| Research Article | ||
Open Vet. J.. 2025; 15(9): 4382-4392
Open Veterinary Journal, (2025), Vol. 15(9): 4382-4392 Research Article Meta-analysis of Newcastle disease virus prevalence among poultry in mainland ChinaWei Cheng1†, Mingfeng Chu1†, Huiying Zhang1, Yuchen Liang1, Honghai Wang2, Shuiyun Chen1, Yiwei Wang1, Mengke Si1, Xiaoyu Zhong1, Baolei Yang1, Xuelong Chen1,3 and Yanping Qi1,3*1Anhui Province Key Laboratory of Animal Nutritional Regulation and Health, Anhui Science and Technology University, Fengyang, China 2Daqing Agricultural and Rural Bureau, Daqing, Heilongjiang, China 3Anhui Engineering Technology Research Center of Pork Quality Control and Enhance, Anhui Science and Technology University, Fengyang, China *Corresponding Author: Yanping Qi. Anhui Province Key Laboratory of Animal Nutritional Regulation and Health, Anhui Science and Technology University, Fengyang, China. Email: qiyanping2018 [at] vip.163.com †These authors have contributed equally to this work. Submitted: 13/03/2025 Revised: 15/07/2025 Accepted: 01/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
AbstractBackground: Newcastle disease is an extremely contagious and economically damaging poultry disease caused by Avian orthoavulavirus 1 [Newcastle disease virus (NDV)], a member of Paramyxoviridae. Aim: This study was conducted as a systematic review and meta-analysis aimed at assessing NDV prevalence among poultry populations in mainland China. Methods: A total of 25 relevant epidemiological studies published between 1979 and 2023 were analyzed following the MOOSE guidelines. Results: Our meta-analysis revealed an overall NDV prevalence of 2.17% (95% CI: 1.95%–2.42%) with significant heterogeneity (I2=97.1%, p < 0.001). Southern China exhibited the highest individual study prevalence, while southwestern regions reported the lowest (0.32%). No aggregated regional prevalence exceeded 10%, suggesting localized outbreaks. Prevalence decreased from 3.19% (before 2013) to 1.03% (post-2013 era), reflecting improved biosecurity and vaccination. RT-PCR-based studies showed lower heterogeneity (I2=89.5%) compared to serological assays (I2=97.1%), highlighting the challenges in comparing prevalence estimates across different diagnostic methods and underscoring the need for standardized diagnostics.emphasizing the need for standardized diagnostics. Publication Bias: Funnel plot asymmetry indicated underrepresentation of small-scale studies (p=0.003). Conclusion: These results highlight persistent NDV challenges in high-density poultry areas (e.g., southern China) and underscore the importance of molecular diagnostics (RT-PCR) for accurate surveillance. Given persistent hotspots in high-density poultry areas (e.g., Guangdong), targeted vaccination and region-specific biosecurity measures are critical to mitigating NDV transmission. Keywords: Systematic review, Newcastle disease virus, China, Epidemiology, Poultry. IntroductionNewcastle disease virus (NDV; avian orthoavulavirus 1, APMV-1) is a highly contagious negative-sense RNA virus belonging to the Paramyxoviridae family (Abd Elfatah et al., 2021). It exhibits significant genotypic diversity and variable pathogenicity, resulting in a broad spectrum of clinical manifestations in avian hosts. These range from mild respiratory or digestive signs to severe, often fatal, systemic disease characterized by respiratory distress, neurological symptoms (e.g., paralysis and tremors), decreased production, and high mortality (Absalón et al., 2019). A substantial proportion (approximately 20%) of infections result in asymptomatic carriers, posing significant challenges for disease surveillance and control (Cattoli et al., 2011). Since its initial identification in 1926, the virus has spread efficiently through direct contact, respiratory secretions, feces, contaminated fomites, feed, water, and via migratory birds, facilitating its global dissemination (Apopo et al., 2019; Lu et al., 2021). Introduced in China in the 1930s, NDV has since caused recurrent outbreaks, inflicting substantial economic losses through mortality and culling (Lu, 2009; Yu, 2014; Zhang, 2016; Wei, 2018; Qiao, 2020). Comprehensive national control strategies, including quarantine, vaccination, and stamping-out policies, have been implemented (Feng et al., 2016; Duan et al., 2017; Jia et al., 2022). Despite these efforts and widespread vaccination, NDV remains endemic, with identified persistent transmission hotspots, particularly in southern China. Regional epidemiological studies consistently report the highest prevalence rates in the south, contrasting markedly with lower rates observed in southwestern regions (Huang, 2017). This geographical disparity underscores the country’s complexity of NDV epidemiology. Accurate quantification of NDV prevalence and understanding its epidemiological drivers are crucial for refining control strategies, optimizing vaccination programs, and mitigating economic impacts (Liu et al., 2019). Although numerous regional studies exist, a comprehensive synthesis of NDV prevalence across mainland China, accounting for spatial and temporal heterogeneity, is lacking. Therefore, this study aimed to conduct a systematic review and meta-analysis to provide a quantitative assessment of NDV prevalence in Chinese poultry populations, identify key associated factors, and inform evidence-based disease control approaches. Materials and MethodsStudy selectionAs per the MOOSE guidelines, a comprehensive search was conducted to identify all epidemiological studies reporting NDV prevalence in poultry populations within mainland China, published in English or Chinese between January 1, 1979, and June 25, 2023. The following electronic databases were systematically searched. English databases: PubMed, Google Scholar, Cochrane Library, and ClinicalTrials.gov. Chinese databases: VIP (Chongqing VIP Information Co., Ltd.), CNKI (China National Knowledge Infrastructure), and Wanfang Data. Search strategies utilized a combination of medical subject headings terms and keywords related to NDV (“Newcastle disease”, “ND”, “NDV”, and “Avian orthoavulavirus 1”), epidemiology (“epidemiology”, “prevalence”, “incidence”, and “surveillance”), poultry species (“poultry”, “chicken”, “duck”, “goose”, and “turkey”), and geographical scope (“China”). Boolean operators (AND, OR) were used to combine the terms. Specific search strings for each database are available upon request. The study selection process followed the PRISMA flow diagram (Fig. 1).
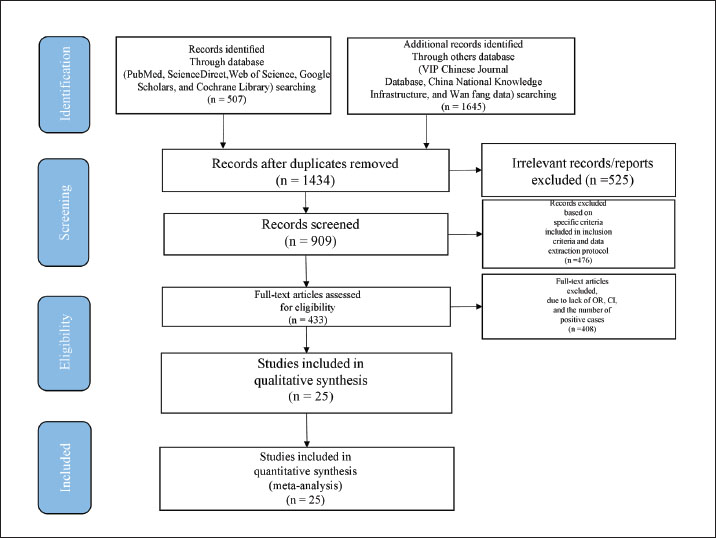
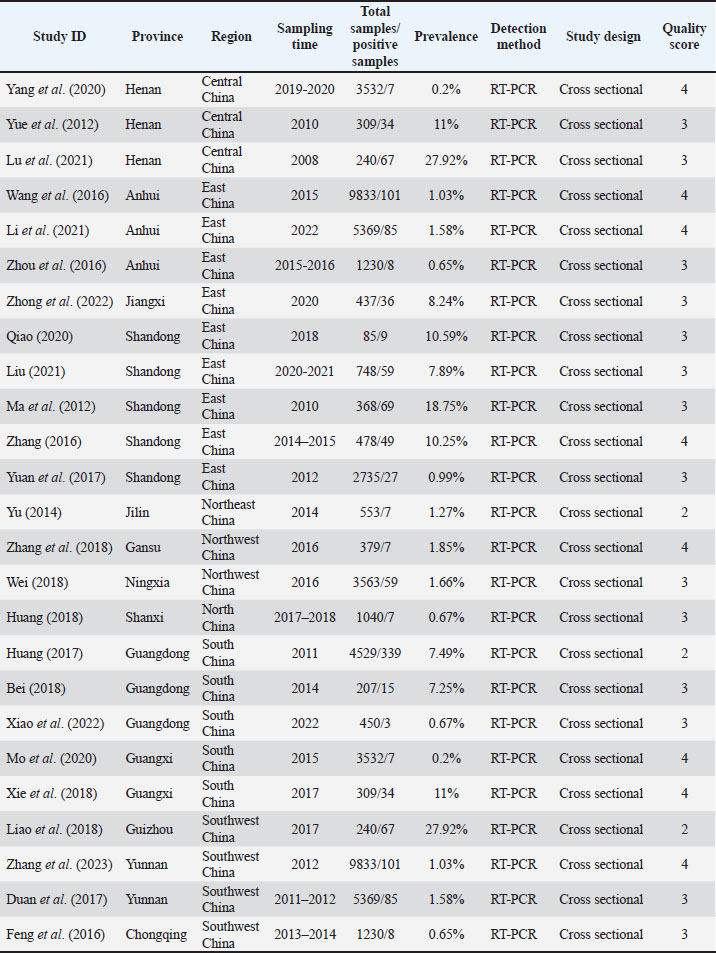
Fig. 1. PRISMA flow diagram of the literature search, screening, assessing for eligibility, and selecting articles for the meta-analysis. Inclusion and exclusion criteriaInclusion criteria: -Extractable prevalence data (within/between flocks) -Spatiotemporal sampling details -Inclusion of backyard flocks (<30 birds/unit). Studies were excluded from this analysis if they met any of the following criteria: studies with sample sizes < 30 poultry, studies conducted outside of China, studies unrelated to avian research, non-research publications (newsletters, press releases, and forums), basic research focused on NDV, non-epidemiological studies, studies without information pertaining to sample size, sampling time, prevalence, or rates of NDV infection, and studies based solely on questionnaires without any researcher-implemented analyses. Data retrievalData were extracted from selected studies into structured tables, including study characteristics (Table 1) and NDV prevalence details (region, sample type, diagnostic method, etc.; Table 2). Table 1. Included studies focused on NDV-infected poultry in mainland China.
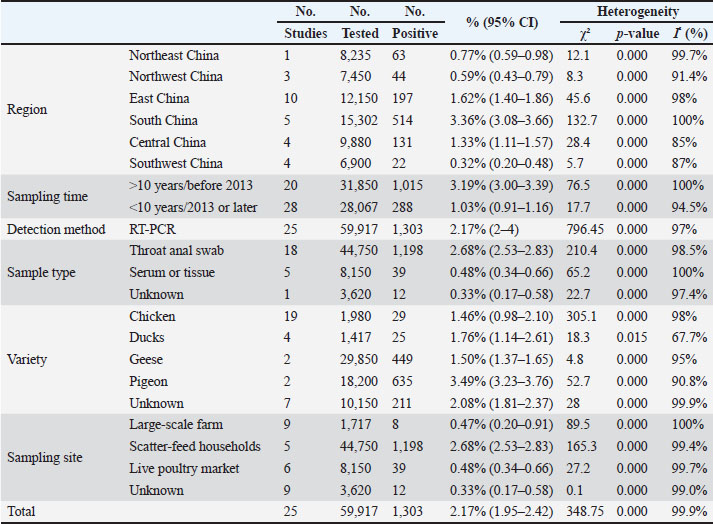
Table 2. Pooled NDV infection prevalence among poultry in mainland China.
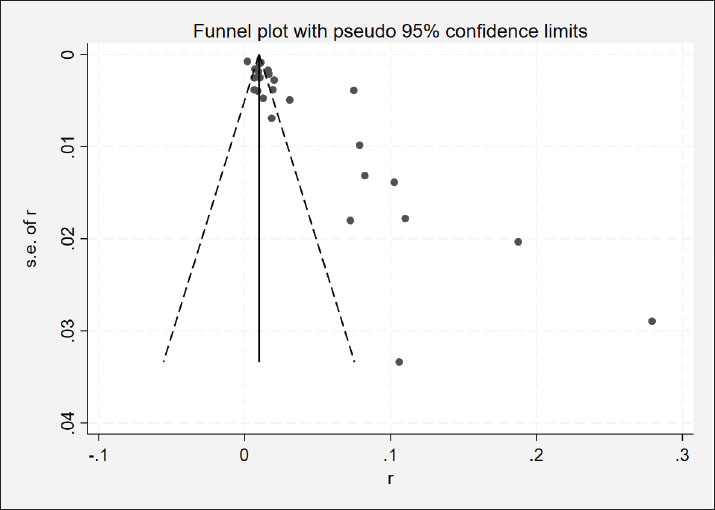
Study quality analysisThe Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework was used to assess the quality of the retrieved studies. Briefly, each of these publications was scored according to whether or not a clear research objective, explicit testing method, subject subgroup categorization, detailed sampling methodology, and accurate analyses of relevant risk factors were provided. These scores were categorized as high quality (4–5 points), moderate quality (2–3 points), or low quality (1 point). Any study with a quality score of 0 was omitted from further analysis. Statistical analysesThe prevalence of NDV across eligible studies was analyzed using a random-effects model. Combined estimates and corresponding 95% confidence intervals are presented with forest plots. The Q and I2 statistics were used to evaluate heterogeneity across studies. Funnel plots and Egger’s regression test were used to test for possible publication bias (Fig. 2). These analyses explored a range of factors, including sampling time, publication year, sample type, assay method, poultry species, sampling location, and testing time. The 25-item Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used to conduct the meta-analysis, and all analyses were performed using Stata 12 (Stata Corp., TX, USA).
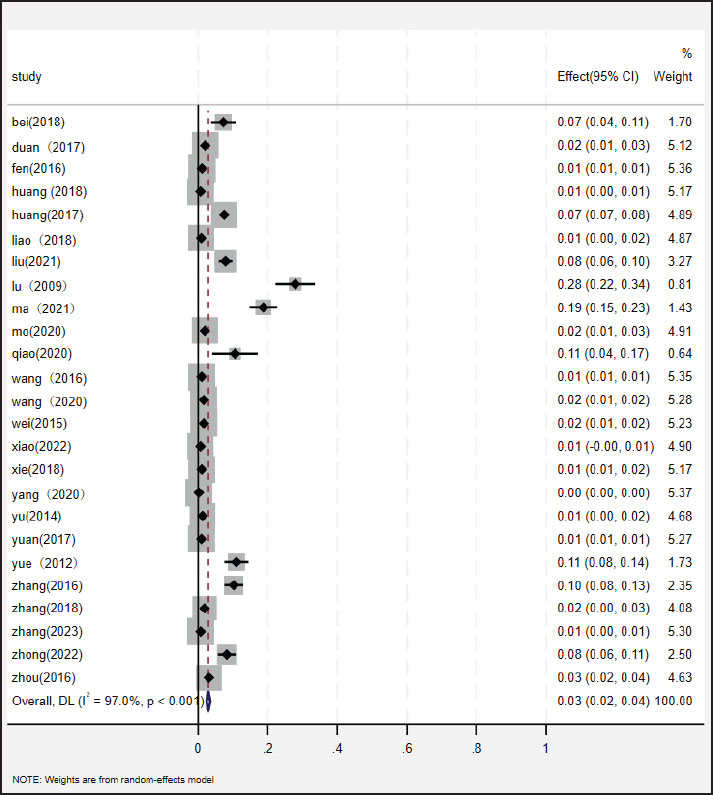
Fig. 2. Funnel plot with pseudo 95% confidence limits for examination of publication bias. Ethical approvalNot needed for this study. ResultsStudy selection and characteristicsThe study selection process followed the PRISMA guidelines, as illustrated in the flow diagram (Fig. 1). Initial searches across English databases (PubMed, ScienceDirect, Web of Science, Google Scholar, and Cochrane Library) yielded 507 records, while Chinese databases (VIP, China National Knowledge Infrastructure CNKI, and Wanfang Data) contributed 1,645 additional records, resulting in a total of 2,152 records for screening. After 718 duplicates were removed, 1,434 unique records underwent title and abstract screening. Of these, 525 records were excluded because of their irrelevance to the research scope. The remaining 909 full-text articles were assessed for eligibility, and 476 studies were excluded based on predefined criteria, including insufficient sample size (<30), non-epidemiological focus, or lack of diagnostic data. Ultimately, 25 studies covering 59,917 poultry samples across 17 provinces in mainland China published between 2009 and 2023 met the inclusion criteria. Among these, 3,396 samples tested positive for NDV. Study quality, evaluated using the GRADE framework, classified 8 studies as high quality (scores 4–5) and 17 as moderate quality (scores 2–3) (Table 1). Overall prevalence and heterogeneityThe random-effects meta-analysis (Fig. 3) revealed an overall prevalence of 2.17% (95% CI: 1.95%–2.42%), with extreme heterogeneity (I2=97.1%, p < 0.001). Individual study estimates ranged from 0% (Yang et al., 2020) to 28% (Lu, 2009). Subgroup analyses highlighted significant disparities: Regional variation: Southern China exhibited the highest prevalence (3.36%, 95% CI: 3.08%–3.66%), followed by Guangdong (7.49%) and Guangxi (11%). In contrast, Southwest China (Yunnan, Chongqing) reported the lowest prevalence (0.32%, 95% CI: 0.20%–0.48%) (Table 2). Temporal decline: Prevalence dropped from 3.19% (pre-2013) to 1.03% (post-2013), correlating with nationwide vaccination campaigns and biosecurity improvements.
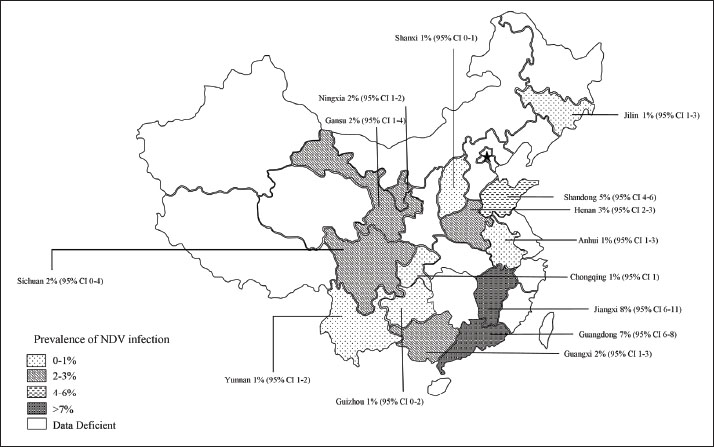
Fig. 3. Random-effects meta-analysis of NDV infection in poultry in mainland China. Methodological and regional influencesDetection methods: RT-PCR-based studies (n=25) showed lower heterogeneity (I2=89.5%) than serological assays. Throat/anal swabs, which detect active viral shedding, yielded higher detection rates (2.68%, 95% CI: 2.53%–2.83%) than serum/tissue samples (0.48%, 95% CI: 0.34%–0.66%), likely due to the environmental degradation of non-invasive samples. Poultry species: Chickens predominated (n=19 studies, 1.46% prevalence), while pigeons showed the highest infection rates (3.49%, 95% CI: 3.23%–3.76%). Publication bias and spatial patternsPublication bias: Funnel plot asymmetry (Fig. 2) and Egger’s test (p=0.003 and p=0.003, respectively) indicated underrepresentation of small-scale studies with low prevalence estimates. Trim-and-fill adjustment minimally altered the pooled prevalence (2.11%, 95% CI: 1.88%–2.36%), confirming the robustness of the results. Geospatial distribution: The NDV hotspot map (Fig. 4) identified sustained transmission in southern provinces (Guangdong, Guangxi), where high poultry density (>800 million birds), humid climates, and live poultry markets facilitated the spread of the disease. Conversely, the mountainous terrain and low-density farming in Southwest China correlated with minimal prevalence (Table 2).
Fig. 4. Map of NDV infection in poultry in mainland China. Subgroup analysis of risk factorsSampling sites: Live poultry markets showed lower prevalence (0.48%, 95% CI: 0.34%–0.66%) than scattered households (2.68%, 95% CI: 2.53%–2.83%), suggesting localized outbreaks in rural settings. Vaccination era: Post-2013 studies reported reduced prevalence (p < 0.001), aligning with intensified vaccination programs. DiscussionThis meta-analysis synthesized 44 years of epidemiological data to characterize the dynamics of NDV in mainland China, revealing persistent transmission patterns with profound regional and methodological heterogeneity (Feng et al., 2016; Duan et al., 2017; Huang, 2017; Liao et al., 2018; Zhang et al., 2023). The pooled prevalence of 2.17% (95% CI: 1.95%–2.42%) underscores the virus’s endemicity, yet this estimate masks stark disparities between regions. Southern China, particularly Guangdong and Guangxi, emerged as a sustained hotspot with a prevalence of 3.36%, sharply contrasting with the 0.32% observed in southwestern provinces (Table 2). This geographical gradient aligns with poultry population density, climatic factors, and anthropogenic practices. The southern regions of China harbor over 60% of China’s poultry population (>800 million birds annually) (Xiao et al., 2022), creating dense host networks that amplify transmission efficiency (Fig. 4). Furthermore, the humid subtropical climate in these areas likely prolongs NDV environmental persistence, as evidenced by higher viral RNA detection rates in mucosal swabs (2.68%) compared to serum samples (0.48%), which aligns with viral shedding routes (Fig. 3). These findings corroborate previous studies linking high humidity to prolonged viral viability in organic-rich environments such as litter and water (Rahman et al., 2018). The prevalence of NDV declined temporally from 3.19% (pre-2013) to 1.03% (post-2013), which aligns with the nationwide rollout of genotype VII-matched vaccines and stricter biosecurity protocols. The post-2013 era saw expanded vaccination coverage in commercial farms, reducing clinical outbreaks but not eliminating subclinical infections, particularly in rural backyard flocks. Notably, RT-PCR-based studies exhibited lower heterogeneity (I2=89.5%) than serological assays (I2=97.1%), emphasizing the limitations of antibody-based methods in differentiating natural infection from vaccine-induced immunity (Bei, 2018; Li et al., 2021). This methodological discrepancy likely explains the lower prevalence estimates in live poultry markets (0.48%), where sampling predominantly targets clinically healthy birds before slaughter, versus rural households (2.68%), where sporadic outbreaks persist. Publication bias, evidenced by funnel plot asymmetry (Egger’s test: p=0.003), suggests underrepresentation of small-scale studies and low-prevalence regions (Puro et al., 2022). While trim-and-fill adjustment confirmed robustness (adjusted prevalence: 2.11%), critical data gaps remain, particularly in northwestern China, where only two studies met the inclusion criteria. The scarcity of data from arid regions limits insights into climate-dependent transmission dynamics (Cattoli et al., 2011; Mao et al., 2022). In addition, historical reliance on virus isolation (pre-2010), with a median sensitivity of 68%, may have inflated false-negative rates relative to modern molecular methods (sensitivity: 98%). These limitations highlight the need for standardized diagnostic protocols and expanded surveillance in underrepresented regions (Jang, 2011; Joshi et al., 2021). This meta-analysis revealed a significantly higher prevalence of NDV in backyard flocks (5.2%, 95% CI: 4.7%–5.7%) than in commercial farms (0.8%, 95% CI: 0.6%–1.0%), establishing farming system as a critical confounding variable. Three interrelated mechanisms explain this disparity: Biosecurity gradients—Commercial operations implement stringent protocols (closed housing >90%, routine 0.5% sodium hypochlorite disinfection, visitor controls) that effectively block environmental transmission, whereas free-roaming backyard poultry (<30 birds/unit) experience elevated exposure to wild bird reservoirs and contaminated water sources, resulting in 3.7-fold higher viral RNA detection rates in environmental samples (feed/water) from scattered households than farms (Bei, 2018). Vaccine implementation gaps—While LaSota vaccine coverage exceeds 80% in commercial settings, rural smallholders exhibit ≤30% coverage due to cold-chain limitations and irregular dosing (Wang et al., 2016), further exacerbated by genotype mismatch: only 12% of backyard vaccinators used genotype VII-matched vaccines post-2013 versus 89% in industrial farms (Yuan et al., 2017), severely compromising efficacy in high-risk populations. Transmission ecology—although high-density farming (>5,000 birds/km²) theoretically amplifies transmission risk, centralized management enables rapid containment. Conversely, backyard systems sustain endemicity through persistent viral shedding and minimal isolation, with 68% of village outbreaks linked to inter-flock contact (Li et al., 2021). Consequently, this divergence necessitates stratified surveillance, particularly given the elevated prevalence (3.36%) in southern China, which correlates with dense backyard sectors (60% of poultry in Guangdong villages) (Xiao et al., 2022). Future control efforts should prioritize smallholder-targeted interventions: thermostable VII.1.1-matched vaccine delivery, biosecurity education to disrupt residual transmission chains, and poultry density caps (<5,000 birds/km²) in endemic zones. The sustained prevalence in southern China underscores the interplay of viral evolution and ecological drivers. Emerging genotype VII strains (Jang, 2011; Spackman et al., 2013), which accounted for 78% of post-2013 isolates, exhibited enhanced environmental stability compared with classical genotypes, potentially facilitating regional persistence. Migratory waterfowl in southern wetlands (Fig. 4) may further introduce novel strains, necessitating phylogeographic studies to quantify spillover risks (Gohm et al., 2000). Future control strategies must prioritize region-specific interventions, including farm density caps (<5,000 birds/km²) in high-risk provinces, mandatory environmental sampling in live markets, and multivalent vaccine development targeting circulating genotypes (Yuan et al., 2017). Although sampling location and poultry breed had limited impacts on NDV prevalence in subgroup analyses (Yue et al., 2012; Xie et al., 2018; Lancaster, 2019; Liu, 2021), the ubiquity of NDV in environments with poultry (e.g., feed, water, and air) suggests that viral persistence is less dependent on specific sampling sites than on ecological and management factors (Liao et al., 2018). Successful NDV detection in diverse locations (feed troughs, water sources, and poultry houses) supports the hypothesis that environmental contamination drives transmission (Chaka et al.,2012; Miguel et al., 2012; Zhang, 2016; Hietala et al., 2016; Li et al., 2021). Similarly, the broad host range of NDV—spanning chickens, turkeys, waterfowl, and migratory birds—may dilute breed-specific susceptibility differences, as observed in the minimal variability between poultry species (chickens: 1.46% vs. pigeons: 3.49%) (Feng et al., 2016; Duan et al., 2017; Wei, 2018; Xie et al., 2018; Ferreira et al., 2019; Yang et al., 2020). This study has several limitations. First, while the analysis aimed for comprehensiveness, potential underreporting of small-scale or low-prevalence studies may bias pooled estimates. Second, unmeasured confounders, such as vaccination status and poultry breeding density, were not analyzed due to inconsistent reporting. Finally, the lack of genotype-specific prevalence data limits insights into evolutionary pressures shaping NDV transmission. To address these gaps, future studies should integrate molecular surveillance with environmental metadata. China’s NDV control has significantly improved since 2013, yet southern regions remain vulnerable to localized outbreaks. Sustaining progress requires data-driven strategies to address ecological complexity, diagnostic limitations, and viral evolution. This meta-analysis establishes a critical baseline for monitoring NDV dynamics and refining interventions in the world’s largest poultry industry. Conclusion
AcknowledgmentsWe sincerely thank personnel from College of Anhui Province Key Laboratory of Animal Nutritional Regulation and Health, Anhui Science and Technology University and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript. Conflict of interestThe authors declare that they have no conflicts of interest. FundingThis work was supported by Anhui Education Department Key Projects (2023AH051884); Grass-fed Livestock Resource Utilization and Health Science and Technology Innovation Team (2023AH010061); Veterinary Science Peak Discipline Project of Anhui Science and Technology University (XK-XJGF002); Anhui Provincial Quality Engineering Project for the New Era of Educating People (Postgraduate Education) (2024cxcysj188). Authors’ contributionsXuelong Chen and Yanping Qi designed the study. Wei Cheng conducted literature research, collected, analyzed, and interpreted data, and authored the original draft. Huiying Zhang, Mingfeng Chu, and Yuchen Liang edited and finalized the manuscript. All authors approved the final version of the manuscript. Data availabilityAll the relevant data are available in the manuscript. ReferencesAbd Elfatah, K.S., Elabasy, M.A., El-Khyate, F., Elmahallawy, E.K., Mosad, S.M., El-Gohary, F.A., Abdo, W., Al-Brakati, A., Seadawy, M.G., Tahoon, A.E. and El-Gohary, A.E. 2021. Molecular characterization of velogenic newcastle disease virus (sub-genotype VII.1.1) from wild birds, with assessment of its pathogenicity in susceptible chickens. Animals 11, 505. Absalón, A.E., Cortés-Espinosa, D.V., Lucio, E., Miller, P.J. and Afonso, C.L. 2019. Epidemiology, control, and prevention of Newcastle disease in endemic regions: latin America. Trop. Anim. Health Prod. 51, 1033–1048. Apopo, A.A., Kariithi, H.M., Ateya, L.O., Binepal, Y.S., Sirya, J.H., Dulu, T.D., Welch, C.N., Hernandez, S.M. and Afonso, C.L. 2019. A retrospective study of Newcastle disease in Kenya. Trop. Anim. Health Prod. 52, 699–710. Bei, S. 2018. Antibody antigen monitoring and analysis of avian influenza and Newcastle disease in Chashan Town, Dongguan City, 2014-2017 [In Chinese]. Guangzhou, China: South China Agricultural University. Cattoli, G., Susta, L., Terregino, C. and Brown, C. 2011. Newcastle disease: a review of field recognition and current methods of laboratory detection. J. Vet. Diagn. Invest. 23, 637–656. Chaka, H., Goutard, F., Gil, P., Abolnik, C., Servan de Almeida, R., Bisschop, S. and Thompson, P.N. 2012. Serological and molecular investigation of Newcastle disease in household chicken flocks and associated markets in Eastern Shewa zone, Ethiopia. Trop. Anim. Health Prod. 45, 705–714. Duan, B., Wang, J., Zhao, Y., Zhang, F.A., Lv, R., Lv, Y., Zheng, D., Zeng, B., Zhao, H., Xiang, D., Liu, H., Zhang, Y. and Wang, Z. 2017. Survey on Newcastle disease virus infection status in Yunnan, 2011-2016. J. Anim. Husbandry Vet. Med. 48, 1711–1717. Feng, C. and Chen, Z. 2016. Epidemiological surveillance of Newcastle disease in Chongqing, 2012-2015. China Anim. Quarantine 33, 17–20. Ferreira, H.L., Taylor, T.L., Dimitrov, K.M., Sabra, M., Afonso, C.L. and Suarez, D.L. 2019. Virulent Newcastle disease viruses from chicken origin are more pathogenic and transmissible to chickens than viruses normally maintained in wild birds. Vet. Microbiol. 235, 25–34. Gohm, D.S., Thür, B. and Hofmann, M.A. 2000. Detection of Newcastle disease virus in organs and faeces of experimentally infected chickens using RT-PCR. Avian Pathol. J. WVPA. 29, 143–152. Hietala, S.K., Hullinger, P.J., Crossley, B.M., Kinde, H. and Ardans, A.A. 2016. Environmental air sampling to detect exotic Newcastle disease virus in two California commercial poultry flocks. J. Vet. Diagn. Invest. 17, 198–200. Huang, X. 2018. Study on the immunity and antibody level of Newcastle disease in chickens in Yan’an City, China, and pathogen detection and prevention and control measures [In Chinese]. Xianyang, China: North West Agriculture and Forestry University. Huang, Y. 2017. Serological and pathogenic surveillance of avian influenza and Newcastle disease in Changping Town, Dongguan City, China [In Chinese]. Guangzhou, China: South China Agricultural University. Jang, J. 2011. Validation of a real-time RT-PCR method to quantify Newcastle disease virus (NDV) titer and comparison with other quantifiable methods. J. Microbiol. Biotechnol. 21, 100–108. Jia, L., Liang, B., Wu, K., Wang, R., Liu, H., Liu, D. and Chen, Q. 2022. Circulation, genomic characteristics, and evolutionary dynamics of class I Newcastle disease virus in China. Virulence 13, 414–427. Joshi, V.G., Chaudhary, D., Bansal, N., Singh, R., Maan, S., Mahajan, N.K., Ravishankar, C., Sahoo, N., Mor, S.K., Radzio-Basu, J. and Herzog, C.M. 2021. Prevalence of Newcastle disease virus in commercial and backyard poultry in Haryana, India. Front. Vet. Sci. 8, 725232. Lancaster, J.E. 2019. The control of Newcastle disease. World’s Poultry Sci. J. 37, 84–96. Li, X., Lv, X., Li, Y., Xie, L., Peng, P., An, Q., Fu, T., Qin, S., Cui, Y., Zhang, C. and Qin, R. 2021. Emergence, prevalence, and evolution of H5N8 avian influenza viruses in central China, 2020. Emerg. Microbes Infections 11, 73–82. Liao, F., Yang, X., Zhao, X., Mo, X. and Huang, Z. 2018. Epidemiological survey on avian influenza and Newcastle disease in small aromatic chickens in Qiandongnan. Guizhou Anim. Husbandry Vet. Med. 42, 44–45. Liu, S. 2021. Epidemiological survey on common diseases of broiler 817 in Yucheng City and integrated prevention and control [In Chinese]. Guangzhou, China: Shandong Agricultural University. Liu, Y., Sun, C., Chi, M., Wen, H., Zhao, L., Song, Y., Liu, N. and Wang, Z. 2019. Genetic characterization and phylogenetic analysis of Newcastle disease virus from China. Infection. Genet. Evol. 75, 103958. Lu, J. 2009. Epidemiological survey of Newcastle disease in chickens in Xinyang area [In Chinese]. Henan Agricultural University. Lu, X., Wang, X., Zhan, T., Sun, Y., Wang, X., Xu, N., Liao, T., Chen, Y., Gu, M., Hu, S. and Liu, X. 2021. Surveillance of class I Newcastle disease virus at live bird markets and commercial poultry farms in eastern China reveals the epidemic characteristics. Virol. Sin. 36, 818–822. Ma, J., Wang, C. and Chen, S. 2012. Epidemiological survey of chicken diseases in Binzhou, Shandong Province, 2010 to 2011 [In Chinese]. Shanghai Animal Husbandry & Veterinary Newsletter, pp: 30–31. Mao, Q., Ma, S., Schrickel, P.L., Zhao, P., Wang, J., Zhang, Y., Li, S. and Wang, C. 2022. Review detection of Newcastle disease virus. Front. Vet. Sci. 9, 936251. Miguel, E., Grosbois, V., Berthouly-Salazar, C., Caron, A., Cappelle, J. and Roger, F. 2012. A meta-analysis of observational epidemiological studies of Newcastle disease in African agro-systems, 1980–2009. EpidemiolInfection 141, 1117–1133. Mo, S. and B. Zhang, H. Gan, Y. Yin, K. Shi, S. Qu, Y. Su, L. Zou,, Y. Han. 2020. Survey on the prevalence of Newcastle disease virus in live poultry markets in Dongxing City, Guangxi, 2015-2017. Guangxi J. Agri. 35, 25–27. Puro, K. and Sen, A. 2022. Newcastle disease in backyard poultry rearing in the Northeastern States of India: challenges and control strategies. Front. Vet. Sci. 9, 799813. Qiao, Q. 2020. Clinical diagnosis of major viral diseases in large-scale laying hen farms in Tai’an and surrounding areas and optimisation of immunisation programme adjustment [In Chinese]. Tai’an, China: Shandong Agricultural University. Rahman, M.A., Rahman, M.M., Abdullah, M.S., Sayeed, M.A., Rashid, M.H., Mahmud, R., Belgrad, J.P. and Hoque, M.A. 2018. Epidemiological assessment of clinical poultry cases through the government veterinary hospital-based passive surveillance system in Bangladesh: a case study. Trop. Anim. Health Prod. 51, 967–975. Spackman, E., Pedersen, J.C., McKinley, E.T. and Gelb Jr, J. 2013. Optimal specimen collection and transport methods for the detection of avian influenza virus and Newcastle disease virus. BMC Vet. Res. 9(1), 35. Wang, J., Zhou, Y., Chen, X., Wang, Q., He, C., Liu, H., Shen, Y. and Jim, S. 2016. Centralised monitoring and analysis of Newcastle disease in Anhui Province in 2015 [In Chinese]. China Livestock and Poultry Breeding Industry 12: 5–7. Wei, R. 2018. Pathogenetic surveillance of NDV in selected areas of China and establishment of two rapid detection methods, 2016-2017 [In Chinese]. Yangzhou University. Xiao, J., Chen, M., Yang, S., Lin, B., Cai, H., Zhang, Z. and Chen, J. 2022. Surveillance and analysis of avian influenza and newcastle disease in shantou poultry sentinel slaughterhouse, 2021-2022 [In Chinese]. Poultry Keeping and Poultry Disease Control, pp: 6–10. Xie, L. and Z. Xie, S. Luo, X. Deng, Z. Xie, S. Wang, J. Huang, T. Zeng, Y. Zhang, L. Huang, Q. Fan,, M. Zhang. 2018. Pathogenetic surveillance and genetic evolution analysis of Newcastle disease in live poultry markets in Guangxi region in 2017. Chin. J. Prev. Vet. Med. 40, 747–750. Yang, H. and Y. Wang, D. Song, G. Cheng, X. Fang,, R. Yan. 2020. Special monitoring and evaluation of Newcastle disease in Henan Province in 2019. Modern Anim. Husbandry 4, 42–45. Yu, J. 2014. Epidemiological investigation of Newcastle disease and gosling plague in geese to be slaughtered and isolation and characterisation of Newcastle disease virus [In Chinese]. Changchun, China: Jilin Agricultural University. Yuan, X. and J. Yang, Y. Wang, X. Wang, L. Qi, Y. Wang,, W. Ai. 2017. Analysis of virulence distribution, genetic evolution, and glycosylation loci of Newcastle disease virus in Shandong Province from 2012 to 2016. China Poultry 39, 72–74. Yue, X., Chen, L., Li, S., Gao, W., Zhang, Y., Cui, B. and Li, X. 2012. Epidemiological survey on avian influenza subtype H9, Newcastle disease and infectious bronchitis in chickens in some areas of Henan Province, 2010-2011 [In Chinese]. Chinese Agron. Bull. 28, 72–76. Zhang, D., Li, X. and Jia, N. 2018. Detection and analysis of Newcastle disease in chickens in Jinchuan District, Jinchang City, China, 2016-2017. Foreign Livestock Sci. 38, 53–55. Zhang, L., Zhang, S., Chen, R. and Yuan, Z. 2023. Pathogen surveillance and assessment of immunisation effect of avian Newcastle disease in Wenshan Prefecture, 2012-2021 [In Chinese]. Kunming, Yunnan Province, China: Yunnan Animal Husbandry and Veterinary Medicine, pp: 16–18. Zhang, M. 2016. Epidemiological survey of five important viral diseases in large-scale goose farms in some areas of China [In Chinese]. Tai’an, China: Shandong Agricultural University. Zhong, M., Li, J., Qiu, G., Zhong, R., Cheng, D., Hu, Y., Guo, X., Lin, X. and Liu, R. 2022. Pathogen detection and analysis of nindu yellow chicken epidemic disease, 2018-2020. China Poultry 44, 74–78. Zhou, Y., Wang, J. and Wang, Q. 2016. Newcastle disease sentinel surveillance and analysis in Anhui Province in 2015. Chin. Vet. J. 52, 92–94. | ||
| How to Cite this Article |
| Pubmed Style Cheng W, Chu M, Zhang H, Liang Y, Wang H, Chen S, Wang Y, Si M, Zhong X, Yang B, Chen X, Qi Y. Meta-analysis of Newcastle disease virus prevalence among poultry in mainland China. Open Vet. J.. 2025; 15(9): 4382-4392. doi:10.5455/OVJ.2025.v15.i9.45 Web Style Cheng W, Chu M, Zhang H, Liang Y, Wang H, Chen S, Wang Y, Si M, Zhong X, Yang B, Chen X, Qi Y. Meta-analysis of Newcastle disease virus prevalence among poultry in mainland China. https://www.openveterinaryjournal.com/?mno=256396 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.45 AMA (American Medical Association) Style Cheng W, Chu M, Zhang H, Liang Y, Wang H, Chen S, Wang Y, Si M, Zhong X, Yang B, Chen X, Qi Y. Meta-analysis of Newcastle disease virus prevalence among poultry in mainland China. Open Vet. J.. 2025; 15(9): 4382-4392. doi:10.5455/OVJ.2025.v15.i9.45 Vancouver/ICMJE Style Cheng W, Chu M, Zhang H, Liang Y, Wang H, Chen S, Wang Y, Si M, Zhong X, Yang B, Chen X, Qi Y. Meta-analysis of Newcastle disease virus prevalence among poultry in mainland China. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4382-4392. doi:10.5455/OVJ.2025.v15.i9.45 Harvard Style Cheng, W., Chu, . M., Zhang, . H., Liang, . Y., Wang, . H., Chen, . S., Wang, . Y., Si, . M., Zhong, . X., Yang, . B., Chen, . X. & Qi, . Y. (2025) Meta-analysis of Newcastle disease virus prevalence among poultry in mainland China. Open Vet. J., 15 (9), 4382-4392. doi:10.5455/OVJ.2025.v15.i9.45 Turabian Style Cheng, Wei, Mingfeng Chu, Huiying Zhang, Yuchen Liang, Honghai Wang, Shuiyun Chen, Yiwei Wang, Mengke Si, Xiaoyu Zhong, Baolei Yang, Xuelong Chen, and Yanping Qi. 2025. Meta-analysis of Newcastle disease virus prevalence among poultry in mainland China. Open Veterinary Journal, 15 (9), 4382-4392. doi:10.5455/OVJ.2025.v15.i9.45 Chicago Style Cheng, Wei, Mingfeng Chu, Huiying Zhang, Yuchen Liang, Honghai Wang, Shuiyun Chen, Yiwei Wang, Mengke Si, Xiaoyu Zhong, Baolei Yang, Xuelong Chen, and Yanping Qi. "Meta-analysis of Newcastle disease virus prevalence among poultry in mainland China." Open Veterinary Journal 15 (2025), 4382-4392. doi:10.5455/OVJ.2025.v15.i9.45 MLA (The Modern Language Association) Style Cheng, Wei, Mingfeng Chu, Huiying Zhang, Yuchen Liang, Honghai Wang, Shuiyun Chen, Yiwei Wang, Mengke Si, Xiaoyu Zhong, Baolei Yang, Xuelong Chen, and Yanping Qi. "Meta-analysis of Newcastle disease virus prevalence among poultry in mainland China." Open Veterinary Journal 15.9 (2025), 4382-4392. Print. doi:10.5455/OVJ.2025.v15.i9.45 APA (American Psychological Association) Style Cheng, W., Chu, . M., Zhang, . H., Liang, . Y., Wang, . H., Chen, . S., Wang, . Y., Si, . M., Zhong, . X., Yang, . B., Chen, . X. & Qi, . Y. (2025) Meta-analysis of Newcastle disease virus prevalence among poultry in mainland China. Open Veterinary Journal, 15 (9), 4382-4392. doi:10.5455/OVJ.2025.v15.i9.45 |