| Research Article | ||
Open Vet. J.. 2025; 15(9): 4412-4417 Open Veterinary Journal, (2025), Vol. 15(9): 4412-4417 Research Article Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, IraqShaimaa A. Majeed1 and Entesar Hussain Madi2*1Parasitology Department, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq 2Department of Zoonoses Research Unit, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Entesar Hussain Madi. Department of Zoonoses Research Unit, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Email: Intisar.h [at] covm.uobaghdad.edu.iq Submitted: 29/04/2025 Revised: 18/07/2025 Accepted: 05/07/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
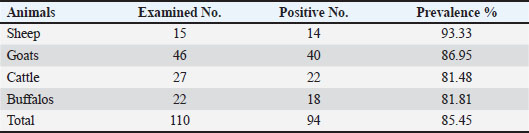
ABSTRACTBackground: Toxoplasma gondii is a zoonotic disease that is widespread worldwide and considered one of the most common tropical diseases in some countries. Accurate serological distribution and risk factors for animal and human parasite infection in Baghdad city/Iraq have not been adequately studied despite the importance of such studies. Aim: This study aimed to identify the Toxoplasma hemoparasite and potential risk factors of Toxoplasma parasite infection in sheep, goats, cattle, and buffaloes in the Abu Guraib area west of Baghdad city using enzyme-linked immunosorbent assay (ELISA). Methods: In total, 110 serum samples were randomly collected from 15 sheep, 46 goats, 27 cattle, and 22 buffalos of different ages during December 1, 2023 to December 1, 2024 in the Abu Guraib area west of Baghdad city. For identification using an indirect ELISA test. Results: The results presented 94 (85.45%) positive serum samples for Toxoplasma gondii in all animals included in this study were in sheep 15 (93.33%), goats 46 (86.95%), cattle 27 (81.48%), and buffalos 22 (81.18%). The highest prevalence rate was observed in sheep, and there was no significant difference between different animals, whereas there was high significant difference at (p ≤ 0.05) between females and males in all animals. Conclusion: This study concluded that the infection rate was high with T. gondii in sheep, goats, cattle, and buffaloes, indicating contamination with the parasite in livestock in the Abu Guraib area west of Baghdad city and may be a risk factor for infection in humans by consumption of meat from these animals. Key words: Toxoplasma gondii, ELISA, Ruminant, Prevalence, Iraq. IntroductionToxoplasmosis occurs in flocks and affects many countries. It is given as one of the main reasons for abortion in many countries (Blewett and Watson, 1984; Dubey and Beattie, 1988; Dubey, 2004). It is an important economic disease that affects herds, mainly sheep and goats, due to Toxoplasma gondii (Mohamad et al., 2010). Toxoplasma infection causes fetal death, resorption, embalming, reproductive failure, newborn death, or birth of weakly and incapable fetuses (Amina et al., 2015; Al-Abodi, 2019), and huge economic losses to the industry of ovine and caprine and was considered one of the most important reasons for abortion of goats and sheep (Buxton et al., 2007). According to Khan et al. (2017), nearly all animal species, including humans, are infected with toxoplasmosis due to its extensive global presence. The severity and progression of an infection are largely determined by the immune system’s strength and response in the affected individual (Muhsin et al., 2013; Al-Abodi, 2017). Toxoplasma gondii is a crescent-shaped cell (~2 μm wide, 5 μm long) with sharp anterior and rounded posterior ends. The cytoskeleton supports structure, motility, and cell invasion, housing organelles like the Golgi, ribosomes, mitochondria, apicoplast, ER, and a plastid-like structure. In warm-blooded animals, T. gondii multiplies asexually, whereas sexual reproduction is confined to felines, which serve as the parasite’s definitive hosts(Weiss and Dubey, 2009; Mercier et al., 2011; Hill and Dubey, 2013; Majeed and Abbas, 2018). Toxoplasmosis is a widespread parasitic infection affecting both humans and livestock, significantly affecting public health and animal productivity (Dubey, 2010). A study by Madi and Al-Samarai (2022) confirmed the presence of toxoplasmosis in milk from Iraqi local and Shami goats, while also examining how infection and various factors impact milk composition in these breeds. It poses a public health concern due to its transmission through consumption of undercooked meat containing tissue cysts, contaminated food or water with oocysts, or accidental ingestion of sporulated oocysts from soil (Alvarado-Esquivel et al., 2011). Issa and Omer (2011) in the northern Iraqi city of Dohuk revealed that the prevalence of T. gondii antibodies was 95.65% and 27.17% using LAT and ELISA, respectively. In ewes, T. gondii antibodies were detected by LAT in 97.4% of samples and ELISA in 33% of samples. In does, 94.33% and 22.64% of samples were positive by the LAT and ELISA, respectively. Toxoplasma gondii, an intracellular parasite causing significant disease in humans and animals, has unknown genetic diversity in Iraqi sheep.in study investigated T. gondii genotypes in sheep from Wasit province, Iraq, by analyzing 315 samples (300 blood and 15 placenta tissue) from aborted ewes. Samples were tested using LAT serology and RT-PCR (B1 gene amplification), confirming 10 T. gondii DNA-positive cases. Genotyping via nested PCR-RFLP of the SAG2 gene revealed that 60% were Type II, 30% Type III, and 10% Type I, with Type II being the dominant genotype in the region (Aaiz, 2016). Madi et al. (2022) found that sequencing of the SAG3 gene in T. gondii isolates from goats and humans revealed high similarity, ranging from 98.65% to 99.90%, specifically for genotypes I and III. In Baghdad, Iraq, study by Rifaat and Jawdat (1998) utilized two diagnostic methods—the complement fixation test and the direct fluorescent antibody test—to detect T. gondii antibodies in serum samples from 143 sheep and 44 goats. CFT identified complement-fixing antibodies in 26.2% of sheep sera (38/143) and 54.5% of goat sera (24/44), while FAT detected antibodies in 18.2% of sheep sera (26/143). Also, study conducted in Erbil city, Iraq, seroprevalence of T. gondii in sheep (25.4%) and goats (28.4%) using latex agglutination, MAT and ELISA confirmed similar positivity rates in latex-positive samples. No significant difference between ELISA and MAT results (Kader and Al-Khayat, 2013). Materials and MethodsCollection of samplesA total of 110 blood samples were randomly collected from animals, comprising 15 sheep, 46 goats, 27 cattle, and 22 buffaloes, specifically targeting females with a history of abortion. These animals, ranging in age, were gathered from various locations in Abu Graib, Baghdad, an area with a high concentration of livestock, between December 1, 2023 and December 1, 2024. Blood was drawn from the jugular vein into 5-ml tubes, maintained in an icebox during transport to the University of Baghdad’s College of Veterinary Medicine. Upon arrival, the samples were centrifuged at 3,000 rpm for 10 minutes to isolate the serum, which was then stored at 20°C for subsequent Toxoplasma antibody testing using indirect ELISA. ImmunoassayToxoplasma antibodies in serum samples were detected using a commercially available indirect ELISA kit (ID Screen Toxoplasmosis indirect multi-species®, IDVET, Montpellier, France) following the manufacturer’s instructions. Positive results were defined as an optical density (OD) greater than 0.350. The color change from blue to yellow after adding the stop solution indicated specific antibodies, whereas no color change suggested their absence. The microplate was read at 450 nm. The presence or absence of antibodies was determined by calculating the S/P ratio, with S/P ≥ 50% considered positive, as per the kit’s manual formula (Table 1). Statistical analysisIndirect ELISA results were statistically analyzed using the chi-square test (χ2), and the SPSS program (SPSS, 2008). Ethical approvalFor animal experiments, all protocols were approved by the Institutional Animal Care and Use Committee Approval Number: (P.G/965 in 23/4/2025), and animals were treated in accordance with the Guidelines for the Care and Use of Animals.” ResultsThe seroprevalence of the Toxoplasma hemoparasite in the serum samples of all animals was 110 (85.45%). The seroprevalence of the infection was 15 (93.33%) in sheep, 46 (86.95%) in goats, 27 (81.48%) in cattle, and 22 (81.81%) in buffalo. There are no significant differences at p ≥ 0.05 (Table 2). Table 1. Results and status of the iELISA of toxoplasmosis.
Table 2. Toxoplasmosis seroprevalence in animals, without significant differences at (p ≥ 0.05).
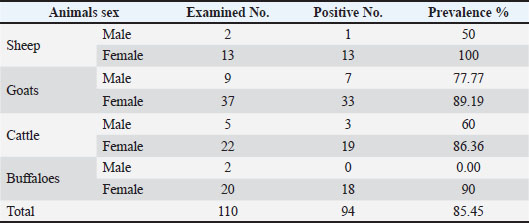
Table 3. Toxoplasmosis seroprevalence in sheep, goats, cattle, and buffaloes according to sex, significant differences (p ≤ 0.05).
The results of this study showed that there were highly statistically significant differences at the level (p ≤ 0.05) in the seroprevalence percentage between males and females of different animals. The prevalence rates of sheep, goats, cattle, and buffalo males were 1/2, 7/9, 3/5, and 0/2, respectively. The prevalence rates in females in all animals of this study were 13/13, 33/37, 19/22, and 18/20, respectively (Table 3). A high prevalence rate was observed in sheep females 13/13 compared with the low prevalence rate in cattle females 19/22, whereas a high prevalence rate was observed in males of goats 7/9 and the lowest prevalence rate in buffaloes 0/2, respectively, without significant differences (p ≥ 0.05) (Table 2). DiscussionThe study’s seroprevalence rates in both sheep (93.33%) and goats (86.95%) were notably higher than those reported in previous studies across various regions, highlighting significant regional variability in T. gondii infection rates. This version is organized by species (sheep and goats), groups comparisons by region, and clearly separates findings from different studies with proper citations. Sheep seroprevalence were Bahia, Brazil (18.75%) Pita Gondim et al. (1999) Uruguay (38.9%) Suzuki et al. (2011) Northeast Brazil (22.1%) Andrade et al. (2013) Algeria (57.89%) Dechicha et al. (2015) North-Eastern Region of Pakistan (26.2%) Ahmed et al. (2016) Erbil city, Iraq (25.4%) Kader and Al-Khayat(2013). Additional studies in sheep reported: 36.08% (149/413) IgM-positive sera using ELISA Mikail and Al-Barwary. (2014) 60% (15/25) seroprevalence in Baghdad using ELISA—Hade et al. (2015)—**11.59% (32 samples) in Algeria using indirect fluorescent antibody test Dechicha et al. (2015). Goat Seroprevalence were Brazil (28.93%) Pita Gondim et al. (1999) Iraq (28.4%) Kader and Al-Khayat (2013). Algeria (13.21%) Dechicha et al. (2015) Southern Tunisia (34.5%) Lahmar et al. (2015) Pakistan (42.8%) Ahmed et al. (2016). Additional studies in goats reported: 54.6% seroprevalence in Iraq using ELISA by Al-Taie and Abdulla (2011). As well as 28.7% (blood) and 21.2% (milk) in Iraqi goats reported by Madi et al. (2022). The seroprevalence of toxoplasmosis in cattle was (81.48%), which was in agreement with da Silva et al. (2015), who recorded that infection in cattle was 83.40% in Northern and Midwestern Brazil, as well as that asymptotic rate with Ibrahim et al. (2014), who mentioned that toxoplasmosis infection was 89.3%, but disagreement with those observed by Pita Gondim et al. (1999), who referred to very low seroprevalence rates in cattle (1.03%); in Sudan, Elfahal et al. (2014) mentioned 44.80% and in Iraq, Akber et al. (2014) recorded 14.6% of seroprevalence rate of Toxoplasma gondii in cattle, respectively. In buffaloes, the seroprevalence for toxoplasmosis detected in this study was 81.81%, which was disagreement with Pita Gondim et al. (1999), who examined 104 heads of water buffaloes, which was very low 3.85%; Persad et al. (2011) and Alvarado-Esquivel et al. (2014) reported lower seropositive for toxoplasmosis (7.8%) and (48.7%), respectively. Also, Akber et al. (2014) reported a T. gondii infection rate of 8.6%, while Jabbari et al. (2024) conducted a study on 150 animals (50 buffaloes and 100 cattle). Their findings revealed that 6.7% of the meat samples were infected with T. gondii, specifically 9% of cattle and 2% of buffalo meat samples. There was a positive association between population density and a high number of animals in an area; additionally, these high percentages referred to contamination of the environment, and the oocyst parasite is widespread in these farms. The observed differences between these infection rates may be related to the serological methods used in these studies, the number of animals examined, the specific hosts, and variables of climatic (Al-Abodi, 2018; Al-Hatami et al., 2018; Dahmane et al., 2024). ELISA testing revealed the highest T. gondii infection rates in sheep compared to goats, cattle, and buffaloes. These findings align with reports by Dubey (2009), Asgari et al. (2010), Sharif et al. (2015), and Tonouhewa et al. (2017), who consistently noted that the parasite is more prevalent in sheep than in goats and cattle. These differences may be due to geographic region, grazing style, and location of definitive host (Majeed and Abbas, 2018), as well as to the natural susceptibility of sheep to infection with the T. gondii parasite (Dahmane et al., 2024). The seroprevalence of toxoplasmosis Azizi et al. (2014) found that sheep were six times more susceptible to T. gondii infection than cattle. This observation aligns with Jittapalapong et al. (2005), who noted that goats primarily consume tree tops and young plants, which may have lower parasite contamination. In contrast, sheep graze on the lower parts of trees, where parasite prevalence is reported to be higher, potentially explaining their increased susceptibility. in male and female sheep was 50% and 100%, respectively, and in goats was 77.77% and 89.19%, respectively. These results are in agreement with Tegegne et al. (2016), who reported higher seroprevalence in female sheep (66.7%) compared to males (46.5%), and in female goats (62.2%) compared to males (42.9%). Similarly, Hanif and Tasawar (2016) and Qazaz and Faraj (2016) observed higher rates in female sheep (28.28% vs. 19.60%) and female goats (87.32% vs. 71.42%), respectively. However, these findings contradict Madi et al. (2025), who reported a significantly higher infection rate in male goats (40.42%) than in female goats (18.88%) (p < 0.01). These differences are likely due to management practices. Females, primarily reared for milk production, are often culled earlier, while males remain in the herd longer, increasing their cumulative exposure to pathogens over time. Additionally, males face heightened risk due to their involvement in mating with multiple females, which further elevates their susceptibility to infection. The results revealed that T. gondii infection in cattle and buffalo was higher in females than in males, as follows: 86.36%, 60% and 90%, 0.00%, respectively. These results are in agreement with Dechicha et al. (2015), who reported the highest rate in females 3.96% compared with males 0.00%, while the results disagree with Elfahal et al. (2013), who observed a higher rate in male cattle (30.8%) than in female cattle (11.9%). Persad et al. (2011) reported a higher rate in female buffaloes (8.3%) than in male buffaloes (6.7%). Alvarado-Esquivel et al. (2014) reported the highest rate in male buffaloes (59.4%) compared with female buffaloes (47.6%). These differences in the results of the current study may be attributed to several factors, such as keeping a small number of males for breeding while others are slaughtered, and hormonal differences related to pregnancy and lactation stress are considered factors that decrease immunity, which might expose the female to toxoplasmosis (Dubey and Lappin, 1998). ConclusionThe research found that toxoplasmosis was prevalent in a benign form among farm animals, with sheep exhibiting the highest infection rates. Female sheep across all breeds were more frequently infected than male sheep, highlighting their role as a significant source of Toxoplasma transmission to humans and a potential public health risk. AcknowledgmentsThe authors thank Prof. Dr. Waffa. A. Ahmed and all staff in the zoonoses research unit at the College of Veterinary Medicine, Baghdad University. Conflict of interestNo conflict of interest. FundingThe authors declare that finance is personal. Authors’ contributionsThe authors declare that they have contributed to the research through working and writing. Data availabilityAll data supporting the research are available within the manuscript. ReferencesAaiz, N.N. 2016. Determination of Toxoplasma gondii lineages of sheep in Wasit. Iraqi J.Vet .Sci, 30, 23–26. Ahmed, H., Malik, A., Arshad, M., Mustafa, I., Khan, M.R., Afzal, M.S., Ali, S., Mobeen, M.and Simsek, S. 2016. Seroprevalence and spatial distribution of toxoplasmosis in sheep and goats in North-Eastern Region of Pakistan. Korean J. Parasitol. 54(4), 439–446; doi:10.3347/kjp.2016.54.4.439 Akber, A.A., Hanna, L.Y., Hussain, A.A., Abod, K., Mohammed, N., Taleb, S. and Awad, A. 2014. Seroprevalence study of Toxoplasmosis in Iraq on some of Ruminant animals. Iraqi J. Agri. Sci. 45(1), 92–98. Available via https://www.iasj.net/iasj/article/86404 Al-Abodi, H.R.J. 2017. Serological and molecular detection of Toxoplasma gondii in Columba livia hunting pigeons of Al- Qadisiyah province. Al-Qadisiyah J. Vet. Med. Sci. 16, 128. Al-Abodi, H.R.J. 2018. Use of rapid cassette test and polymerase chain reaction technique to investigate toxoplasmosis in Columba livia birds in Al-Muthanna governorate. Proceeding of 6th International Scientific Conference, College of Veterinary Medicine University of Basrah, Iraq. Basrah J. Vet. Res. 17(3), 45–50. Al-Abodi, H.R.J. 2019. Use of immunological methods to the detection of toxoplasmosis and heat shock protein HSP70 in men . J. Parasit. Dis. 43, 234. Al-Hatami, A.O., Al-Kardhi, I.K. and Al-Mosa, M.A. 2018. Prevalence of seropositive Toxoplasma cases in association with the frequency of abortion in sheep and goat. Kufa J. Vet. Med. Sci. 9(1), 1–10; doi:10.36326/kjvs/2018/v9i14096 Al-Taie, L.H. and Abdulla, S.H. 2011. Seroprevalance of Toxoplasmosis in sheep and goat: Iraq/Sulaimania. Iraqi J. Vet. Med. 35(1), 16–24. Alvarado-Esquive, C., Estrada-Martínez, S., Pizarro-Villalobos, H., Arce-Quiñones, M. and Liesenfeld, O. 2011. Seroepidemiology of Toxoplasma gondii infection in general population in a northern Mexican city. J. Parasitol. (97), 40–43. Alvarado-Esquivel, C., Dora, R., Zeferino, G., Anabel, C., Álvaro, P., Nelly, I., Mariel, A., Adalberto, A.P. and Dubey, P.J. 2014. Seroprevalence of Toxoplasma gondii infection in water buffaloes (Bubalus bubalis) in Veracruz State, Mexico and its association with climatic factors. BMC Vet. Res. 10(232), 2–5. Available via http://www.biomedcentral.com/1746-6148/10/232 Amina, S.D., Fatma, B., Ismail, G., Edmee, G., Djamila, B., Mohamed, B. and Djame, G. 2015. Sero-epidemiological survey on toxoplasmosis in cattle, sheep, and goats in Algeria. African J. Agri. Res. 10(20), 2113–2119. Andrade, M.M.C., Mariangela, C., Andrea, D.M., Valter, A.N. and Ricardo, W.A.V. 2013. Seroprevalence and risk factors associated with ovine toxoplasmosis in Northeast Brazil. Parasite J. 20(20), 1–5. Available via www.parasite-journal.org Asgari, Q., Kalantari, M., Motazedian, M. and Shahriari, B. 2010. Molecular survey of Toxoplasma infection in sheep and goat from Fars province, Southern Iran. Trop. Anim. Health. Prod. 43(2), 389–392; doi:10.1007/s11250-010-9704-1 Azizi, H., Shiran, B., Boroujeni, A. and Jafari, M. 2014. Molecular survey of Toxoplasma gondii in sheep, cattle and meat products in Chaharmahal va Bakhtiari Province, Southwest of Iran. Iranian J. Parasitol. 9, 429–434. Blewett, D.A. and Watson, W.A. 1984. The epidemiology of ovine toxoplasmosis. III. Observations on outbreaks of clinical toxoplasmosis in relation to possible mechanisms of transmission. Br. Vet. J. 140, 54–63. Buxton, D., Maley, S., Wright, S., Rodger, S., Bartley, P. and Innes, E. 2007. Toxoplasma gondii and ovine toxoplasmosis: new aspects of an old story. Vet. Parasitol. 149, 25–28. da Silva, J.B., de Santana Castro, G.N., dos Santos, P.N., da Fonseca, A.H., da Silva Lima, D.H., dos Anjos Bomjardim, H., Reis, A.D.S.B., de Oliveira Soares, S. and Barbosa, J.D. 2015. Detection of a high prevalence of antibodies against Toxoplasma gondii in cattle in Northern and Midwestern Brazil. Rev. Salud Anim. 37(1), 52. Dahmane, A., Almeida, D., Reghaissia, N., Baroudi, D., Samari, H., Abdelli, A., Laatamna, A. and João R. Mesquita, J.R. 2024. Seroprevalence assessment and risk factor analysis of Toxoplasma gondii infection in goats from Northeastern Algeria. Animals 2024, 14, 883; doi:10.3390/ani14060883 Dechicha, A.S., Bachi, F., Gharbi, I., Gourbdji, E., Baazize-Ammi, D. and Guetarni, D. 2015. Sero-epidemiological survey on toxoplasmosis in cattle, sheep and goats in Algeria. African J. Agric. Res. 10(20), 2113–2119. Dubey, J.P. 2004. Toxoplasmosis-awaterborne zoonosis. Vet. Parasitol. 126, 57–72. Dubey, J.P. 2009. Toxoplasmosis of animals and humans, 2nd ed. Boca Raton, FL: CRC Press. Dubey, J.P. 2010. Toxoplasmosis of animals and humans, 2nd ed. Beltsville: CRC Press; pp. 1–338. Dubey, J.P. and Beattie, C.P. 1988. Toxoplasmosis of animals and man. Boca Raton, FL: CRC Press; pp. 200. Dubey, J.P. and Lappin, M.R. 1998. Toxoplasmosis and neosporosis. In Infectious diseases of the dog and cat. Ed., Greene, C.E. Philadelphia: WB Saunders; pp. 493–509. Elfahal, A.M., Elhassan A.M., Hussein, M.O., Enan, K.A., Musa, A.B. and El Hussien, A.M. 2014. Seroprevalence of Toxoplasma gondii in cattle in Gezira and Khartoum States: a comparison between ELISA and latex agglutination tests. Sudan J. Vet. Res. 29, 15–19. Elfahal, A.M., Elhassan, A.M., Hussien, M.O., Enan, K.A., Musa, A.B. and El Hussein, A.M. 2013. Seroprevalence of Toxoplasma gondii in dairy cattle with reproductive problems in Sudan. ISRN Vet. Sci. 2013(1), 895165. Hade, B.F., Ghareeb, A.M. and Kawan, M.H. 2015. Direct amplification of B1 gene of Toxoplasma gondii DNA using nested polymerase chain reaction following microwave treatment for whole blood samples. Iraqi J. Vet. Med. 39(1), 23–27. Hanif, M. and Tasawar, Z. 2016. Seroprevalence and risk factors associated with toxoplasmosis in sheep in Multan and Khanewal districts of Punjab (Pakistan), J. Animal Plant Sci. 26(6), 1620–1627. Hill, D. and Dubey, J. 2013. Toxoplasma gondii prevalence in farm animals in the United States. Int. J. Parasitol. 43, 107–113. Ibrahim, A.M., Ismail, A.A., Angara, T.E.E. and Osman, O.M. 2014. Serological survey on Toxoplasma gondii in dairy cows from the Sudan using ELISA. GJASLPAB, 2(3), 114–118. Available via http://www.globalscienceresearchjournals.org/ Issa, N.A. and Omer, L.T. 2011. Prevalence of Toxoplasma gondii in aborted ewes and does in Duhok province of Iraq. Res. Opin. Anim. Vet. Sci. 1(10), 627–630. Jabbari, J., Hajipour, N., Hassanzadeh, P. and Ketzisc, J. 2024. Detection of Toxoplasma gondii infection in buffaloes (Bubalus bubalis) and cattle (Bos taurus) at the Tabriz abattoir, Iran. Vet. Med. Sci. 10, e1511; doi:10.1002/vms3.1511 Jittapalapong, S., Sangvaranond, A., Pinyopanuwat, N., Chimnoi, W., Khachaeram, W., Koizumi, S. and Maruyama, S. 2005. Seroprevalence of Toxoplasma gondii infection in domestic goats in Satun Province, Thailand. Vet. Parasitol. 127(1), 17–22. Kader, J.M. and Al-Khayat, Z.A.Y. 2013. Serodiagnosis of toxoplasmosis in sheep and goats in Erbil city, Iraq. Iraqi J. Vet. Sci. 27(1), 21–23; doi:10.33899/IJVS.2013.82947 Khan, M.U., Rashid, I., Akbar, H., Islam, S., Riaz, F., Nabi, H., Ashraf, K. and Singla, L.D. 2017. Seroprevalence of Toxoplasma gondii in south Asian countries. Rev. Sci. Tech. Off. Int. Epiz. 36(3), 1–36. Lahmar, I., Lachkhem, A., Slama, D., Sakly, W., Haouas, N., Gorcii, M., Pfaff, A.W., Candolfi, E. and Babba, H. 2015. Prevalence of Toxoplasmosis in sheep, goats and cattle in Southern Tunisia, J. Bact. Parasitol. 6, 1–4. Madi, E.H, Al-Samarai, F.R, Maaeni, Y.M. and Gangwar, S.K. 2022. Comparison between nested-PCR and ELISA for the detection of Toxoplasma gondii in blood and milk and its genotyping in lactating goats and aborted women in Iraq. Iraqi J. Vet. Med. 46(2), 53–59; doi:10.30539/znnp2y07 Madi, E.H., Al-Samarai, F.R., Maaeni, Y.M.A. and Abdel-Ghany, A.M. 2025. Detection of toxoplasmosis and some risk factors with the infection rate in local and shami goats. Iraqi J. Agri. Sci. 56(3), 976–984. Madi, E.H. and Al-Samarai, F.R. 2022. Using elisa and nested PCR for detection of the Toxoplasmosis in milk and the influence of infection and some factors on milk composition in the Iraqi local and shami goats. IJHS. 6(S7), 3280–3288; doi:10.53730/ijhs.v6nS7.12456 Majeed, B. and Abbas, W.H. 2018. Serological and molecular detection of Toxoplasma gondii in meat and minced meat in Basra city. Basrah J. Vet. Res. 17, 491–505. Mercier, A., Ajzenberg, D., Devillard, S., Demar, M.P., De Thoisy, B., Bonnabau, H., Collinet, F., Boukhari, R., Blanchet, D., Simon, S., Carme, B. and Dardé, M.L. 2011. Human impact on genetic diversity of Toxoplasma gondii: example of the anthropized environment from French Guiana. Infect. Genet. Evol. 11, 1378–1387. Mikail, F.B. and Al-Barwary, L.T.O. 2014. Seroprevalence of toxoplasmosis in aborted ewes by using different immunologic tests in Duhok governorate, Kurdistan region, Iraq. Iraqi J. Vet. Sci. 28(1), 11–15. Mohamad, A.A., Mustafa, M.A., Nektarios, D.G. and Shawkat, Q.L. 2010. Ovine and caprine Toxoplasmosis (Toxoplasma gondii), Iranian J. Vet. Sci.and Tech. 2(2), 61–76. Muhsin, S.S., Jafar, E.H. and Jafar, N.S. 2013. Biochemical study on the effect of Toxoplasma gondii on liver function in women. Iraqi J. Vet. Med. 37(2), 257–260. Persad, A., Charles, R. and Adesiyun, A.A. 2011. Frequency of Toxoplasmosis in Water Buffalo (Bubalus bubalis ) in Trinidad. Vet. Med. Int. 1–4; doi:10.4061/2011/705358 Pita Gondim, L.F.P., Barbosa, H.V., Ribeiro Filho, C.H.A. and Saeki, H. 1999. Serological survey of antibodies to Toxoplasma gondii in goats, sheep, cattle and water buffaloes in Bahia State, Brazil. Vet. Parasitol. 82, 273–276. Qazaz, E.A. and Faraj, A.A. 2016. Seroprevalence of Toxoplasmosis in goat in Baghdad governorate, mirror of rese. Vet. Sci. Animals 5(2), 58–66. Available via http://mrvsa.com/ Rifaat, K.H. and Jawdat, S.Z. 1998. Prevalence of toxoplasmosis among sheep and goats in Baghdad area. Iraqi J. Vet. Med. 22, 43–49. Sharif, M., Sarvi, S., Shokri, A., Hosseini Teshnizi, S., Rahimi, M.T., Mizani, A., Ahmadpour, E. and Daryani, A. 2015. Toxoplasma gondii infection among sheep and goats in Iran: a systematic review and meta-analysis. Parasitol. Res. 114, 1–16. SPSS. 2008. Statistical package for the social sciences, versions 16 and 17 (Win/Mac/Linux), user’s guide SPSS Inc., Chicago III, USA. Suzuki, K., Corva, S.G., Travería, G.E., Cattáneo, M., Puentes, R. and Martinicorena, M. 2011. Seroprevalence of Toxoplasma gondii and Neospora caninum in sheep in Uruguay. Analecta Vet. 31(2), 28–32. Tegegne, D., T.; kelifa, A. ., Abdurahaman, M. and Yohannes, M. 2016. Seroepidemiology and associated risk factors of Toxoplasma gondii in sheep and goats in Southwestern Ethiopia. BMC Vet. Res. 12(280), 1–6; doi:10.1186/s12917-016-0906-2 Tonouhewa, A.B.N., Akpo, Y., Sessou, P., Adoligbe, C., Yessinou, E., Hounmanou, Y.G., Assogba, M.N., Youssao, I. and Farougou, S. 2017. Toxoplasma gondii infection in meat animals from Africa: Systematic review and meta-analysis of sero-epidemiological studies. Vet. World 10(2), 194–208. Weiss, L.M. and Dubey, J.P. 2009. Toxoplasmosis: a history of clinical observations. Int. J. Parasitol. 39, 895–901. | ||
| How to Cite this Article |
| Pubmed Style Majeed SA, Madi EH. Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, Iraq. Open Vet. J.. 2025; 15(9): 4412-4417. doi:10.5455/OVJ.2025.v15.i9.48 Web Style Majeed SA, Madi EH. Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, Iraq. https://www.openveterinaryjournal.com/?mno=255056 [Access: January 26, 2026]. doi:10.5455/OVJ.2025.v15.i9.48 AMA (American Medical Association) Style Majeed SA, Madi EH. Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, Iraq. Open Vet. J.. 2025; 15(9): 4412-4417. doi:10.5455/OVJ.2025.v15.i9.48 Vancouver/ICMJE Style Majeed SA, Madi EH. Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, Iraq. Open Vet. J.. (2025), [cited January 26, 2026]; 15(9): 4412-4417. doi:10.5455/OVJ.2025.v15.i9.48 Harvard Style Majeed, S. A. & Madi, . E. H. (2025) Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, Iraq. Open Vet. J., 15 (9), 4412-4417. doi:10.5455/OVJ.2025.v15.i9.48 Turabian Style Majeed, Shaimaa A., and Entesar Hussain Madi. 2025. Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, Iraq. Open Veterinary Journal, 15 (9), 4412-4417. doi:10.5455/OVJ.2025.v15.i9.48 Chicago Style Majeed, Shaimaa A., and Entesar Hussain Madi. "Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, Iraq." Open Veterinary Journal 15 (2025), 4412-4417. doi:10.5455/OVJ.2025.v15.i9.48 MLA (The Modern Language Association) Style Majeed, Shaimaa A., and Entesar Hussain Madi. "Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, Iraq." Open Veterinary Journal 15.9 (2025), 4412-4417. Print. doi:10.5455/OVJ.2025.v15.i9.48 APA (American Psychological Association) Style Majeed, S. A. & Madi, . E. H. (2025) Seroprevalence of toxoplasmosis in sheep, goat, cattle, and buffaloes in Baghdad, Iraq. Open Veterinary Journal, 15 (9), 4412-4417. doi:10.5455/OVJ.2025.v15.i9.48 |