| Research Article | ||
Open Vet. J.. 2025; 15(8): 3571-3579 Open Veterinary Journal, (2025), Vol. 15(8): 3571-3579 Research Article Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewesMaha A. Al-Obaidi1, Ibrahim Q. Naji2 and Ali A. Abd3*1Department of Remote Sensing, Laser and Photonics Center, Al-Hamdaniya University, Hamdaniya, Iraq 2Department of Physiology and Pharmacology, College of Veterinary Medicine, Tikrit University, Tikrit, Iraq 3Department of Surgery and Obstetric, College of Veterinary Medicine, Tikrit University, Tikrit, Iraq *Corresponding Author: Ali Aziz Abd. Department of Surgery and Obstetric, College of Veterinary Medicine, Tikrit University, Tikrit, Iraq. Email: aliaziz2235 [at] tu.edu.iq Submitted: 29/04/2025 Revised: 28/06/2025 Accepted: 09/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
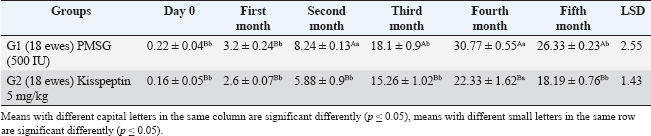
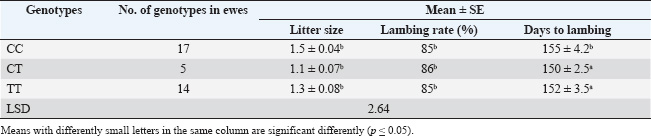
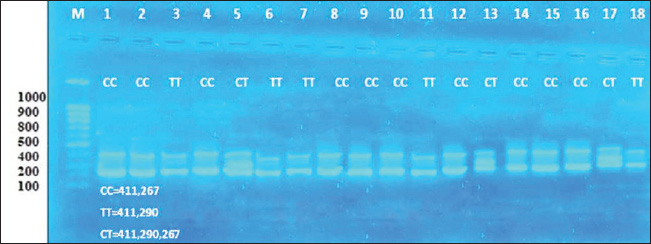
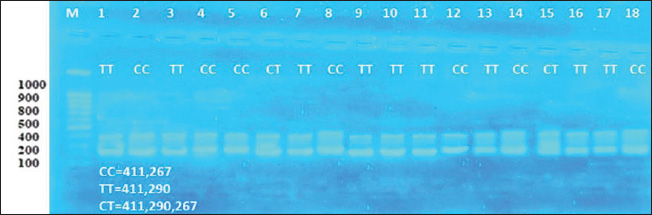
ABSTRACTBackground: One of the most important problems in sheep is seasonal anestrus, which limits the reproductive efficiency of the sheep. Estrous synchronization is considered the first plan for reproductive performance in sheep due to the pregnancy time is limited, and parturition as well as an increase in twining and reached good genetic characteristics. Aim: This study aimed to manipulate seasonality that limits fertility in ewes by induction estrus during seasonal anestrous in sheep by using Pregnant Mare Serum Gonadotropin (PMSG), Kisspeptin hormone, and study the impact of MTNR1A gene on reproduction efficiency in ewes. Methods: This study examined 36 Awassi ewes divided into two groups, each containing 18 ewes, 2–3 years old, and two fertile rams aged 3–4 years and weighing 60–65 kg. All non-pregnant ewes were synchronized using vaginal sponges (60 mg Medroxy Progesterone acetate) for 10 days. The injection of treatment when sponges are draws. The first group (G1) received 500 IU of PMSG injection, and the second group (G2) injection a Kisspeptin hormone 5 μg/kg B.W. Results: The results showed the G1 treated by PMSG 500IU were higher significantly (p ≤ 0.05) of estrus response, induction estrus and pregnancy (89%, 89%, and 89%), respectively, comparative with G2 treated by (Kisspeptin 5 mg/kg) were (72%, 72%, and 66%), respectively, and non-significant changes in estrus were observed in all groups. The average peripheral progesterone concentration significantly increased from day 0 to 5th month in G1 comparative with G2 in pregnant ewes. Serum progesterone levels were significantly p < 0.05 during of 4th month in G1 treatment by (PMSG 500IU) compared with day 0 and all other months during pregnancy of ewes. The average days to lambing in genotypes CT and TT (150 ± 2.5 and 152 ± 3.5 days), respectively, were significant comparative with CC genotype; however, the litter size and lambing rate observed in the enrolled ewes were non-significant in all genotypes. CC, CT, and TT represent three possible genotypes at a specific location (locus) in the genome, often referring to a single nucleotide polymorphism (SNP). These genotypes indicate the combination of alleles an individual inherits from their parents for a particular gene. They refer to the presence of two alleles for a particular nitrogenous base: C and T are nitrogenous bases: C=Cytosine and T=Thymine. Conclusion: In conclusion, the application of PMSG and Kisspeptin was effective in achievement good higher significantly of reproduction efficiency in this study. The genotypes CT and TT of the MTNR1A gene polymorphism were connected with a short significant of days to lambing in genotypes. Keywords: Induction, Kisspeptin, MTNR1A gene, PMSG, Progesterone. IntroductionIraqi sheep are seasonality reproductive, experience a period of reproductive rest during early summer and spring (Al-Hamedawi, 1990; Al-Shati, 2019). Ewes are considered to have low reproductive efficiency because they achieve a long lambing interval due to the seasonal reproduction for most of them (Noakes et al., 2009; Alkass et al., 2021). One of the most important problems in sheep is seasonal anestrus, which limits the reproductive efficiency in the sheep (Knights et al., 2001). The reproductive season in sheep is a succession of estrous cycles, starting of the summer or early autumn because of the short day length, with increases of estrous activation and ovulation rate occurring in the autumn and finishing in early spring or late winter (Rosa and Bryant, 2003; Abecia et al., 2012; Thomson et al., 2021). Estrous synchronization is considered the first plan for reproductive performance in sheep due to the pregnancy time being limited and parturition as well as an increase in twining and reached to good genetic characteristics (Makawi and Manhail, 2007; Garoussi et al., 2020). The estrous synchronization in farms can decrease the impacts of seasonality factors on the sheep reproduction, prolong the reproductive seasonal of ewes, regulation the birth time of ewes, shorten the lambing cycles and achievement two to three births in a 2-year (De et al., 2015; Wei et al., 2016; Biehl et al., 2019), that way decrease the economic cost of reproduction, increasing the numbers of lambs, and obtaining more economics benefit (Kumar et al., 2015; Rosasco et al., 2019). In sheep, estrous synchronization is achievement either by equine chorionic gonadotropin (Abdullah et al., 2002), progesterone (Emsen et al., 2011), Kisspeptin (Clarke et al., 2015), prostaglandins (Fierro et al., 2013), melatonin (Mura et al., 2019a, b), Pregnant Mare Serum Gonadotropin (PMSG) (Al-Shati, 2019), human chorionic gonadotropin (Dias et al., 2020) and sulpiride (Abd and Ibrahim, 2023; Al-Mousawe and Ibrahim, 2024; Abd et al., 2025). Kisspeptin, a neuropeptide produced by the kisspeptin 1 gene, plays a vital role in reproduction through its effects on the hypothalamic-pituitary-gonadal axis. Kisspeptins are necessary for the development and proper functioning of the reproductive systems in both males and females (Padda et al., 2021). The kisspeptin is a substance that increases gonadotropin-releasing hormone production, which in turn triggers the release of luteinizing hormone (LH). The anterior pituitary produces LH as the last neuroendocrine regulator of reproduction in ruminants. Kisspeptin has garnered significant attention because of its involvement in the regulation of reproduction across various species, including ruminants. Recently, kisspeptin has been implicated in the integration of metabolic control of reproductive (Daniel et al., 2015). Kisspeptin provides a new strategy for the manipulation of gonadotropin secretion and can cause ovulation in noncyclical females (Caraty et al., 2007). PMSG is a glycoprotein hormone that is secreted in high concentrations in the blood of young pregnant mares (40–130 days) (Cole and Hart, 1930). Interestingly, in one gonadotrophin hormone molecule, there are biological activities of the follicle-stimulating hormone (FSH) and LH (Stamatiades and Kaiser, 2018). FSH functions to control the sexual characteristics of males and females, stimulate, follicular maturation, and produce estrogen hormone. The FSH hormone effect on appearance of estrus signs in ewes, and the LH hormone plays a role with FSH in the development of follicular and ovulation. The LH hormone affects the secretion of the progesterone hormone and the maturation of eggs (Andriyanto et al., 2015; Laith et al., 2020). PMSG biological activity, such as FSH and LH, can be used as a superovulation agent in a single dose (Somanjaya et al., 2021). A recent study by (Al-Marzani and Barwary, 2025) reported that exogenous hormonal treatments in a combination of fluorogestone acetate sponge using PMSG improved reproduction parameters (estrous rate, fertility rate, and pregnancy length) in ewes. Among the melatonin receptor types (MTNR 1A, 1B, and 1C), only MTNR1A appears effect in regulating seasonal reproductive activity at ewes (Dubocovich et al., 2003). Several studies at the polymorphism of the MTNR1A gene were associated with reproduction activity in sheep (Pelletier et al., 2000; Faigl et al., 2008). The MTNR1A position shows several polymorphism sites, which are associated with seasonal breeding activity in ewes (Pelletier et al., 2000; Carcangiu et al., 2009; Younis et al., 2019). The polymorphism sites found in the MTNR1A gene affect reproduction performances in general and effect on season of reproduction resumption in various sheep breeds (Starič et al., 2020; Cosso et al., 2021). This study aimed to manipulate seasonality that limits fertility in ewes by induction estrus during seasonal anestrous in sheep by using PMSG, Kisspeptin hormone, and study the impact of MTNR1A gene on reproduction efficiency in ewes. Materials and MethodsExperimental animalsA total of 36 anestrus ewes aged 2–3 years, with a ranged weight (35–40 kg), and two fertility ram aged (3–4 years and weight 60–65 kg). The study was carried out between June 1 and 15 of 2024, which is the period accepted as outside the breeding season in Iraq. This study was conducted on farm animals in Wasit. The randomly divided of ewes into two groups, each one contains 18 ewes. Detection of estrus was done twice a day using two fertile males on the ewes and measurement of progesterone concentration was measured to assure anestrus. The ewe that failed to appear in estrus with a progesterone concentration of decrease than 1 ng/ml was ewes in anestrus. Used intravaginal progesterone impregnated sponge Medroxy Progesterone acetate 60mg (SYNTEX S.A, Bunos Aris-Argentina) for 10 days for all anestrus ewes and non-lactating ewes. The treatment gives an injection into the muscle during the sponge withdrawal. The first group (G1) injection of PMSG 500 IU (Follimag® Mosagrogen, Russia), and the second group (G2) injection of Kisspeptin hormone 5 μg/kg B.W (Wuhan Senwayer Century Chemical Co., Ltd, China). Progesterone assay assessmentThe blood sample collection (10 ml) from jugular vena puncture and evacuated in gel tubes at days 0 before treatment and monthly for determination of progesterone levels. Serum was collected after centrifuging at 3,000 rpm for 10 minutes, and the serum was kept in a spinoff tube in −20°C until analysis of progesterone hormone levels using the Abbott TECTplus immunoassay analyzer (Zarkawi and Soukouti, 2001; Li et al., 2019). PCR-restriction fragment length polymorphism (RFLP)and genotypingTen milliliters of blood collection from the jugular vein after being treated at ethylenediaminetetraacetic acid coated tubes, samples of blood transport to laboratory by cool box and store at −18 until DNA extraction. Total DNA was extracted using a Genomic DNA from whole blood, using a commercial (Geneaid Biotech Ltd GMB 100) Geneius TM Micro gDNA Extraction Kit/ Korea, and stored at −18°C. The MTNR1A gene polymorphism was identified using the PCRRFLP method, examined after enzymatic treatment of the resulting amplification (PCR products 10 μl) with RsaI restriction enzyme (Thermo scientific) (Saxena et al., 2014). The digestion reaction was conducted at a finally volume; in 37°C for 4 hours. Post digestion of the amplified PCR fragments revealed CC, TT (Homozygote), and CT (Heterozygote) genotypes sheep breed. The symbols CC, CT, and TT are commonly used in genetics (molecular genetics) to indicate genotypes at a specific location on DNA. They refer to the presence of two alleles for a particular nitrogenous base: C and T are nitrogenous bases: C=cytosine and T=thymine. The reaction was conducted at a 30 μl volume containing 10 μl of amplicon, 1 μl of enzyme, 2 μl of 10 buffer, and 17 μl Nuclease-Free Water at 37°C for 90 minutes, followed by deactivation at 65°C for 20 minutes. The digestion results were visualized by 2% gel agarose electrophoresis and smearing with Red stain. Statistical analysisThe analysis of data by conducted using SAS (version 9.1). Two-way ANOVA and least significant difference post hoc test were completely valued significantly different among means (p < 0.05) is considered statistically significant (SAS, 2010). Ethical approvalEthical approval of the processes was carried out under animal care guidelines at Tikrit University, Iraq. tu.vet.95. 2025. ResultsIraqi Awassi ewes treated with PMSG at a dose of 500 IU during the non-cycling period had a higher significant (p ≤ 0.05) of estrus response, induction of estrus, and pregnancy. Table 1 shows the response ewes and duration of estrus in different treated groups. The ewes exhibited signs of estrus in 16 ewes in G1 (PMSG 500 IU) and 13 ewes in G2 of (Kisspeptin 5 mg/kg). The rates of estrus response, induction of estrus, and pregnancy were 89%, 89%, and 89% in G1 and 72%, 72%, and 66% in G2, respectively, and non-significant changes in estrus duration between the groups. The hormone assays were pooled monthly. Table 2 below serum levels of progesterone (ng/ml) in different treated groups, giving progesterone results in treatment groups of ewes were showed cyclic estrous and subsequently became pregnant and produced normal new borne. The average peripheral progesterone level significantly increased from (0.22 ± 0.04 ng/ml on day 0 to 26.33 ± 0.23 ng/ml on 5th month) in G1 and (0.16 ± 0.05 on day 0 to 18.19 ± 0.76 on 5th month) in G2 in pregnant ewes. Table 2 the detected serum progesterone levels were significant (p < 0.05) during the 4th month (30.77 ± 0.55 ng/ml) in G1 treatment by (PMSG 500IU) compared with day 0 and all other months during pregnancy of ewes. All single-nucleotide polymorphisms were analyzations to assess their associations with litter size, lambing rate, and days to lambing. According to the data presented in Table 3, the relationship between the days to lambing, and the litter size, and lambing rate with genotype MTNR1A, the average days to lambing in genotypes CT and TT (150 ± 2.5 and 152 ± 3.5 days), respectively, were significant comparative with CC genotype, litter size and lambing rate observed in the enrolled ewes were non-significant in all genotypes. After DNA extraction from the whole blood, 36 samples with DNA and by means of PCR-RFLP and RsaI enzyme digestion analysis two allele variant of MTNR1A genes (T and C) and all three genotypes were identified: CC (267, 411 bp), TT (290, 411 bp), and CT (267, 290, 411 bp), as shown in Figure 1. The digestion of 824 bp fragments of MTNR1A gene was performed using RsaI enzyme in a 1.5% agarose gel, treated with 500 IU PMSG. As shown in Figure 2, the digestion of 824 bp fragments of MTNR1A gene using RsaI enzyme in a 1.5% agarose gel. CT (267, 290, 411 bp), CC (267, 411 bp), and TT (290, 411 bp) in G2 (18 ewes) treated by Kisspeptin 5 mg/kg. DiscussionIraqi Awassi ewes treated by PMSG 500IU during the non-cycling was higher significantly (p ≤ 0.05) comparative with treatment by Kisspeptin 5 mg/kg to estrus response, induction of estrus, with pregnancy rate, and non-significant changes in estrus duration in groups. The results of the estrus duration in this study agreement with studies (Hashemi et al., 2006) that reported 36 hours of estrus duration. Also, agreement with the result of the estrus duration Santa ewes was 38 hours, and in Santa/Dorper crossbred, it was 36 hours, while 31 hours in synchronized ewes of the same breeds (Cavalcanti et al., 2012). The result of Nasser et al. (2012), who reported 34.4 hours of duration of estrus, convention with this study. The results of the present study convention with a higher percentage of successful rates of the estrus response, induction of estrus, and pregnancy percentages when 500 IU of PMSG was used in ewes (Tirpan et al., 2019; Hussein et al., 2021). The results of this study agreement with the previous studies that used PMSG hormone as the most successful hormone for estrus synchronization program out of the reproduction season (Quintero-Elisea et al., 2011; Naderipour et al., 2012). The impact of PMSG hormone was as gonadotropins action, higher ovulation rate, litter size, and number of growth follicles (Koyuncu and Alticekic, 2010; Takci and Dinc, 2023; Abd, 2024). The action of kisspeptin affects several processes, including steroidogenesis, follicular maturation, and ovulation (Szeliga and Meczekalski, 2022; Dai et al., 2022). The result of kisspeptin treatment in this study convention with Qayssar (2024), who reported a non-significant estrus response and duration, and the estrus duration was not significant in kisspeptin treatment. These findings align with (Texeira et al., 2016). This treatment induces ovulation in females without a regular breeding cycle (Smith et al., 2014). Qayssar (2024) reported that the Controlled Internal Drug Release (CIDR)-kisspeptin regime in this study had non-significantly impact on reproduction efficiency in comparison with CIDR alone regarding the percentage of responded animals, conception rate, and fecundity rate, which result similarity with the present study. Abdulkareem et al. (2021) reported that the Kisspeptin treatment enhanced the overall reproductive performance was significantly higher in fertility 90%, estrus 100%, lambing 90%, and conception 100%. This result disagreement with the result of this study. The result of progesterone concentration was significantly increased from day 0 to 5th month in G1 and G2 of pregnant ewes. Serum progesterone levels were significantly p < 0.05 during the 4th month in G1 treatment by (PMSG 500IU) compared with day 0 and all other months during pregnancy of ewes. The present study was similarity result with of the level increasing steadily from period 16–30 to 61–75 and then rose significantly to a maximum level (14 ng/ml) between the periods 121–135, then declined in the last 2 weeks until lambing (Ganaie et al., 2009). The result of the present study agrees with the progesterone concentrations of pregnant ewes and does, which steadily increased during 4th month and declined after the birth of ewes (Bono et al., 1983; Alwan et al., 2010). These results were in line with those of Alwan et al. (2010) and Mukasa-Mugerwa and Viviani (1992), who reported an increase in progesterone concentration in mid-pregnancy and a decrease as parturition progressed. In addition, Pasciu et al. (2022) reported a significant increase in progesterone levels in ewes with twins compared with ewes with single lambs during late pregnancy (110–140 days), while a non-significant relationship was recorded in early and mid-pregnancy (40–80 days). The increase in progesterone concentration in the mid-gestation period is attributed to additional release from the placenta and additional extra-ovarian sources (Khatun et al., 2011; Khan et al., 2020). The results of genotypes in this study were similarity with result who reported it Chu et al. (2006) and Saxena et al. (2014). The RsaI enzyme used for digestion, evidenced three splitting sites, which resulted in the resolution of three bands at positions 267, 290, and 411 bp. The observed three genotypes in the Chokla ewes breed are CC, TT, and CT. Rahawy et al. (2016) reported three cleavage sites in Iraqi local ewes: CC (411/267), CT (411/290/267), and TT (411/290). This result agreement with the result in this study. The result of this study disagreement with of the genotypes distributed of the MTNR1A genes, the CT genotypes had a higher frequency in Rahmani and Ossimi. This result was similar to Falk (2013), who reported in Swedish sheep. Also, Hristova et al. (2012) reported a similar result in Bulgarian sheep. We note from the results of this study that the genotype CC was dominant compared with the genotypes TT and CT. The CC genotype has a positive impact on out-of-breeding season activity in Iraqi Awassi ewes. According to Notter (2003), out-of-season breeding is associated with the CC genotype. As a result, scientists discovered that animals with higher frequencies of the C gene are more likely to breed year-round or out of season. The results of genotypes in this study were the average days to lambing in genotypes CT and TT were significant comparative with CC genotype, and litter size, and lambing rate observed in the enrolled ewes were non-significant in all genotypes. This result similarity with Alkhammas et al. (2023), who reported that the single-nucleotide polymorphism CT was associated with reproductive performance in ewes, and that CT-carrying ewes had significant decreased litter sizes, rates of lambing, and increased days to birth of lambs comparative with ewes carrying the CC and TT genotypes. Alkhammas et al. (2023) who reported the genotype CC show significantly association with shorter days to lambing, increased lambing rates, twinning rate, and litter sizes comparative with ewes carrying the TT and CT genotypes. The ewes carrying CT and CC genotypes showed the greatest numbers of lambing comparative with genotype TT in ewes (Cosso et al., 2021; Mura et al., 2022). Abuzahra et al. (2025) reported a significant association between the litter size and GC genotype, exhibiting a higher average litter size than the GG genotype. This result disagreement with present study. Also, Antonopoulou et al. (2025) reported that the MTNR1A is the main gene that can influence reproductive seasonality in sheep. This study similarity with Mura et al. (2014) and Giantsis et al. (2016) explains that ewes with CC genotypes had significantly higher fertility out of season reproduction, and genotypes CC at Bovska and Sarda were association with great fertility (Pulinas et al., 2022). Table 1. Response of ewes and estrus duration in different treated groups.
Table 2. Serum progesterone levels (ng/ml) in different treated groups.
Table 3. Relationship between days to lambing, litter size and lambing rate with genotype MTNR1A.
Fig. 1. The digestion of 824 bp fragments of MTNR1A gene using RsaI enzyme in 1.5% agarose gel. CT (267, 290, 411 bp), CC (267, 411 bp), and TT (290, 411 bp) in G1 (18 ewes) treated with 500 IU PMSG.
Fig. 2. The digestion of 824 bp fragments of MTNR1A gene using RsaI enzyme in 1.5% agarose gel. CT (267, 290, 411 bp), CC (267, 411 bp), and TT (290, 411 bp) in G2 (18 ewes) treated by Kisspeptin 5 mg/kg. ConclusionIn conclusion, the application of PMSG and Kiss was effective in achieving good higher significantly of estrus response, induction of estrus, and pregnancy in this study. The genotype CT and TT of the MTNR1A gene polymorphism were significantly associated with short significantly of days to lambing in genotypes. AcknowledgmentsWe acknowledge the help and support provided by the Veterinary Medicine College/University of Tikrit for conducting this research work. Conflict of interestThe authors did not declare any conflicts of interest. FundingNo grant was received for this study, and it has been supported by self-funding. Authors’ contributionsAssi. Prof. Dr. Ali aziz abd was the corresponding author for this article and was responsible for animal care, clinical parameters, and blood sample collection. Maha Abdul-Karim Al-Obaidi was responsible for the study observations and research management. The article was written equally between the authors. Data availabilityAll data are provided in the manuscript. ReferencesAbd, A. 2024. Impact of sulpiride and pmsg hormone treatment on estrus and number of resulted offsprings in female rats. Assiut Vet. Med. J. 70(183), 84–89. Abd, A.A., Al-Juhaishi, O.A. and Jumma, Q.S. 2025. Effects of sulpiride on the reproductive system of male rats after puberty. World Vet. J. 15(1), 42–48. Abd, A.A. and Ibrahim, N.S. 2023. Induction of oestrus by Sulpiride and measurement of estrogen hormone in Iraqi AwassiEwes during the out of breeding season. Ind. Vet. J. 100(12), 15–18. Abdulkareem, T.A., Muhammad, S.J. and Yousif, A.N. 2021. Effect of kispeptin-10 as an alternative to ECG in estrus synchronization protocol on improving the reproductive performance of karadi ewes. Iraqi J. Agric. Sci. 52(3), 535–546. Abdullah, A.Y., Husein, M.Q. and Kridli, R.T. 2002. Protocols for estrus synchronization in Awassi ewes under arid environmental conditions. Asian-Aust. J. Anim. Sci. 15, 957–962. Abecia, J., Forcada, F. and González-Bulnes, A. 2012. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 130, 173–179. Abuzahra, M., Al-Shuhaib, M.B.S., Wijayanti, D., Effendi, M.H., Mustofa, I. and Moses, I. B. 2025. A novel p.127Val>Ile single nucleotide polymorphism in the MTNR1A gene and its relation to litter size in Thin-tailed Indonesian ewes. Anim. Biosci. 38(2), 209–222. Al-Hamedawi, T.M. 1990. Induction of two births with twins in a year in ewes by using hormonal treatment. M.Sc. Thesis. College of Veterinary Medicine Baghdad University, Baghdad, Iraq. Alkass, J.E., Hermiz, M. and Mevan, I.B. 2021. Some aspects of reproductive efficiency in awassi ewes. Iraqi J. Agri. Sci. 52(1), 20–27. Alkhammas, A.H., Al-Thuwaini, T.M., Al-Shuhaib, M.B.S. and Khazaal, N.M. 2023. Association of novel C319T variant of PITX2 gene 3’UTR region with reproductive performance in Awassi sheep. Bioinform. Biol. Insights. 5, 17. Al-Marzani, E.A.H. and Barwary, M.S. 2025. Impact of different estrus synchroniation methods on reproductive parameters of karadi and arabi ewes in erbil region. Iraqi J. Agric. Sci. 56(2), 727–735. Al-Mousawe, A. and Ibrahim, N. 2024. Diagnosis of pregnancy in Iraqi Awassi ewes through progesterone hormone measurement and ultrasonography following induction of fertile estrus with Sulpiride. Egypt. J. Vet. Sci. 55(4), 945–953. Al-Shati, I.R. 2019. Comparative study between the role of vaginal SpongesPMSG and liposome incorporated progesterone-PMSG in the reproductive performance in Iraqi ewes. Thesis, PhD. Veterinary Medicine/University of Baghdad in Theriogenology, Baghdad, Iraq. Alwan, A.F., Amin, F.A.M. and Ibrahim, N.S. 2010. Blood progesterone and estrogen hormones level during pregnancy and after birth in Iraqi sheep and goat. Bas. J. Vet. Res. 10(2), 153–157. Andriyanto, M., Amrozi, M., Rahminiwati, A. Boediono, and W. Manalu. 2015. Korelasi folikel dominan akibat penyuntikan hormon pregnant mare serum gonadotropin (PMSG) dengan peningkatan respons berahi pada kambing kacang. J. Kedokt. Hewan. 9(1), 20–23. Antonopoulou, D., Symeon, G., Zaralis, K., Avdi, M., Frydas, I.S. and Giantsis, I.A. 2025. Genome-wide association study (GWAS) on reproductive seasonality in indigenous greek sheep breeds: insights into genetic integrity. Curr. Issues Mol. Biol. 47(4), 279. Biehl, M., Ferraz, J., Barroso, J., Susin, I., Ferreira, E. and Polizel, D.T. 2019. The reused progesterone device has the same effect on short or long estrus synchronization protocols in tropical sheep. Trop. Anim. Health Prod. 51(6), 1545–1549. Bono, G., Cairoli, F., Tamanini, C. and Abrate, L. 1983. Progesterone, 414 estrogen, LH, FSH and PRL concentrations in plasma during the 415 estrous cycle in goats. Reprod. Nutr. Develop. 23(2A), 217–416. Caraty, A., Smith, J.T., Lomet, D., Ben Saïd, S., Morrissey, A., Cognie, J., Doughton, B., Baril, G., Briant, C. and Clarke, I.J. 2007. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 148(11), 5258–5267. Carcangiu, V., Mura, M.C., Vacca, G.M., Pazzola, M., Dettori, M.L., Luridiana, S. and Bini, P.P. 2009. Polymorphism of the melatonin receptor MT1 gene and its relationship with seasonal reproductive activity in the Sarda sheep breed. Anim. Reprod. Sci. 116(1–2), 65–72. Cavalcanti, A.S., Brandão, F.Z., Nogueira, L.A.G. and Fonseca, J.F. 2012. Effects of GnRH administration on ovulation and fertility in ewes subjected to estrous synchronization. Rev. Bras. Zootec. 41(6), 1412–1418. Chu, M.X., Cheng, D.X., Liu, W.Z., Fang, L. and Ye, S.C. 2006. Association between melatonin receptor 1A gene and expression of reproductive seasonality in sheep. Asian-Australas. J. Anim. Sci. 19, 1079–1084. Clarke, H., Dhillo, W.S. and Jayasena, C.N. 2015. Comprehensive review on kisspeptin and its role in reproductive disorders. Endocrinol. Metab. 30(2), 124–141. Cole, H.H. and Hart, G.H. 1930. The potency of blood serum of mares in progressive stages of pregnancy in effecting the sexual maturity of the immature rat. Am. J. Physiol. Content. 93(1), 57–68. Cosso, Nehme, M., Luridiana, S., Pulinas, L., Curone, G., Hosri, C., Carcangiu, V. and Mura, M.C. 2021. Detection of polymorphisms in the MTNR1A gene and their association with reproductive performance in Awassi ewes. Animals 11(2), 583. Dai, T., Kang, X., Yang, C., Mei, S., Wei, S., Guo, X. and Dan, X. 2022. Integrative analysis of miRNA-mRNA in ovarian granulosa cells treated with Kisspeptin in Tan sheep. Animals 12(21), 2989. Daniel, J.A., Foradori, C.D., Whitlock, B.K., and Sartin, J.L. 2015. Reproduction and beyond, kisspeptin in ruminants. J. Animal Sci. Biotechnol. 6(23), 1–6. De, K., Kumar, D., Sethi, D., Gulyani, R. and Naqvi, S.M. 2015. Estrus synchronization and fixed-time artificial insemination in sheep under field conditions of a semi-arid tropical region. Trop. Anim. Health Prod. 47, 469–472. Dias, J.H., Miranda, V.O., Oliveira, F.C., Junior, S.V., Haas, C.S., Costa, V.G.G. and Gasperin, B.G. 2020. Treatment with eCG and hCG to induce onset of estrous cycles in ewes during the non-breeding season: Effects on follicular development and fertility. Anim. Reprod. Sci. 212, 106232. Dubocovich, M.L., Rivera-Bermudez, M.A., Gerdin, M.J. and Masana, M.I. 2003. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front. Biosci. 8, 1093–1108. Emsen, E., Gimenez-Diaz, C., Kutluca, M. and Koycegiz, F. 2011. Reproductive response of ewes synchronized with different lengths of MGA treatments in intrauterine insemination program. Anim. Reprod. Sci. 126(1-2), 57–60. Faigl, V., Arnyasi, M., Keresztes, M., Kulcsar, M., Reiczigel, J., Danko, G., Javor, A., Cseh, S. and Huszeniccza, G. 2008. Seasonality of reproduction and MT1 receptor gene polymorphism in Awassi sheep. Reprod. Domest. Anim. 43, 11. Falk, A. 2013. Variation in frequency of alleles in the MTNR1A gene with possible impact on ability of ewes to show oestrus out of season. Thesis of Master science in Department of Animal Breeding and Genetics, Swedish University of Agricultural Sciences, Uppsala, Sweden. Fierro, S., Gil, J., Viñoles, C. and Olivera-Muzante, J. 2013. The use of prostaglandins in controlling estrous cycle of the ewe: a review. Theriogenology 79(3), 399–408. Ganaie, B.A., Khan, M.Z., Islam, R., Makhdoomi, D.M., Qureshi, S. and Wani, G.M. 2009. Evaluation of different techniques for pregnancy diagnosis in sheep. Small Rumin. Res. 85(2–3), 135–141. Garoussi, M.T., Mavadati, O., Bahonar, M. and Ragh, M.J. 2020. The effect of medroxyprogesterone acetate with or without eCG on conception rate of fat-tail ewes in out of breeding season. Trop. Anim. Health Prod. 52, 1617–1622. Giantsis, I.A., Laliotis, G.P., Stoupa, O. and Avdi, M. 2016. Polymorphism of the melatonin receptor 1A (MNTR1A) gene and association with seasonality of reproductive activity in a local Greek sheep breed. J. Biol. Res. 29, 23–29. Hashemi, M., Safdarian, M. and Kafi, M. 2006. Estrous response to synchronization of estrus using different progesterone treatments outside the natural breeding season in ewes. Small Rumin. Res. 65, 279–283. Hristova, D., Georgieva, S., Yablanski, T., Tanchev, S., Slavov, R. and Bonev, G. 2012. Genetic polymorphism of the melatonin receptor MT1 gene in four Bulgarian sheep breeds, J. Agri. Sci. Tech. 4, 187–192. Hussein, E.K. Naoman, U.T. and Al-Ajeli, R.R. 2021. Induction of estrus using human menopausal gonadotrophin in Iraqi Awassi ewes. Iraqi J. Vet. Sci. 35(3), 529–533. Khan, D., Khan, H., Nazir, A., Tunio, M.T., Tahir, M., Khan, M.S. and Khan, R.U. 2020. Early pregnancy diagnosis using pregnancy associated glycoproteins in the serum of pregnant ruminants. Pak. J. Zool. 52(2), 785. Khatun, A., Wani, G.M., Bhat, J.I.A., Choudhury, A.R. and Khan, M.Z. 2011. Biochemical indices in sheep during different stages of pregnancy. Asian. J. Anim. Vet. Adv. 6, 175–181. Knights, M., Hoehn, T., Lewis, P.E. and Inskeep, E.K. 2001. Effectiveness of intravaginal progesterone inserts and FSH for inducing synchronized estrus and increasing lambing rate in anestrous ewes. J. Anim. Sci. 79, 1120–1131. Kumar, D., Saxena, V.K., De, K., Naqvi, S.M.K., Krishnaswamy, N. and Tiwari, A.K. 2015. Induction of ovulation in anestrus ewes using a dopamine receptor antagonist. Theriogenology 84(8), 1362–1366. Laith, S.Y., Saad, T.R., Qusay, M.A., Mustafa, S.H. and Ali, A.A. 2020. Identification the effect of inhibin ßA/activin A genes polymorphism on superovulation (Calving Rate) in Holstein Friesian cows. Sys. Rev. Pharm. 11(2), 471–481. Li, L., Lu, S., Ma, Q., Pengcheng, W., Liu, C., Yang, H. and Shi, G. 2019. The comparison of reproductive hormone receptor expressions of the sheep ovary and hormone concentrations in two Chinese breeds. Reprod. Dom. Anim. 54, 892–901. Makawi, A.S. and Manhail, A.Z. 2007. Fertility response of desert ewes to hormonal estrus synchronization and artificial insemination using fresh diluted semen. J. Anim. Vet. Adv. 6(3), 385–391. Koyuncu, M.A.F. and Alticekic, S.O. 2010. Effects of progestagen and PMSG on estrous synchronization and fertility in Kivircik ewes during natural breeding season. Asian Australian J. Anim. Sci. 23(3), 308–311. Mukasa-Mugerwa, E. and Viviani, P. 1992. Progesterone concentrations in peripheral plasma of Menz sheep during gestation and parturition. Small Rumin. Res. 8(1-2), 47–53. Mura, M.C., Cosso, G., Pulinas, L., Carcangiu, V. and Luridiana, S. 2022a. Reproductive resumption in winter and spring related to MTNR1A gene polymorphisms in Sarda sheep. Animals 12(21), 2947. Mura, M., Luridiana, S., Pulinas, L., Bizzarri, D., Cosso, G. and Carcangiu, V. 2019. Melatonin treatment and male replacement every week on the reproductive performance in Sarda sheep breed. Theriogenology 135, 80–84. Mura, M.C., Luridiana, S., Bodano, S., Daga, C., Cosso, G. and Diaz, M.L. 2014. Influence of melatonin receptor 1A gene polymorphisms on seasonal reproduction in Sarda ewes with different body condition scores and ages. Anim. Reprod. Sci. 149, 173–177. Mura, M.C., Luridiana, S., Pulinas, L., di Stefano, M.V. and Carcangiu, V. 2019b. Reproductive response to male joining with ewes with different allelic variants of the MTNR1A gene. Anim. Reprod. Sci. 200, 67–74. Naderipour, H., Yadi, J., Shad, A. and Sirjani, M.A. 2012. The effects of three methods of synchronization on estrus induction and hormonal profile in Kalkuhi ewes: a comparison study. Afr. J. Biotechnol. 11, 530–533. Nasser S.O., Wahid H., Aziz A.S., Zuki A.B., Azam M.K., Jabbar A.G. and Mahfoz M.A. 2012. Effect of different oestrus synchronizations protocols on the reproductive efficiency of Dammar ewes in Yemen during winter. African J. Biotechnol. 11, 9156–9162. Noakes, D.E., Parkinson, D.J. and England, G.W. 2009. Pregnancy and parturition. In Arthurs veterinary reproduction and obstetrics. Eds., Noakes, D.E., Parkinson, T.J. and Arthur, G.H. Edinburgh, UK: Saunders; pp: 950. Notter, D.R. 2008. Genetic aspect of reproduction in sheep. Reprod. Domest. Anim. 43, 122–128. Padda, J., Khalid, K., Moosa, A., Syam, M., Kakani, V., Imdad, U. and Jean-Charles, G. 2021. Role of kisspeptin on hypothalamic-pituitary gonadal pathology and its effect on reproduction. Cureus 13(8), e17600. Pasciu, V., Nieddu, M., Baralla, E., Porcu, C., Sotgiu, F. and Berlinguer, F. 2022. Measurement of progesterone in sheep using a commercial ELISA kit for human plasma. J. Vet. Diagn. Invest. 34(1), 90–93. Pelletier, J., Bodin, L., Hanocq, E., Malpaux, B., Teyssier, J., Thimonier, J. and Chemineau, P. 2000. Association between expression of reproductive seasonality and alleles of the gene for Mel(1a) receptor in the ewe. Biol. Reprod. 62(4), 1096–1101. Pulinas, L., Starič, J., Cosso, G., Curone, G., Mura, M.C., Carcangiu, V. and Luridiana, S. 2022. MTNR1A gene polymorphisms and reproductive recovery after seasonal Anoestrus in different Mediterranean sheep breeds. Anim. Reprod. Sci. 236, 106905. Qayssar, J.K. 2024. Effect of CIDR and kisspeptin with investigation of kisspeptin r genotype on reproductive performance in Awassi ewes. Doctoral dissertation, PhD thesis. College of Veterinary Medicine, University of Baghdad College of/University of Baghdad. Theriogenology, Baghdad, Iraq. Quintero-Elisea, J.A. 2011. The effects of time and dose of pregnant mare serum gonadotropin (PMSG) on reproductive efficiency in hair sheep ewes. Trop. Anim. Health Prod. 43(8), 1567–1573. Rahawy, M.A., AL-Timimi, I.H. and Najwa S.A. 2016. Analysis of polymorphism of melatonin receptor type 1A (MTNR1A) gene, in Iraqi local sheep using PCR-RFLP technique. Iraqi J. Biotechnol. 15(3), 85–95. Rosa, H.J.D. and Bryant, M.J. 2003. Seasonality of reproduction in sheep. Small Rumin. Res. 48, 155–171. Rosasco, S.L., Beard, J.K., Hallford, D.M. and Summers, A.F. 2019. Evaluation of estrous synchronization protocols on ewe reproductive efficiency and profitability. Anim. Reprod. Sci. 210, 106191. SAS. 2010. SAS/STAT users guide for personal computer. Release 9.13. Cary, NC: SAS Institute, Inc. Saxena, V.K., Jha, B.K., Meena, A.S. and Naqvi, S.M.K. 2014. Sequence analysis and identification of new variations in the coding sequence of melatonin receptor gene (MTNR1A) of Indian Chokla sheep breed. Meta Gene 2, 450–458. Smith, J.T., Hawken, P.A., Lehman, M.N. and Martin, G.B. 2014. The role of kisspeptin in reproductive function in the ewe. In Reproduction in domestic ruminants VIII: Proceedings of the Ninth International Symposium on Reproduction in Domestic Ruminants. Madison, WI: Context Publishing, pp: 105–116. Somanjaya R, Fuah AM, Rahayu S, Setiadi MA, Abdullah L. 2021. PMSG in ewes: a practical and efficient step for superovulation. IOP Conf. Ser.: Earth Environ. Sci. 748, 012010. Stamatiades, G.A. and Kaiser, U.B. 2018. Gonadotropin regulation by pulsatile GnRH: signaling and gene expression. Mol. Cell Endocrinol. 463:131–141. Starič, J., Farci, F., Luridiana, S., Mura, M.C., Pulinas, L., Cosso, G. and Carcangiu, V. 2020. Reproductive performance in three Slovenian sheep breeds with different alleles for the MTNR1A gene. Anim. Reprod. Sci. 216, 106352. Szeliga, A. and Meczekalski, B. 2022. Kisspeptin modulation of reproductive function. Endocrines 3(3), 367–374. Takci, A. and Dinc, D.A. 2023. Stimulation of estrus and ovulation by resynchronization in Kangal sheep during early anestrus. Vet. Sci. 10(8), 499. Texeira, T.A., Da Fonseca, J.F., de Souza-Fabjan, J.M.G., de Rezende Carvalheira, L., de Moura Fernandes, D.A. and Brandao, F.Z. 2016. Efficiency of different hormonal treatments for estrus synchronization in tropical Santa Ines sheep. Trop. Anim. Health Prod. 48, 545–551. Thomson, B.C., Smith, N.B. and Muir, P.D. 2021. Effect of birth rank and age at first lambing on lifetime performance and ewe productivity. N. Z. J. Agric. Res. 64, 529–539. Tirpan, M.B., Tekin, K., Cil, B., Alemdar, H., Inanc, M.E., Olgac, K.T., Stelletta, C. and Daskin, A. 2019. The effects of different PMSG doses on estrus behavior and pregnancy rate in Angora goats. Animal 13(3), 564–569. Wei, S., Chen, S., Wei, B., Liu, Z., Bai, T. and Lin, J. 2016. Estrus synchronization schemes and application efficacies in anestrus lanzhou fat-tailed ewes. J. Appl. Anim. Res. 44(1), 466–473. Younis, L.S., Abid, A.A. and Rasheed, S.T. 2019. Effect of G(129)R polymorphism in growth differentiation factor 9 gene on awassi ewes that breed out of season. Malaysian J. Biochem. Mol. Biol. 22(2), 69 –73. Zarkawi, M. and Soukouti, A. 2001. Serum progesterone levels using radioimmunoassay during oestrous cycle of indigenous Damascus does. N. Zealand J. Agric. Res. 44(2–3), 165–169. | ||
| How to Cite this Article |
| Pubmed Style Al-obaidi MA, Naji IQ, Abd AA. Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewes. Open Vet. J.. 2025; 15(8): 3571-3579. doi:10.5455/OVJ.2025.v15.i8.19 Web Style Al-obaidi MA, Naji IQ, Abd AA. Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewes. https://www.openveterinaryjournal.com/?mno=255093 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.19 AMA (American Medical Association) Style Al-obaidi MA, Naji IQ, Abd AA. Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewes. Open Vet. J.. 2025; 15(8): 3571-3579. doi:10.5455/OVJ.2025.v15.i8.19 Vancouver/ICMJE Style Al-obaidi MA, Naji IQ, Abd AA. Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewes. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3571-3579. doi:10.5455/OVJ.2025.v15.i8.19 Harvard Style Al-obaidi, M. A., Naji, . I. Q. & Abd, . A. A. (2025) Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewes. Open Vet. J., 15 (8), 3571-3579. doi:10.5455/OVJ.2025.v15.i8.19 Turabian Style Al-obaidi, Maha A., Ibrahim Q. Naji, and Ali A. Abd. 2025. Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewes. Open Veterinary Journal, 15 (8), 3571-3579. doi:10.5455/OVJ.2025.v15.i8.19 Chicago Style Al-obaidi, Maha A., Ibrahim Q. Naji, and Ali A. Abd. "Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewes." Open Veterinary Journal 15 (2025), 3571-3579. doi:10.5455/OVJ.2025.v15.i8.19 MLA (The Modern Language Association) Style Al-obaidi, Maha A., Ibrahim Q. Naji, and Ali A. Abd. "Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewes." Open Veterinary Journal 15.8 (2025), 3571-3579. Print. doi:10.5455/OVJ.2025.v15.i8.19 APA (American Psychological Association) Style Al-obaidi, M. A., Naji, . I. Q. & Abd, . A. A. (2025) Manipulating seasonality by using PMSG and Kisspeptin hormones and the impact of the MTNR1A gene on reproduction efficiency in ewes. Open Veterinary Journal, 15 (8), 3571-3579. doi:10.5455/OVJ.2025.v15.i8.19 |