu et al., 2020). Our results do not agree with those of Fernández-Maestú et al. (2025), who indicated that fish fed a plant-based diet consumed significantly less feed compared to those fed a commercial-like diet, regardless of fish age or the size of the aquafeeds. This reduction in feed intake led to a lower SGR and relative growth rate in fish fed a plant-based diet. These results may be attributed to lower palatability or preference for plant-based diets, possibly due to variations in certain raw materials (Geurden et al., 2005) or nutrients (Roy et al., 2023). Additionally, plant-based diets may contain secondary metabolites that function as antinutritional factors, such as alkaloids found in legumes (e.g., peas and lupins) and saponins present in soybeans (Balasubramanian et al., 2016). The optimal inclusion level appears to be approximately 0.02 from the feed weight, a beneficial balance in the diet. These results are consistent with modern research that highlights the advantages of plant-based feeds in aquaculture, particularly for enhancing growth and feed efficiency. Al-Turaih et al. (2023) indicated an enhancement in growth performance after feeding the common carp on T. cordifolia. This might be attributed to T. cordifolia being a rich source of polysaccharides and polyphenols that support intestinal immunity and digestive enzyme function. The use of a natural phytobiotic mixture (Syrena Boost) has led to a significant improvement in performance, intestinal health, and immune physiology. It also improved certain blood hematological parameters, while decreasing the levels of aspartate aminotransferase, alanine aminotransferase, cholesterol, triglycerides, glucose, and albumin in Nile tilapia (Oreochromis niloticus) fingerlings (Mabrouk et al., 2025). Antioxidants and immune responseFish fed a diet supplemented with pumpkin seeds demonstrated improved biochemical parameters compared with those on the control diet. Al-Turki et al. (2008) highlighted that phenolic and flavonoid compounds possess immune-stimulating and antioxidant properties. During normal metabolism, fish produce reactive oxygen species (ROS); under typical physiological conditions, the antioxidant defense system mitigates the harmful effects of ROS through free radical scavenging. Oxidative stress occurs when the production of free radicals exceeds the capacity of the antioxidant defense system (Kumar and Pandey, 2013). This study compares these findings with recent aquaculture literature. Pumpkin seed oil has antioxidant, antibacterial, and anti-inflammatory properties, making it a promising ingredient for pharmaceutical and food applications (Mukherjee et al., 2022). Additionally, it has been found to protect against liver damage by reducing oxidative stress induced by sodium nitrate in rats (Rouag et al., 2020). Pumpkin seeds flavonoids exhibit anti-inflammatory and antimicrobial activities (Roger and Geissman, 2011). Consistent with these findings, other studies have reported the antioxidant potential of various pumpkin parts, particularly the seeds, using different assessment methods. The enrichment of phenolic compounds, sterols, tocopherols, and polyunsaturated fatty acids has been shown to enhance free radical scavenging activity (Mateos et al., 2003). Pumpkin seed oil contains significant amounts of tocopherols, which may contribute to its ability to neutralize free radicals (Amin et al., 2019). Similarly, a study by Dubey et al. (2010) demonstrated that the methanolic extract of C. pepo fruit exhibited moderate to strong antibacterial activity against various bacterial strains, including Bacillus subtilis, Escherichia coli, Enterobacter aerogenes, Salmonella enteritidis, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Cryptococcus meningitis. The antioxidant response in carp fed with pumpkin seeds, as measured by SOD and GPX activity, underscores the seeds’ role in enhancing fish health and resilience under stress conditions, such as infection. Pumpkin seed supplementation significantly increased SOD and GPX enzyme activity compared with the control diet. This effect is attributed to the phytochemicals present in pumpkin seeds (Jakab et al., 2003). Pumpkin seeds are also rich in unsaturated and essential fatty acids, including oleic acid (Omega-9), linoleic acid (Omega-6), and alpha-linolenic acid (Omega-3), which support hormone synthesis and immune regulation (Martins et al., 2023). Pumpkin seed extracts also combat oxidative stress, offering antioxidative benefits in cardiovascular diseases, triglyceride metabolism, and energy regulation (Oh et al., 2024). In fish, LYZ is a critical immune component that effectively dissolves bacterial cell wall peptidoglycans and activates the complement system and phagocytosis (Valipour et al., 2018). Maintaining a balance between oxidants and antioxidants is essential for cell viability, organ function, and overall health (Birben et al., 2012), according to the results reached by Ahmed et al. (2023). SOD activityBefore infection, SOD levels significantly increased with higher seed inclusions. The control group had the lowest SOD activity (50.81 IU/l), whereas the T2 (72.96 IU/l) and T3 (70.84 IU/l) groups exhibited the highest values, indicating that higher levels of seed incorporation enhance the antioxidant defense mechanism in carp. This is consistent with the findings of Negm et al. (2024), who demonstrated that dietary antioxidants could significantly enhance SOD activity in fish, promoting better oxidative stress management. After infection, SOD levels in the positive control and T1 groups remained relatively low (52.85 and 53.16 IU/l, respectively), while T2 maintained a notable increase (62.25 IU/l), demonstrating a robust antioxidant response. T3 showed a decrease to 58.06 IU/l but still indicated an improvement compared with the control groups. The ability of T2 to maintain higher SOD activity post-infection is supported by the work of Shabanzadeh et al. (2023), who revealed that fish fed diets supplemented with astaxanthin showed improved serum antioxidant levels. This improvement was evidenced by researchers through a measurable reduction in MDA levels, an established marker of oxidative stress, accompanied by an elevation in total antioxidant activity. This study agreed with Abbas et al. (2024), who used prickly pear fruits to feed tilapia fish and pointed out the high rate of SOD, as prickly pear fruits contain protein, fats, and fibers, which are also found in pumpkin seeds. This is an indication of the importance of the presence of these elements in fish feed to raise their immunity. GPX activityThe increased GPX activity with higher seed inclusion suggests an enhanced ability to neutralize ROS, supporting findings from Sánchez-Nuñoo et al. (2019). Moreover, dietary components can impact redox function in fish, highlighting the critical role of antioxidants such as SOD, CAT, and GPX in preserving redox balance and reducing oxidative damage (Sánchez-Nuño et al., 2019). GPX activity after infection reflected similar trends, with T2 showing the highest level at 14.82 IU/l, followed by T3 at 11.39 IU/l. The negative and positive control groups exhibited lower GPX values (6.01 and 6.05 IU/l, respectively), underscoring the benefits of pumpkin seed inclusion in diets. The results of the antioxidant response in carp fed on pumpkin seeds, as indicated by MPO, NBT reduction, phagocytosis, and LYZ activity, reveal valuable insights into the fish’s immune and antioxidant capacities. Myeloperoxidase activityBefore infection, MPO activity was significantly higher in T2 (0.48%) than in the control (0.17%). T1 (0.25%) and T3 (0.30%) also exhibited increased MPO activity, although not as pronounced as that of T2. Elevated MPO levels indicate enhanced neutrophil activity, which is crucial for the innate immune response. Similar findings by Kadhim (2022) that diets enriched with plant-based ingredients can boost antioxidant enzyme activities, enhancing the response of fish through their immune system. After infection, T2 exhibited the highest MPO activity (0.50%), indicating a sustained immune response. The increase in MPO post-infection reflects the fish’s ability to enhance their immune functions when challenged. This aligns with the observations of Lau et al. (2005) and Srivastava and Pandey (2015), who highlighted the role of dietary antioxidants in regulating immune responses in aquaculture species. They noted that neutrophils and monocytes secrete this compound during infections, which significantly contributes to innate immunity and boosts neutrophil activity in the bloodstream. NBT reductionBefore infection, NBT reduction, a measure of superoxide production, was highest in T2 (36.3%) and was significantly greater than that in the control (25.3%). This aligns with research by Khosravi et al. (2023), who found that antioxidant-rich diets could stimulate ROS production, necessary for effective pathogen defense. After infection, the NBT reduction was also the highest in T2 (38.24%), further indicating enhanced ROS production in response to infection. The positive control group showed a moderate increase (28.0%) in antioxidant capacity, highlighting that dietary influences can enhance the antioxidant capacity during stress. The results corroborate the findings of Kadhim (2022) that antioxidant-rich diets help maintain redox balance during immune challenges. PhagocytosisThe percentage of phagocytosis remained consistent across groups, with no significant differences observed. However, the control group exhibited the highest phagocytic activity (9.3%). This could indicate that while the antioxidant response improved, phagocytic activity may depend on other factors beyond diet. Phagocytic activity in fish can be influenced by overall health and stress levels. This improvement underscores the role of diet in bolstering immune function. This may be attributed to the increased phagocytic activity resulting from natural immune stimulants that stimulate the fish’s immune system. Probiotics adhering to the fish intestine can stimulate mucosal immunity, triggering signals that activate phagocytic cells within the intestinal mucosa and bloodstream (Pérez et al., 2010). This observation aligns with findings from studies on probiotic use in Nile tilapia (Tachibana et al., 2020). LYZ activityThis study highlights the role of pumpkin seeds in strengthening the connection between dietary antioxidants and improved immune function. The results from this study indicate that feeding carp on pumpkin seeds significantly enhances antioxidant responses and immune functions, as evidenced by increased MPO, NBT reduction, and LYZ activity, particularly at the optimal inclusion level of 0.02 (T2). This is in agreement with the findings of Shadrack et al. (2023), who observed significant improvements in liver LYZ activity, SOD, peroxidase activity, and biological antioxidant potential in fish that were supplemented with probiotics. Conclusion• The inclusion of pumpkin seeds in fish feed represents a valuable addition due to several nutritional and environmental advantages. Pumpkin seeds are rich in high-quality proteins that support muscle development in fish. They also contain essential fatty acids, such as omega-6 (linoleic acid) and omega-3 (alpha-linolenic acid), which contribute to skin and fin health and enhance immune function because they contain phenolics, selenium, and phytosterols. In addition, they are a source of important micronutrients, including zinc, magnesium, and vitamin E, which support various physiological processes and reduce oxidative stress. Improved nutrient use minimizes organic waste accumulation in culture systems, contributing to improved water quality. Additionally, the use of plant-based ingredients, such as pumpkin seeds, supports the reduction of dependence on animal-derived proteins, promoting sustainable aquafeed development. Pumpkin seeds are locally available in many regions, offering a cost-effective ingredient that may reduce overall feed expenses. They are rich in essential nutrients, which enhance the overall nutritional profile of the feed and may support fish growth and health. • PSF is highly digestible, allowing fish to use nutrients efficiently. • In this study, fish fed with diets containing pumpkin seeds showed better growth performance compared with those on conventional diets. Recommendations• Future research should focus on long-term field trials under commercial farming conditions, optimal inclusion levels across different developmental stages, and mechanistic studies to clarify how pumpkin seed bioactive compounds interact with fish immune pathways. Investigating their synergistic effects with probiotics or other phytogenics may also enhance formulation strategies for functional aquafeeds. AcknowledgmentsWe would like to thank the Aquaculture Unit, College of Agriculture, University of Basrah, for their assistance in obtaining fish. Conflict of interestsThe authors confirm the absence of any conflicts of interest related to the publication of this paper. Additionally, no financial support or funding was received that could have affected the results or interpretations outlined in this study. FundingThis research did not receive any specific grants from public, commercial, or nonprofit funding agencies. All research materials were provided by the primary researcher (Al-Hasson), and the study is part of a doctoral thesis. Authors’ contributionsHAH.A-H: Proposed the subject, wrote the manuscript, edited, laboratory work, and provided funding. KSA-N: Designed the study protocol, performed laboratory work supervision, and reviewed the manuscript. MElB: Supervised the laboratory work, reviewed the manuscript, and provided assistance and guidance throughout the research. Data availabilityAll data are provided within the article, included in the supplementary materials, or referenced accordingly. ReferencesAbbas, M.M.M., Amer, M.A., Al Malki, J.S., Mohammadein, A., Metwally, M.G., Waheed, R.M., Elraey, S.M.A. and Radwan, M. 2024. Elucidating the role of prickly pear fruits (Opuntia littoralis) in mitigation of cadmium toxicity in Nile tilapia: impacts on haemato-biochemical and immunological responses. Aquacult. Int. 32, 8877–8898; doi:10.1007/s10499-024-01596-z Abdelaziz, M., Ibrahem, M.D., Ibrahim, M.A., Abu-Elala, N.M. and Abdel-Moneam, D.A. 2017. Monitoring of different Vibrio species affecting marine fishes in Lake Qarun and Gulf of Suez: phenotypic and molecular characterization. Egypt. J. Aquat. Res. 43, 141–146. Abd-Elnoor, A.V. 2019. Hypoglycemic and hypolipidemic effects of pumpkin seeds powder and oil on alloxan-induced diabetic rats. Egypt. J. Food Sci. 47(2), 255–269. Abdel-Razek, N., Awad, S.M. and Abdel-Tawwab, M. 2019. Effect of dietary purslane (Portulaca oleracea L.) leaves powder on growth, immunostimulation, and protection of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila infection. Fish Physiol. Biochem. 45, 1907–1917; doi:10.1007/s10695-019-00685-8 Abdel-Tawwab, M. and Abbass, F.E. 2017. Turmeric powder (Curcuma longa L.) in common carp (Cyprinus carpio L.) diets: growth performance, innate immunity, and challenge against pathogenic Aeromonas hydrophila infection. J. World Aquac. Soc. 48, 303–312; doi:10.1111/jwas.12349 Aebi, H. 1984. Catalase. Methods Enzymol. 105, 121–126. Ahmed, A.R., Al-Hasson, H.A.H. and Al-Niaeem, K.S. 2023. The effect of Shilajit on growth performance, blood parameters, and key liver enzymes of the common carp (Cyprinus carpio). Egypt. J. Aquat. Biol. Fish. 27(4), 683–693; doi:10.21608/ejabf.2023.312094 Al-Shammari, N.A.H. 2024. Isolation and identification of some pathogenic bacteria and the effect of Aeromonas hydrophila on antioxidant enzymes in common carp (Cyprinus carpio L.) and Nile tilapia (Oreochromis niloticus L.) in Basrah Governorate, Iraq. PhD Thesis, University of Basrah, College of Agriculture, Basrah, Iraq. Al-Turaihi, Z.M.R., Ahmed, A.R. and Al-Niaeem, K.S. 2023. Effect of dietary Tinospora cordifolia supplementation on growth performance and hemato-biochemical parameters of the common carp (Cyprinus carpio). Egypt. J. Aquat. Biol. Fish. 27(4), 677–688. Al-Turki, A.I., El-Ziney, M.G. and Abdel-Salam, A.M. 2008. Chemical and anti-bacterial characterization of aqueous extracts of oregano, marjoram, sage, and licorice and their application in milk and labneh. J. Food Agric. Environ. 6(1), 39–44. Amend, D.F. 1981. Potency testing of fish vaccines. In Fish biologics: serodiagnostics and vaccines. Basel, Switzerland: Karger, pp: 447–454. Amin, M.Z., Islam, T., Mostofa, F., Uddin, M.J., Rahman, M.M. and Satter, M.A. 2019. Comparative assessment of the physicochemical and biochemical properties of native and hybrid varieties of pumpkin seed and seed oil (Cucurbita maxima Linn.). Heliyon 5, e02994; doi:10.1016/j.heliyon.2019.e02994 Balasubramanian, M.N., Panserat, S., Dupont-Nivet, M., Quillet, E., Montfort, J., Le Cam, A., Médale, F., Kaushik, S.J. and Geurden, I. 2016. Molecular pathways associated with the nutritional programming of plant-based diet acceptance in rainbow trout following an early feeding exposure. BMC Genomics. 17, 449; doi:10.1186/s12864-016-2804-1 Birben, E., Sahiner, U.M., Sackesen, C., Erzurum, S. and Kalayci, O. 2012. Oxidative stress and antioxidant defense. World Allergy Organ. J. 5, 9–19. Caruso, D., Schlumberger, O., Dahm, C. and Proteau, J.P. 2002. Plasma LYZ levels in sheatfish Silurus glanis (L.) subjected to stress and experimental infection with Edwardsiella tarda. Aquac. Res. 33, 999–1008. Ceclu, L., Mocanu, D.G. and Nistor, O.V. 2020. Pumpkin – health benefits. J. Agroaliment. Process Technol. 26(3), 241–246. Dadras, F., Velisek, J. and Zuskova, E. 2023. An update about beneficial effects of medicinal plants in aquaculture: a review. Vet. Med. 68(12), 449–463; doi:10.17221/96/2023-VETMED Diler, O., Gormez, O., Diler, I. and Metin, S. 2017. Effect of oregano (Origanum onites L.) essential oil on growth, LYZ and antioxidant activity, and resistance against Lactococcus garvieae in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Nutri. 23(4), 844–851. Dubey, A., Mishra, N. and Singh, N. 2010. Antimicrobial activity of some selected vegetables. Int. J. Appl. Biol. Pharm. 1(3), 995–999. Dytham, C. 2011. Choosing and using statistics: a biologist’s guide. Chichester, UK: Blackwell Science Ltd. Elgendy, M.Y., Ali, S.E., Dayem, A.A., Khalil, R.H., Moustafa, M.M. and Abdelsalam, M. 2024. Alternative therapies recently applied in controlling farmed fish diseases: mechanisms, challenges, and prospects. Aquacu. Int. 32(6), 9017–9078; doi:10.1007/s10499-024-01603-3 FAO. 2024. Making aquafeed more sustainable and accessible . Rome, Italy: Food and Agriculture Organization (FAO); doi:10.4060/cd1542en Fernandez-Maestú, C., Calo, J., Martinat, M., Soengas, J.L., Roy, J. and Blanco, A.M. 2025. Effects of a plant-based diet from first feeding on the intestinal expression of nutrient sensors in rainbow trout (Oncorhynchus mykiss). Aquaculture 599, 1–14. Geurden, I., Cuvier, A., Gondouin, E., Olsen, R., Ruohonen, K., Kaushik, S. and Boujard, T. 2005. Rainbow trout can discriminate between feeds with different oil sources. Physiol. Behav. 85, 107–114; doi:10.1016/j.physbeh.2005.03.010 Hassaan, M.S., El-Sayed, A.M.I., Mohammady, E.Y., Zaki, M.A., Elkhyat, M.M., Jarmołowicz, S. and El-Haroun, E.R. 2021. Eubiotic effect of a dietary potassium diformate (KDF) and probiotic (Lactobacillus acidophilus) on growth, hemato-biochemical indices, antioxidant status, and intestinal functional topography of cultured Nile tilapia (Oreochromis niloticus) fed diet free fishmeal. Aquaculture 533, 736147. Hepher, B. 1988. Nutrition of pond fishes. Cambridge, UK: Cambridge University Press, p: 388. Herwig, N., Garibaldi, L. and Walke, R.E. 1979. Handbook of drugs and chemicals used in the treatment of fish disease. Charles C. Thomas Publisher. Hwihy, H.M., Zeina, A.F., Abu Husein, M.Sh. and El-Tabakh, M.A.M. 2025. Biofloc biosecurity: a revolutionary paradigm in augmenting aquaculture health and resilience against Aeromonas hydrophila. Aquac. Fish. 10, 608–616; doi:10.1016/j.aaf.2024.03.001 Ibrahim, R.E., Amer, S.A., Rhouma, N.R., Younis, E.M., Abdelwarith, A.A., Abdel-Ghany, H.M., Elshobaky, G.E., El-Saber, M.M., Osman, A., Davies, S.J. and Rahman, A.N.A. 2024. Using moringa (Moringa oleifera) seed protein hydrolysate as a dietary protein supplement modulates hemato-biochemical indices, antioxidant response, and tissue histo-morphology of Nile tilapia (Oreochromis niloticus). Aquac. Int. 32(6), 9157–9178; doi:10.1007/s10499-024-01608-y Jakab, A., Jablonkai, I. and Forgács, E. 2003. Quantification of the ratio of positional isomer dilinoleoyl-oleoyl glycerols in vegetable oils. Rapid Commun. Mass. Spectrom. 17(20), 2295–2302. Kadhim, A.H. 2022. Utilize Origanum majorana and Brassica oleracea to enhance the common carp Cyprinus carpio L. resistance against Staphylococcus lentus and their effect on some health parameters. PhD thesis, University of Basrah, College of Agriculture, Basrah, IRAQ, p 195. Karatas, T., Sokmen, T.O. and Akgol Gur, S.T. 2025. Effects of pumpkin seed extract on growth, blood biochemistry, antioxidant status, immunity, and digestive enzymes of common carp (Cyprinus carpio L.) fingerlings. Aquac. Rep. 43, 1028902; doi:10.1016/j.aqrep.2025.102890 Keskin Çavdar, H. 2019. Active compounds, health effects, and extraction of unconventional plant seed oils. In Plant and human health, Volume 2. Eds., Ozturk, M. and Hakeem, K. Cham, Switzerland: Springer, pp: 245–285; https://doi.org/10.1007/978-3-030-03344-6_10 Khosravi, M., Asl, S.T.S., Anamag, A.N., Langaroudi, M.S., Moharami, J., Ahmadi, S., Ganjali, A., Ghiasi, Z., Nafeli, M. and Kasaeiyan, R. 2023. Parenting styles, maladaptive coping styles, and disturbed eating attitudes and behaviors: a multiple mediation analysis in patients with feeding and eating disorders. PeerJ 11, e14880; doi:10.7717/peerj.14880 Kumar, S. and Pandey, A.K. 2013. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013, 1–16; doi:10.1155/2013/162750 Lau, D., Mollnau, H., Eiserich, J.P., Freeman, B.A., Daiber, A., Gehling, U.M., Brümmer, J., Rudolph, V., Münzel, T., Heitzer, T. and Meinertz, T. 2005. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. U S A. 102(2), 431–436; doi:10.1073/pnas.0405193102 Lee, Y.K., Chung, W.I. and Ezura, H. 2003. Efficient plant regeneration via organogenesis in winter squash (Cucurbita maxima Duch). Plant Sci. 164(3), 413–418; doi:10.1016/S0168-9452(02)00433-4 Mabrouk, S.G., El-Nokrashy, A.M., Ebied, N.A., Abdella, B.H., Zayed, M.M., Aboleila, S.M. and Mohamed, R.A. 2025. A blend of natural phytobiotics enhances growth performance, feed efficiency, and the immuno-health status of fingerlings of Nile tilapia (Oreochromis niloticus). Open Vet. J. 15(2), 746–764; doi:10.5455/OVJ.2025.v15.i2.24 Martins, N., Magalhães, R., Vieira, L., Couto, A., Serra, C.R., Maia, M.R.G., Fonseca, A.J.M., Cabrita, A.R.J., Pousão-Ferreira, P., Castro, C., Peres, H. and Oliva-Teles, A. 2023. Dietary oleic acid supplementation improves feed efficiency and modulates fatty acid profile and cell signaling pathway in European sea bass (Dicentrarchus labrax) juveniles fed high-lipid diets. Aquaculture 576, 739870; doi:10.1016/j.aquaculture.2023.739870 Mateos, R., Domínguez, M.M., Espartero, J.L. and Cert, A. 2003. Antioxidant effect of phenolic compounds, alpha-tocopherol, and other minor components in virgin olive oil. J. Agric. Food Chem. 51(24), 7170–7175; doi:10.1021/jf034415q McCord, J.M. and Fridovich, I. 1969. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Bio.l Chem. 244, 6049–6055. Mohammadi, G., Rafiee, G., El Basuini, M.F., Van Doan, H., Ahmed, H.A., Dawood, M.A. and Abdel-Latif, H.M. 2020. Oregano (Origanum vulgare), St John’s-wort (Hypericum perforatum), and lemon balm (Melissa officinalis) extracts improved the growth rate, antioxidative, and immunological responses in Nile tilapia (Oreochromis niloticus) infected with Aeromonas hydrophila. Aquac. Rep. 18, 100445; doi:10.1016/j.aqrep.2020.100445 Monsang, S.J., Acharya, A., Choudhury, T.G. and Kamilya, D. 2025. Immunoadjuvant potential of Asparagus racemosus ethanolic root extract on protection and immune response of Labeo rohita immunized with inactivated Aeromonas hydrophila vaccine. Aquac. Int. 33, 26; doi:10.1007/s10499-024-01695-x Mukherjee, D., Saha, S., Chukwuka, A.V., Ghosh, B., Dhara, K., Saha, N.C., Pal, P. and Faggio, C. 2022. Antioxidant enzyme activity and pathophysiological responses in the freshwater walking catfish, Clarias batrachus Linn under sub-chronic and chronic exposures to the neonicotinoid, Thiamethoxam. Sci. Total Environ. 836, 155716; doi:10.1016/j.scitotenv.2022.155716 Negm, A.E., Abo-Raya, M.H., Gabr, A.M., Baloza, S.H., El-Nokrashy, A., Prince, A., Arana, D., Wang, Y., Abdelazeem, S., Albadrani, G.M. and Al-Ghadi, M.Q. 2024. Effects of phytase enzyme supplementation on growth performance, intestinal morphology and metabolism in Nile tilapia (Oreochromis niloticus). J. Anim. Physiol. Anim. Nutr. 1–18; doi:10.1111/jpn.13917 Nor-Amalina, Z., Dzarifah, M.Z., Mohd-Termizi, Y., Amal, M.N.A., Zamri-Saad, M. and Ina-Salwany, M.Y. 2017. Phenotypic and genotypic characterization of Vibrio species isolates from Epinephelus species in Selangor, Malaysia. In Proceedings of the International Conference on Advances in Fish Health, April 4–6, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, 2017. Oftebro, T.L., Veylit, L., Tiller, R., Strand, A.V., Misund, A. and Thorvaldsen, T. 2025. What’s next for Norwegian salmon farming? Stakeholder perceptions on what influences industry development. Aquaculture 599, 1–11; doi:10.1016/j.aquaculture.2025.740102 Oh, J., Hong, S., Ko, S.-H. and Kim, H.-S. 2024. Evaluation of antioxidant effects of pumpkin (Cucurbita pepo L.) seed extract on aging- and menopause-related diseases using Saos-2 cells and ovariectomized rats. Antioxidants 13, 241; doi:10.3390/antiox13020241 Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358; doi:10.1016/0003-2697(79)90738-3 Oke, I.O., Dada, A.A. and Adebayo, A.O. 2017. Dietary effects of Brassica oleracea on growth performance and haematological parameters of Oreochromis niloticus fingerlings. J. Food Agric. Environ. 13, 133–136. Öztürk, T. and Turhan, S. 2020. Physicochemical properties of pumpkin (Cucurbita pepo L.) seed kernel flour and its utilization in beef meatballs as a fat replacer and functional ingredient. J. Food Process. Preserv. 44, e14695; doi:10.1111/jfpp.14695 Paglia, D.E. and Valentine, W.N. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70, 158–169. Pérez, T., Balcázar, J.L., Ruiz-Zarzuela, I., Halaihel, N., Vendrell, D., de Blas, I. and Múzquiz, J.L. 2010. Host–microbiota interactions within the fish intestinal ecosystem. Mucosal Immunol. 3(4), 355–360; doi:10.1038/mi.2010.12 Perez-Gutierrez, R.M. 2016. Review of Cucurbita pepo (pumpkin), its phytochemistry and pharmacology. Med. Chem. 6, 12–21. Peteri, A. 2009. Cultured aquatic species information programme. Cyprinus carpio. Rome: FAO Fisheries and Aquaculture Department. Quade, M. and Roth, J.A. 1994. A rapid direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 58, 239–289. Rašković, B., Stanković, M., Markelić, M. and others. 2024. Growth, feed utilization, and quantitative histological assessment of the distal intestine and liver of common carp (Cyprinus carpio L.) fed formulated diets containing grains of different soybean cultivars. Aquac. Int. 32, 6903–6921; doi:10.1007/s10499-024-01494-4 Roger, A. and Geissman, T.A. 2011. Chemistry of flavonoid compounds. Macmillan J. Am. Chem. Soc. 61(8), 132–212. Rook, G.A.W., Steele, J., Umar, S. and Dockrell, H.M. 1985. A simple method for the solubilization of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by γ-interferon. J. Immunol. Methods, 82, 161–167. Rouag, M., Berrouague, S., Djaber, N., Khaldi, T., Boumendjel, M. and Taibi, F. 2020. Pumpkin seed oil alleviates oxidative stress and liver damage induced by sodium nitrate in adult rats: biochemical and histological approach. Afr. Health. Sci. 20(1), 413–425. Roy, J., Baranek, E. and Marandel, L. 2023. Characterization of free fatty acid receptor family in rainbow trout (Oncorhynchus mykiss): towards a better understanding of their involvement in fatty acid signalisation. BMC Genom. 24, 130; doi:10.1186/s12864-023-09181-z Sánchez-Nuño, S., Carbonell, T. and Valls, A.I. 2019. Redox balance affects fish welfare. Intech Open, pp: 1–18; doi:10.5772/intechopen.89842 Sayed-Lafi, R.M., Shtewi, H.H., Sultan, F.A. and Al-Shammari, N.A. 2024. Pomegranate peel extract diet enhances health and immunity of common carp (Cyprinus carpio) against Aeromonas veronii. Open Vet. J. 14(11), 2762–2773; doi:10.5455/OVJ.2024.v14.i11.5 Schäperclaus, W. 1992. Fish diseases. Rotterdam, Netherland: CRC Press. Seden, M.E.A., Abbass, F.E. and Ahmad, M.H. 2009. Effect of Origanum vulgare as a feed additive on growth performance, feed utilization, and whole-body composition of Nile tilapia (Oreochromis niloticus) fingerlings challenged with pathogenic Aeromonas hydrophila. J. Anim. Poult. Prod. 34(3), 1683–1695. Shabanzadeh, S., Vatandoust, S., Hosseinifard, S.M., Sheikhzadeh, N. and Shahbazfar, A.A. 2023. Dietary astaxanthin (Lucantin Pink) mitigated oxidative stress induced by diazinon in rainbow trout (Oncorhynchus mykiss). Vet. Res. Forum. 14, 97–104. Shadrack, R.S., Kotra, K.K., Gereva, S.D., Teiba, I.I., El-Ratel, I.T. and El Basuini, M.F. 2023. Utilizing dietary probiotics can boost amberjack (Seriola dumerili) LYZ activity, antioxidant capacity, and gut microbiota. Sci. African 22, e01491; doi:10.1016/j.sciaf.2023.e01491 Srivastava, P.K. and Pandey, A.K. 2015. Role of immunostimulants in immune responses of fish and shellfish. Biochem. Cell. Arch. 15(1), 47–73. Swain, S., Sawant, P.B., Chadha, N.K., Chhandaprajnadarsini, E.M. and Katare, M. 2020. Significance of water pH and hardness on fish biological processes: a review. Int. J. Chem. Stud. 8(4), 315–320; doi:10.22271/chemi.2020.v8.i4e.9710 Szlaminska, M. and Przybyl, A. 1986. Feeding of carp (Cyprinus carpio L.) larvae with an artificial dry food, living zooplankton and mixed food. Aquaculture 54(1–2), 77–82; doi:10.1016/0044-8486(86)90115-3 Tachibana, L., Telli, G.S., Dias, D.C., Gonçalves, G.S., Ishikawa, C.M., Cavalcante, R.B., Natori, M.M., Ben Hamed, S. and Ranzani-Paiva, M.J.T. 2020. Effect of feeding strategy of probiotic Enterococcus faecium on growth performance, hematologic, biochemical parameters, and non-specific immune response of Nile tilapia. Aquac. Rep. 16, 100277; doi:10.1016/j.aqrep.2020.100277 Tiyaprasertkul, P., Phungkeha, P., Srikijkasemwat, K., Philatha, A., Rassmidatta, K., Ruangpanit, Y., Siwapirunthep, P., Yan, F., Romero-Sanchez, H. and Chaosap, C. 2025. Thymol-carvacrol supplementation in broilers: impact on performance, blood biomarkers, and gut health. Int. J. Agric. Tech. 21(2), 741–752; doi:10.63369/ijat.2025.21.2.741-752 Valipour, A., Heidari, B., Hadavi, M. and Yousefi, A. 2018. Changes in immune parameters (LYZ, IgM, C3) in early life stages and broodstock of Siberian sturgeon, Acipenser baerii. Fish Aqua. Life 26, 21–30. Velasco-Tirado, V., Romero-Alegría, Á., Belhassen-García, M., Alonso-Sardón, M., Esteban-Velasco, C., López-Bernús, A., Carpio-Perez, A., Jimenez López, M.F., Muñoz Bellido, J.L., Muro, A. and Cordero-Sanchez, M. 2017. Recurrence of cystic echinococcosis in an endemic area: a retrospective study. BMC Infect. Dis. 17, 455; doi:10.1186/s12879-017-2553-8 Wanna Vijit, P., Outama, C., Le Xuan, C.M., Fontana, M., Paolucci, M.A., Ahmed Sumon, E., El-Haroun, H. and Van Doan, H. 2024. Evaluation of longan (Dimocarpus longan) peel powder as fruit by-product additive in Nile tilapia (Oreochromis niloticus) feed: effects on growth, immunity, and immune-antioxidant gene expressions. Heliyon 11, e41609; doi:10.1016/j.heliyon.2024.e41609 | ||
| How to Cite this Article |
| Pubmed Style Al-hasson HA, Al-niaeem KS, Elbour M. Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpio. Open Vet. J.. 2025; 15(9): 4418-4431. doi:10.5455/OVJ.2025.v15.i9.49 Web Style Al-hasson HA, Al-niaeem KS, Elbour M. Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpio. https://www.openveterinaryjournal.com/?mno=256444 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.49 AMA (American Medical Association) Style Al-hasson HA, Al-niaeem KS, Elbour M. Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpio. Open Vet. J.. 2025; 15(9): 4418-4431. doi:10.5455/OVJ.2025.v15.i9.49 Vancouver/ICMJE Style Al-hasson HA, Al-niaeem KS, Elbour M. Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpio. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4418-4431. doi:10.5455/OVJ.2025.v15.i9.49 Harvard Style Al-hasson, H. A., Al-niaeem, . K. S. & Elbour, . M. (2025) Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpio. Open Vet. J., 15 (9), 4418-4431. doi:10.5455/OVJ.2025.v15.i9.49 Turabian Style Al-hasson, Hayder A.h., Khalidah S. Al-niaeem, and Monia Elbour. 2025. Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpio. Open Veterinary Journal, 15 (9), 4418-4431. doi:10.5455/OVJ.2025.v15.i9.49 Chicago Style Al-hasson, Hayder A.h., Khalidah S. Al-niaeem, and Monia Elbour. "Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpio." Open Veterinary Journal 15 (2025), 4418-4431. doi:10.5455/OVJ.2025.v15.i9.49 MLA (The Modern Language Association) Style Al-hasson, Hayder A.h., Khalidah S. Al-niaeem, and Monia Elbour. "Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpio." Open Veterinary Journal 15.9 (2025), 4418-4431. Print. doi:10.5455/OVJ.2025.v15.i9.49 APA (American Psychological Association) Style Al-hasson, H. A., Al-niaeem, . K. S. & Elbour, . M. (2025) Effects of dietary pumpkin seed flour (Cucurbita pepo) supplementation on growth, physiological condition, and resistance to pathogens challenge in Cyprinus carpio. Open Veterinary Journal, 15 (9), 4418-4431. doi:10.5455/OVJ.2025.v15.i9.49 |