| Research Article | ||
Open Vet. J.. 2025; 15(8): 3598-3607 Open Veterinary Journal, (2025), Vol. 15(8): 3598-3607 Research Article Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccineHaider Hamza Alfatly and Noor Idan Jarad*Department of Veterinary Microbiology, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah City, Iraq *Corresponding Author: Noor Idan Jarad. Department of Veterinary Microbiology, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah City, Iraq. Email: noor.jarad [at] qu.edu.iq Submitted: 27/04/2025 Revised: 28/06/2025 Accepted: 12/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
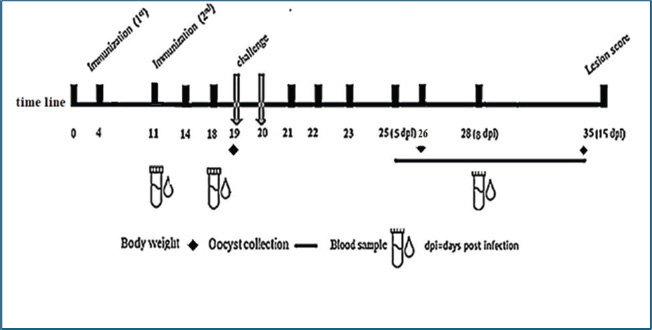
ABSTRACTBackground: Coccidiosis is an economically important disease caused by Eimeria species in poultry. Aim: This study aimed to design, express, and evaluate a recombinant 19-kDa sporozoite protein (E19kDa) as a vaccine candidate against mixed Eimeria infection in broiler chickens. Methods: The study used bioinformatic analysis, protein expression analysis, immunization trials, and immune response evaluation were performed. Protein properties were predicted using bioinformatics tools. Gene 838 (Accession No. PV347147) was cloned into a pET-28b plasmid and expressed in Escherichia coli BL21(DE3). IPTG induction levels were optimized, and expression was confirmed via quantitative PCR. The protein was purified using Ni-NTA chromatography and validated by SDS-PAGE. Twenty Ross 308 chickens were divided into negative control, positive control (infected), and challenge group. Birds in the vaccine group received two subcutaneous doses of E19kDa with aluminum hydroxide adjuvant. The challenge was performed using a mixed Eimeria oocyst suspension (1 × 10⁴ oocysts per species). Immunity was assessed through live weight, oocyst counts, white blood cell counts, and IgY levels. Results: The results showed high expression of E19kDa at 1 mM IPTG. SDS-PAGE confirmed the purified protein at ~29kDa. Immunized birds exhibited better weight gain, lower oocyst counts (10,245 versus 22,755), and increased white blood cell counts and IgY titers compared with infected controls. Enzyme-linked immunosorbent assay confirmed a peak antibody response on day 28 post-vaccination. The vaccine significantly reduced clinical symptoms and mortality. Conclusion: This study highlights the potential of the recombinant E19kDa protein as a promising candidate vaccine against coccidiosis. The approach combining bioinformatics, molecular expression, and immunological testing offers a cost-effective solution for the disease. Keywords: Adjuvant, ELISA, Expression, Immune response, Oocyst count. IntroductionAvian coccidiosis is a widespread intestinal disease caused by protozoan parasites of the genus Eimeria, which invade and destroy epithelial cells in the intestinal tract of chickens. This results in poor nutrient absorption, decreased weight gain, and in severe cases, mortality (Fatemi Motlagh and Mousavi Gargari, 2022). The economic burden of coccidiosis on the global poultry industry is enormous, with annual estimated losses exceeding 3 billion US dollars. These losses stem not only from bird mortality, reduced feed efficiency, poor growth, and high treatment costs. The impact of coccidiosis is especially pronounced in broiler production systems, where birds are reared in high densities and under intensive conditions (Venkatas and Adeleke, 2019; Bozkurt and Savaş, 2024; Shahininejad et al., 2024). In such systems, subclinical infections often go unnoticed while continuously impairing performance (Blake et al., 2021; El-Shall et al., 2022; van Eerden et al., 2022; da Silva Giacomini et al., 2023; Bora et al., 2024). In the past, coccidiosis was mainly controlled by adding anticoccidial drugs to feed or water. However, drug-resistant Eimeria strains appeared. At the same time, new rules and customer demand for antibiotic-free meat pushed the poultry industry to find other solutions (Fatoba and Adeleke, 2018; Cai et al., 2022; Gaghan et al., 2022; Lee et al., 2022; Rizwan et al., 2022; Liu et al., 2023; Oryasin and Eren, 2025). Today, live and live attenuated vaccines are the most common tools against coccidiosis. These vaccines expose birds to small amounts of real or weakened parasites. This teaches the birds’ immune systems to fight back (da Silva Giacomini et al., 2023; Liu et al., 2023). Although effective, live vaccines are associated with problems. Some birds may lose weight, suffer intestinal damage, or develop uneven immune responses, especially if the vaccine cycles are not managed well (Sokale et al., 2021; Zheng et al., 2023). The variety of Eimeria species adds more difficulty. At least seven species can infect chickens. Each one has different levels of virulence, immune response, and gut targeting. Therefore, one vaccine often cannot protect against all types. Researchers are currently working on recombinant and subunit vaccines to fix these issues. These vaccines use specific parasite proteins to make birds immune without the need for live parasites (Yu et al., 2022). Many of these target proteins are synthesized during the sporozoite and merozoite stages. The sporozoite stage occurs when the parasite first meets the host after eating contaminated oocysts. Proteins from this early stage are good vaccine targets because the immune system can spot them immediately (Cai et al., 2022). New advances in bioinformatics and molecular biology help researchers find and design these proteins faster and cheaper (Lee et al., 2022). Fatemi Motlagh and Mousavi Gargari (2022) found that a bivalent vaccine could protect up to 84% of birds from necrotic enteritis and coccidiosis. This vaccine worked better than older treatments such as live vaccines or antibiotics. Live attenuated vaccines also work better when combined with organic feed additives, such as essential oils or organic acids. Some scientists are also testing herbal extracts and plant-based supplements. However, the success of these natural treatments can change depending on the parasite strain (Rizwan et al., 2022). Other new methods, like in ovo vaccination (injecting vaccines into eggs) and nanocarrier systems, are being studied. These may improve vaccine delivery and trigger earlier immune responses in chicks (Yu et al., 2022). Despite these advances, scientists still do not fully understand how subunit and recombinant vaccines work inside the body. Molecular vaccine design, supported by immune-response studies, was recently highlighted by Gaghan et al. (2022), who demonstrated significant reductions in oocyst shedding post-challenge, even in the absence of complete immunity. This study focuses on a recombinant 19-kDa sporozoite surface protein that was designed using bioinformatics tools and tested for its protective potential against mixed Eimeria infection in broiler chickens. This strategy may offer a safer, scalable alternative to conventional vaccines and contribute to the ongoing effort to achieve drug-free poultry farming. We selected the 19-kDa sporozoite protein because of its strong antigenicity, sporozoite-stage specificity, and role in early host invasion. Materials and MethodsBioinformatic analysis of the sporozoite protein, 19 kDaThe gene encoding the 19-kDa sporozoite protein (gene ID: 838; Accession Number PV347147) was selected for vaccine development. Bioinformatic tools were used to assess the immunological and physicochemical properties of the proteins. The ProtParam tool (https://web.expasy.org/protparam/) was used to compute the molecular weight, theoretical pI, aliphatic index, and GRAVY score. Antigenicity was predicted using VaxiJen v2.0 (https://www.ddg-pharmfac.net/vaxijen/VaxiJen/VaxiJen.html), using the default threshold of 0.5 for protective antigens. Solubility prediction was done using Protein-Sol (https://protein-sol.manchester.ac.uk/), which estimates the likelihood of soluble expression in E. coli systems. This analysis confirmed that the protein was stable, antigenic, and suitable for expression in bacterial systems. Gene synthesis, cloning, and transformationThe 19-kDa gene was synthesized and cloned into the pET-28b(+) vector using BamHI and XhoI restriction enzymes by GenScript (USA). The vector includes a His-tag for purification. The recombinant plasmid Eimeria_pET-28b was used to transform E. coli BL21 (DE3) competent cells. For transformation, 1–5 µL of plasmid DNA was added to 50 µl of competent cells on ice for 30 minutes. The mixture was heat-shocked at 42°C for 30 seconds, then returned to ice immediately. Transformed cells were shaken in LB broth for 1 hour at 37°C with shaking, then spread onto LB agar plates containing 50 µg/ml kanamycin. Protein expression and inductionColonies containing the recombinant plasmid were grown in LB broth supplemented with kanamycin. When the optical density at 600 nm (OD600) reached 0.6–0.8, protein expression was induced by adding IPTG at different concentrations: 0.25, 0.5, 0.75, and 1.0 mM. Cultures were incubated for 4 hours at 37°C for 4 hours with shaking. Samples were collected for quantitative PCR (qPCR) and SDS-PAGE analysis. The expression levels were compared across IPTG concentrations. Quantitative PCR confirmationTotal ribonucleic acid (RNA) was extracted from the induced bacterial cells using a commercial RNA extraction kit. RNA quality was checked using Nanodrop, and cDNA was synthesized using a reverse transcription kit. qPCR was carried out using the following specific primers: Forward: 5′-TCC GCA AGA AGA TGG GAC TTC-3′ Reverse: 3′-TTT GAT GTA CAC GCC GTT CG-5′. The reaction was performed using the SYBR Green Master Mix. Each 20 µl reaction contained 10 µl of mix, 1 µl each of forward and reverse primers, 1 µl of cDNA, and 7 µl of nuclease-free water. qPCR was performed for 40 cycles with annealing at 60°C. Expression was confirmed using melt curve analysis and Cq values. Cell lysis and protein purificationAfter induction, cells were harvested by centrifugation at 4,000 ×g for 15 minutes at 4°C. The pellet was resuspended in 5 ml of lysis buffer (50 mM Tris-HCl, 300 mM NaCl, 10 mM imidazole, pH 8.0). Cells were lysed by sonication in short bursts of 30 seconds followed by 30-s pauses for a total of 5–10 minutes. The lysate was centrifuged at 12,000 ×g for 30 minutes at 4°C to remove cell debris. Clear supernatants containing soluble His-tagged protein were collected. Protein purification was performed using Ni-NTA agarose resin under fast protein liquid chromatography (FPLC). The system used was manufactured by KNAUER (Berlin, Germany). Eluted fractions were collected and stored at −20°C. SDS-PAGE analysisProtein samples were prepared by mixing with Laemmli sample buffer and heating at 95°C for 5 minutes. Samples were loaded onto 12% polyacrylamide gels prepared using the Mini-PROTEAN Tetra Cell casting system (Bio-Rad, USA). Electrophoresis was performed at 100 V for 90 minutes. The gels were stained with Coomassie Brilliant Blue R-250 and then destained with a methanol-acetic acid solution. Protein bands were visualized, and the presence of a ~29 kDa band was used to confirm the recombinant protein expression. Animal experiment and study designTwenty one-day-old Ross 308 broiler chicks were purchased from Al-Atta Hatchery. The birds were housed in controlled experimental cages under standard conditions and monitored for 35 days. The birds were randomly grouped, and the initial weights on day 11 showed no significant differences (Fig. 1). The chicks were randomly divided into three groups: Group 1: Negative control (non-immunized, non-infected) Group 2: Positive control (non-immunized, infected) Group 3: Challenge group (immunized, then infected) Each bird in Group 3 received two subcutaneous doses of 50 µg purified recombinant protein mixed with 30 µg aluminum hydroxide adjuvant. Injections were administered using insulin syringes on days 11 and 18. Challenge and collection of samplesOn day 21, birds in Groups 2 and 3 were orally challenged with 1 × 10⁴ oocysts per species of Eimeria tenella, Eimeria acervulina, and Eimeria necatrix. Oocysts were identified and quantified using the McMaster counting method. Stool samples were collected from each cage for 10 days post-challenge (days 25–34). Blood samples were collected from the wing vein on days 11, 18, and 28 using gel tubes. Serum was separated for Enzyme-linked immunosorbent assay (ELISA), and whole blood was used for leukocyte count. Weight and clinical observationsLive body weight was measured using a digital scale on days 19, 26, and 34. Clinical signs, such as lethargy, loss of appetite, bloody diarrhea, and mortality, were recorded daily. Deaths were noted and compared among the groups. The average weight gain was calculated for each group at each time point. Oocyst count oocysts per gram (OPG)Oocysts per gram of feces were determined using a McMaster counting chamber. A known weight of fecal sample was mixed with saturated salt solution and filtered. The suspension was loaded into the chamber, and the oocysts were counted under a light microscope. Data were collected on days 25–34. White blood cell count (WBC)The leukocyte levels were assessed using a Mindray 2800 BC automated hematology analyzer (Mindray, China). Three birds were sampled per group at each time point. WBC values were recorded and compared with normal chicken ranges (9.0–21.0 × 10³/mm³) as reported by Iheanacho et al. (2024).
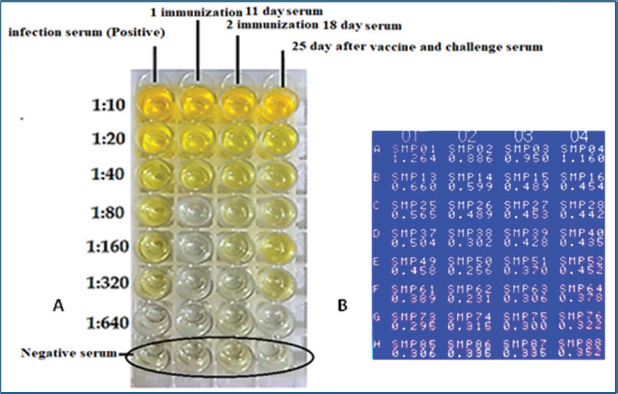
Fig. 1. Experimental timeline of the E19Kda vaccine. Indirect ELISA for IgY measurementELISA plates were coated with 5 µg of recombinant antigen in 100 µl PBS per well and incubated at 4°C overnight. The wells were blocked with 200 µl of 1% BSA for 1 hour at room temperature. Plates were washed three times with PBS-Tween. Serial dilutions of serum (1:10–1:640) were added and incubated for 1 hour. After washing, goat anti-chicken IgY conjugated to HRP (1:5000) was added for another hour. TMB substrate was added for 30 minutes to develop color, then the reaction was stopped with 50 µl of stop solution. The absorbance was read at 450 nm using a microplate reader. The cut-off value was defined as the mean OD of negative sera + 2×SD. Table 1. Physicochemical and immunological features of the recombinant E19kDa protein.
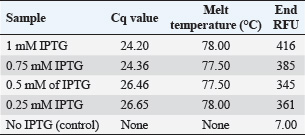
Table 2. qPCR analysis of E19kDa gene expression in IPTG-induced cultures.
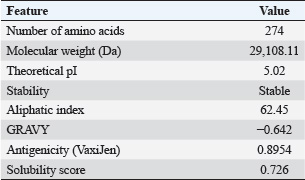
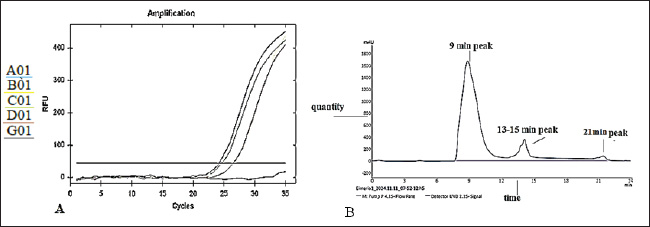
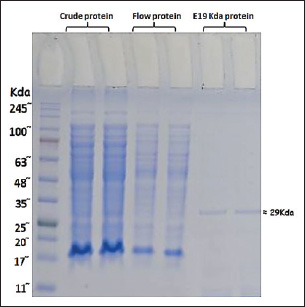
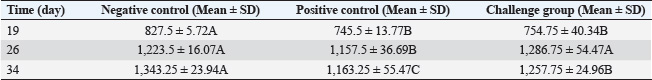
Fig. 2. Melt curve shows a single peak at ~78°C, confirming specific amplification. The fluorescence was strongest in the 1 mM IPTG sample, with a Cq <25. Statistical analysisData were analyzed using one-way ANOVA with SPSS software. Significant differences were defined at p < 0.05. Post hoc tests were used to compare the values between the groups. The mean values are reported with standard deviation (Mean ± SD). Differences were marked by different letters in the tables to show statistical variation among the groups. Ethical approvalThe experiment was conducted according to the protocol approval for research ethics (1890 on 28-8-2023). ResultsBioinformatic analysis of the E19kDa proteinBioinformatic prediction of the E19kDa protein showed promising properties for vaccine development. The recombinant protein was composed of 274 amino acids with a molecular weight of 29,108.11 Da. Its theoretical isoelectric point (pI) was calculated as 5.02. The instability index classified the protein as stable, with an aliphatic index of 62.45 and a hydropathicity value (GRAVY) of −0.642. The antigenicity score was 0.8954, and the solubility prediction was 0.726 (Table 1). Expression and quantification of recombinant proteinsAfter IPTG induction, qPCR was used to evaluate gene expression. The highest expression was recorded at 1 mM IPTG, with a Cq of 24.20 and end RFU of 416. The expression levels decreased with lower IPTG concentrations. The control group (no IPTG) showed no amplification (Table 2). Figure 2 shows the qPCR melt curve and fluorescence intensity confirming the expression of the E19kDa gene in the IPTG-treated samples. SDS-PAGE analysis of the recombinant proteinSDS-PAGE revealed a clear protein band at approximately 29 kDa in the induced samples. This matched the size predicted by bioinformatics. No band was observed in the uninduced sample or the vector-only control (Fig. 3). Figure 3 shows the SDS-PAGE image of the expressed recombinant E19kDa protein. FPLC purification profileThe purification profile obtained by FPLC showed one main peak between minutes 13 and 16, indicating the successful isolation of the His-tagged E19kDa protein. Clinical signs and mortalityBy day 28, the birds in the positive control group showed signs of infection, including bloody feces, lethargy, and reduced feed intake. Mortality reached 6 birds between days 30 and 35. The vaccinated group had only 1 death in the same period. The birds in the negative control group remained healthy. Growth performanceLive weight measurements showed that vaccinated birds had higher average weights than infected controls on all recorded days. On day 34, vaccinated birds (challenge group) had a mean weight of 1257.75 ± 24.96 g, which was significantly higher than that of the positive control group (1163.25 ± 55.47 g, p < 0.05) (Table 3).
Fig. 3. Lane M: molecular weight marker; Lane 1: uninduced control; Lane 2: 0.25 mM IPTG; Lane 3: 0.5 mM; Lane 4: 0.75 mM; Lane 5: 1 mM. A distinct band at ~29 kDa appears in Lane 5, confirming the optimal expression of 1 mM IPTG. Table 3. Live body weight of the chickens during the study period.
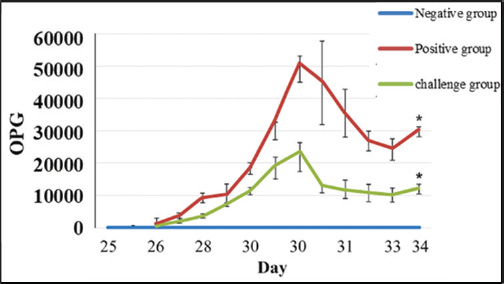
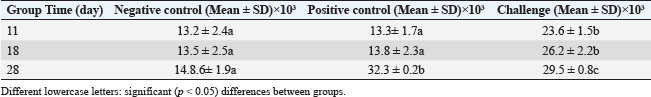
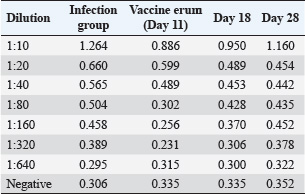
Oocyst sheddingOocyst counts were measured from day 25 to day 34. A significant difference was observed on day 30. The vaccinated group had an average OPG of 10,245, whereas the infected control group had an average OPG of 22,755 (Fig. 4). White blood cell countWBC levels were monitored to assess the immune response. The challenge group had elevated counts on days 14, 18, and 28 compared with both controls. On day 28, the WBC count reached 17.3 ± 0.8 × 10⁹/l in the challenge group, significantly higher than that in the negative control (Table 4). Antibody detection by ELISAIgY levels were measured using indirect ELISA. Serum from vaccinated birds showed higher absorbance values, especially at 1:10 dilution on day 28 (OD=1.160). The cutoff value was calculated as 0.365. All vaccinated samples exceeded this value (Table 5 and Fig. 5). DiscussionCoccidiosis remains one of the most economically damaging diseases in poultry, affecting birds globally and causing significant losses through mortality, poor weight gain, and decreased feed efficiency (da Cunha et al., 2020; Jeon et al., 2022; Hauck and Macklin, 2024). The emergence of drug resistance and regulatory pressure against chemical coccidiostats has driven interest in vaccine-based alternatives. In this study, the immunoprotective efficacy of a recombinant 19-kDa multiepitope protein derived from Eimeria tenella was evaluated. The recombinant protein was expressed and purified from E. coli, and its immunological and protective impact was tested in vaccinated broiler chickens. The bioinformatic design of the multiepitope construct was based on conserved sequences from the PV347147 accession. The selected epitopes exhibited favorable physicochemical properties, including stability, solubility, and high antigenicity, making the construct suitable for use in bacterial expression systems (Cai et al., 2023). These factors might explain the variations observed in other field studies and support the controlled design used here (Muthamilselvan et al., 2016; Saeed and Alkheraije, 2023).
Fig. 4. Oocyst shedding per gram (OPG) of feces over time. Bar graph comparing OPG among groups on days 28, 30, and 34. The peak shedding occurred on day 30. Vaccinated birds shed fewer oocysts, confirming partial protection. Data represent means ± SD with significance determined by ANOVA (p < 0.05). Table 4. WBC counts at different time points (Mean ×10⁹/l ± SD).
Table 5. ELISA OD values at different dilutions across groups.
A consistent finding in this trial was improved growth performance in the vaccinated group. By day 34, the birds had significantly higher mean body weight compared to the positive control group. Similar growth improvements have been reported in trials using multivalent and recombinant vaccines (Behnamifar et al., 2019). Vaccinated birds also exhibited significantly lower oocyst output. On day 30 post-infection, the oocyst counts were nearly 50% lower in the vaccinated group than in the positive control. The reduction in oocyst shedding helps lower environmental contamination. It also interrupts the parasite life cycle. Lower oocyst shedding is an important sign that the vaccine was effective. Other studies have found similar drops in oocyst output after subunit or DNA vaccine use (Ritzi et al., 2016; Savary et al., 2021). These results show that recombinant proteins can slow the spread of parasites. Blood tests showed a rise in white blood cell counts in the vaccinated birds. This was clear on day 28, which matched the peak immune response after the challenge. The rise in white cells indicates that the immune system is active and fighting (Karim et al., 2018; Yaseen et al., 2020; Abd-Alhassen et al., 2021; Maty, 2023). The early increase in leukocytes indicates both quick and long-term immune responses. Other studies found the same pattern, showing that leukocytes move and the spleen gets involved early in infection (Fatoba and Adeleke, 2020). A few studies have reported that infections sometimes cause a short drop in leukocytes because the parasite weakens the immune system. However, in this study, the birds maintained strong blood cell levels, suggesting that their immune systems stayed ready (Zaheer et al., 2022). The humoral (antibody) response was tested using indirect ELISA. Birds that received the vaccine had much higher IgY antibody levels by day 28 compared with the controls. After the second vaccine dose, the antibody response grew even more. This indicates a normal “memory” response by the immune system. A strong IgY response is important for halting the early stages of Eimeria, especially sporozoites and merozoites. Other studies using ELISA tests also found that recombinant vaccines boost antibody production (Chen et al., 2023). In this study, the recombinant protein was made with several B and T cell parts. This wide targeting helps trigger a stronger immune reaction. Many new subunit vaccines use the same idea, and other studies agree that it is important (Peebles, 2018). This study also showed that using an adjuvant with the recombinant protein boosted the immune response (Zhao et al., 2022; Saied and Hameed, 2023; Qui, 2023; Hassan et al., 2024; Rahawi et al., 2024). Aluminum hydroxide was used as the adjuvant. It is a long-trusted and safe ingredient in poultry vaccines. This trial played a key role in improving the results. Subunit vaccines offer many benefits over live vaccines. They are safer, easier to standardize, and simpler to produce. They do not cycle inside the host. They cannot become dangerous again or disturb the gut bacteria (Blake et al., 2017).
Fig. 5. ELISA titration curve for the vaccinated and infected groups. A line graph displaying the OD values across serum dilutions. The vaccine group showed the highest titer at 1:10 dilution on day 28. All values are above the cut-off value of 0.365, confirming seroconversion. Although not directly tested in this study, future trials could evaluate the gut microbiota composition following vaccination. Some reports suggest that gut health improves following vaccination or probiotic use, which could further enhance performance outcomes (Mansilla and Capozzo, 2017). Botanicals and plant-derived compounds have also shown promise in modulating immune responses and reducing coccidiosis severity (Saeed and Alkheraije, 2023). However, their variability and lack of standardization limit their use as standalone devices. Integrating probiotics with vaccination strategies may offer synergistic benefits. Several studies have shown that combining probiotics with coccidiosis vaccines boosts immunity and reduces lesion scores and oocyst counts (Mustafa et al., 2024). In terms of vaccine development, many recent efforts have targeted Eimeria proteins, such as profilin, GAPDH, AMA1, and EtROP17, with promising results (Vereecken et al., 2021). The protein used in this study shares conceptual design principles with these strategies. Molecular vaccine platforms are advancing rapidly. Transgenic Eimeria parasites, DNA vaccines, and recombinant Lactobacillus-based vaccines are being tested and show strong potential for future commercialization (Zhang et al., 2024). However, the widespread adoption of recombinant vaccines in commercial settings requires clearer regulatory frameworks and standardized evaluation criteria, which are currently lacking. In ovo vaccination is another growing field. Although traditionally used for live vaccines, the use of recombinant proteins in in ovo systems is under active investigation, potentially allowing for earlier immune priming. Nanoparticle-based delivery systems are being explored to increase antigen stability and improve immune cell uptake. These findings could enhance the efficacy of recombinant vaccines in the future (Mustafa et al., 2024). Another factor for consideration is the emergence of Eimeria operational taxonomic units, which complicate diagnostics and raise questions about cross-protection among strains. Multiepitope vaccines may address this issue by targeting conserved sequences (Alkhashb et al., 2024; Ghazi et al., 2024; Haydar, 2024; Khayoon et al., 2024; Khidhir et al., 2024). The sustained IgY response in the vaccinated group, stable oocyst output, and higher final body weights support the efficacy of this recombinant antigen. This confirms the ability of the protein to trigger memory responses after a challenge (Wang and Suo, 2020). Validation by Western blot using anti-His antibodies is planned for future work. ConclusionDespite its small sample size, this study presents clear evidence that bioinformatically designed recombinant vaccines can reduce clinical signs, mortality, and environmental contamination during Eimeria outbreaks. Future studies should expand sample sizes and compare different adjuvants and delivery systems. Exploring mucosal vaccination and combining recombinant proteins with microbiota-friendly additives could optimize protection and support antibiotic-free poultry production. Conflict of interestThe authors have no competing interests to declare. FundingNo funding was received. Authors’ contributionsAll researchers participated in the current study. Data availabilityThe data are available upon request. ReferencesAbd-Alhassen, J.K., Janabi, A.H.D. and Aboktifa, M.A. 2021. Antioxidant and antimicrobial evaluation of lycopene isolated from watermelon. Biochem. Cell Arch. 21, 2905–2910. Alkhashb, A., Alhaji, T. and Thalij, K. 2024. Effectiveness of chitosan and ag-nanoparticle films on the quality of chicken meat. Mesopotamia J. Agric. 52(2), 14–26; doi: 10.33899/mja.2024.145729.011337 Behnamifar, A.R., Rahimi, S., Kiaei, M.M. and Fayazi, H. 2019. Comparison of the effect of probiotic, prebiotic, salinomycin and vaccine in control of coccidiosis in broiler chickens. Iran J. Vet. Res. 20(1), 51–54. Blake, D.P., Marugan-Hernandez, V. and Tomley, F.M. 2021. Spotlight on avian pathology: eimeria and the disease coccidiosis. Avian Pathol. 50, 1–5; doi: 10.1080/03079457.2021.1912288 Blake, D.P., Pastor-Fernández, I., Nolan, M.J. and Tomley, F.M. 2017. Recombinant anticoccidial vaccines - a cup half full? Infect. Genet. Evol. 55, 358–365; doi: 10.1016/j.meegid.2017.10.009 Bozkurt, M. and Savaş, N.N. 2024. Effects of monensin sodium and live attenuated oocyst vaccine as coccidiosis management programs on productive performance, bone quality and mineral utilisation in broiler chickens. Br. Poult. Sci. 65(1), 87–96; doi: 10.1080/00071668.2023.2287726 Bora, C.A.F., Kumar, V.J.A. and Mathivathani, C. 2024. Prevalence of avian coccidiosis in India: a review. J. Parasit. Dis. 48(2), 181–188; doi: 10.1007/s12639-024-01661-7 Cai, H., Liao, S., Li, J., Liu, Q., Luo, S., Lv, M., Lin, X., Hu, J., Zhang, J., Qi, N. and Sun, M. 2022. Single and combined effects of clostridium butyricum and coccidiosis vaccine on broiler chickens. Front. Microbiol. 13, 811428; doi: 10.3389/fmicb.2022.811428 Cai, H., Luo, S., Zhou, Q., Yan, Z., Liu, Q., Kang, Z., Liao, S., Li, J., Lv, M., Lin, X. and Hu, J. 2022. Effects of bacillus subtilis and coccidiosis vaccine on growth indices and intestinal microbiota of broilers. Poult. Sci. 101(11), 102091; doi: 10.1016/j.psj.2022.102091 Cai, H., Luo, S., Liu, Q., Zhou, Q., Yan, Z., Kang, Z., Liao, S., Li, J., Lv, M., Lin, X. and Hu, J. 2023. Effects of a complex probiotic preparation, Fengqiang Shengtai and coccidiosis vaccine on the performance and intestinal microbiota of broilers challenged with Eimeria spp. Parasit. Vectors. 16(1), 253; doi: 10.1186/s13071-023-05855-5 Chen, C., Su, J., Lu, M., Xu, L., Yan, R., Li, X. and Song, X. 2023. Protective efficacy of multiepitope vaccines constructed from common antigens of Eimeria species in chickens. Vet. Res. 54(1), 119; doi: 10.1186/s13567-023-01253-y da Cunha, A.F., Santin, E. and Kogut, M. 2020. Editorial: poultry coccidiosis: strategies to understand and control. Front. Vet. Sci. 7, 599322; doi: 10.3389/fvets.2020.599322 da Silva Giacomini, L., Fernandes, F.D., Guerra, R.R., de Avila Botton, S., Sangioni, L.A. and Vogel, F.S.F. 2023. Production performance and economic analysis of broiler chickens after vaccination with a live attenuated vaccine against avian coccidiosis. Parasitol. Res. 122(7), 1677–1683; doi: 10.1007/s00436-023-07879-3 El-Shall, N.A., Abd El-Hack, M.E., Albaqami, N.M., Khafaga, A.F., Taha, A.E., Swelum, A.A., El-Saadony, M.T., Salem, H.M., El-Tahan, A.M., AbuQamar, S.F. and El-Tarabily, K.A. 2022. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 101(1), 101542; doi: 10.1016/j.psj.2021.101542 Fatemi Motlagh, M. and Mousavi Gargari, S.L. 2022. A bivalent vaccine against avian necrotic enteritis and coccidiosis. J. Appl. Microbiol. 132(1), 113–125; doi: 10.1111/jam.15178 Fatoba, A.J. and Adeleke, M.A. 2018. Diagnosis and control of chicken coccidiosis: a recent update. J. Parasit. Dis. 42(4), 483–493; doi: 10.1007/s12639-018-1048-1 Fatoba, A.J. and Adeleke, M.A. 2020. Transgenic Eimeria parasite: a potential control strategy for chicken coccidiosis. Acta Trop. 205, 105417; doi: 10.1016/j.actatropica.2020.105417 Gaghan, C., Adams, D., Mohammed, J., Crespo, R., Livingston, K. and Kulkarni, R.R. 2022. Characterization of vaccine-induced immune responses against coccidiosis in broiler chickens. Vaccine 40(28), 3893–3902; doi: 10.1016/j.vaccine.2022.05.043 Ghazi, A.M., Al-Bayati, M.A. and Janabi, A.H. 2024. Metabolomics-detected alterations generated by phytosomal propolis and phytosomal lycopene in male rats with induced benign prostatic hyperplasia. Iraqi J. Vet. Sci. 38(Suppl I-IV), 7–15; doi: 10.33899/ijvs.2024.147764.3531 Giacomini, L. da S., Fernandes, F.D., Guerra, R.R., de Ávila Botton, S., Sangioni, L.A. and Vogel, F.S.F. 2023. Production performance and economic analysis of broiler chickens after vaccination with a live attenuated vaccine against avian coccidiosis. Parasitol. Res. 122(7), 1677–1683; doi: 10.1007/s00436-023-07879-3 Gaghan, C., Adams, D., Mohammed, J., Crespo, R., Livingston, K. and Kulkarni, R.R. 2022. Characterization of vaccine-induced immune responses against coccidiosis in broiler chickens. Vaccine 40(28), 3893–3902; doi: 10.1016/j.vaccine.2022.05.043 Hassan, W.S., Abdulrazzaq, K.M., Al-Obaidi, Q.T. and Al-Azow, K.A. 2024. Molecular detection of Anaplasma platys in dogs in Nineveh province, Iraq. Iraqi J. Vet. Sci. 38(3), 677–682; doi: 10.33899/ijvs.2024.148465.3592 Haydar, H. 2024. The effect of adding sweet bean seed powder (Foeniculum vulgare) and roselle (Hibiscus sabdariffa) seed powder on the growth performance of common carp cyprinus carpio L. Mesopotamia J. Agric. 52(2), 58–67; doi: 10.33899/mja.2024.146824.1374 Jeon, Y.S., Kim, Y.B., Lee, H.G., Park, J., Heo, Y.J., Chu, G.M. and Lee, K.W. 2022. Effect of dietary organic and inorganic sulfur on the performance of coccidiosis vaccine challenged broiler chickens. Animals (Basel) 12(9), 1200; doi: 10.3390/ani12091200 Karim, S.M., Mansour, K.A., Janabi, A.H.D. and Al-Nakeeb, N.K.M. 2018. First phylogenetic characterization of pseudocowpox virus from cattle in Al-Qadisiyah province, Iraq. Iraqi J. Vet. Sci. 33(1), 123–126; doi: 10.33899/ijvs.2019.125525.1047 Khayoon, T., Abbas, R. and Abdullah, F. 2024. Effects of feeding various levels of postbiotics produced by lactic acid bacteria on growth performance, gastrointestinal microbiota count, and digestibility of some nutrients in broiler chickens. Mesopotamia J. Agric. 52(2), 68–81; doi: 10.33899/mja.2024.145531.132019 Lee, Y., Lu, M. and Lillehoj, H.S. 2022. Coccidiosis: recent progress in host immunity and alternatives to antibiotic strategies. Vaccines (Basel) 10(2), 215; doi: 10.3390/vaccines10020215 Liu, Q., Liu, X., Zhao, X., Zhu, X.Q. and Suo, X. 2023. Live attenuated anticoccidial vaccines for chickens. Trends Parasitol. 39(12), 1087–1099; doi: 10.1016/j.pt.2023.09.002 Mansilla, F.C. and Capozzo, A.V. 2017. Apicomplexan profilins in vaccine development applied to bovine neosporosis. Exp. Parasitol. 183, 64–68; doi: 10.1016/j.exppara.2017.10.009 Maty, H.N. 2023. Impact of sorbitol and l-carnitine on stimulating thyroid hormone, triiodothyronine, and adenosine triphosphate levels in broilers. Iraqi J. Vet. Sci. 37(3), 589–590; doi: 10.33899/ijvs.2022.135305.2464 Mustafa, S., Abbas, R.Z., Saeed, Z., Baazaoui, N. and Khan, A.M.A. 2024. Use of metallic nanoparticles against Eimeria—the coccidiosis-causing agents: a comprehensive review. Biol. Trace Elem. Res. 203, 3412–3431; doi: 10.1007/s12011-024-04399-8 Muthamilselvan, T., Kuo, T.F., Wu, Y.C. and Yang, W.C. 2016. Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. Evid. Based Complement. Alternat. Med. 2016, 2657981; doi: 10.1155/2016/2657981 Oryasin, A.G. and Eren, H. 2025. Investigation of betaine and vaccine efficacy for coccidiosis prevention in broilers. Acta Parasitol. 70(1), 25; doi: 10.1007/s11686-024-00967-z Peebles, E.D. 2018. In ovo applications in poultry: a review. Poult. Sci. 97(7), 2322–2338; doi: 10.3382/ps/pey081 Qui, N.H. 2023. Baker’s yeast (Saccharomyces cerevisiae) and its application on poultry’s production and health: a review. Iraqi J. Vet. Sci. 37(1), 213–221; doi: 10.33899/ijvs.2022.132912.2146 Rahawi, A.M., Al-Taee, S.K., Ali, F.F., Altaey, O.Y. and Abdullah, D.A. 2024. Protective role of biosynthetic silver nanoparticles in broilers with aflatoxicosis through histopathological study of spleen. Iraqi J. Vet. Sci. 38(3), 565–572; doi: 10.33899/ijvs.2024.146024.3414 Ritzi, M.M., Abdelrahman, W., van-Heerden, K., Mohnl, M., Barrett, N.W. and Dalloul, R.A. 2016. Combination of probiotics and coccidiosis vaccine enhances protection against an Eimeria challenge. Vet. Res. 47(1), 111; doi: 10.1186/s13567-016-0397-y Rizwan, H.M., Khan, M.K., Mughal, M.A.S., Abbas, Z., Abbas, R.Z., Sindhu, Z.U.D., Sajid, M.S., Ain, Q.U., Abbas, A., Zafar, A. and Imran, M. 2022. A new insight in immunomodulatory impact of botanicals in treating avian coccidiosis. J. Parasit. Dis. 46(4), 1164–1175; doi: 10.1007/s12639-022-01519-w Saeed, Z. and Alkheraije, K.A. 2023. Botanicals: a promising approach for controlling cecal coccidiosis in poultry. Front. Vet. Sci. 10, 1157633; doi: 10.3389/fvets.2023.1157633 Saied, M.Q. and Hameed, H.M. 2023. Bioceutical role of nano and organic selenium on certain reproductive value of laying hen during force molting. Iraqi J. Vet. Sci. 37(2), 325–331; doi: 10.33899/ijvs.2022.134401.2364 Savary, R.K., Fiss, T.A., Abbott, D.A., Nicholds, J.A., Van Kessel, A.G. and Classen, H.L. 2021. Development of a coccidiosis disease challenge model using a commercially available live oocyst vaccine. Avian Dis. 65(1), 149–158; doi: 10.1637/aviandiseases-D-20-00105 Shahininejad, H., Rahimi, S., Karimi Torshizi, M.A., Arabkhazaeli, F., Ayyari, M. and Behnamifar, A. 2024. Comparing the effect of phytobiotic, coccidiostat, toltrazuril, and vaccine on the prevention and treatment of coccidiosis in broilers. Poult. Sci. 103(5), 103596; doi: 10.1016/j.psj.2024.103596 Sokale, A.O., Williams, C.J., Hoerr, F.J., Collins, K.E.C. and Peebles, E.D. 2021. Effects of administration of an in ovo coccidiosis vaccine at different embryonic ages on vaccine cycling and performance of broiler chickens. Poult. Sci. 100(3), 100914; doi: 10.1016/j.psj.2020.11.078 Van Eerden, E., Santos, R.R., Molist, F., Dardi, M., Pantoja-Millas, L.A., Molist-Badiola, J., Baratelli, M. and Pages, M. 2022. Efficacy of an attenuated vaccine against avian coccidiosis in combination with feed additives based on organic acids and essential oils. Poult. Sci. 101(6), 101848; doi: 10.1016/j.psj.2022.101848 Venkatas, J. and Adeleke, M.A. 2019. Emerging threat of eimeria operational taxonomic units (OTUs) on poultry production. Parasitology 146(13), 1615–1619; doi: 10.1017/S0031182019001100 Vereecken, M., Dehaeck, B., Rathinam, T., Schelstraete, W., De Gussem, K. and Chapman, H.D. 2021. Restoration of the sensitivity of Eimeria acervulina to anticoccidial drugs in the chicken following use of a live coccidiosis vaccine. Vet. Parasitol. 292, 109416; doi: 10.1016/j.vetpar.2021.109416 Wang, S. and Suo, X. 2020. Still naive or primed: anticoccidial vaccines call for memory. Exp. Parasitol. 216, 107945; doi: 10.1016/j.exppara.2020.107945 Yaseen, M.M., Karawan, A.C., Alfatlawi, M.A.A. and Janabi, A.H.D. 2020. The role of gut bacterial cytochrome-P450 of mosquito larvae in degradation of temephos insecticide. Ann. Trop. Med. Public Health 23(1), S412; doi: 10.36295/ASRO.2020.23126 Yu, Z., Chen, S., Huang, J., Ding, W., Chen, Y., Su, J., Yan, R., Xu, L., Song, X. and Li, X. 2022. A multiepitope vaccine encoding four Eimeria epitopes with PLGA nanospheres: a novel vaccine candidate against coccidiosis in laying chickens. Vet. Res. 53(1), 27; doi: 10.1186/s13567-022-01045-w Zhang, T., Qu, H., Zheng, W., Zhang, Y., Li, Y., Pan, T., Li, J., Yang, W., Cao, X., Jiang, Y. and Wang, J. 2024. Oral vaccination with a recombinant Lactobacillus plantarum expressing the Eimeria tenella rhoptry neck 2 protein elicits protective immunity in broiler chickens infected with E. tenella. Parasit. Vectors 17(1), 277; doi: 10.1186/s13071-024-06355-w Zhao, D., Suo, J., Liang, L., Liang, R., Zhou, R., Ding, J., Liu, X., Suo, X., Zhang, S. and Tang, X. 2024. Innovative prevention and control of coccidiosis: targeting sporogony for new control agent development. Poult. Sci. 103(12), 104246; doi: 10.1016/j.psj.2024.104246 Zhao, H., Li, C., Zhu, S., Zhao, Q., Dong, H., Huang, B., and Han H. 2022. Molecular characterization and immune protection by cystathionine β-synthase from Eimeria tenella. J. Eukaryot. Microbiol. 69(2), e12876; doi: 10.1111/jeu.12876 Zheng, L., Zhang, L., Tan, F., Wang, C., Lv, X., Bai, R., Huo, N. and Zheng, M. 2023. Prevention and control of chicken coccidiosis using recombinant Lactococcus lactis expressing chicken IL-4 and IL-2 fusion protein. Poult. Sci. 102(4), 102530; doi: 10.1016/j.psj.2023.102530 | ||
| How to Cite this Article |
| Pubmed Style Alfatly HH, Jarad NI. Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccine. Open Vet. J.. 2025; 15(8): 3598-3607. doi:10.5455/OVJ.2025.v15.i8.22 Web Style Alfatly HH, Jarad NI. Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccine. https://www.openveterinaryjournal.com/?mno=254787 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.22 AMA (American Medical Association) Style Alfatly HH, Jarad NI. Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccine. Open Vet. J.. 2025; 15(8): 3598-3607. doi:10.5455/OVJ.2025.v15.i8.22 Vancouver/ICMJE Style Alfatly HH, Jarad NI. Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccine. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3598-3607. doi:10.5455/OVJ.2025.v15.i8.22 Harvard Style Alfatly, H. H. & Jarad, . N. I. (2025) Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccine. Open Vet. J., 15 (8), 3598-3607. doi:10.5455/OVJ.2025.v15.i8.22 Turabian Style Alfatly, Haider Hamza, and Noor Idan Jarad. 2025. Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccine. Open Veterinary Journal, 15 (8), 3598-3607. doi:10.5455/OVJ.2025.v15.i8.22 Chicago Style Alfatly, Haider Hamza, and Noor Idan Jarad. "Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccine." Open Veterinary Journal 15 (2025), 3598-3607. doi:10.5455/OVJ.2025.v15.i8.22 MLA (The Modern Language Association) Style Alfatly, Haider Hamza, and Noor Idan Jarad. "Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccine." Open Veterinary Journal 15.8 (2025), 3598-3607. Print. doi:10.5455/OVJ.2025.v15.i8.22 APA (American Psychological Association) Style Alfatly, H. H. & Jarad, . N. I. (2025) Harnessing bioinformatics and recombinant technology to combat avian coccidiosis: A 19-kDa sporozoite protein-based vaccine. Open Veterinary Journal, 15 (8), 3598-3607. doi:10.5455/OVJ.2025.v15.i8.22 |