| Research Article | ||
Open Vet. J.. 2025; 15(8): 3608-3617 Open Veterinary Journal, (2025), Vol. 15(8): 3608-3617 Research Article Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in ThailandKosal Phourng1, Ratiwan Sitdhibutr2, Yared Beyene Yohannes3, Yoshinori Ikenaka3,4,5 and Aksorn Saengtienchai1*1Department of Pharmacology, Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand 2Kasetsart University Raptor Rehabilitation Unit, Faculty of Veterinary Medicine, Kasetsart University, Kamphaeng Saen, Thailand 3Laboratory of Toxicology, Department of Environmental Veterinary Science, Faculty of Veterinary Medicine, Hokkaido University, Sapporo, Japan 4Veterinary Teaching Hospital, Graduate School of Veterinary Medicine, Hokkaido University, Sapporo, Japan 5One Health Research Center, Hokkaido University, Sapporo, Japan *Corresponding Author: Aksorn Saengtienchai, Department of Pharmacology, Faculty of Veterinary Medicine, Kasetsart University, 50 Ngamwongwan Road, Bangkok, Thailand. Email: fvetasc [at] ku.ac.th Submitted: 26/04/2025 Revised: 05/07/2025 Accepted: 23/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
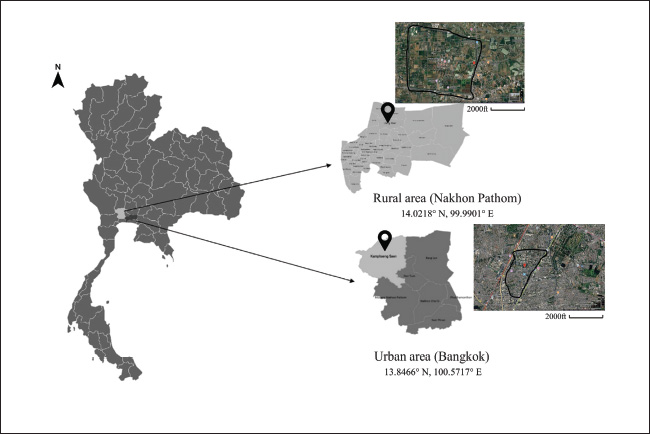
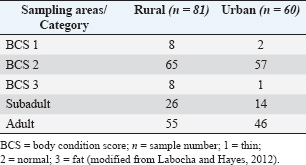
ABSTRACTBackground: Anticoagulant rodenticides (ARs) are widely used worldwide to control rodents. Currently, many countries are considering the use of ARs, which results in widespread accumulation and poisoning of nontarget animals. Nontarget pigeons (Columba livia) typically live near human communities and agricultural areas. These birds are at high risk of accidental daily exposure to ARs. Aim: This study aimed to evaluate ARs in the liver and plasma of pigeons living in rural and urban areas of Thailand. Methods: Bird samples were characterized by sampling location, age, body weight (BW), and body condition score. Liver and plasma samples were extracted using liquid–liquid extraction with diethyl ether and analyzed for coumatetralyl and warfarin by liquid chromatography–mass spectrometry. Results: Coumatetralyl and warfarin were detected in pigeons from both rural and urban areas. Both subadult and adult birds showed the presence of these two compounds, which were mainly detected in rural areas. The increase in BW was compared with the accumulation of coumatetralyl and warfarin, and a significant difference was observed. In addition, the concentration of coumatetralyl was significantly higher in the liver than in the plasma. The plasma concentrations of coumatetralyl and warfarin were 0.19–0.50 and 0.12–2.41 ng/ml, respectively. In the liver, coumatetralyl levels were higher than those of warfarin, with concentrations ranging from 0.07- to 3.33-ng/g wet weight and from 0.12- to 0.50-ng/g wet weight. Conclusion: AR use in human settlements and agricultural areas poses a high risk of accumulation in nontarget animals, such as pigeons. The findings of this study indicate coumatetralyl and warfarin in various concentrations in pigeon hepatic and plasma samples. Keywords: Coumatetralyl, Pigeons, Rural, Urban, Warfarin. IntroductionAnticoagulant rodenticides (ARs) are widely used worldwide to control rodent populations in both agricultural and urban areas. These chemicals include warfarin and its derivatives, which are more toxic and have long-lasting effects on animals and humans. Although ARs are effective in targeting rodents, their use poses significant risks to nontarget species, such as humans, pets, and wildlife. In ecosystems, the continued use of ARs can lead to secondary poisoning, where nontarget animals are exposed to these toxicants through contaminated prey or environmental residues (Erickson and Urban, 2004; Bradbury, 2008; Thomas et al., 2011; Nakayama et al., 2019). Different AR classes are regulated for various applications in each country. In California, only first-generation ARs (FGARs) are approved for field use, whereas second-generation ARs (SGARs) are available to human communities, structural pest control operators, and others for controlling commensal rodents (Martínez-Padilla et al., 2017). The Ministry of Public Health of Thailand has established criteria for the prevention and control of insects and disease-carrying animals that pose health hazards, recommending the use of both biological and chemical methods, including ARs (Sapbamrer et al., 2023). The widespread use of ARs has resulted in significant poisoning of nontarget animals, with increasing toxicity and accumulation reported in wildlife worldwide. This is primarily due to the death of rodents poisoned by rodenticides, which are then consumed by predators such as snakes, carnivorous mammals, and predatory birds. These predators are at high risk of accumulating ARs in their bodies, which may lead to toxicity and mortality. For instance, research conducted from 1998 to 2015 found that over 2,500 predatory birds had accumulated ARs, particularly in the liver and kidney (Rattner et al., 2014). Several bird species, especially predatory birds, have been reported to be affected by ARs, including the great horned owl, barn owls, and barred owls in Canada (Albert et al., 2010), the common buzzard and peregrine falcon in Scotland (George et al., 2024), the golden eagle and eagle owl in Norway (Langford et al., 2013), Bonelli’s eagle in Spain (Vicedo et al., 2024), predatory and scavenging birds in France (Fourel et al., 2024), and native birds in New Zealand (Hoare and Hare, 2006). Recently, not only predatory birds, insectivores, reptiles, and carnivores have been reported in various European countries such as Australia, Italy, Germany, Slovenia, and the United Kingdom (Dowding et al., 2010; Lettoof et al., 2020; Cerkvenik-Flajs et al., 2024; Musto et al., 2024; Regnery et al., 2024). Furthermore, AR poisoning has been reported less frequently in domestic and water birds, such as the gray heron, waterfowl, pigeons, and partridges (Sarabia et al., 2008; Guitart et al., 2010). The population of pigeons in Thailand is increasing, and they are commonly found in both urban and rural areas. Their behavior and habitats are closely associated with human communities and agricultural areas. Pigeons often feed on the ground, and as a result, they may ingest various contaminants, including pesticides such as ARs (Katevorn et al., 2020). Pigeons living together with feed behavior are a high risk to contaminant ARs, which might be a one biomonitoring as nontarget species. In addition, data on AR toxicity in pigeons in Thailand are still limited. Agriculture is a major sector in Thailand, a leading exporter of rice, rubber, and cassava. Pesticides, including rodenticides, are widely used in agricultural areas. Over the period from 2012 to 2021, the trend of pesticide imports into Thailand has shown a gradual increase from 2012 to 2021 (Sapbamrer et al., 2023). Herbicides, fungicides, and insecticides are the top three pesticides used for agricultural purposes in Thailand. According to the Office of Agricultural Regulation, rodenticides rank among the top 10 products (Sapbamrer et al., 2023). FGARs, SGARs, and non-AR products are commonly described as baits. The chemicals most frequently found include coumatetralyl and warfarin; however, some SGARs and non-ARs, such as bromadiolone, brodifacoum, flocoumafen, and zinc phosphide, were found in some places (Sapbamrer et al., 2023). The lack of knowledge and awareness about pesticides, including rodenticides, among agricultural workers and the environmental contamination resulting from weak enforcement of existing regulations have contributed to the misuse and overuse of these chemicals. This has led to increased environmental contamination and increased exposure to both humans and animals (Panuwet et al., 2012). A retrospective cohort study found that more than 1,000 Thai people were exposed to rodenticides. Most rodenticides involved were zinc phosphide, warfarin, and long-acting anticoagulants (Wananukul et al., 2007; Trakulsrichai et al., 2017). Furthermore, a retrospective histopathological study conducted in 2011 found coumarin-induced hemorrhagic lesions in various organs of dogs, with the liver being the most affected organ, showing the highest hemorrhagic score (Kaewamatawong et al., 2011). Additionally, a recent case report described suspected AR poisoning in Patagonian maras (Dolichotis patagonum) based on clinical history, symptoms, gross pathological lesions, and chemical analysis. ARs were suspected to have been contaminated in their feed (Chansiripornchai et al., 2025). Owing to a lack of information on AR contamination, especially coumatetralyl and warfarin, in pigeons, nontarget animals in Thailand, this study aimed to evaluate the levels of these compounds that accumulate in the liver and plasma of pigeons across urban and rural areas in Thailand. Materials and MethodsChemicalsThe chemicals used in this study included warfarin sodium, coumatetralyl, and warfarin-D5 (Wako Pure Chemical, Osaka, Japan), sodium acetate, and ammonium acetate (Kanto Chemicals, Tokyo, Japan). Methanol (HPLC grade), diethyl ether, and heparin were obtained from the CT Laboratory (Bangkok, Thailand). AnimalsBetween January and February 2023, 141 pigeons were captured from two locations in the Central and Western regions of Thailand, representing both urban (city) and rural (agricultural) areas, using a net trap (Fig. 1 and Table 1). The health condition and physical examination of the pigeons were approved by a veterinarian, which was modified from Jones (2009) as follows: 1) body weight (BW) together with palpation to estimate body condition score (BCS); 2) lung sound and breath pattern; 3) mucus membrane color; 4) normal mucus; 5) fecal pattern and white urine; and 6) diphtheritic membrane and screening for pox lesions in their mouths. The BW was expressed in grams (g), and BCS was categorized as follows: 1) thin; 2) normal (230–370 g); and 3) fat, as modified from Labocha and Hayes (2012). The BCS of pigeons were identified based on the morphometric indices of body condition as proxies for the mass of body fat from four indicators: 1) the skeletons are of similar size and proportions (birds are similar in structure size); 2) the gut contents are similar in mass; 3) hydration; and 4) the organs and muscle are similar in mass (pectorals muscles and digestive organs). Estimates of age as subadult and adult were recorded in the study by Schroeder and Robb (2005). Birds were classified as subadult and adult following two major indicators: 1) behavioral and 2) general morphology and appearance, as accurate classification of an animal’s age is fundamental to wildlife research and management. The data from the bird sampling are summarized in Table 1. Plasma samples were collected from the basilic vein, and liver tissue samples were collected after euthanasia using the cervical dislocation technique. All samples were stored at 20°C until analysis.
Fig. 1. Sampling areas. Table 1. Information on the pigeon samples.
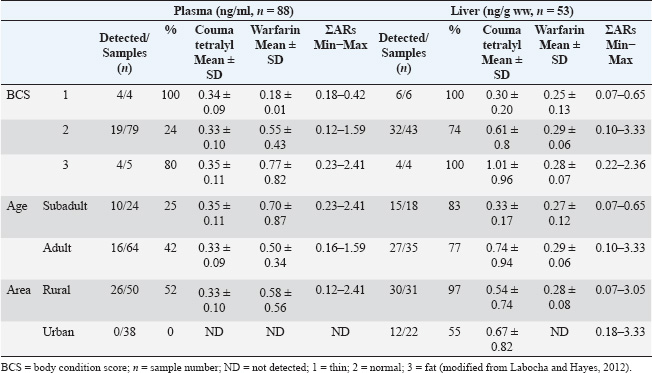
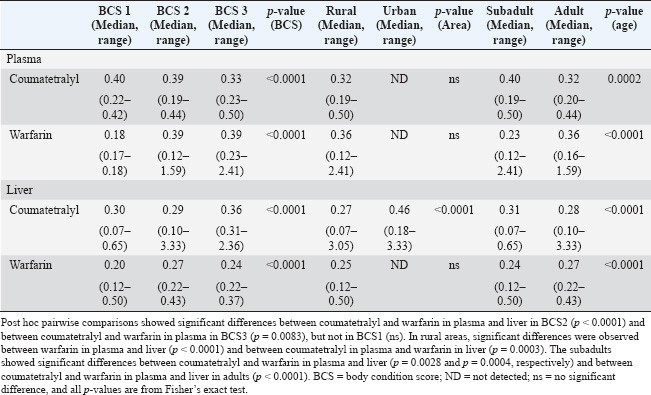
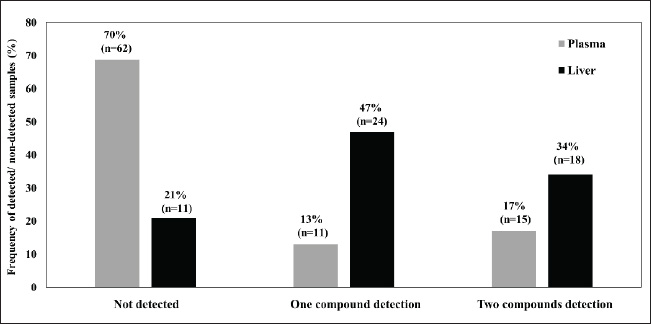
Liquid-liquid extractionThe extraction and clean-up of the liver and plasma samples followed a previous description by Takeda et al. (2016). In brief, plasma sample aliquots (10–20 µl) and small pieces of liver tissue (0.5 ± 0.1 g wet weight, ww) were added to 10-ml glass tubes, followed by 2 ml of 0.1-M sodium acetate. Then, 10 ng of the internal standard (warfarin-D5) was added and vortexed for 10 seconds. The ARs were extracted from the sample twice with 5 ml of diethyl ether by vortexing for 3 minutes on a multitube shaker and then centrifuged at 3,000 rpm for 10 minutes. The supernatants were combined and evaporated to near dryness under a gentle stream of nitrogen gas at 40°C. The residue was dissolved in 50% methanol (100 µl), filtered using a 0.2-µm centrifugal disposable ultrafilter (Kurashiki Hosho, Osaka, Japan), and then transferred to the liquid chromatography vial. Liquid chromatography–mass spectrometry (LC-MS/MS) analysisThe ARs were analyzed using an Agilent 6495 Triple Quadrupole LC/MS system (Agilent, USA). The LC system was equipped with an Agilent Poroshell 120 EC, C18 (1.9 µm, 3.0 × 100 mm) column. The mobile phase consisted of 2-mM ammonium acetate in Deuterium-depleted water for phase A and 100% methanol for phase B. The gradient profile was as follows: 0–10.0 minutes, 20% B to 100% B; 10.01–13.00 minutes, 100% B; and 13.01–15.00 minutes, 20% B. The flow rate was 0.7 ml/min, the column temperature was 45°C, and the injection volume was 40 μl. The CE and MS parameters are presented in Table 2. Quality control and assuranceThe recovery test and spike samples were used to investigate the recovery rate. The recovery rates of coumatetralyl and warfarin were performed for three different concentrations of 0.1 (low concentration), 1.0 (middle concentration), and 5.0 (high concentration) ng/ml. The recovery rates from the spike of low to high coumatetralyl concentrations were 88.50 ± 3.25, 61.13 ± 1.41, and 60.55 ± 5.67. The recovery rates from the spike of low to high warfarin concentrations were 60.38 ± 1.17, 69.88 ± 4.45, and 77.25 ± 0.92. A signal-to-noise ratio (S/N) of 3:1 and S/N of 10:1 was determined for the limit of detection (LOD) and the LODquantification (LOQ), respectively. The LODs of coumatetralyl and warfarin were 0.023 and 0.018 ng/ml, respectively, and the LOQs of coumatetralyl and warfarin were 0.070 and 0.055 ng/ml, respectively. Statistical analysisStatistical significance and data visualization were conducted using nonparametric statistical tests. Comparisons of AR concentrations among BCSs, sampling areas, and ages were performed for both coumatetralyl and warfarin concentrations in the liver and plasma of pigeons using JMP Statistical Discovery LLC version 18 (JMP 18) with a significance level set at p < 0.001. Ethical approvalThis study was approved by the Kasetsart Institutional Animal Care and the Animal Ethics Research Committee of the Faculty of Veterinary Medicine (approval number: ACKU66-VET-013). ResultsAR accumulation in plasma and liver of pigeonAll pigeon samples from urban and rural areas were evaluated for health status, BCS, and estimated age. The pigeons were within the range of normal body weight and showed a similar distribution of subadult and adult individuals. The patients were categorized into BCSs 1–3 (Table 1), and all exhibited normal health without any clinical signs or visible lesions, which were approved by the clinician. The presence of coumatetralyl and warfarin in both plasma and liver samples was investigated. Coumatetralyl was detected in 20% and 64% of plasma and liver samples, respectively. The highest residual level of coumatetralyl observed in liver tissue was 3.33 ng/g ww (Table 3). The range of coumatetralyl in plasma and liver was 0.19–0.50 ng/ml and 0.07–3.33 ng/g ww, respectively. Warfarin was found in 26% of plasma samples and 47% of liver samples. Its concentration in plasma ranged from 0.12 to 2.41 ng/ml, which was higher than that in liver tissue, where levels ranged from 0.12 to 0.50 ng/g ww (Table 4). A significant difference was found when comparing the warfarin level between the plasma and the liver, with p < 0.0001. Furthermore, Fisher’s exact test indicated a statistically significant difference in coumatetralyl and warfarin concentrations between plasma and liver samples (p < 0.0001). Furthermore, the frequency of ARs detected in liver samples indicated at least one AR compound in both sampling areas (Fig. 2). Approximately 47% of the liver samples contained either coumatetralyl or warfarin, whereas approximately 34% showed the accumulation of both compounds. In contrast, only 30% of the plasma samples contained one or both compounds; specifically, coumatetralyl and warfarin were detected in 13% and 17% of the plasma samples, respectively. AR accumulation in relation to age, BCS, and sample areaARs were detected across different characteristics of pigeons, such as BCS, age, and sampling areas. Coumatetralyl and warfarin were detected in both plasma and liver samples across all BCS categories. Similar AR accumulation patterns were observed in both subadult and adult pigeons, with at least one AR compound detected in 11% of plasma samples and 47% of liver samples. Fisher’s exact test revealed significant differences among BCSs 1–3. In plasma, the concentration of coumatetralyl differed significantly from that of warfarin in pigeons with BCS 2 (p < 0.001) and BCS 3 (p=0.0083). In the liver, pigeons with BCS 2 showed a significant difference between coumatetralyl and warfarin concentrations (p < 0.001) (Table 4). Although both compounds were detected in pigeons with BCS 1, no significant differences were found between compound concentrations in either sample type. Both subadult and adult pigeons exhibited a high prevalence of AR accumulation in the liver (83% and 77%, respectively). Statistically significant differences were observed between age groups when comparing compounds across sample types (Table 4). Significant differences were observed between coumatetralyl and warfarin levels in both plasma (p=0.0028) and liver (p=0.0004) in subadult pigeons. A statistically significant association was found between coumatetralyl and warfarin levels in the plasma and liver of adult pigeons (p < 0.001). Both coumatetralyl and warfarin were found in plasma and liver samples from rural pigeons. However, in urban pigeons, only coumatetralyl was detected in liver samples, with concentrations ranging from 0.18 to 3.33 ng/g ww (Table 4). A significant difference was observed between the two compounds and sample types in the rural pigeons (p < 0.001). Table 2. Collision energies and mass spectrometry parameters for AR analysis.
Table 3. Characteristics of coumatetralyl and warfarin accumulation in pigeons.
DiscussionAccumulation of ARsIn Thailand, ARs are an approved method for controlling rodents in both agricultural and urban environments. A wide range of AR products, including both FGARs and SGARs, are available on the market (Sapbamrer et al., 2023). Although SGARs are more potent and effective in controlling rodents, as shown in the previous studies (Martínez-Padilla et al., 2017), FGARs, such as coumatetralyl and warfarin, are more commonly available and accessible in Thailand. The prices of different brands do not vary significantly; however, certain brands and products are more prevalent in the market. The uneven distribution of these products across different regions may be attributed to factors such as cost, availability, consumer preference, and frequency of use. With the increasing use of rodenticides, concerns about environmental contamination and potential adverse effects on nontarget wildlife species, including their health and survival, are growing (Panuwet et al., 2012). Nontarget animals, such as carnivores, insectivores, reptiles, and predatory birds, living in backyards, human communities, and agricultural areas are most likely susceptible to unintentional ingestion of ARs through contaminated food sources and the environment (Dowding et al., 2010; Lettoof et al., 2020; Cerkvenik-Flajs et al., 2024; Regnery et al., 2024). In the present study, pigeons, one of the nontarget animals commonly found in both urban and rural areas, were shown to accumulate ARs, with both coumatetralyl and warfarin detected in their liver and plasma samples. As a primary organ involved in xenobiotic metabolism, the liver is often used as a key target organ in the accumulation of environmental pollutants. Previous studies from Thailand have reported similar findings, where coumarin derivatives were detected in the livers of nontarget animals, including dogs, cats, and Patagonian mara (Kaewamatawong et al., 2011; Chansiripornchai et al., 2025). However, previous studies focusing on the diagnosis of AR toxicosis used thin layer chromatography and spectral analysis via derivative spectrophotometry, together with histological evaluation and observation of hemorrhagic lesions, to determine the cause of death in these animals. Meanwhile, these techniques do not provide precise quantification or identification of specific compounds, as is the case with LC-MS/MS in the present study. In contrast, the pigeons examined in this study showed no clinical signs indicative of AR toxicity. However, their exposure concerns that AR contamination may be widespread in Thailand’s urban and rural areas. This aligns with previous findings indicating that sublethal exposure is more common than lethal poisoning among nontarget species (Dowding et al., 2010). Although many countries, including Thailand, regulate the use of chemicals in agriculture, rodent control using ARs remains less strictly regulated. The import and distribution of ARs, particularly SGARs, are not well controlled, which contributes to their widespread use. Rodents are one of the pest species that have a high impact on crop damage, damage in lowland/upland areas, and disease carriers. Agricultural production in Thailand is strongly requesting for rodent control, not only zinc phosphide, mechanical or physical methods, but also chemical control using ARs (both FGARs and SGARs), which are popular for agriculture and big cities (rural and urban areas) (Boonsong et al., 1999). As a result, the continuous use of these compounds has led to reports of rodent resistance to certain ARs (Ishizuka, et al. 2007; Takeda, et al. 2016). SGARs, while effective, pose a significant risk to nontarget animals and have been linked to notable ecological impacts in European countries (Lettoof et al., 2020). Table 4. Comparison of coumatetralyl and warfarin concentrations in the plasma and liver of pigeons according to BCS, age, and sampling area.
Fig. 2. Number of ARs detected in the plasma and liver of urban and rural pigeons The continued use of AR raises significant concerns regarding unintended exposure in nontarget animals, particularly predatory and domestic birds such as eagles, barn owls, red kites, and kestrels, as reported by Nakayama et al. (2019), including pigeons in the present study. These birds are vulnerable to accidental AR exposure through their feeding habits. Many countries use multiple AR compounds for rodent control, increasing the likelihood of such exposure. Several studies have reported the presence of multiple AR compounds, including both SGARs and FGARs, in the tissues of nontarget animals (Moriceau et al., 2022; Cooke et al., 2023). Recently, high concentrations of bromadiolone and warfarin were detected in the liver, heart, kidney, and lungs of rooks and carrion crows in Poland (Kobylarz et al., 2024). Similarly, both coumatetralyl and warfarin were found to accumulate in the liver and plasma of pigeons. According to Cooke et al. (2023) and Lohr (2018), the liver tissue concentration of ARs in predatory birds can be categorized as: 1) lethal (> 700 ng/g), 2) probably lethal (from 500 to <700 ng/g); 3) possibly lethal and likely toxic (from 200 to <500 ng/g); 4) possibly lethal and likely toxic (from 100 to <200 ng/g); 5) possibly toxic but unlikely lethal (from 10 to <100 ng/g); 6) probably no toxicity (LOD < 10 ng/g); and 7) no detected rodenticide (below LOD). Though the AR concentrations in liver tissue in this study were below the established toxicity thresholds, the findings ƩARs in liver <10 ng/g still indicate a potential toxicological risk to nontarget animals. Both predatory and domestic birds, including pigeons, play vital roles in food webs from an ecological perspective. Therefore, the widespread use of ARs raises serious environmental concerns, highlighting the importance of further study into their toxicological impacts on avian species. In the present study, AR residues, notably coumatetralyl and warfarin, were found in nearly 50% of the pigeon samples. This study highlights the presence of AR contamination in pigeons across diverse environments, including agricultural and livestock-farming regions and urban environments surrounded by buildings and residential areas. Similar to previous studies, nontarget species, such as raptors, small birds, foxes, hedgehogs, dogs, and cats, residing in backyards, scrublands, and communities are at risk of exposure due to the widespread and often uncontrolled use of ARs (Erickson and Urban, 2004; Nakayama et al., 2019). Given these concerns, this study examined pigeons from two locations representing different ecosystems and human activities. Despite their contrasting characteristics, both locations share a common factor, i.e., AR use. The metabolization of ARs in mammals, including rodents, involves phase I oxidation and phase II conjugation, followed by excretion through feces and urine. However, some AR compounds accumulate in the body, particularly in the liver (Newton et al., 1990; Shore et al., 2003; Walker et al., 2008; Rattner et al., 2013; Nakayama et al., 2019; Khidkhan et al., 2024; Kobylarz, et al., 2024;). Similarly, previous studies have demonstrated hepatic accumulation of ARs and their metabolites in nontarget animals, including birds (Ishizuka et al., 2007; Khidkhan et al., 2024). Additionally, the interspecies differences in AR metabolism among birds, including pigeons, are likely due to variations in the expression and activity of phase I enzymes involved in AR breakdown. Recent findings indicate that pigeons have a shorter plasma warfarin half-life for warfarin (19.9 ± 6.6 hours) than chickens (97.9 ± 24.0 hours) (Khidkhan et al., 2024). The abovementioned kinetic parameters supported the possibility of detecting ARs such as coumatetralyl and warfarin in pigeons. Although AR residues were detected in the livers of pigeons in the present study, these concentrations were lower than those previously reported in predatory birds (Nakayama et al., 2019; Cooke et al., 2023; Vicedo, et al., 2024). This lower accumulation may be attributed to several factors: 1) the low contamination of the environment; 2) the least amount of contamination in their food; and 3) the feeding habitats of pigeons. Pigeons commonly forage in the same areas on the ground and often feed in flocks, which may restrict their exposure to AR-contaminated prey. These behavioral patterns, along with their trophic level as mid-level consumers, could contribute to the observed relatively low AR accumulation. The potential for habitual exposure through repeated foraging in contaminated areas remains a concern. In this study, pigeons with a BCS of 1–3 and ranging from subadult to adult were potentially exposed to ARs. There is a significant association between BCSs and AR compounds. BCSs, especially BCS levels 2 and 3, might be at risk of AR accumulation in the long term in their liver tissues. Since the liver is the main organ for AR accumulation, some deposition also occurs in fat tissues (Tasheva, 1995). These findings underscore the importance of monitoring physiological conditions in exposed birds. Both subadult and adult pigeons appear to be susceptible to accidental AR exposure, which might be influenced by their inhabitants. Recently, Vicedo et al. (2024) highlighted that the widespread use of ARs in agricultural and urban areas has been linked to increased accumulation in nontarget animals. Thailand is characterized by dense agricultural and livestock regions and frequently uses pesticides, including ARs, for pest and rodent control. The agricultural sampling area was crowded with cattle and pig farms in the present study. Meanwhile, higher concentrations of coumatetralyl and warfarin were detected in pigeons from agricultural areas than in those from urban environments. This trend may be due to the intensive use of AR aimed at controlling rodent populations to protect agricultural yields. It is plausible that the sampling sites in this study experienced careless and frequent application of ARs in agricultural areas, which may contribute to food source contamination. This is the first study to report the presence of ARs in pigeons, representing a nontarget avian species. Although the overall concentrations detected were relatively low, AR contamination was evident in both agricultural and urban environments. Future studies should include additional bird species and broader geographical sampling to gain a more comprehensive understanding of AR exposure in nontarget animals. This would allow for a more detailed analysis of the relationships among AR contamination, species susceptibility, and environmental context. ConclusionThis study provides the accumulation of ARs that are commonly used in Thailand; coumatetralyl and warfarin were present in pigeons, nontarget species living in both rural and urban areas. The presence of these compounds in both plasma and liver samples raises chronic and ongoing environmental exposure. In particular, the liver serves as a reliable biomarker for long-term accumulation of ARs. These findings raise concerns regarding the ecological risks of the use of ARs and potential secondary exposure in nontarget species. This study emphasizes the importance of appropriate AR management and public awareness to mitigate unintentional exposure. Future research should focus on expanding sample sizes and geographical coverage to assess the extent of AR contamination and provide evidence that can be carefully considered to promote safe usage. AcknowledgementsThe authors would also like to acknowledge the Kasetsart University Raptor Rehabilitation Unit, Kamphaeng Saen, for sample collection; the Central Laboratory, Faculty of Veterinary Medicine, Kasetsart University, for sample preparation; and the Laboratory of Toxicology and OHRC, Hokkaido University, for liquid chromatography–mass spectrometry (LC-MS/MS) analysis. Conflicts of interestThe authors have no competing interests to declare. FundingGraduate Development Research Grants and Research Potential Development of Faculty of Veterinary Medicine Researcher Grants by the Department Ent of Pharmacology, Faculty of Veterinary Medicine, Kasetsart University and Prof. Aksorn Saengtienchai, PhD Authors' contributionsConceptualization: Phourng K and Saengtienchai A; data curation: Phourng K; formal analysis: Phourng K and Saengtienchai A; funding acquisition: Saengtienchai A; investigation: Saengtienchai A; methodology: Phourng K, Saengtienchai A, Yohannes YB, and Ikenaka Y; project administration: Saengtienchai A; supervision: Saengtienchai A; validation: Saengtienchai A; visualization: Phourng K and Saengtienchai A; writing–original draft: Phourng K; writing–review and editing: Yohannes YB and Saengtienchai A. Data availabilityAll data supporting this study’s findings are available within the manuscript. ReferencesAlbert, C.A., Wilson, L.K., Mineau, P., Trudeau, S.. and Elliott, J.E. 2010. Anticoagulant rodenticides in three owl species from Western Canada, 1988–2003. Arch. Environ. Contam.ination Toxicol. 58, 451–459. Boonsong, P., Hongnark, S., Suasa-ard, K., Khoprasert, Y., Promkerd, P., Hamarit, G., Nookarn, P. and Jakel, T. 1999. Rodent management in Thailand In: Ecologically-based rodent management. Eds., Singleton, G.R., Hinds, L.A., Leirs, H and Zhang, Z. Bruce, Australia: ACIAR, pp: 338–357. Bradbury, S. 2008. Risk mitigation decision for ten rodenticides. Washington, DC: United States Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substance. Cerkvenik-Flajs, V., Schenke, D., Žele-Vengušt, D., Korenjak-Černe, S., Perpar, A. and Vengušt, G. 2024. Exposure assessment of anticoagulant rodenticides in the liver of red foxes (Vulpes vulpes) in Slovenia. Sci. Total Environ. 918, 170400. Chansiripornchai, P., Hunprasit, V.. and Techangamsuwan, S.. 2025. First report on the occurrence of anticoagulant rodenticides toxicosis in nontarget animals in Thailand. BMC Vet. Res. 21(1), 337. Cooke, R., Whiteley, P., Death, C., Weston, M.A., Carter, N., Scammell, K., Yokochi, K., Nguyen, H. and White, J.G. 2023. Silent killers? The widespread exposure of predatory nocturnal birds to anticoagulant rodenticides. Sci. Total Environ. 904, 166293. Dowding, C.V., Shore, R.F., Worgan, A., Baker, P.J. and Harris, S. 2010. Accumulation of anticoagulant rodenticides in a non-target insectivore, the European hedgehog (Erinaceus europaeus). Environ. Pollut. 158(1), 161–166. Erickson, W. and Urban, D. 2004. Potential risks of nine rodenticides to birds and non-target mammals: comparative approach. US Environmental. Washington DC, USA: Protection Agency, p 225. Fourel, I., Roque, F., Orabi, P., Augiron, S., Couzi, F.X., Puech, M.P., Chetot, T. and Lattard, V. 2024. Stereoselective bioaccumulation of chiral anticoagulant rodenticides in the liver of predatory and scavenging raptors. Sci. Total Environ. 917, 170545. George, S., Sharp, E., Campbell, S., Giela, A., Senior, C., Melton, L.M., Vyas, D., Mocogni, L.. and Galloway, M.. 2024. Anticoagulant rodenticide exposure in common buzzards: impact of new rules for rodenticide use. Sci. Total Environ. 944, 173832. Guitart, R., Sachana, M., Caloni, F., Croubels, S., Vandenbroucke, V. and Berny, P. 2010. Animal poisoning in Europe. Part 3: wildlife. Vet. J. 183(3), 260–265. Hoare, J.M. and Hare, K.M. 2006. The impact of brodifacoum on non-target wildlife: gaps in knowledge. New Zealand J. Ecol. 30(2), 157–167. Ishizuka, M., Okajima, F., Tanikawa, T., Min, H.W., Tanaka, K.D., Sakamoto, K.Q. and Fujita, S. 2007. Elevated warfarin metabolism in warfarin-resistant roof rats (Rattus rattus) in Tokyo. Drug Metab. Dispos.ition 35, 62–66. Jones, A.K. 2009. The physical examination: handbook of Avian Medicine, 2nd ed. Ohio, USA: W.B. Saunders, pp: 56–76. Kaewamatawong, T., Lohavanijaya, A., Charoenlertkul, P., Srichairat, S. and Srichairat, S. 2011. Retrospective histopathological study of hemorrhagic lesion of coumarin intoxication in dogs. Thai J. Vet. Med. 41(2), 239–244. Katevorn, T., Denduang, N., Seamsuan, S., Homsawat, S., Juntaropakorn, M. and Jadejaroen, J. 2020. Abundance, factor affecting abundance, and breeding behavior of rock pigeons Columba livia in Kasetsart University, Sriracha Campus. The 3rd Academic Conference, Kasetsart University Sriracha Campus, August 28, 2020 at Kasetsart University Sriracha Campus Chonburi Province, Thailand, pp 78–87. Khidkhan, K., Yasuhira, F., Saengtienchai, A., Kasorndorkbua, C., Sitdhibutr, R., Ogasawara, K., Adachi, H., Watanabe, Y., Saito, K., Sakai, H., Horikoshi, K., Suzuki, H., Kawai, Y.K., Takeda, K., Yohannes, Y.B., Ikenaka, Y., Rattner, B.A., Ishizuka, M. and Nakayama, S.M.M. 2024. Evaluation of anticoagulant rodenticide sensitivity by examining in vivo and in vitro responses in avian species, focusing on raptors. Environ. Pollut. 341, 122837. Kobylarz, D., Paprotny, Ł., Wianowska, D., Gnatowski, M. and Jurowski, K. 2024. Silent bird poisoning in Poland: reconfirmation of bromadiolone and warfarin as the proximal causes using GC-MS/MS-based methodology for forensic investigations. Pharmaceuticals 17(6), 764. Labocha, M.K. and Hayes, J.P. 2012. Morphometric indices of body condition in birds: a review. J. Ornithol. 153(1), 1–22. Langford, K.H., Reid, M.. and Thomas, K.V. 2013. The occurrence of second generation anticoagulant rodenticides in non-target raptor species in Norway. Sci. Total Environ. 450-–451(451), 205–208. Lettoof, D.C., Lohr, M.T., Busetti, F., Bateman, P.W. and Davis, R.A. 2020. Toxic time bombs: frequent detection of anticoagulant rodenticides in urban reptiles at multiple trophic levels. Sci. Total Environ. 724, 138218. Lohr, M.T. 2018. Anticoagulant rodenticide exposure in an Australian predatory bird increases with proximity to developed habitat. Sci. Total Environ. 643, 134–144. Martínez-Padilla, J., López-Idiáquez, D., López-Perea, J.J., Mateo, R., Paz, A. and Viñuela, J. 2017. A negative association between bromadiolone exposure and nestling body condition in common kestrels: management implications for vole outbreaks. Pest Manage. Sci. 73(2), 364–370. Moriceau, M.A., Lefebvre, S., Fourel, I., Benoit, E., Buronfosse-Roque, F., Orabi, P., Rattner, B.A. and Lattard, V. 2022. Exposure of predatory and scavenging birds to anticoagulant rodenticides in France: exploration of data from French surveillance programs. Sci. Total Environ. 810, 151291. Musto, C., Cerri, J., Capizzi, D., Fontana, M.C., Rubini, S., Merialdi, G., Berzi, D., Ciuti, F., Santi, A.., Rossi, A., Barsi, F., Gelmini, L., Fiorentini, L., Pupillo, G., Torreggiani, C., Bianchi, A., Gazzola, A., Prati, P., Sala, G., Apollonio, M., Delogu, M., Biancardi, A., Uboldi, L., Moretti, A. and Garbarino, C. 2024. First evidence of widespread positivity to anticoagulant rodenticides in grey wolves (Canis lupus). Sci. Total Environ. 915, 169990. Nakayama, S.M.M., Morita, A., Ikenaka, Y., Mizukawa, H. and Ishizuka, M. 2019. A review: poisoning by anticoagulant rodenticides in non-target animals globally. J. Vet. Med. Sci. 81(2), 298–313. Newton, I., Wyllie, I. and Freestone, P. 1990. Rodenticides in British barn owls. Environ. Pollut. 68(1–2), 101–117. Panuwet, P., Siriwong, W., Prapamontol, T., Ryan, P.B., Fiedler, N., Robson, M.G. and Barr, D.B. 2012. Agricultural pesticide management in Thailand: situation and population health risk. Environ. Sci. Policy 17, 72–81. Rattner, B.A., Horak, K.E., Lazarus, R.S., Goldade, D.A. and Johnston, J.J. 2014. Toxicokinetics and coagulopathy threshold of the rodenticide diphacinone in eastern screech-owls (Megascops asio). Environ. Toxicol. Chem. 33(1), 74–81. Regnery, J., Rohner, S., Bachtin, J., Möhlenkamp, C., Zinke, O., Jacob, S., Wohlsein, P., Siebert, U., Reifferscheid, G. and Friesen, A. 2024. First evidence of widespread anticoagulant rodenticide exposure of the Eurasian otter (Lutra lutra) in Germany. Sci. Total Environ. 907, 167938. Sapbamrer, R., Kitro, A., Panumasvivat, J. and Assavanopakun, P. 2023. Important role of the government in reducing pesticide use and risk sustainably in Thailand: current situation and recommendations. Front. Public Health 11, 1141142. Sarabia, J., Sánchez-Barbudo, I., Siqueira, W., Mateo, R., Rollán, E. and Pizarro, M. 2008. Lesions associated with the plexus venosus subcutaneus collaris of pigeons with chlorophacinone toxicosis. Avian Dis. 52(3), 540–543. Schroeder, M.A. and Robb, L.A. 2005. Criteria for gender and age: techniques for wildlife investigations and management. 6th ed. Wildlife Society. Ed., Braun, C.E. Bethesda, MD: Wildlife Society, pp: 303–338. Shore, R.F., Birks, J.D.S., Afsar, A., Wienburg, C.L. and Kitchener, A.C. 2003. Spatial and temporal analysis of second-generation anticoagulant rodenticide residues in polecats (Mustela putorius) from throughout their range in Britain, 1992–1999. Environ. Pollut. 122(2), 183–193. Takeda, K., Ikenaka, Y., Tanikawa, T., Tanaka, K.D., Nakayama, S.M.M., Mizukawa, H. and Ishizuka, M. 2016. Novel revelation of warfarin resistant mechanism in roof rats (Rattus rattus) using pharmacokinetic/pharmacodynamic analysis. Pestic. Biochem. Physiol. 134, 1–7. Tasheva, M. 1995. Environmental health criteria 175: anticoagulant rodenticides. World Health Organization Geneva. Thomas, P.J., Mineau, P., Shore, R.F., Champoux, L., Martin, P.A., Wilson, L.K., Fitzgerald, G. and Elliott, J.E. 2011. Second generation anticoagulant rodenticides in predatory birds: probabilistic characterisation of toxic liver concentrations and implications for predatory bird populations in Canada. Environ. Int. 37(5), 914–920. Trakulsrichai, S., Kosanyawat, N., Atiksawedparit, P., Sriapha, C., Tongpoo, A., Udomsubpayakul, U., Rittilert, P. and Wananukul, W. 2017. Clinical characteristics of zinc phosphide poisoning in Thailand. Ther. Clin. Risk Manag. 13, 335–340. Vicedo, T., Navas, I., María-Mojica, P. and García-Fernández, A.J. 2024. Widespread use of anticoagulant rodenticides in agricultural and urban environments. A menace to the viability of the endangered Bonelli's eagle (Aquila fasciata) populations. Environ. Pollut. 358, 124530. Walker, L.A., Turk, A., Long, S.M., Wienburg, C.L., Best, J.. and Shore, R.F. 2008. Second generation anticoagulant rodenticides in tawny owls (Strix aluco) from Great Britain. Sci. Total Environ. 392(1), 93–98. Wananukul, W., Sriapha, C., Tongpoo, A., Sadabthammarak, U., Wongvisawakorn, S. and Kaojarern, S. 2007. Human poisoning in Thailand: the Ramathibodi Poison Center's experience (2001–2004). Clin. Toxicol. 45(5), 582–588. | ||
| How to Cite this Article |
| Pubmed Style Phourng K, Sitdhibutr R, Yohannes YB, Ikenaka Y, Saengtienchai A. Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in Thailand. Open Vet. J.. 2025; 15(8): 3608-3617. doi:10.5455/OVJ.2025.v15.i8.23 Web Style Phourng K, Sitdhibutr R, Yohannes YB, Ikenaka Y, Saengtienchai A. Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in Thailand. https://www.openveterinaryjournal.com/?mno=254517 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.23 AMA (American Medical Association) Style Phourng K, Sitdhibutr R, Yohannes YB, Ikenaka Y, Saengtienchai A. Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in Thailand. Open Vet. J.. 2025; 15(8): 3608-3617. doi:10.5455/OVJ.2025.v15.i8.23 Vancouver/ICMJE Style Phourng K, Sitdhibutr R, Yohannes YB, Ikenaka Y, Saengtienchai A. Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in Thailand. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3608-3617. doi:10.5455/OVJ.2025.v15.i8.23 Harvard Style Phourng, K., Sitdhibutr, . R., Yohannes, . Y. B., Ikenaka, . Y. & Saengtienchai, . A. (2025) Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in Thailand. Open Vet. J., 15 (8), 3608-3617. doi:10.5455/OVJ.2025.v15.i8.23 Turabian Style Phourng, Kosal, Ratiwan Sitdhibutr, Yared Beyene Yohannes, Yoshinori Ikenaka, and Aksorn Saengtienchai. 2025. Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in Thailand. Open Veterinary Journal, 15 (8), 3608-3617. doi:10.5455/OVJ.2025.v15.i8.23 Chicago Style Phourng, Kosal, Ratiwan Sitdhibutr, Yared Beyene Yohannes, Yoshinori Ikenaka, and Aksorn Saengtienchai. "Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in Thailand." Open Veterinary Journal 15 (2025), 3608-3617. doi:10.5455/OVJ.2025.v15.i8.23 MLA (The Modern Language Association) Style Phourng, Kosal, Ratiwan Sitdhibutr, Yared Beyene Yohannes, Yoshinori Ikenaka, and Aksorn Saengtienchai. "Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in Thailand." Open Veterinary Journal 15.8 (2025), 3608-3617. Print. doi:10.5455/OVJ.2025.v15.i8.23 APA (American Psychological Association) Style Phourng, K., Sitdhibutr, . R., Yohannes, . Y. B., Ikenaka, . Y. & Saengtienchai, . A. (2025) Anticoagulant rodenticide accumulation in the liver and plasma of pigeons (Columba livia) in Thailand. Open Veterinary Journal, 15 (8), 3608-3617. doi:10.5455/OVJ.2025.v15.i8.23 |