| Research Article | ||
Open Vet. J.. 2025; 15(8): 3459-3467 Open Veterinary Journal, (2025), Vol. 15(8): 3459-3467 Research Article Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, BanyuwangiAmung Logam Saputro1,2*, Ragil Angga Prastiya1,2, Muhammad Thohawi Elziyad Purnama1,2, Ratih Novita Praja1,2, Wiwik Misaco Yuniarti3, Salipudin Tasil Maslamama4, Azhar Burhanuddin1, Deborah Michelle Immanuela1 and Siti Nur Fadhila11Study Program of Veterinary Medicine, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia 2Animal Biomedical and Conservation Research Group 3Department of Veterinary Clinic, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Agricultural Biotechnology, Faculty of Agriculture, Eskişehir Osmangazi Üniversitesi, Eskişehir, Turkey *Corresponding Author: Amung Logam Saputro. Study Program of Veterinary Medicine, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia. Email: amunglogamsaputro [at] fkh.unair.ac.id Submitted: 22/04/2025 Revised: 01/07/2025 Accepted: 07/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
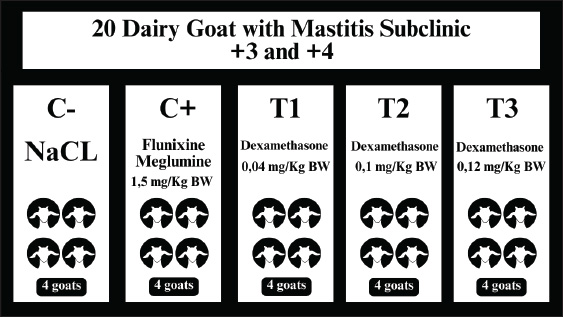
ABSTRACTBackground: Dairy goats are small ruminants with high potential in the livestock industry but are susceptible to mastitis infection, which can reduce milk productivity. Dexamethasone is one therapy used in the treatment of mastitis. Its excessive use can cause oxidative stress, inhibit follicle development, and interfere with reproduction. The stress experienced due to mastitis also increases cortisol hormone levels and decreases LH levels. Aim: This study aimed to evaluate the effect of dexamethasone administration on luteinizing hormone (LH) and cortisol levels in dairy goats with subclinical mastitis. Method: This research is a laboratory experimental study with completely randomized design. A total of 20 dairy goats with subclinical mastitis were divided into five treatment groups. C- group was given physiological NaCl 0.9%; C+ was given Flunixin Meglumine® 1,5 mg/kgBW, whereas the treatment group was given dexamethasone at a dose of 0.04 mg/kgBW (T1), 0.1 mg/kgBW (T2), and 0.12 mg/kgBW (T3) via intramuscular for five consecutive days. Blood samples were taken from the jugular vein on the sixth day. LH and cortisol levels were tested using an enzyme-linked immunosorbent assay. Result: The administration of dexamethasone at a toxic dose of 0.12 mg/kgBW and Flunixin Meglumine® at a therapeutic dose of 50 mg/kgBW significantly reduced LH levels. In addition, statistical analysis with the one-way ANOVA test showed that dexamethasone administration decreased cortisol levels (p < 0.05). Duncan’s post hoc test showed significant differences between treatment groups and negative and positive controls (p < 0.005). Conclusion: Dexamethasone administration to dairy goats with subclinical mastitis can reduce LH and cortisol levels, potentially affecting livestock reproduction and productivity. Keywords: Dairy goats, Subclinical mastitis, Dexamethasone, LH hormone, Cortisol. IntroductionThe Siliragung District in the southern region of Banyuwangi Regency has emerged as a leading area for dairy goat farming. According to data from the Banyuwangi Regency Agriculture and Food Service in 2020, the population of dairy goats reached 3,992 (BPS, 2021; Putri et al., 2021). Goats are highly adaptable and productive compared with other livestock, making them an excellent choice for cultivation in Indonesia (Mardian et al., 2020). Goat milk has less fat than cow milk; therefore, it is a nutritional option for dietary and fat reduction (Radiati et al., 2022). Mastitis or udder inflammation, one of the most common infections, can negatively affect dairy goat productivity (Pribadi et al., 2020). Significant farm economic repercussions because more than 60% milk production which poses (Suwito, 2014). An optimal biosecurity system on a goat farm can minimize the risk of diseases (Putri et al., 2023). Mastitis is inflammation of the mammary glands that can be recognized by physical changes in the udder or alterations in milk secretion due to physical trauma or infectious agents (Pradika et al., 2019). Increased somatic cells in the milk characterize subclinical mastitis without noticeable udder swelling. It is caused by inadequate farming, milking management, and bacterial contamination from feces, bedding, feed, or milking equipment (Sevitasari et al., 2019; Sukoco et al., 2022). This condition impacts the mammary glands and triggers a systemic immune response, leading to endocrine changes that induce stress in the animals and increase cortisol production by the adrenal cortex (Ibrahim et al., 2023). The prevalence of subclinical mastitis in goat farms is approximately 30%, potentially resulting in substantial losses due to decreased milk yield. This disease can only be identified through the California Mastitis Test (CMT), which detects bacterial infections such as Staphylococcus aureus and Escherichia coli in the udder (Suwito, 2014). The prevalence rate of subclinical mastitis has reached 64.51% in the Siliragung District (Faizah et al., 2023). Treatment of inflammation in livestock generally uses anti-inflammatory drugs such as corticosteroids and nonsteroidal anti-inflammatory drugs (NSAIDs) can effectively relieve inflammatory reactions. However, the long-term use of these drugs causes adverse health side effects (Neman et al., 2022). NSAIDs, including flunixin meglumine, function by inhibiting the enzyme cyclooxygenase, thereby blocking the production of inflammatory mediators such as eicosanoids (Knych, 2017). Additionally, NSAID administration during ovulation may disrupt the follicular environment and the dominant follicle’s response to luteinizing hormone (LH) stimulation, potentially impacting oocyte maturity (Von-Wolff et al., 2024). Corticosteroids are commonly used for their capacity to modulate the body’s immune response (Ferrara et al., 2019). Stress in livestock, such as that caused by mastitis, can elevate the production of glucocorticoid hormones, particularly cortisol, which is released during periods of stress. This stress can negatively affect livestock milk production and reproductive performance (Herman et al., 2016). However, treatments often prove ineffective when conducted without veterinary supervision, such as administering medications without a prescription or neglecting to consider the appropriate dosage, a common practice in the field (Panie et al., 2022; Detha et al., 2021). One of the synthetic glucocorticoids with high anti-inflammatory potential is dexamethasone (Restuti, 2014). As an adrenocortical steroid, dexamethasone is used to treat inflammation at a relatively more affordable price than other corticosteroids. However, long-term excessive use can trigger hormonal disorders (Cendekiawan, 2019). Dexamethasone administration in livestock is often given to relieve inflammation due to pathogenic infections, including mastitis, by inhibiting inflammatory mediators and repairing damaged tissue (Sipka et al., 2013). High doses of dexamethasone can cause side effects such as heart damage and stress response disorders (Pratama et al., 2018; Harjanti et al., 2023). Dexamethasone can inhibit ovulation mediated by LH, affecting oocyte production under stressful conditions (Zhang et al., 2022). The cortisol inhibitory effect of dexamethasone has been reported in several animals, including dogs, horses, and buffalo, through a negative feedback mechanism on the hypothalamic–pituitary–adrenal (HPA) axis that suppresses adrenocorticotropic hormone (ACTH) secretion (Abraham et al., 2009; Pessina et al., 2009; Sathya et al., 2018). Decreased cortisol levels due to dexamethasone administration can reduce mastitis-related stress and increase dairy goats’ productivity (Ponchon et al., 2017). Luteinizing hormone plays an important role in gonadal function and works with follicle-stimulating hormone (FSH) to stimulate follicle growth and ovulation. FSH stimulates follicles to produce estrogen, which then triggers the release of LH from the anterior pituitary to trigger ovulation. LH also triggers luteinization of granulosa cells into the corpus luteum, producing progesterone (Raju et al., 2013; Rahmi et al., 2021). Low LH levels can cause reproductive disorders, such as delayed ovulation and repeat breeding (Bhattacharyya and Hafiz, 2009). Based on the described background, it is necessary to conduct research on the effect of dexamethasone administration on cortisol and LH hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi. Until now, no research has been conducted on the effect of dexamethasone on LH levels in inhibiting the rate of ovulation that has the potential to cause repeat breeding in dairy goats with subclinical mastitis in the region. Therefore, this study aimed to evaluate the effect of dexamethasone on cortisol and LH levels to understand its impact on the reproduction of dairy goats with subclinical mastitis. Materials and MethodsEthical approvalAn ethical permit has been issued and approved by ethical certificate No. 2.KEH.133.09.2024 on 23 September 2024 from the Animal Ethics Commission, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya. PreparationThe research materials consisted of BOVIVET CMT Liquid (Kruuse®), paddle, 3 cc tuberculin syringes (Onemed®), 5 cc syringes (Terumo® 103), 75% alcohol, plain vacuum tubes (Vaculab®), dexamethasone (Glucortin®), Flunixin Meglumine®, microcentrifuge tubes, NaCl (Otsuka®), and Venoject needle. The Venoject holder, toolbox, ice gel, and gauge (Rondo®) were used. Mastitis subclinical detectionThe materials needed to obtain goat milk indicated as subclinical mastitis through the CMT test include CMT solution (Kruuse® BOVIVET CMT Liquid), paddle, and 70% alcohol. Milk samples were taken directly from dairy goats with subclinical mastitis that tested positive for +++ and ++++ after the CMT test. CMT collection and milk testing were carried out at the farm using sterile gloves; the goats were standing upright, and the teats were cleaned with 70% alcohol (Artdita et al., 2020). The first milk collection was discarded, and the next milking was collected on a paddle with 1:1 CMT reagent and then mixed by shaking for 10 s until the milk and CMT reagent were mixed for 10 seconds. The reaction of this test is characterized by the presence or absence of changes in the viscosity of the milk in the form of a score, starting from + (no thickened mass), ++ (there is a little thickened mass), +++ (the formation of a thickened mass), and ++++ (the formation of a mass resembling gelatin). Treatment designThis research used 20 female dairy goats with subclinical mastitis infection in the lactation period +++ and ++++ in four farms around the Siliragung area, Banyuwangi. The goats were provided with free access to water and a concentrated diet. Goats were randomly assigned to 5 groups of treatment and great injection treatment daily for 5 days, as shown in Figure 1. Each treatment has 4 goats. T1 was the negative control with injection of 0,03 ml/kg BW of NaCl. T2 is a positive control with injection of Flunixin Meglumine® dosage 1,5 mg/kg BW. T3 received an injection administered dexamethasone at a dose 0,04 mg/kg BW, T4 received an injection administered dexamethasone at a dose 0,1 mg/kg BW, and T5 received injection administered dexamethasone at a dose 0,12 mg/kg BW. Serum collectionBlood sampling for hormone level analysis was performed on the sixth day by taking 5 ml of blood through the jugular vein in the lateral neck that had been cleaned using cotton soaked in 70% alcohol. The collected blood was put into a plain vacutainer blood tube, which did not contain additives, for examination. After being left for 10 minutes at room temperature, the blood was centrifuged at 3,500 rpm for 15 minutes to separate the serum in the Integrated Laboratory 3 of the Faculty of Health, Medicine, and Life Science, Universitas Airlangga, Banyuwangi. LH and cortisol measurementSerum testing occurred at the Satwa Sehat Indonesia Research and Diagnostic Laboratory, Malang. The serum was stored in a cooler box at 8°C. This serum measured cortisol and LH levels using the enzyme-linked immunosorbent assay (ELISA) method. Cortisol levels were examined by competitive ELISA using the Diatek brand ELISA READER type DR-200 BC. LH levels were examined by indirect ELISA using the LABTRON brand ELISA READER type LMPR-A12. The results of both examinations are expressed in ng/ml units. Statistical analysisThe data obtained were analyzed using the SPSS 25 application with a one-way ANOVA test and Duncan’s post hoc test if the data were normally distributed. If the data were not normally distributed, the analysis was performed using the Kruskal–Wallis test and the Mann–Whitney post hoc test to determine significant differences.
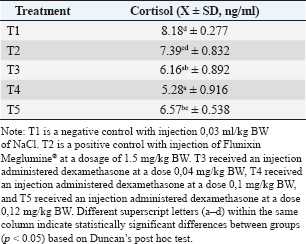
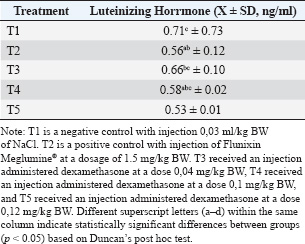
Fig. 1. Treatment design. ResultsThe Shapiro–Wilk normality test results for cortisol and LH indicated that the data were normally distributed, with a significance value of p > 0.05. The post hoc Duncan test results showed that cortisol levels, as shown in Table 1, in the C-group were significantly different (p < 0.05) from the T1, T2, and T3 groups but not significantly different from the C+ group. Cortisol levels in the C+ group were significantly different from T1 and T2 but not from C- and T3. Cortisol levels in T1 were significantly different from C- and C+ but not from T2 and T3. The T2 group showed significant differences from C-, C+, and T3 but not from T1. Finally, the T3 group exhibited significantly different cortisol levels from C- and T2 but not from C+ and T1. The statistical analysis using one-way ANOVA and Duncan’s post hoc test in Table 2 demonstrated that dexamethasone administration significantly affected LH levels in dairy goats with subclinical mastitis (p=0.00; p < 0.05). C- was not significantly different from T1 and T2 but was significantly different from C+ and T3. C+ was not significantly different from T1, T2, or T3 but was significantly different from C-. T1 was not significantly different from C-, C+, and T2 but was significantly different from T3. T2 showed no significant difference with C-, C+, T1, or T3. T3 was not significantly different from C+ and T2 but significantly different from C-. DiscussionPhysiological saline lacks therapeutic properties to alleviate inflammation or stress. Anti-inflammatory agents can reduce cortisol concentrations by inhibiting pro-inflammatory and pain mediators, thereby minimizing stress associated with mastitis (Dalanezi et al., 2020; Alhussien et al., 2021). A low dose of dexamethasone may not have effectively inhibited the secretion of corticotropin-releasing hormone (CRH) from the hypothalamus and ACTH from the pituitary gland, allowing cortisol synthesis and release from the adrenal cortex to continue, albeit at a lower level (Kageyama et al., 2021). Dexamethasone at the appropriate dose significantly reduced cortisol levels through a negative feedback mechanism on the HPA axis. Dexamethasone inhibits the secretion of CRH from the hypothalamus and ACTH from the pituitary gland, thereby reducing the stimulation of cortisol synthesis and release from the adrenal cortex. Endocrine glucocorticoid changes occur primarily during the early phase of inflammation (Lea et al., 2024). Table 1. Cortisol levels (ng/ml) with a treatment in female dairy goats with subclinical mastitis (n=20).
Administering toxic doses of dexamethasone resulted in higher cortisol levels than low-dose dexamethasone, although the difference was not statistically significant. Toxic doses of dexamethasone produced more pronounced immunosuppressive effects and cortisol suppression than flunixin meglumine. Significant cortisol suppression was due to glucocorticoid activity, which directly reduces HPA axis activity (Kleinhenz et al., 2017). Dexamethasone can work optimally when administered at the recommended dosage. Higher doses may increase the risk of toxicity to the body. The main side effects of excessive dexamethasone use include a decrease in white blood cell count, thymus and spleen atrophy, and reduced adrenal gland weight. The effects of toxic-dose dexamethasone may trigger significant stress responses in goats, including excessive activation of the HPA axis (Niu et al., 2018). Dexamethasone suppresses cortisol production through harmful feedback mechanisms. Toxic doses can lead to endocrine system dysregulation, resulting in persistently high cortisol levels (Sic et al., 2024). Table 2. The mean luteinizing hormone levels (ng/ml) with a treatment in female dairy goats with subclinical mastitis (n=20).
As a primary stress indicator, cortisol becomes imbalanced, increasing the animal’s susceptibility to infections and other inflammatory conditions (Shimba and Ikuta, 2020). Dexamethasone can heighten vulnerability to infections due to its residues being detected in body tissues, which may affect the immune system and hormonal balance, especially under conditions of excessive dosing. These effects are predicted to result from the glucocorticoid action of dexamethasone (Chicoine et al., 2024). Glucocorticoids are hormones secreted by the adrenal cortex and released following the activation of the HPA axis. In mammals, glucocorticoids are generally classified as cortisol in ruminants and corticosterone in rodents (Hua et al., 2018). Glucocorticoids are key regulators of metabolism and stress, playing vital roles in various physiological functions, including tissue development, energy balance regulation, immune system modulation, stress adaptation, and behavioral regulation. Modern synthetic glucocorticoids, such as dexamethasone, are structurally based on cortisol and are used alone or in combination with other drugs to treat mastitis, an inflammatory or infectious disease. Glucocorticoids exert their physiological effects by binding to glucocorticoid receptors, which subsequently initiate interactions with DNA or proteins to regulate gene expression (Ma et al., 2023). Activation of the HPA axis in response to physical or psychological stress triggers glucocorticoid secretion. Initial stress signals stimulate the release of CRH and pressor neurons within the paraventricular nucleus of the hypothalamus, which subsequently induce the secretion of ACTH by the anterior pituitary, ultimately leading to the production of adrenocortical glucocorticoids (Mbiydzenyuy and Qulu, 2024). Sustained high levels of endogenous glucocorticoids can have harmful effects; therefore, glucocorticoid-mediated rapid negative feedback regulation is essential to terminate HPA axis activation. The activity of the HPA axis is often attenuated during gestation through autoregulatory mechanisms to prevent the adverse effects of endogenous glucocorticoid secretion on lactation suppression and maternal behavior in animals (Ma et al., 2023). Endogenous glucocorticoid release is part of a generalized stress response to adverse conditions. Milk production typically decreases when animals experience stress, and this acute effect may involve the release of adrenaline (Ortega et al., 2022). Exposure to glucocorticoids, such as dexamethasone, can inhibit the release of Ggonadotropin-Rreleasing Hhormone (GnRH), which affects the function of the hypothalamus and pituitary gland adverse effects on hormone balance given the potential negative impact on reproductive function (Saputro et al., 2025). GnRH is a hormone that stimulates the secretion of LH and FSH, both of which are essential for follicular development and ovulation (Rejeki et al., 2017). Low LH and FSH levels in the body can prevent follicular development. Disruption in the release of FSH and LH may lead to failure in follicle maturation, resulting in fewer growing follicles and an increased rate of follicular atresia. Follicular atresia can occur at any stage of follicular development (Rafian et al., 2023). Follicle maturation failure, which hinders the ovulation process, has also been observed using Flunixin Meglumine®, a drug that has been shown to block ovulation strongly (Vernunft et al., 2023). NSAIDs inhibit cyclooxygenase (COX) enzymes like COX-1 and COX-2, which are involved in converting arachidonic acid into prostaglandins, including the luteolytic hormone Prostaglandin F2α. NSAIDs work by preventing ovulation (Hartinah et al., 2023). The inhibition of ovulation and suppression of LH release is caused by the lack of positive estrogen feedback and negative progestin feedback on the hypothalamus, which can inhibit GnRH. Reduces the secretion of FSH and LH from the anterior pituitary (Adiesti and Wari, 2020). The measurement of LH levels in this study assessed the ovulation rate in dairy goats with subclinical mastitis. The results showed a decrease in LH levels following the administration of 0.12 mg/kg BW of a toxic dose of dexamethasone. Luteinizing hormone plays a key role in stimulating follicular growth and activating ovarian function, leading to the onset of estrus. The early follicular phase is characterized by low LH levels maintained throughout the mid-follicular phase. An LH surge occurs toward the end of the follicular phase and at the beginning of ovulation, causing the dominant follicle to rupture and release an oocyte, a process known as ovulation (Zeleznik and Plant, 2015). A reduction in the number of developing follicles can suppress the response of FSH, which in turn prevents the LH surge, ultimately leading to the failure of ovulation (Danus et al., 2020). Follicle-stimulating hormone and LH are essential hormones that regulate reproductive processes and are synthesized in the pituitary gland. The hypothalamus releases GnRH, which stimulates the anterior pituitary gland to secrete gonadotropins with LH and FSH. In female mammals, GnRH secretion occurs in two modes. Pulsatile secretion regulates the basal release of LH and FSH for follicular development, and surge secretion induces the LH surge necessary for ovulation (Sukarjati and Nugroho, 2021). GnRH secretion that triggers the LH surge is crucial for ovulation and corpus luteum formation. Inflammatory stress is believed to act on the hypothalamic–pituitary–gonadal (HPG) axis, suppressing the release of GnRH. Inflammatory diseases, such as follicular cysts and inactive ovaries, which severely disrupt reproductive efficiency, are often associated with ovarian dysfunction. Follicular cysts are thought to result from the absence of the LH surge, leading to failed ovulation. In contrast, weakened pulsatile LH secretion may cause inactive ovaries (Magata et al., 2023). This study showed a non-significant decrease in the average LH levels in dairy goats with mastitis who were given therapeutic doses of dexamethasone at 0.04 and 0.1 mg/kg BW compared with the control group. The use of glucocorticoids such as dexamethasone at therapeutic doses affects the pituitary gland, resulting in a non-significant reduction in LH levels (Whirledge and Cidlowski, 2010). Glucocorticoids can reduce the GnRH pulse generator center activity. This indicates that the primary site of glucocorticoid inhibition of gonadotropin secretion is at the suprapituitary level, where GnRH release from the hypothalamus is disrupted. Glucocorticoids like dexamethasone decrease the expression of GnRH messenger RNA in the hypothalamus and alter the number of gonadotrope cells (Negic et al., 2007). Easy accessibility has led to the misuse of corticosteroids, often without appropriate indications, proper dosing, or administration duration. Glucocorticoid exposure affects gonadal function at various levels within the HPG axis by reducing the synthesis and release of GnRH in the hypothalamus, inhibiting the synthesis and release of LH and FSH from the pituitary gland, and directly modulating steroidogenesis and/or gametogenesis in the testes or ovaries (Odetayo et al., 2024). Excessive glucocorticoid exposure has been shown to increase the production of reactive oxygen species and reduce mitochondrial activity, leading to oxidative stress (Tang et al., 2009). Oxidative stress is a major factor that directly impacts oocyte quality and limits reproductive outcomes in females across several mammalian species (Prasad et al., 2016). Excessive glucocorticoids, combined with oxidative stress, contribute to female reproductive dysfunction. According to Wagenmaker et al. (2009), the administration of glucocorticoids such as dexamethasone affects estradiol concentrations, resulting in decreased LH levels due to estrogen’s negative feedback on LH secretion. An LH surge is essential for ovulation to occur. Luteinizing hormone-releasing hormone, which depends on estradiol levels, triggers this preovulatory surge (Khaki et al., 2009). Low LH levels in the blood can lead to delayed ovulation or follicular cyst formation. As a result, the follicular phase becomes prolonged, and the luteal phase may be delayed or may not occur at all (Suharyati and Hartono, 2016). This research has practical implications for the treatment of mastitis in dairy goats, particularly in high-prevalence areas, such as Siliragung District, where subclinical mastitis affects over 60% of the population (Faizah et al., 2023). Although dexamethasone offers a cost-effective and potent anti-inflammatory option (Restuti, 2014; Cendekiawan, 2019), its use must be carefully regulated to avoid compromising reproductive health, particularly in the absence of veterinary oversight (Detha et al., 2021; Panie et al., 2022). Although dexamethasone effectively reduces cortisol levels associated with subclinical mastitis, its suppressive effect on LH levels necessitates careful dosage management to prevent long-term reproductive consequences in dairy goats. Further studies are recommended to explore the optimal therapeutic window that maximizes anti-inflammatory benefits while minimizing hormonal disruption. ConclusionBased on the research results, it can be concluded that the administration of dexamethasone at doses of 0.04, 0.1, and 0.12 mg/kg BW reduces cortisol hormone levels, and a dose of 0.12 mg/kg BW can reduce LH levels in these dairy goats suffering from subclinical mastitis in Siliragung District. Conflict of interestThe authors declare that there are no conflicts of interest that might be interpreted as potentially biasing the objectivity of the presented research. FundingFunding: This work was not supported. Authors contributionsALS: Conceptualization, methodology, writing, and reviewing the original draft; DMI: Conceptualization, methodology, and writing the original draft; ALS: Writing—review and editing; RAP: Writing—review and editing; MTEP: visualization; RNP: resources and data curation; WMY: resources, data curation, and review; STM: Editing and review; AB: writing, editing, and review; SNF: Conceptualization, methodology, and writing the original draft; DMI: Conceptualization, methodology, and writing the original draft. Data availabilityAll data are provided in the manuscript. Any extra data needed can be provided upon reasonable request from the corresponding author. ReferencesAbraham, G., Allersmeier, M., Gottschalk, J., Schusser, G. F., Hoppen, H.O. and Ungemach, F.R. 2009. Effects of dermal dexamethasone application on ACTH and both basal and ACTH-stimulated cortisol concentration in normal horses. J. Vet. Pharmacol. Ther. 32(4), 379–387. Alhussien, M.N., Panda, B.S.K. and Dang, A.K., 2021. A comparative study on changes in total and differential milk cell counts, activity, and expression of milk phagocytes of healthy and mastitic indigenous sahiwal cows. Front. Vet. Sci., 8, 1–12. Artdita, C.A., Andityas, M., Prihanani, N.I. and Budiyanto, Y.W. 2020. Bacterial detection causing subclinical mastitis on etawah crossbreed goat in Kokap, Kulon Progo, Yogyakarta Province. J. Sain Vet. 38(1), 37–44. Bhattacharyya, H.K. and Hafiz, A. 2009. Treatment of delayed ovulation in dairy cattle. Indian J. Ani. Res. 43(3), 209–210. BPS 2021. Populasi Ternak Menurut Jenis Ternak dan Kecamatan di Kabupaten Banyuwangi 2020. Available via https://banyuwangikab.bps.go.id/statictable/2021/10/25/205/populasi-ternak-menurut-jenis-ternak-dan-kecamatan-di-kabupaten-banyuwangi-2020.html (Accessed 26 Januari 2025) Cendekiawan, K.A., Winarso, S. and Marchianti, A.C.N. 2019. Surveilans Penyalahgunaan Bahan Kimia Sintetis Deksametason pada Jamu Pegal Linu Menggunakan Metode Near Infra Red dan Kemometrik. Multi. J. 2(1), 30–36. Chicoine, A., Renaud, D.L., Enouri, S.S., Dowling, P. M., Gu, Y. and Johnson, R.J. 2024. Depletion of dexamethasone in cattle: food safety study in dairy and beef cattle. J. Vet. Pharmacol. Ther.. 47(2), 80-86. Dalanezi, F.M., Joaquim, S.F., Cerri, R.L.A., Langoni, H., Guerra, S.T., Lopes, B.C. and Schmidt, E. M. S. 2020. Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J. Dairy Sci.103(4), 3648–3655. Danus, M. and Rusiyantono, Y. 2020. Identifikasi Gangguan Reproduksi Pada Pelaksanaan Inseminasi Buatan Sapi Donggala. Mitra Sains. 8(1), 19–31. Detha, A., Wuri, D.A., Ramos, F., Biru, D., Meha, M.M. and Lakapu, A. 2021. Penggunaan Antibiotik Yang Kurang Tepat Pada Peternakan Babi Di Kota Kupang, Nusa Tenggara Timur. J.Vet. 22(2), 162–167. Faizah, A.N., Setiawan, B., Saputro, A.L., Warsito, S.H., Praja, R.N. and Fikri, F. 2023. Isolation, identification, and risk factors of Staphylococcus aureus bacteria in dairy goat milk with subclinical mastitis in Siliragung District, Banyuwangi Regency. J. Basic Med. Vet. 12(2), 68–72. Ferrara, G., Petrillo, M.G., Giani, T., Marrani, E., Filippeschi, C., Oranges, T., Simonini, G. and Cimaz, R. 2019. Clinical use and molecular action of corticosteroids in the pediatric age. Int. J. Mol. Sci. 20, 444. Harjanti, D.W., Solehah, D.A. and Setiatin, E.T. 2023. Mastitis sebagai Indikator Kesejahteraan Sapi Perah yang Dipelihara secara Zero Grazing di Daerah Tropis. J Agripet. 23(2), 114–120. Herman, J.P., Mcklveen, J.M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., Scheimann, J. and Myers, B., 2016. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol., 6(2), 603–621. Hua, C., Geng, Y., Chen, Q., Niu, L., Cai, L., Tao, S., Ni, Y. and Zhao, R. 2018. Dexamethasone impacts zinc levels in goats by regulating zinc transportation in the colon and the metabolism in the liver. Anim. Sci. J. 89(9), 1296–1301. Ibrahim, N., Regassa, F., Yilma, T. and Tolosa, T. 2023. Impact of subclinical mastitis on uterine health, reproductive performances and hormonal profile of Zebu × Friesian crossbred dairy cows in and around Jimma town dairy farms, Ethiopia. Heliyon. 9(6), E16793. Kageyama, K., Iwasaki, Y. and Daimon, M. 2021. Hypothalamic regulation of corticotropin-releasing factor under stress and stress resilience. Int. J. Mol. Sci. 22, 12242. Khaki, A., Fathiazad, F., Nouri, M., Khaki, A.A., Khamenehi, H.J. and Hamadeh, M. 2009. Evaluation of androgenic activity of Allium Cepa on spermatogenesis in the rat. Folia Morphol. 68(1), 45–51. Knych, H. K. 2017. Nonsteroidal anti-inflammatory drug use in horses. Vet. Clin. North Am. Equine Pract.. 33(1), 1–15. Lea, A.J., Trible, B.R., Grum, D.S., Sewell, A.D., Sewell, J.R. and Raghavendra Rao, M.M. 2024. The development of a dexamethasone challenge model for evaluating feed additives in sheep. Am. J. Vet. Res. 85(10), ajvr.24.02.0035. Ma, X., Liu, H., Jia, Q., Zheng, Y., Li, W., Chang, M., Fu, H. and Zhu, H. 2023. Diverse roles of glucocorticoids in the ruminant mammary gland: modulation of mammary growth, milk production, and mastitis. Stress, 26(1), 2252938. Magata, F., Tsukamura, H. and Matsuda, F. 2023. The impact of inflammatory stress on hypothalamic kisspeptin neurons: mechanisms underlying inflammationassociated infertility in humans and domestic animals. Peptides. 162, 170958. Mardian, N.Z.N., Soeharsono, S., Harijani, N., Budiarto, B., Hermadi, H.A. and Wurlina, W. 2020. Kejadian Mastitis Subklinis pada Kambing Perah Peranakan Etawa di Desa Bangelan Kecamatan Wonosari Kabupaten Malang. Ovozoa J. An. Rep. 9(3), 60–63. Mbiydzenyuy, N.E. and Qulu, L.A. 2024. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab. Brain Dis. 39, 1613–1636. Negić, N., Nestorović, N., Manojlović-Stojanoski, M., Filipović, B., Šošić-Jurjević, B., Trifunović, S., Milošević, V. and Sekulić, M. 2007. Pregnancy and deksametason: effects on morphometric parameters of gonadotropic ells in rats. Acta Histochem. 109(3), 185–192. Neman, A.I., Maarisit, W. and Karauwan, F. 2022. Uji Ekstrak Etanol Daun Benalu Kersen (Dendropthoe Pentrandra L.) Terhadap Tikus Putih (Ratus Norvegicus) Sebagai Anti Inflamasi. Trop. J. Biopharma. 5(1), 55–59. Niu, L., Chen, Q., Hua, C., Geng, Y., Cai, L., Tao, S., Ni, Y. and Zhao, R. 2018. Effects of chronic dexamethasone administration on hyperglycemia and insulin release in goats. J. Anim. Sci. Biotechnol. 9, 26. Odetayo, A.F., Akhigbe, R.E., Bassey, G.E., Hamed, M.A. and Olayaki, L.A. 2024. Impact of stress on male fertility: role of gonadotropin inhibitory hormone. Front. Endocrinol. 14, 1329564. Ortega, J., de Juan, L., Sevilla, I. A., Garrido, J. M., Roy, Á., Velasco, C., Romero, B., Domínguez, M., Pérez de Val, B., Nebot, C., Sáez-Llorente, J. L., Álvarez, J. and Bezos, J. 2022. Effect of a recent parenteral dexamethasone and ketoprofen administration on the immunological diagnosis of tuberculosis in goats. Front. Vet. Sci. 9, 1042428. Panie, P.B.A., Detha, A.I.R. and Wuri, D.A. 2022. Kajian Penggunaan Antibiotik Pada Peternak Babi Di Kabupaten Rote Ndao. J.K.Vet. 10(1), 51–60. Pessina, P., Fernández-Foren, A., Cueto, E., Delucchi, L., Castillo, V. and Meikle, A., 2009. Cortisol secretion after adrenocorticotrophin (ACTH) and deksametason tests in healthy female and male dogs. Acta. Vet. Scand. 51(1), 33. Ponchon, B., Zhao, X., Ollier, S. and Lacasse, P., 2017. Relationship between glucocorticoids and prolactin during mammary gland stimulation in dairy cows. J. Dairy Sci. 100(2), 1521–1534. Pradika, A. Y., Chusniati, S., Purnama, M.T.E., Effendi, M.H., Yudhana, A. and Wibawati, P.A. 2019. Uji Total Escherichia coli Pada Susu Sapi Segar Di Koperasi Peternak Sapi Perah (KPSP) Karyo Ngremboko Kecamatan Purwoharjo Kabupaten Banyuwangi. JMV. 2(1), 1. Pratama, A.P.C., Berata, I.K., Samsuri, S. and Merdana, I.M. 2018. Pengaruh Pemberian Vitamin E dan Deksametason Terhadap Gambaran Histopatologi Jantung Tikus Putih Jantan. Bull. Vet. Udayana. 10(2), 147. Pribadi, A.D., Yudhana, A. and Chusniati, S. 2020. Isolation and identification Streptococcus Sp. from dairy cattle with subclinical mastitis in purwoharjo banyuwangi. JMV. 3(1), 51–56. Putri, L.R.S., Lamid, M., Lokapirnasari, W.P., Saputro, A.L., Wibawati, P.A. and Prastiya, R. A. 2023. Detection Brucella sp. antibody in Sapera (Saanen í— Peranakan Etawa) goat using Rose Bengal Test (RBT) method in Siliragung Subdistrict Banyuwangi Regency. JBMV. 12(2), 53–56. Putri, R.A.A., Tyasningsih, W. and Faisal Fikri. 2021. Uji cemaran Salmonella Sp. pada susu segar kambing sapera di kecamatan siliragung kabupaten Banyuwangi. Proc. Semnas Pemb. Pend. Vok. Pertanian. 2(1), 186–197. Radiati, L.E., Hati, D.L., Andarini, S., Handayani, D. and Rosyidi, D. 2022. Potensi Whey Kefir Susu Kambing Sebagai Anti-Obesitas Melalui Penghambatan Sintesis Lipid Dan Aktivitas Phosphoenolpyruvate Carboxykinase (PEPCK) Pada Sel Model Adiposit 3T3-L1. Indo J. Hum Nutr. 9(2), 207. Rafian, T., Lase, J. A. and Bilyaro, W. 2023. Potensi Hormon Dan Gen Prolaktin Sebagai Metode Seleksi Performa Produksi Telur Itik Lokal Di Indonesia. J. Peternak. Silampari (JPS). 2(2), 59–64. Rahmi, C.A., Harijani, N., Suwarno, S., Budiarto, B., Arif, M.A.A. and Warsito, S.H. 2021. Perbandingan Produksi Dan Kualitas Susu Kambing Peranakan Ettawa Pada Dua Peternakan Yang Berbeda Di Kota Batu Berdasarkan Komposisi Pakan. Ovozoa J. Anim. Reprod. 10(3), 90. Raju, G.A.R., Chavan, R., Deenadayal, M., Gunasheela, D., Gutgutia, R., Haripriya, G., Govindarajan, M., Patel, N.H. and Patki, A.S. 2013. Luteinizing hormone and follicle stimulating hormone synergy: a review of role in controlled ovarian hyper-stimulation. J. Hum. Reprod. Sci. 6(4), 227–234. Restuti, R.D. 2014. Pengaruh Deksametason Terhadap Proliferasi Sel, Kadar IL-Α, Dan TNF-Α Pada Biakan Kolesteatoma. Oto Rhino Laryng. Ind. 44(1), 11–18. Rejeki, R. T., Harjana, T. and Sukiya, S. 2017. Pengaruh Ekstrak Daun Kenari (Canarium indicum, L.) Terhadap Perkembangan Folikel Ovarium Tikus Putih Betina (Rattus norvegicus, L.). Kingdom. J. Bio. Stud. 6(3), 194–203. Prasad, S., Tiwari, M., Pandey, A., Shrivastav, T. and Chaube, S. 2016. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 23, 36. Saputro, A.L., Prastiya, R.A., Purnama, M.T.E., Praja, R.N., Yuniarti, W.M., Maslamama, S.T., Burhanuddin, A., Santoso, D.C.A., Kusuma, E.Z.W., Annisa, J., Praja, S.S., Wijaya, W.A. and Jaladara, W.T. 2025. Characterization of the effect of graded doses of dexamethasone administration on the functional system and maternal prenatal Wistar rat (Rattus Norvegicus). Open Vet. J. 15(5), 1982–1989. Sathya, A., Prabhakar, S. and Ghuman, S P S. 2018. Effect of deksametason administration on cortisol concentration and biochemical profile in buffaloes suffering from dystocia. Anim. Repro. 2(4), 233–239. Sevitasari, A.P., Effendi, M.H. and Wibawati, P. A., 2019. Deteksi mastitis subklinis pada kambing Peranakan Etawah di Kelurahan Kalipuro, Banyuwangi. JMV. 2(2), 72. Shimba, A. and Ikuta, K. 2020. Control of immunity by glucocorticoids in health and disease. Semin. Immunopathol. 42(6), 669–680. Sic, A., Cvetkovic, K., Manchanda, E. and Knezevic, N.N. 2024. Neurobiological implications of chronic stress and metabolic dysregulation in inflammatory bowel diseases. Diseases. 12, 220. Sipka, A., Gurjar, A., Klaessig, S., Duhamel, G.E., Skidmore, A., Swinkels, J., Cox, P. and Schukken, Y. 2013. Prednisolone and cefapirin act synergistically in resolving experimental Escherichia coli mastitis. J. Dairy Sci. 96(7), 4406–4418. Suwito, W., Nugroho, W.S., Sumiarto, B. and Wahyuni, A.E.T.H. 2014. Faktor-faktor risiko mastitis subklinis pada kambing Peranakan Etawah di Kabupaten Sleman, Yogyakarta. J. Vet. 15(1), 130–138. Tang, L., Carey, L. C., Bi, J., Valego, N., Sun, X., Deibel, P., Perot, J., Figueroa, J.P., Chappell, C. M. and Rose, J. C. 2009. Gender differences in the effects of antenatal betamethasone exposure on renal function in adult sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296(2), R309–R317. Vernunft, A., Lapp, R., Viergutz, T. and Weitzel, J. M. 2022. Effects of different cyclooxygenase inhibitors on prostaglandin E2 production, steroidogenesis and ovulation of bovine preovulatory follicles. J. Reprod. Dev. 68(4), 246–253. Von-Wolff, M., Reid, G., Stute, P., Kohl-Schwartz, A.S., Roumet, M. and Fink, A. 2024. Ibuprofen delays ovulation by several hours: a prospective controlled study in spontaneous cycles with Hcg triggered ovulation. Reprod. BioMed. Online. 49(3), 103975. Wagenmaker, E.R., Breen, K.M., Oakley, A.E., Pierce, B.N., Tilbrook, A.J., Turner, A.I. and Karsch, F.J. 2009. Cortisol interferes with the estradiol-induced surge of luteinizing hormone in the ewe. Biol. Reprod. 80(3), 458–463. Whirledge, S. and Cidlowski, J.A. 2010. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 35(2), 109. Zhang, X., Wei, Y., Li, X., Li, C., Zhang, L., Liu, Z., Cao, Y., Li, W., Zhang, X., Zhang, J., Shen, M. and Liu, H. 2022. The corticosterone–glucocorticoid receptor–AP1/CREB axis inhibits the luteinizing hormone receptor expression in mouse granulosa cells. Int. J. Mol. Sci. 23(20), 12454. Zeleznik, A.J., and Plant, T.M. 2015. Control of the Menstrual Cycle. Knobil and Neill’s Physiology of Reproduction. Fourth Edition. Vol. 2. USA. Elsevier Inc. | ||
| How to Cite this Article |
| Pubmed Style Saputro AL, Prastiya RA, Purnama MTE, Praja RN, Yuniarti WM, Maslamama ST, Burhanuddin A, Immanuela DM, Fadhila SN. Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi. Open Vet. J.. 2025; 15(8): 3459-3467. doi:10.5455/OVJ.2025.v15.i8.9 Web Style Saputro AL, Prastiya RA, Purnama MTE, Praja RN, Yuniarti WM, Maslamama ST, Burhanuddin A, Immanuela DM, Fadhila SN. Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi. https://www.openveterinaryjournal.com/?mno=253816 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.9 AMA (American Medical Association) Style Saputro AL, Prastiya RA, Purnama MTE, Praja RN, Yuniarti WM, Maslamama ST, Burhanuddin A, Immanuela DM, Fadhila SN. Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi. Open Vet. J.. 2025; 15(8): 3459-3467. doi:10.5455/OVJ.2025.v15.i8.9 Vancouver/ICMJE Style Saputro AL, Prastiya RA, Purnama MTE, Praja RN, Yuniarti WM, Maslamama ST, Burhanuddin A, Immanuela DM, Fadhila SN. Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3459-3467. doi:10.5455/OVJ.2025.v15.i8.9 Harvard Style Saputro, A. L., Prastiya, . R. A., Purnama, . M. T. E., Praja, . R. N., Yuniarti, . W. M., Maslamama, . S. T., Burhanuddin, . A., Immanuela, . D. M. & Fadhila, . S. N. (2025) Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi. Open Vet. J., 15 (8), 3459-3467. doi:10.5455/OVJ.2025.v15.i8.9 Turabian Style Saputro, Amung Logam, Ragil Angga Prastiya, Muhammad Thohawi Elziyad Purnama, Ratih Novita Praja, Wiwik Misaco Yuniarti, Salipudin Tasil Maslamama, Azhar Burhanuddin, Deborah Michelle Immanuela, and Siti Nur Fadhila. 2025. Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi. Open Veterinary Journal, 15 (8), 3459-3467. doi:10.5455/OVJ.2025.v15.i8.9 Chicago Style Saputro, Amung Logam, Ragil Angga Prastiya, Muhammad Thohawi Elziyad Purnama, Ratih Novita Praja, Wiwik Misaco Yuniarti, Salipudin Tasil Maslamama, Azhar Burhanuddin, Deborah Michelle Immanuela, and Siti Nur Fadhila. "Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi." Open Veterinary Journal 15 (2025), 3459-3467. doi:10.5455/OVJ.2025.v15.i8.9 MLA (The Modern Language Association) Style Saputro, Amung Logam, Ragil Angga Prastiya, Muhammad Thohawi Elziyad Purnama, Ratih Novita Praja, Wiwik Misaco Yuniarti, Salipudin Tasil Maslamama, Azhar Burhanuddin, Deborah Michelle Immanuela, and Siti Nur Fadhila. "Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi." Open Veterinary Journal 15.8 (2025), 3459-3467. Print. doi:10.5455/OVJ.2025.v15.i8.9 APA (American Psychological Association) Style Saputro, A. L., Prastiya, . R. A., Purnama, . M. T. E., Praja, . R. N., Yuniarti, . W. M., Maslamama, . S. T., Burhanuddin, . A., Immanuela, . D. M. & Fadhila, . S. N. (2025) Impact of dexamethasone on cortisol and luteinizing hormone levels in dairy goats with subclinical mastitis in Siliragung District, Banyuwangi. Open Veterinary Journal, 15 (8), 3459-3467. doi:10.5455/OVJ.2025.v15.i8.9 |