| Research Article | ||
Open Vet. J.. 2025; 15(9): 4346-4353 Open Veterinary Journal, (2025), Vol. 15(9): 4346-4353 Research Article Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imagingVeysel Delibaş1*, Zafer Soygüder1 and Cemil Göya21Faculty of Veterinary Medicine, Department of Anatomy, Van Yuzuncu Yil University, Van, Turkey 2Faculty of Medicine, Department of Radiodiagnostics, Van Yuzuncu Yil University, Van, Turkey *Corresponding Author: Veysel Delibaş. Department of Anatomy, Faculty of Veterinary Medicine, Van Yuzuncu Yil University, Van, Turkey. Email: veyseldeibas [at] yyu.edu.tr Submitted: 24/04/2025 Revised: 23/07/2025 Accepted: 08/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
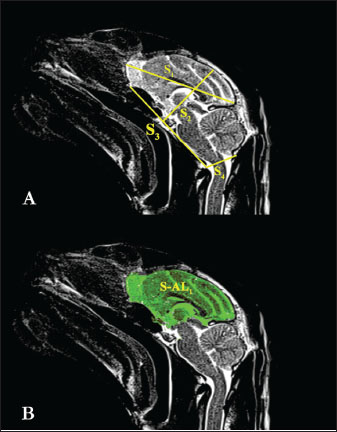
AbstractBackground: Brain asymmetry is defined as neuroanatomical or functional differences between the brain’s left and right hemispheres. These differences can be explained as morphometric changes in regional brain size or functional changes resulting from neuronal density. Aim: This study aimed to use magnetic resonance imaging (MRI) to visualize the brain tissue of Van cats, obtain anatomical morphometric measurements, and examine brain asymmetry. Methods: A total of 20 healthy adult Van cats, including 10 females and 10 males, who had not undergone any surgical procedures, were included in the study. The animals were anesthetized using a combination of ketamine and xylazine. Midsagittal and dorsal cross-sectional images were acquired using an MRI scanner. Images were transferred to a workstation and processed in digital imaging and communications in medicine format. Morphometric measurements were obtained using the Enlil picture archiving and communication system radiological image processing and archiving program. Results: Statistical analysis of the morphometric measurements revealed that, with the exception of encephalon height, most parameters were significantly higher in male Van cats (p < 0.05). Additionally, the study assessed brain asymmetry in Van cats and found a general asymmetry favoring the right hemisphere (hemispherium cerebri dexter). In female Van cats, regional brain asymmetry was observed specifically toward the right frontal cortex (cortex frontalis dexter) (p < 0.05). Conclusion: This study provides a valuable tool for defining some morphometric measurements of the sagittal and dorsal planes of the brain of Van cats and demonstrating brain asymmetry in cats. Keywords: Brain asymmetry, Magnetic resonance imaging, Morphometric, Cat. IntroductionThe van cat is a cat breed that grows in Turkey. It has a triangular head, a small nose, and its entire body is covered with white hair (Odabaşıoğlu and Ateş, 2000). The most typical feature of Van cats is that one eye is blue and the other eye is light amber yellow (Odabaşıoğlu and Ateş, 2000). Brain asymmetry refers to neuroanatomical or functional differences between the left and right hemispheres of the brain (Le May, 1977). These differences may be attributed to variations in neuronal density, regional brain size, and larger-scale torsion that reflect skull shape (Le May, 1977). Since the initial discovery of structural and functional asymmetry in the human brain, various in vivo methods, including volumetric measurements and traditional approaches, have been employed to study anatomical brain asymmetries (Kennedy et al., 1999; Preis et al., 1999; Amunts et al., 2000; Yücel et al., 2001). The relationship between neuroanatomy and human behavior has been a subject of scientific interest for over a century (Pilcher et al., 2001). Neuroanatomical asymmetries have major effects on grammatical organization and limb use (Geschwind and Levitsky, 1968; Eggert, 1977). Neuroanatomical asymmetries were considered to be unique to humans and directly related to language acquisition until recently (Toga and Thompson, 2003). However, Groves and Humphrey (1973) stated that a mountain gorilla (Gorilla beringei beringei) exhibited asymmetry of skull length to the left and that this issue should be investigated in mammals other than humans. In addition, the existence of brain asymmetry in dogs has been reported in previous studies (Siniscalchi et al., 2011). A review of the veterinary anatomy literature reveals numerous studies focusing on the comparative examination of brain anatomy in various domestic mammals, such as sheep (Olopade et al., 2005), goats (Yoshikawa, 1968), horses (Yoshikawa, 1968; Getty, 1975; Çağdaş and Hazıroğlu, 2009), pigs (Félix et al., 1999), and dogs (De Rycke et al., 2005). However, studies focusing on anatomical brain measurements in cats are scarce. Furthermore, research addressing brain asymmetry in felines is lacking. The aims of this study were to perform morphometric measurement analyses of brain tissue in normal Van cats, especially in sagittal and dorsal sections, using magnetic resonance imaging (MRI), and to investigate the presence of brain asymmetry in Van cats and to evaluate potential sex-related differences. Materials and MethodsAnimalsA total of 20 healthy adult Van cats, 10 female and 10 male, aged 4 (3 minutes -5 max) on mean, were used in the study. Female Van cats weighed a mean of 3,300 g, and male Van cats weighed a mean of 4,340 g. All cats were selected from Van cats that had not undergone any surgical procedures, including neutering, had no visible anomalies, and had no physical or neurological disorders. The cats used in the study were taken to a separate observation room approximately 1 month before the MRI scan, and a controlled feeding program was applied. AnesthesiaIn accordance with the local ethics committee’s guidelines for animal welfare, all cats were fed ad libitum until the MRI procedure. Cats were made to stop eating and drinking approximately 2 hours before anesthesia to prevent aspiration pneumonia. Subsequently, the patients were restrained and transferred to the anesthesia room. During the MRI scan, general anesthesia was induced to immobilize the cats. The anesthesia procedure included an intramuscular (IM) injection of a combination of ketamine (15 mg/kg body weight, IM, Ketasol® 10% injectable, Interhas Veterinary Pharmaceuticals, Ankara) and Xylazine (1–2 mg/kg body weight, IM, Alfazyne® 2% injectable, Ege Vet Veterinary Pharmaceuticals, Izmir). The depth of anesthesia was monitored during the anesthesia of the cats. In this context, pupillary reflexes and respiratory movements were periodically examined. MRI image acquisitionAnesthetized Van cats were positioned in supine orientation, and imaging was performed on the head region using a 1.5 T MRI scanner. T1 and T2-weighted dorsal and sagittal sequences were obtained. The parameters for T1-weighted dorsal images were as follows: repetition time (TR): 480 ms; echo time (TE): 11 ms; slice thickness: 3 mm; field of view (FoV): 230 mm; gap: 30%, averages: 1; matrix: 886 × 886. For T2-weighted dorsal images: TR: 4,030 ms, TE: 87 ms; slice thickness: 3 mm, FoV: 230 mm, gap: 30%, averages: 1, matrix: 894 × 885. For T2-weighted sagittal images: TR: 4,160 ms, TE: 120 ms; slice thickness: 3 mm, FoV: 240 mm, gap: 30%, averages: 1, matrix: 894 × 885. Morphometric measurementsMorphometric measurements were taken from the MRI based on methods described by Carrera et al. (2009), Lewis et al. (2016), Taştemur et al. (2017), Reinstrup et al. (2019), and Metwally et al. (2021). The measurements were as follows: Sagittal measurementsS1: Prosencephalon length (Fig. 1A).
Fig. 1. S1, S2, S3, S4, and S-AL1 measurement parameters in T2 sagittal MRI images of the Van cat. (A) S1: length of the prosencephalon, S2: height of the encephalon, S3: base length of the encephalon, S4: dorsoventral length of the foramen magnum. (B) S-AL1: midsagittal surface area of the prosencephalon. S2: Encephalon height (Fig. 1A). S3: Encephalon base length (Fig. 1A). S4: Dorsoventral length of the magnum of the foramen (Fig. 1A). S-AL1: Prosencephalon’s midsagittal surface area (Fig. 1B). Dorsal measurementsD1: Encephalon width (Fig. 2A).
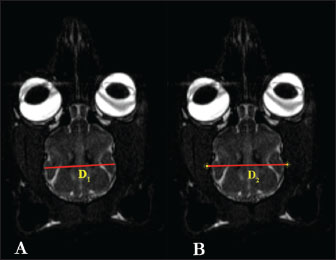
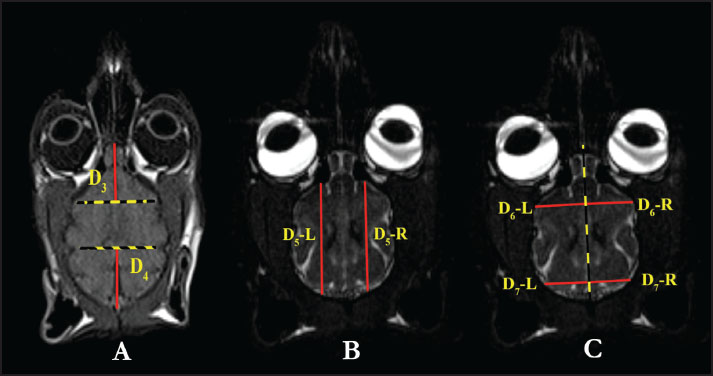
Fig. 2. D1 and D2 measurement parameters in T2 dorsal MRI images in the Van cat. (A) D1: encephalon width. (B) D2: cavum cranii width. D2: The width of the cavum cranii (Fig. 2B). D3: The longest distance of the fissura longitudinalis cerebri cranialis (Fig. 3A).
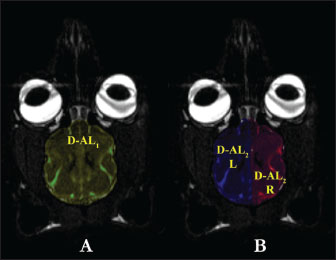
Fig. 3. D3, D4 measurement parameters in T1 dorsal MRI images and D5, D6, and D7 measurement parameters in T2 dorsal MRI images in the Van cat. (A) D3: longest distance of the fissura longitudinalis cerebri cranialis, D4: longest distance of the fissura longitudinalis cerebri caudalis. (B) D5-L: longest craniocaudal distance of the hemispherium cerebri sinister, D5-R: longest craniocaudal distance of the hemispherium cerebri dexter. (C) D6-R: distance indicating the longest frontal width of the hemispherium cerebri dexter, D6-L: distance indicating the longest frontal width of the hemispherium cerebri sinister, D7-R: distance indicating the longest occipital width of the hemispherium cerebri dexter, D7-L: distance indicating the longest occipital width of the hemispherium cerebri sinister. D4: The longest distance fissura longitudinalis cerebri caudalis (Fig. 3A). D5-L: The longest craniocaudal distance of the left hemisphere (hemispherium cerebri sinister) (Fig. 3B). D5-R: The longest craniocaudal distance in the right hemisphere (hemispherium cerebri dexter) (Fig. 3B). D6-R: The longest frontal width of the right hemisphere (hemispherium cerebri dexter) (Fig. 3C). D6-L: The longest frontal width of the left hemisphere (hemispherium cerebri sinister) (Fig. 3C). D7-R: The longest occipital width of the right hemisphere (hemispherium cerebri dexter) (Fig. 3C). D7-L: The longest occipital width of the left hemisphere (hemispherium cerebri sinister) (Fig. 3C). D-AL1: Total dorsal surface area of the right and left hemispheres (Fig. 4A).
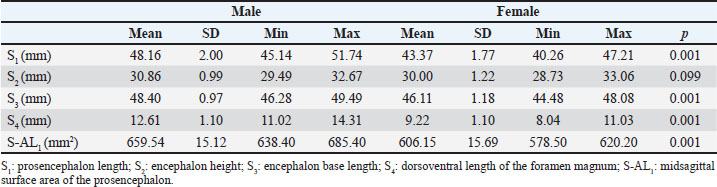
Fig. 4. D-AL1 and D-AL2 measurement parameters in T2 dorsal MRI images in the Van cat. (A) D-AL1: total dorsal surface area measurement of the right and left hemispherium cerebri. (B) D-AL2/R: dorsal surface area of the hemispherium cerebri dexter, and D-AL2/L: dorsal surface area of the hemispherium cerebri sinister. D-AL2/R: Dorsal surface area of the right hemisphere (H. cerebri dexter) (Fig. 4B). D-AL2/L: Dorsal surface area of the left hemisphere (H. cerebri sinister) (Fig. 4B). Statistical analysisDescriptive statistics were used, and data are presented as mean and SD. Differences in measurement parameters between sexes were assessed using the Mann–Whitney U test. The Wilcoxon test was used to analyze the directional differences between the right and left hemispheres. The Mann–Whitney U and Wilcoxon tests indicate that there is sufficient evidence to support the hypothesis that there is a difference between groups if the p < 0.05. Specifically, a p of 0.05 suggests a statistically significant difference between the groups. All statistical analyses were performed using the IBM® SPSS® Statistics V21 software package. Ethical approvalThis study was approved by the Animal Experiments Local Ethics Committee of the Van Yuzuncu Yil University, dated November 25, 2021, under decision number 2021/11-12. ResultsSagittal measurement findingsTable 1 shows the analysis server of the study, prosencephalon length, encephalon base length, and dorsoventral length of the foramen magnum in male Van cats. These measurement values exhibited a statistically positive difference (p < 0.05) in male Van cats (Table 1). Contrary to these measurements, the mean encephalon height was 30.86 mm in males and 30.00 mm in females, and no significant difference was observed between the sexes (p=0.099) (Table 1). The prosencephalon surface area was also determined to be 659.54 mm2 in male Van cats and 606.15 mm2 in females. In this context, it was determined that male cats had a larger prosencephalon surface area than female cats (p=0.001) (Table 1). Table 1. The mean values and SD sagittal MRI measurement parameters in Van cats according to the sexes.
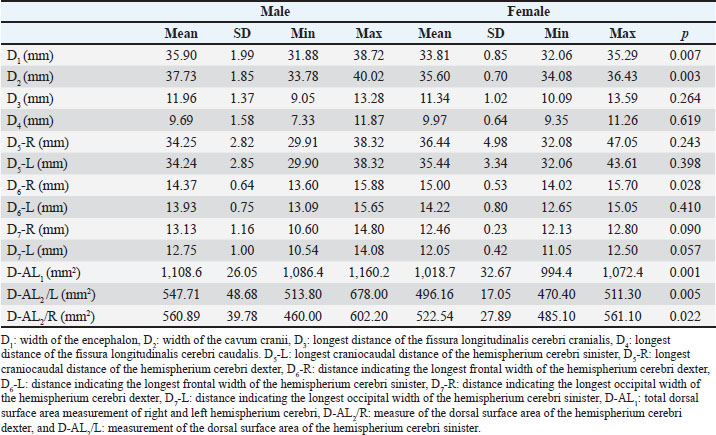
Dorsal measurement findingsTo evaluate whether the dorsal measurements of the encephalon differ according to sex, parameters such as the encephalon, the width, cavum cranii width, and fissura longitudinalis cranialis and caudalis length were analyzed. The widths and craniocaudal lengths of both hemispheres were examined to examine the anatomical brain asymmetry in Van cats. The regional frontal and occipital widths and dorsal surface areas of both hemispheres were measured and evaluated. In this context, the mean widths of the encephalon and cavum cranii of male Van cats were determined as 35.90 and 37.73 mm, respectively, while these values were determined as 33.81 and 35.60 mm, respectively, in female Van cats (Table 2). Table 2. The mean values and SD of the of the dorsal MRI measurement parameters in Van cats according to sex.
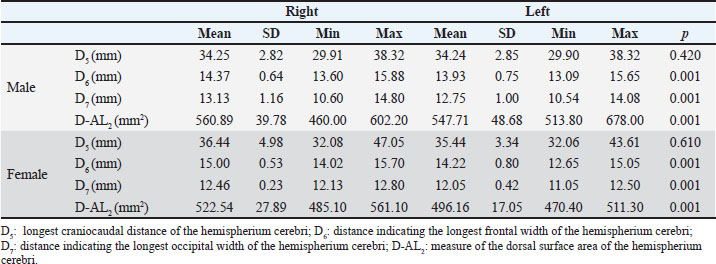
Results showed a significant increase in the mean width of the encephalon (p=0.007) and cavum cranii (p=0.003) in male Van cats compared with female cats (Table 2). Other dorsal measurements, including D3, D4, D5-L, D5-R, D6-L, D7-R, and D7-L did not reveal significant sex differences (p > 0.05) (Table 2). In addition, the longest frontal width of the right hemisphere (H. cerebri dexter) was determined to be 15.00 mm in female cats, whereas it was 14.37 mm in male Van cats, indicating a wider frontal cortex in female cats (p=0.028) (Table 2). The total dorsal surface area measurements revealed that the total dorsal surface area of the right and left hemispherium cerebri’s, hemispherium cerebri dexter’s dorsal surface area (p=0.022), and hemispherium cerebri sinister’s dorsal surface area (p=0.005) measurements had higher values in male cats (Table 2). Measurements of brain asymmetryMeasurements of the right and left hemispheres were compared to evaluate brain asymmetry. There was no significant difference in the longest craniocaudal distance between the right and left hemispheres for both sexes (p > 0.05) (Table 3). In contrast, the longest frontal and occipital widths of the right hemisphere in male Van cats were determined as 14.37 and 13.12 mm, respectively. Similarly, the widths of the left hemisphere were 13.92 and 12.75 mm, respectively. When these measurement values were examined in female cats, the longest frontal and occipital widths of the right hemisphere were determined as 15.00 and 12.46 mm, respectively, while the widths of the left hemisphere were determined as 14.22 and 12.05 mm, respectively. In this context, the presence of a right-sided positive brain asymmetry in both sexes in Van cats was revealed (p=0.001) (Table 3). Table 3. The mean values and SD of dorsal MRI measurement parameters in Van cats according to the right and left directions.
Additionally, to reveal the brain asymmetry status in Van cats, the hemispherium cerebri dexter and sinister surface areas were evaluated separately in male and female sexes. In this context, the dorsal surface area of the right hemisphere was significantly larger than that of the left hemisphere in both male and female Van cats (p=0.001) (Table 3). DiscussionThis study aimed to determine some morphometric measurements of brain tissue in Van cats using Turbo spin echo (TSE)-T2 and T1-weighted sagittal and dorsal MRI, and to evaluate brain asymmetry in Van cats using standard MRI sequences. Previous studies have applied MRI to examine brain tissues in various species, including sheep (Lee et al., 2015), horses (Arencibia et al., 2001), camels (Emam et al., 2020), rabbits (Müllhaupt et al., 2015), foals (Chaffin et al., 1997), and dogs (Lipsitz et al., 2001; Leigh et al., 2008). MRI, especially TSE-T2-weighted imaging, plays an important role in neuroanatomical assessments due to its capacity to provide detailed structural information (Leigh et al., 2008). Therefore, our study was conducted in Van cats by obtaining sagittal and dorsal TSE-T2 and T1 sequence images using a 1.5 T MRI. According to the results of our sagittal measurements, the mean prosencephalon length of male Van cats was 48.16 mm, whereas that of female Van cats was 43.37 mm. Male Van cats have a significantly longer prosencephalon than female Van cats. In addition, male Van cats have a longer encephalon base and a larger foramen magnum dorsoventral diameter compared to female Van cats. These measurements show a clear sex-related difference in brain size. Similar trends have also been observed in humans; male brains are generally larger (Reinstrup et al., 2019). We believe that the possible reason for this is that male Van cats of the same age weigh more than female Van cats. In a study conducted by Kavoi and Jameela (2011), the average brain length was reported to be 168.86 mm in humans, 66.67 mm in dogs, and 66.50 mm in goats. Our data contribute to the literature by providing detailed morphometric measurements of Van cats. Again, within the scope of our sagittal measurements, we determined the encephalon height between male and female Van cats. The encephalon height in male Van cats was determined to be 30.86 mm, and in female Van cats was 30.86 and 30.00 mm, respectively, and we did not find a statistically significant difference between the sexes (p=0.099). This finding is consistent with that by Przyborowska et al. (2017), who reported similar results for the European Shorthair breed. Although there are differences in encephalon height between cat breeds, our data show that sex does not significantly affect this measurement in Van cats. In our study, the dorsal width of the encephalon and the dorsal diameter of the cavum cranii, which are included in the dorsal measurements of the brain, were determined to be an mean of 35.90 ± 1.99 and 37.73 ± 1.85 mm in male Van cats, respectively, and an mean of 33.81 ± 0.85 and 35.60 ± 0.70 mm in female Van cats, respectively. The encephalon width and cavum cranii diameter were larger in males than in females. This gender difference reported in our study is similar to the results of studies conducted in humans (Karakaş et al., 2011; Taştemur et al., 2017; Polat et al., 2019). This sex difference reported in our study is similar to the results of studies conducted in humans (Karakaş et al., 2011; Taştemur et al., 2017; Polat et al., 2019). Furthermore, when looking at the results of dorsal measurements, our study found that H. cerebri dexter exhibited larger frontal and occipital widths than H. cerebri sinister in both sexes. This is consistent with the findings of Siniscalchi et al. (2011) in dogs, who reported that most subjects had a larger hemispherium cerebri dexter. In our study, within the scope of surface area results, the dorsal surface area of H. cerebri sinister, dorsal surface area of H. cerebri dexter, and the total surface area of encephalon measurement parameters were compared in terms of sex. In this context, it was observed that these measurement parameters were 547.71 ± 48.63, 560.89 ± 39.78, and 1,108.60 ± 26.05 mm2 on mean in male Van cats, and 496.16 ± 17.05, 522.54 ± 27.89, and 1,018.70 ± 17.05 mm2 on mean in female Van cats, respectively. The results of our study are statistically consistent with the results of the study by Yücel (2019), and a positive difference was detected in terms of male sex. Since it was determined that the human brain is asymmetric in terms of structure and function, anatomical brain asymmetries have been studied in vivo using methods such as volumetric measurements of certain anatomical structures (Amunts et al., 2000; Yuecel et al., 2001). Recently, with the advancing technological developments, it is suggested to examine brain asymmetry status with new techniques such as basic brain imaging. The most important of these techniques are voxel-based morphometry and MRI measurement analyses (Ashburner and Friston, 2000; Davatzikos and Resnick, 2002). When our study was examined, it was seen that the average widths of the Lobus frontalis and occipitalis dexter in male Van cats were 14.37 ± 0.64 and 13.13 ± 1.16 mm, respectively, and in female Van cats, the means were 15.00 ± 0.53 and 12.46 ± 0.23 mm, respectively. The mean widths of the lobus frontalis and occipitalis sinister in male Van cats were 13.93 ± 0.75 and 12.75 ± 1.00 mm, respectively, and in female Van cats, the means of these values were 14.22 ± 0.80 and 12.05 ± 0.42 mm, respectively. In addition, the mean surface area of H. cerebri sinister in male and female Van cats was 547.71 ± 48.68 and 496.16 ± 17.05 mm2, respectively, whereas the mean surface area of H. cerebri dexter in male and female Van cats was 560.89 ± 39.78 and 522.54 ± 27.89 mm2, respectively. In line with the results, it was concluded that brain asymmetry showed a statistically positive difference on the right side in both male and female Van cats, in accordance with the study of Le May (1977). The results of our study confirm the existence of structural asymmetry in Van cats; the width and surface area of the right hemisphere are larger. This is consistent with previous studies showing rightward asymmetry in several species (Tan and Çalşikan, 1987; Siniscalchi et al., 2011). However, we also observed that female Van cats have a larger frontal lobe width than males and regional brain asymmetry. This observation contradicts Siniscalchi et al. (2011), who reported no significant difference in frontal lobe width between the sexes in dogs. The limitations of this study include the lack of cat body measurements, insufficient reference studies for species-specific comparisons, and potential species-specific variability in measurement points. These factors may affect our results’ objectivity and applicability. To confirm and extend our findings, future studies should include larger sample sizes and multiple breeds. ConclusionIn conclusion, except for the height of the encephalon, the length of the encephalon base, and the dorsoventral diameter of the foramen magnum were found to be greater in male Van cats. In surface area examinations, the sagittal surface area of the prosencephalon was found to be greater in male Van cats than in female Van cats. Cerebral asymmetry was detected in the hemispheric cerebri dexter in both sexes of Van cats. Regional cerebral asymmetry was detected at the frontal lobe level in female Van cats. These findings contribute to our understanding of brain morphometry and asymmetry in Van cats and may inform future research in feline neuroanatomy. AcknowledgmentThis study was obtained from the PhD thesis of Dr. Veysel DELİBAŞ. This research was supported by the Scientific Research Projects Directorate of Van Yuzuncu Yil University (project number TDK-2022-10085). The authors would like to thank Van Yuzuncu Yil University Scientific Research Projects Coordination Unit. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis study did not receive any specific grants. Authors’ contributionConceptualization: V.D., Z.S., and C.G.; methodology: V.D., Z.S., and C.G.; formal analysis: V.D., Z.S., and C.G.; data curation: V.D., Z.S., and C.G.; drafted the manuscript: V.D., Z.S., and C.G.; revised the manuscript: V.D., Z.S., and C.G.; translated the manuscript: V.D., Z.S., and C.G. All the authors have read and agreed with the published version of the manuscript. Data availabilityAll data supporting this study’s findings are available within the manuscript. ReferencesAmunts, K., Jäncke, L., Mohlberg, H., Steinmetz, H. and Zilles, K. 2000. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia 38, 304–312. Arencibia, A., Vazquez, J.M., Ramirez, J.A., Ramirez, G., Vilar, J.M., Rivero, M.A. and Gil, F. 2001. Magnetic resonance imaging of the normal equine brain. Vet. Radiol. Ultrasound. 42, 405–408. Ashburner, J. and Friston, K.J. 2000. Voxel-based morphometry-the methods. Neurolmage 11, 805–821. Çağdaş, O.T.O. and Hazıroğlu, R.M. 2009. Macro-anatomical investigation of encephalon in donkey. Ankara Üniv. Vet. Fak. Derg. 56, 159–164. Carrera, I., Dennis, R., Mellor, D.J., Penderis, J. and Sullivan, M. 2009. Use of magnetic resonance imaging for morphometric analysis of the caudal cranial fossa in Cavalier King Charles Spaniels. Am. J. Vet. Res. 70, 340–345. Chaffin, M.K., Walker, M.A., Mcarthur, N.H., Perris, E.E. and Matthews, N.S. 1997. Magnetic resonance imaging of the brain of normal neonatal foals. Vet. Radiol. Ultrasound. 38, 102–111. Davatzikos, C. and Resnick, S.M. 2002. Degenerative age changes in white matter connectivity visualized in vivo using magnetic resonance imaging. Cereb. Cortex. 12, 767–771. De Rycke, L.M., Gielen, I.M., Van Meervenne, S.A., Simoens, P.J. and van Bree, H.J. 2005. Computed tomography and cross-sectional anatomy of the brain in clinically normal dogs. Am. J. Vet. Res. 66, 1743–1756. Eggert, G.H. 1977. Wernicke’s works on aphasia: a sourcebook and review. The Hague, Netherlands: Mouton. Emam, H., Aref, M., Abdelbaset-Ismail, A., Abdelaal, A., Gouda, S. and Gomaa, M. 2020. Description of normal head structures of the one-humped camel (Camelus dromedarius) by magnetic resonance imaging, computed tomography, and cross-sectional anatomy. Vet. World 13, 1581. Félix, B., Léger, M.E., Albe-Fessard, D., Marcilloux, J.C., Rampin, O., Laplace, J.P. and Duclos, N. 1999. Stereotaxic atlas of the pig brain. Brain Res. Bull. 49, 1–137. Geschwind, N. and Levitsky, W. 1968. Human brain: left-right asymmetries in temporal speech region. Science 161, 186–187. Getty, R., Sisson, S., Grossman, J.D., Rosenbaum, C.E., Ghoshal, N.G. and Hillman, D. 1975. Sisson and Grossman’s the anatomy of the domestic animals. Philadelphia, PA: WB Saunders Company, 1211 p. Groves, C.P. and Humphrey, N.K. 1973. Asymmetry in gorilla skulls: evidence of lateralized brain function? Nature 244, 53–54. Karakaş, P., Koç, Z., Koç, F. and Gülhal Bozkır, M. 2011. Morphometric MRI evaluation of corpus callosum and ventricles in normal adults. Neurol. Res. 33, 1044–1049. Kavoi, B.M. and Jameela, H. 2011. Comparative morphometry of the olfactory bulb, tract and stria in the human, dog and goat. J. Morphol. 29, 939–946. Kennedy, D.N., O’Craven, K.M., Ticho, B.S., Goldstein, A.M., Makris, N. and Henson, J.W. 1999. Structural and functional brain asymmetries in human situs inversus totalis. Neurology 53, 1260–1260. Le May, M. 1977. Asymmetries of the skull and handedness. J. Neurol. Sci. 32, 243. Lee, W., Lee, S.D., Park, M.Y., Foley, L., Purcell-Estabrook, E., Kim, H. and Yoo, S.S. 2015. Functional and diffusion tensor magnetic resonance imaging of the sheep brain. BMC. Vet. Res. 11, 1–8. Leigh, E.J., Mackillop, E., Robertson, I.D. and Hudson, L.C. 2008. Clinical anatomy of the canine brain using magnetic resonance imaging. Vet. Radiol. Ultrasound. 49, 113–121. Lewis, M.J., Olby, N.J., Early, P.J., Mariani. C.L., Muñana, K.R., Seiler, G.S. and Griffith, E.H. 2016. Clinical and diagnostic imaging features of brain herniation in dogs and cats. J. Vet. Intern. Med. 30, 1672–1680. Lipsitz, D., Levitski, R.E. and Berry, W.L. 2001. Magnetic resonance imaging features of multilobular osteochondrosarcoma in 3 dogs. Vet. Radiol. Ultrasound. 42, 14–19. Metwally, M.I., Basha, M.A.A., Abdel Hamid, G.A., Nada, M.G., Ali, R.R., Frere, R.A.F. and Elshetry, A.S.F. 2021. Neuroanatomical MRI study: reference values for the measurements of brainstem, cerebellar vermis, and peduncles. Br. J. Radiol. 94, 20201353. Müllhaupt, D., Augsburger, H., Schwarz, A., Fischer, G., Kircher, P., Hatt, J.M. and Ohlerth, S. 2015. Magnetic resonance imaging anatomy of the rabbit brain at 3 T. Acta. Vet. Scand. 57, 1–9. Odabaşıoğlu, F. and Ateş, C.T. (Eds.). 2000. Van kedisi. 1. Baskı. Konya, Turkey: Selçuk Üniversitesi Basımevi. Olopade, J.O., Onwuka, S.K., Balogun, B.A. and Oke, B.O. 2005. Morphometric investigation of the brain of West African dwarf sheep in Nigeria. Int. J. Morphol. 23, 99–104. Pilcher, D.L., Hammock, E.A. and Hopkins, W.D. 2001. Cerebral volumetric asymmetries in nonhuman primates: a magnetic resonance imaging study. Laterality 6, 165–179. Polat, S., Oksuzler, F., Oksuzler, M., Kabakçı, A. and Yücel, A. 2019. Morphometric MRI study of the brain ventricles in healthy Turkish subjects. Int. J. Morphol. 37, 554–560. Preis, S., Jancke, L., Schmitz-Hillebrecht, J. and Steinmetz, H. 1999. Child age and planum temporale asymmetry. Brain Cogn. 40, 441–452. Przyborowska, P., Adamiak, Z. and Zhalniarovich, Y. 2017. Quantification of cerebral lateral ventricular volume in cats by low-and high-field MRI. J. Feline Med. Surg. 19, 1080–1086. Reinstrup, P., Unnerbäck, M., Marklund, N., Schalen, W., Arrocha, J.C., Bloomfield, E.L. and Hesselgard, K. 2019. Best zero level for external ICP transducer. Acta. Neurochir. 161, 635–642. Siniscalchi, M., Franchini, D., Pepe, A.M., Sasso, R., Dimatteo, S., Vallortigara, G. and Quaranta, A. 2011. Volumetric assessment of cerebral asymmetries in dogs. Brain Cogn. 16, 528–536. Tan, U. and Çalşikan, S. 1987. Allometry and asymmetry in the dog brain: the right hemisphere is heavier regardless of paw preference. Int. J. Neurosci. 35, 189–194. Taştemur, Y., Sabanciogullari, V., Salk, I., Sönmez, M. and Cimen, M. 2017. The relationship of the posterior cranial fossa, the cerebrum, and cerebellum morphometry with tonsiller herniation. Iran. J. Radiol. 14, 13461. Toga, A.W. and Thompson, P.M. 2003. Mapping brain asymmetry. Nat. Rev. Neurosci. 4, 37–48. Yoshikawa, T. 1968. Atlas of the brains of domestic animals. Tokyo, Japan: University of Tokyo Press, 196 p. Yücel, M., Stuart, G.W., Maruff, P., Velakoulis, D., Crowe, S.F., Savage, G. and Pantelis, C. 2001. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb. Cortex. 11, 17–25. Yücel, N. 2019. Morphometric analysis of hippocampus and intracranial formations on MR images in patients with major cognitive impairment. Konya, Turkey: Necmettin Erbakan University Health Sciences Institute. Yuecel, M., Stuart, G.W., Maruff, P., Velakoulis, D., Crowe, S.F., Savage, G., and Pantelis, C. 2001. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: an MRI morphometric study. Cereb. Cortex. 11, 17–25. | ||
| How to Cite this Article |
| Pubmed Style Delibaş V, Soyguder Z, Goya C. Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imaging. Open Vet. J.. 2025; 15(9): 4346-4353. doi:10.5455/OVJ.2025.v15.i9.41 Web Style Delibaş V, Soyguder Z, Goya C. Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imaging. https://www.openveterinaryjournal.com/?mno=254242 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.41 AMA (American Medical Association) Style Delibaş V, Soyguder Z, Goya C. Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imaging. Open Vet. J.. 2025; 15(9): 4346-4353. doi:10.5455/OVJ.2025.v15.i9.41 Vancouver/ICMJE Style Delibaş V, Soyguder Z, Goya C. Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imaging. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4346-4353. doi:10.5455/OVJ.2025.v15.i9.41 Harvard Style Delibaş, V., Soyguder, . Z. & Goya, . C. (2025) Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imaging. Open Vet. J., 15 (9), 4346-4353. doi:10.5455/OVJ.2025.v15.i9.41 Turabian Style Delibaş, Veysel, Zafer Soyguder, and Cemil Goya. 2025. Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imaging. Open Veterinary Journal, 15 (9), 4346-4353. doi:10.5455/OVJ.2025.v15.i9.41 Chicago Style Delibaş, Veysel, Zafer Soyguder, and Cemil Goya. "Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imaging." Open Veterinary Journal 15 (2025), 4346-4353. doi:10.5455/OVJ.2025.v15.i9.41 MLA (The Modern Language Association) Style Delibaş, Veysel, Zafer Soyguder, and Cemil Goya. "Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imaging." Open Veterinary Journal 15.9 (2025), 4346-4353. Print. doi:10.5455/OVJ.2025.v15.i9.41 APA (American Psychological Association) Style Delibaş, V., Soyguder, . Z. & Goya, . C. (2025) Brain asymmetry and morphometric measurements of the encephalon in Van cats by magnetic resonance imaging. Open Veterinary Journal, 15 (9), 4346-4353. doi:10.5455/OVJ.2025.v15.i9.41 |