| Research Article | ||
Open Vet. J.. 2025; 15(9): 4362-4374
Open Veterinary Journal, (2025), Vol. 15(9): 4362-4374 Research Article Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis miceMoch. Ma’roef1,2, Hendy Hendarto3*, Meddy Setiawan4 and Arifa Mustika51Doctoral Program of Medical Science, Faculty of Medicine, Airlangga University, Surabaya, Indonesia 2Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Muhammadiyah Malang, Malang, Indonesia 3Department of Obstetrics and Gynecology, Faculty of Medicine, Airlangga University, ,Surabaya, Indonesia 4Department of Internal Medicine, Faculty of Medicine, Universitas Muhammadiyah Malang, Malang, Indonesia 5Department of Pharmacology, Faculty of Medicine Airlangga University, Surabaya, Indonesia *Corresponding Author: Hendy Hendarto. Department of Obstetrics and Gynecology, Faculty of Medicine, Airlangga University, Surabaya, Indonesia. Email: hendy.hendarto [at] fk.unair.ac.id Submitted: 22/04/2025 Revised: 27/07/2025 Accepted: 14/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
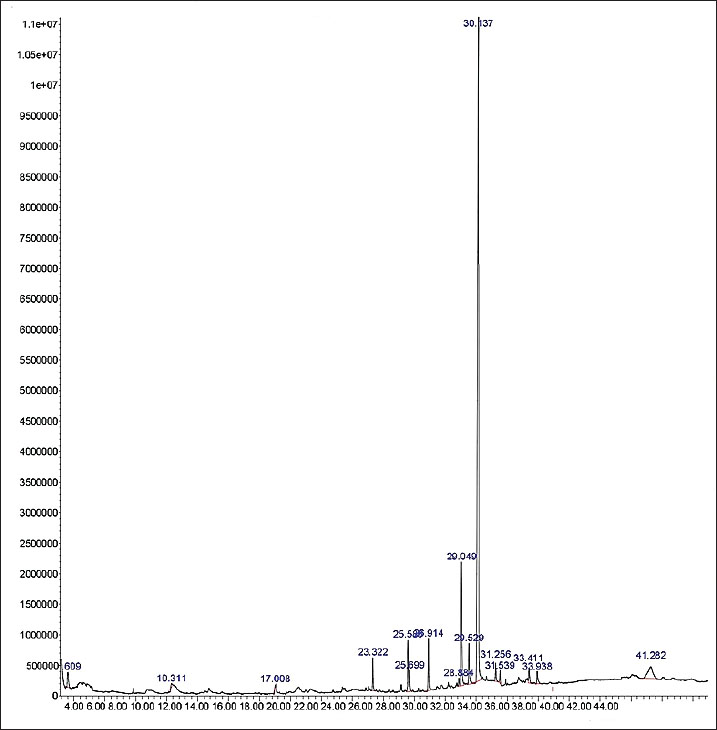
AbstractBackground: Endometriosis can disrupt folliculogenesis due to elevated levels of pro-inflammatory cytokines, free radicals, and the apoptotic response in granulosa cells (GCs). It can cause fertility issues in women. To date, the most effective management strategy to address it has not been identified. The root extract of Saussurea lappa, also known as puck or kush (Indonesia), can reduce tumor necrosis factor (TNF-α) levels, inhibit reactive oxygen species, and prevent apoptosis in GCs, thereby enhancing folliculogenesis. Aim: This study aimed to assess the effects of a 70% ethanol extract of S. lappa on F2-isoprostane (FiSOP), TNF-α, and apoptosis in the GCs of primary, secondary, and tertiary follicles in a mice model of endometriosis, potentially improving folliculogenesis. Methods: Desiccated S. lappa root was transformed into a 70% ethanol extract. Thirty-two mice were utilized to establish the endometriosis model and were divided into four groups: one control group (K1) and three treatment groups administered oral S. lappa extract at doses of 200 (K2), 400 (K3), and 600 (K4) mg/kg. FiSOP levels, TNF-α, and apoptosis in GCs were assessed, along with ovarian follicles (primary, secondary, and tertiary). Results: The research findings indicate (p < 0.05) that the concentrations of FiSOP, TNF-α, and apoptosis in the GCs of primary, secondary, and tertiary follicles diminished, whereas the follicle count augmented in all treatment groups (K2, K3, and K4) administered S. lappa extract compared with the control group (K1). Conclusion: Saussurea lappa reduces the concentrations of FiSOP, TNF-α, and apoptosis in the GCs of primary, secondary, and tertiary follicles, consequently enhancing folliculogenesis, characterized by an increase in the quantity of these follicles in a mice model of endometriosis. Keywords: Endometriosis, Granulosa cells, Folliculogenesis, Saussurea lappa. IntroductionThe presence of endometrial tissue outside the uterine cavity is the defining characteristic of endometriosis. Epidemiological data indicate that 5%–10% of women of reproductive age are afflicted by endometriosis, resulting in various complications, such as discomfort, irregular menstruation, dysmenorrhea, and infertility (Moradi et al., 2021; Harder et al., 2024). Additionally, 75% of these women experience difficulties in conceiving, often due to disruptions in the process of folliculogenesis (Smolarz et al., 2021; Yin et al., 2021; Cano et al., 2024). Seventy-five percent of these women experience difficulties in conceiving, occasionally due to issues with the folliculogenesis process. The pathophysiological mechanisms underlying endometriosis remain ambiguous. Additionally, the peritoneal fluid (PF) exhibits increased concentrations of reactive oxygen species (ROS), interleukin-1β (IL-1β), and tumor necrosis factor (TNF)-α (Oală et al., 2024). This increases the concentration of these chemicals in the ovaries. Increased oxidative stress and lipid peroxidation in follicular fluid can negatively impact oocyte quality and potentially contribute to infertility in endometriosis. The use of F2-isoprostanes (FiSOPs) through immunohistochemical examination can be used as a biomarker for lipid peroxidation and oxidative stress in tissues or cells (Dymanowska et al., 2024; Moretti et al., 2025). The increase in inflammatory cytokines and ROS will accelerate apoptosis in the follicles via both extrinsic and intrinsic routes, thereby disrupting the process of folliculogenesis (Smolarz et al., 2021; Bonavina and Taylor, 2022; Dymanowska et al., 2024). Elevated apoptosis in the ovaries, resulting in follicular abnormalities, contributes to a decline in oocyte quality (Bonavina and Taylor, 2022; Didziokaite et al., 2023). Treatment to address the disruption of the folliculogenesis process in endometriosis has not yet been successful (Dymanowska et al., 2024). Saussurea lappa is a significant medicinal plant commonly employed in traditional medicine in many developing Asian countries. It is used in its entirety, as rhizomes, or as roots. Various chemical constituents, including costunolide, dehydrocostus lactone, and santamarin, are present after extraction (Hassan and Masoodi, 2020; Okubo et al., 2021; Fitrianingsih et al., 2024). Saussurea lappa is a widely used medicinal plant in traditional medicine across Asia. It contains bioactive compounds such as costunolide, dehydrocostus lactone, and santamarin. These compounds are believed to reduce the levels of pro-inflammatory cytokines TNF-α, IL-1β, IL-6), ROS, and caspase-3, thereby suppressing apoptosis and potentially promoting folliculogenesis (Hassan and Masoodi, 2020; Jo et al., 2020; Nadda et al., 2020; Semwal et al., 2020; Abdul et al., 2023; Elshaer et al., 2024). However, the processes and effects of the promotion of folliculogenesis by S. lappa remain unknown, necessitating further investigation for a thorough understanding. This study aimed to elucidate the mechanism by which S. lappa extract influences ovarian follicle growth in a mouse model of endometriosis, with the potential to enhance folliculogenesis in ovaries affected by endometriosis. Materials and MethodsExtract preparationDry roots of S. lappa were obtained from local herbs in Indonesia, and taxonomic identification was performed using the deoxyribose nucleotide acid (DNA) sequencing method in the BRIN laboratory (Indonesian National Research and Innovation Agency) cleaned with running water to remove attached dirt. Samples were dried without being exposed to direct sunlight, cut into small pieces, and ground until they became powder. Then, 200 g of powder was dissolved in 1000 ml of 70% ethanol in a 1,000 ml Erlenmeyer flask, and sonicated with an ultrasonicator for 30 minutes at 45°C, and repeated three times. The sample was filtered using Whattman filter paper, concentrated using a rotary vacuum evaporator at 45°C, and macerated for 5 days at room temperature until it was colorless and a thick extract was obtained (Abubakar and Haque, 2020). Analysis of S. lappa ethanol extract using gas chromatography–mass spectrometryGas chromatography-mass spectrometry (GC-MS) has emerged as an essential analytical technique across various fields, including environmental science, food quality assessment, and pharmaceutical research. Components in the 70% ethanol extract of S. lappa were qualitatively identified based on peak areas from GC-MS analysis with the Agilent 5977B Series MSD (Omer et al., 2019; Mohsen et al., 2022; Sukmawati and Muflihunna, 2024). MethodsThe research methodology used is a true experimental study employing a post-test-only control group design. This study used 32 female mice (Mus musculus, BALB/c strain), aged approximately 12 weeks and weighing between 25 and 30 g, to determine the sample size using the resource equation method. This research was conducted in the research laboratory of the Faculty of Veterinary Medicine at Airlangga University. On the first day, the mice were administered an intramuscular injection of cyclosporine A (Sandimmune; Novartis, Basel, Switzerland) at a dose of 10 mg/kg, along with 5.4 g of estrogen (continued until day 5) at a dose of 5.4 µg. Subsequently, 0.1 ml of an endometrial isolate (human endometrial tissue) was intraperitoneally injected to establish an endometriosis model. The administration of cyclosporine A as an immunosuppressant during the first few days (up to day 5) was intended to provide time for the endometrial tissue to adhere and establish a blood supply before the immune response from the mice begins to work. The immune system of mice that reacts after the initial implantation period was expected to resemble the immune system changes that occur in endometriosis (Burns et al., 2022). On day 14, the endometriosis model was finalized, characterized by the proliferation of endometriotic tissue in the peritoneum (Dwiningsih et al., 2021; Burjiah et al., 2022). This study included four groups: one control group (K1) and three treatment groups (K2, K3, and K4). On day 15, S. lappa extract was orally administered at doses of 200 (K2), 400 (K3), and 600 (K4) mg/kg for 21 days. This dose was obtained from several published studies that have some desired effects, and no toxicity was found in the animal models (Mammate et al., 2023; Elshaer et al., 2024). After treatment completion, the experimental animals underwent ovariectomy, with the specimens preserved in 10% formalin. A visual macroscopic examination was conducted to determine the occurrence of endometriosis in mice, which shows images such as nodules or implants and areas of bleeding or reddish discoloration, found on the peritoneal wall, uterus, and ovaries (Yan et al., 2019; Burns et al., 2022). Immunohistochemistry and TUNEL assay were conducted to assess the concentrations of FiSOP, TNF-α, and apoptosis in granulosa cell (GCs). A visual/manual scoring semi-quantitative method was used to assess the immunohistochemistry (Taylor and Levenson, 2006; Crowe and Yue, 2019). Furthermore, hematoxylin-eosin staining was used to quantify primary, secondary, and transcription factor across all groups. Subsequently, a statistical study was conducted to evaluate the impact of S. lappa extract on folliculogenesis. Ethical approvalThe Research Ethics Committee of the Faculty of Medicine at Airlangga University in Surabaya, Indonesia, approved the experimental protocol no. 2.KEH.010.01.2024. ResultsAll documented data were meticulously analyzed over three trials. Data were analyzed using SPSS version 26.0 and Smart PLS version 3. A one-way analysis of variance (ANOVA) was conducted, followed by a Bonferroni post hoc test to minimize the occurrence of false positives. ANOVA showed a significant difference in this parameter with a p-value of 0.000. The post hoc Bonferroni test indicated that K1 was significantly different from K3 and K4, with a p-value of 0.000, as was the difference between K2 and K3 and K4, with p-values of 0.003 and 0.000, respectively. The best treatment for the reduction of TNF-α, FiSOP, and apoptosis in GCs is K4, which showed the lowest results. The normality test for FiSOP, TNF-α, and apoptosis data indicated a normal distribution (p > 0.05). Furthermore, statistical analysis revealed that S. lappa extract significantly affected the levels of FiSOP, TNF-α, and apoptosis in GCs at all stages of follicle development. The GC/MS analysis of the 70% ethanol extract from the dried root of S. lappa (Fig. 1) showed a 99% concordance in the qualitative profile. The analysis revealed the following compounds: 1. dehydrocostus lactone, 2. dehydrosausurea lactone, 3. beta-cyclodihydrocostunolide, 4. beta-cyclocostunolide, 5. alpha-costol, 6. beta-costol, and 7. Santamarin (Fig. 2). All constituent chemicals belong to the sesquiterpene lactone category.
Fig. 1. GC/MS analysis results of a 70% ethanol extract from S. lappa. The graph shows the GC-MS analysis extract (x-axis: retention time and y-axis: abundance).
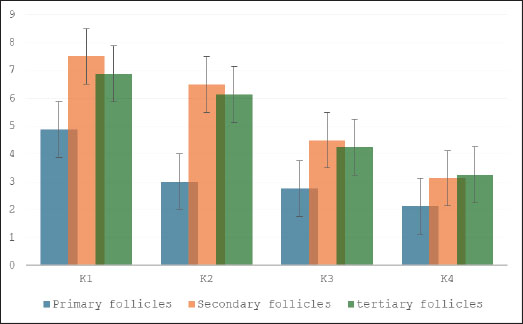
Fig. 2. Chemical structures of the compounds present in the 70% ethanol extract of S. lappa. Comparison of FiSOP levels (Fig. 3) in GCs between the control group (K1) and treatment groups administered with S. lappa 70% ethanol extract at dosages of 200 (K2), 400 (K3), and 600 (K4) mg/kg. The primary follicles in the groups that received 400 (K3) and 600 (K4) mg/kg had lower levels, while the secondary follicles in the 600 mg/kg group (K4) also had lower levels, and the tertiary follicles (Fig. 4) at 400 (K3) and 600 mg/kg (K4) had lower levels compared to the control group (K1).
Fig. 3. Effect of S. lappa extract on FiSOP granulosa cells of primary, secondary, and tertiary follicles. *p < 0.05. Mean ± SD. K-1 (control group), treatment group with 70% ethanol extract of S. lappa: doses K-2 (200 mg/kg), K-3 (400 mg/kg), and K-4 (600 mg/kg). The number of primary, secondary, and tertiary follicles in each group (primary follicles): (K1) 6.25 ± 1.67; (K2) 4.38 ± 2.39; (K3) 3.00 ± 2.00; (K4) 2.75 ± 1.49. Secondary follicles: (K1) 10.88 ± 1.81; (K2) 8.75 ± 1.67; (K3) 5.75 ± 1.98; (K4) 3.25 ± 1.39. Tertiary follicles: (K1) 9.13 ± 1.81; (K2) 8.63 ± 1.69; (K3) 5.50 ± 1.31; (K4) 4.25 ± 1.04.
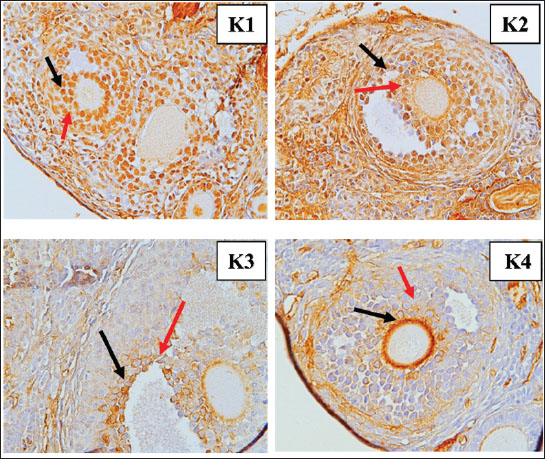
Fig. 4. FiSOP results in the granulosa cells of tertiary follicles, magnifier 400×. Red arrow: positive; black arrow: negative. Figure 5 contrasts the TNF-α levels in GCs from the control group (K1) with those in the treatment groups receiving S. lappa 70% ethanol extract dosages of 200 (K2), 400 (K3), and 600 (K4) mg/kg. The primary follicles in the 600 mg/kg group (K4) exhibited reduced levels, and tertiary follicles (Fig. 6) displayed decreased levels at 200 mg/kg (K2) compared with the control group (K1). The secondary follicles at the 600 mg/kg dosage (K4) demonstrated reduced levels relative to the control group (K1).
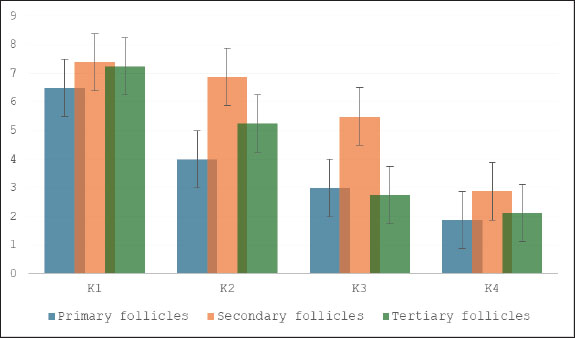
Fig. 5. Effect of S. lappa extract on TNF-α granulosa cells of primary, secondary, and tertiary follicles. *p < 0.05. Mean ± SD. K1 (control group), treatment group with 70% ethanol extract of S. lappa: doses K2 (200 mg/kg), K3 (400 mg/kg), and K4 (600 mg/kg). Number of primary, secondary, and tertiary follicles in each group. Primary follicles: (K1) 4.88 ± 1.46; (K2) 3.00 ± 1.31; (K3) 2.75 ± 1.28; (K4) 2.13 ± 1.13. Secondary follicles: (K1) 7.5 ± 1.2; (K2) 6.50 ± 1.77; (K3) 4.50 ± 1.20; (K4) 3.13 ± 1.46. Tertiary follicles: (K1) 6.88 ± 1.46; (K2) 6.13 ± 1.13; (K3) 4.25 ± 1.39; (K4) 3.25 ± 1.67.
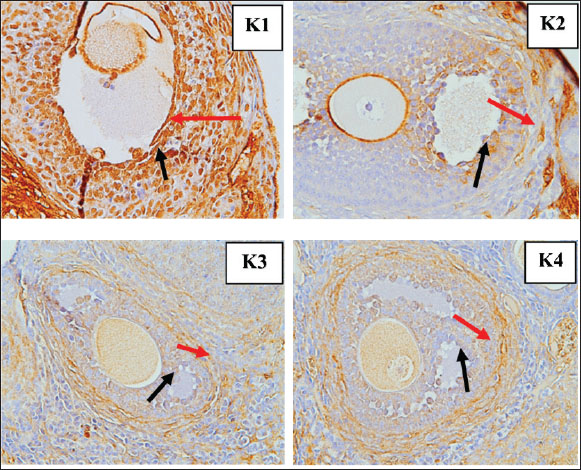
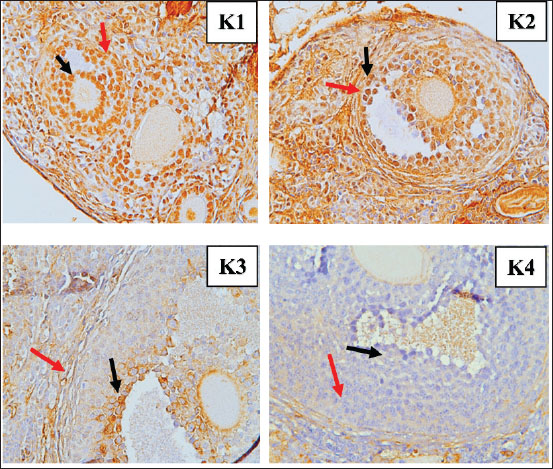
Fig. 6. Immunohistochemistry results TNF-α in the granulosa cells of tertiary follicles, magnifier 400×. Red arrow: positive; black arrow: negative. Apoptosis levels (Fig. 7) in GCs were compared between the control group (K1) and the treatment groups with S. lappa 70% ethanol extract at dosages of 200 (K2), 400 (K3), and 600 (K4) mg/kg. In all treatment groups (K2, K3, and K4) administered with S. lappa extract, the primary and secondary follicles exhibited reduced apoptosis levels compared with the control group (K1). The tertiary follicles (Fig. 8) at the 600 mg/kg dosage (K4) demonstrated reduced levels relative to the control group (K1).
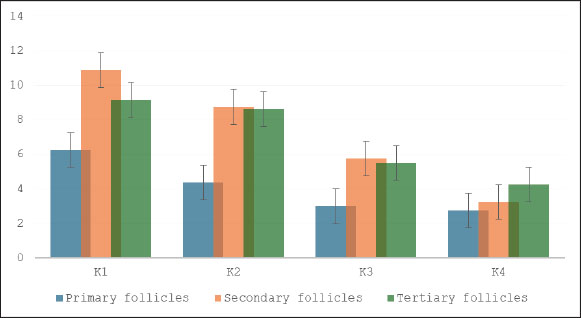
Fig. 7. Effect of S. lappa extract on apoptosis of granulosa cells of primary, secondary, and tertiary follicles. *p < 0.05. Mean ± SD. K1 (control group), treatment group with 70% ethanol extract of S. lappa: doses K2 (200 mg/kg), K3 (400 mg/kg), and K4 (600 mg/kg). Number of primary, secondary, and tertiary follicles in each group. Primary follicles: (K1) 6.50 ± 1.60; (K2) 4.00 ± 1.85; (K3) 3.00 ± 1.85; (K4) 1.88 ± 1.13. Secondary follicles: (K1) 7.38 ± 1.41; (K2) 6.88 ± 1.81; (K3) 5.50 ± 1.31; (K4) 2.88 ± 1.46. Tertiary follicles: (K1) 7.25 ± 1.57; (K2) 5.25 ± 1.28; (K3) 2.75 ± 1.28; (K4) 2.13 ± 1.13.
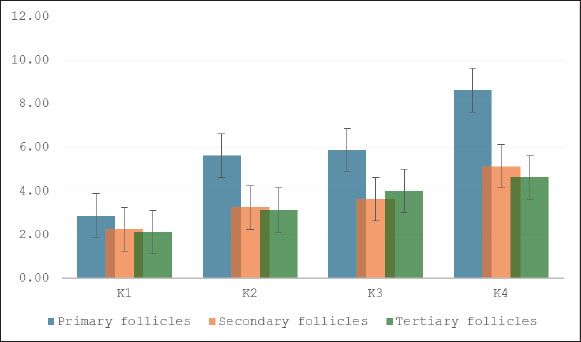
Fig. 8. Tunnel results apoptosis in the granulosa cells of tertiary follicles magnifier 400×. Red arrow: positive; black arrow: negative. The number of primary, secondary, and tertiary follicles (Fig. 9) was higher in all groups that received S. lappa extract (K2, K3, and K4) than in the control group (K1). The treatment group with a dose of 600 mg/kg (K4) had the highest number of primary, secondary, and tertiary follicles compared with the treatment groups receiving doses of 200 (K2) and 400 (K3) mg/kg, as well as the control group (K1).
Fig. 9. Effect of S. lappa extract on the number of primary, secondary, and tertiary follicles. *p < 0.05. Mean ± SD. K-1 (control group), treatment group with 70% ethanol extract of S. lappa: doses K-2 (200 mg/kg), K-3 (400 mg/kg), and K-4 (600 mg/kg). Number of primary, secondary, and tertiary follicles in each group. Primary follicles: (K1) 2.88 ± 1.46; (K2) 5.63 ± 1.06; (K3) 5.88 ± 1.25; (K4) 8.63 ± 1.41. Secondary follicles: (K1) 2.25 ± 1.04; (K2) 3.25 ± 1.04; (K3) 3.63 ± 1.30; (K4) 5.13 ± 1.25. Tertiary follicles: (K1) 2.13 ± 1.13; (K2) 3.13 ± 1.13; (K3) 4.00 ± 1.07; (K4) 4.63 ± 1.19. DiscussionThe pathophysiology of endometriosis is still not fully understood, but increasing evidence suggests that a mix of hormonal, immunological, genetic, and environmental factors play a role in the initiation and progression of endometriosis (Tian et al., 2021; Bonavina and Taylor, 2022; Esmaeilzadeh et al., 2022; Fan et al., 2023; Dymanowska et al., 2024; Oală et al., 2024). Evidence indicates that individuals with endometriosis exhibit atypical immunological and inflammatory responses. Elevated levels of proinflammatory cytokines, such as TNF-α, IL-6, IL-1β, and IL-17A, have been observed in blood serum, PF, and ectopic lesions (Bonavina and Taylor, 2022; Dymanowska et al., 2024; Oală et al., 2024). The GC/MS analysis of S. lappa extract qualitatively identified several sesquiterpene lactone group compounds that have significant therapeutic effects, particularly in relation to anti-inflammatory and oxidative stress inhibition, both of which are crucial for managing various chronic diseases. This inhibition reduces the levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, indicating that it could be useful in treating inflammation-related issues. Sesquiterpene lactones, especially dehydrocostus lactone and costunolide, have anti-inflammatory, antioxidant, and anti-apoptotic effects (Paço et al., 2022). In the follicular fluid of women with endometriosis, vascular endothelial growth factor levels diminish, whereas TNF-α, IL-1, IL-8, IL-6, monocyte chemoattractant protein-1, and endothelin-1, along with various natural killer cells, B lymphocytes, and monocytes, exhibit elevated levels that impair folliculogenesis, resulting in infertility (Prins et al., 2020; Tian et al., 2021; Kacem et al., 2023; Brinca et al., 2024). Heme and iron compounds induce oxidative stress, which significantly contributes to endometriotic lesions during retrograde menstruation. Excessive oxidative stress can induce iron influx that modifies lipids and proteins, resulting in DNA damage and cellular apoptosis. Consequently, it will enhance the apoptosis process in the ovaries, especially within the follicles (Galaris et al., 2019; Yan et al., 2022; Gensluckner et al., 2024). ROS cause mitochondrial membrane depolarization and activate Bax/Bak channels on the outer mitochondrial membrane, promoting the release of cytochrome c and Smac/Diablo into the cytoplasm. Cytochrome c subsequently forms an apoptosome complex with APAF-1 in the cytosol and activates procaspase-9 into caspase-9, which is essential for the conversion of procaspase-3 into caspase-3 (Mustafa et al., 2024; Xu et al., 2024). FiSOPs are recognized as precise indicators of oxidative stress, reflecting the extent of lipid peroxidation in organisms. The measurement method is crucial for comprehending numerous diseases, particularly those resulting from oxidative stress that harms tissues and organs (Saleem et al., 2023; Ahmad et al., 2024). GCs in the primary, secondary, and tertiary follicles exhibited reduced levels of FiSOP upon treatment with a 70% ethanol extract of S. lappa (Fig. 3). The treatment groups with doses of 400 (K3) and 600 (K4) mg/kg had the most pronounced decrease in FiSOP expression compared with the other groups. The impact of oxidative stress is an essential factor in GC apoptosis. An imbalance exists between ROS production and antioxidant defenses, leading to cellular damage. This occurrence is referred to as oxidative stress (Regan et al., 2018). TNF-α will bind to tumor necrosis factor receptor (TNFR-1), thereby activating the TNFR-associated death domain (TRADD). The construction of the complex, including TNF-α, TNFR-1, and TRADD/Fas-associated protein with death domain (FADD), will trigger nuclear factor kappa-light-chain-enhancer of activated B cells activation. Cellular apoptotic signaling triggered by TNF-α is facilitated by TNFR-1. The engagement of TNFR-1 facilitates the assembly of the intracellular death-inducing signaling complex, which comprises TRADD, FADD, and TNFR-associated factor-1. The complex activates pro-caspase 8 into caspase 8 and influences the permeability of the mitochondrial membrane, resulting in the release of cytochrome c. Caspase 8 cleaves BH3-interacting death (BID) into truncated BID and activates pro-caspase 3 into caspase 3 (Webster et al., 2020; Vachliotis and Polyzos, 2023; Guerrache and Micheau, 2024). GCs in the primary, secondary, and tertiary follicles had reduced TNF-α levels when exposed to a 70% ethanol extract of S. lappa (Fig. 5). The GCs of primary follicles in the treatment group receiving a dosage of 600 mg/kg (K4) had the lowest levels of TNF-α. The GCs of secondary follicles exhibited no significant reduction, whereas the GCs of tertiary follicles demonstrated the lowest levels at 200 mg/kg (K2). GC apoptosis is essential for ovarian function, affecting follicular growth, hormone release, and general reproductive health. Apoptosis, or programed cell death in these cells, is a vital mechanism that can profoundly influence fertility. The mechanisms and conditions that affect apoptosis in GCs, emphasize the complexity of this phenomenon in both healthy and pathological circumstances (Carlberg et al., 2000; Regan et al., 2018). Apoptosis in GCs is a complex process by multiple molecular pathways, including caspases, miRNAs, and signaling systems. Malfunctioning or excessive apoptosis can result in follicular atresia and infertility (Liu et al., 2023). The lowest level of GCs apoptosis was found in primary follicles receiving a dose of 600 mg/kg (K4); secondary follicles showed the lowest levels at doses of 200 (K2) and 400 (K3) mg/kg, while tertiary follicles showed the lowest levels at a dose of 600 mg/kg (K4). During the later stages of folliculogenesis, the oocyte is surrounded by several layers of GCs that differentiate into mural and cumulus GCs. GCs supply nutrients and substances essential for the maturation and developmental competence of the oocyte, while also safeguarding the oocyte from oxidative stress damage through their intrinsic antioxidant system throughout maturation (Cavalcanti et al., 2023). The GC/MS examination was a qualitative analysis. Therefore, to achieve reproducibility, an accurate determination of the material species was required, which was done through a DNA sequencing examination, followed by the preparation of extracts according to the established protocol, and then a qualitative GC/MS examination (Monagas et al., 2022). The extract of S. lappa contains many constituents that can reduce ROS, TNF-α activity, and apoptosis (El-Rahman et al., 2020; Ashry et al., 2022). Active metabolites of S. lappa, including costunolide and dehydrocostus lactone, exhibit anti-inflammatory properties. Sesquiterpene exhibits anti-inflammatory properties in vitro by facilitating the release of nitric oxide (NO) and TNF-α from their complexes, whereas santamarin demonstrates anti-inflammatory effects by promoting the expression of mRNA heme oxygenase, inhibiting NO reduction, which subsequently leads to the production of inducible NO synthase, suppressing cyclooxygenase-2, decreasing prostaglandin E2, and inhibiting the synthesis of TNF-α, IL-1β, and IL-6, which are critical mediators of inflammation (El-Rahman et al., 2020, Liu et al., 2021, Gu et al., 2024, Xu et al., 2024, Vu et al., 2024). The application of S. lappa extract in endometriosis, particularly for enhancing folliculogenesis, remains unavailable. The results of this study are anticipated to facilitate the use of S. lappa extract as a treatment for GC disruption in follicles associated with endometriosis, which impairs follicle development. ConclusionEndometriosis elevates the levels of FiSOP, TNF-α, and apoptosis in ovarian follicle GCs, thereby impairing folliculogenesis. Oral administration of a 70% ethanol extract from dried S. lappa root at doses of 400 (K3) and 600 (K4) mg/kg significantly decreased the levels of FiSOP, TNF-α, and apoptosis in the GCs of primary, secondary, and tertiary follicles, while simultaneously enhancing the follicle count, thus facilitating folliculogenesis. AcknowledgmentThis research is part of the dissertation for the doctoral program at the Faculty of Medicine, Airlangga University. We extend our highest appreciation to the Faculty of Veterinary Medicine, Airlangga University, Indonesia, which has greatly assisted in the implementation of this study. Conflict of interestThe authors declare no conflict of interest. FundingThis study received no specific grant. Authors’ contributionsMoch. Ma’roef, Hendy Hendarto, Meddy Setiawan, and Arifa Mustika were preparing materials, collecting data, and conducting analysis. Moch. Ma’roef wrote the initial draft, and all the authors provided comments on the previous version of the manuscript. All the authors have read and approved the final version of the manuscript. Data availabilityThe data supporting the findings of this study are not publicly available due to sensitivity reasons and can be obtained from the corresponding author upon reasonable request. ReferencesAbdul, M.R., Rahim, S.M. and Saleh, A.H. 2023. Cardioprotective activity of costus root ethanol extract in experimentally-induced hypothyroidism in female albino rats. HAYATI J. Biosciences. 30, 1054–1060; doi:10.4308/hjb.30.6.1054-1060 Abubakar, A.R. and Haque, M. 2020. Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied. Sci. 12(1), 1–10; doi:10.4103/jpbs.jpbs_175_19 Ahmad, S., Yang, W., Orellana, A., Frölich, L., De Rojas, I., Cano, A., Boada, M., Hernández, I., Hausner, L., Harms, A.C., Bakker, M.H.M., Cabrera-Socorro, A., Amin, N., Ramírez, A., Ruiz, A., Van Duijn, C.M. and Hankemeier, T. 2024. Association of oxidative stress and inflammatory metabolites with Alzheimer’s disease cerebrospinal fluid biomarkers in mild cognitive impairment. Alzheimer’s Res. Therapy 16, 171; doi:10.1186/s13195-024-01542-4 Ashry, M., Galal ELSahra, D., Abdel-Wahhab, K.G., Abdelsalam, M.E., Mourad, H.H., El-Bitar, A.M. and Gomaa, H.F. 2022. Saussurea costus extract has anti-inflammatory, antioxidant, and hormonal effects against testicular toxicity induced by oxaliplatin in male albino rats. IJT 16(2), 83–90; Bonavina, G. and Taylor, H.S. 2022. Endometriosis-associated infertility: from pathophysiology to tailored treatment. J. Clin. Exp. Ther. 13, 1020827; doi:10.3389/fendo.20221020827 Brinca, A.T., Peiró, A.M., Evangelio, P.M., Eleno, I., Oliani, A.H., Silva, V., Vicente, L.F., Ramalhinho, A.C. and Gallardo, E. 2024. Follicular fluid and blood monitorization of infertility biomarkers in women with endometriosis. Int. J. Mol. Sci. 25(13), 7177; doi:10.3390/ijms25137177 Burjiah, A., Adi, A. and Widjiati, W. 2022. Vitamin D inhibited endometriosis development in mice model through interleukin 17 modulation. Open Vet. J. 12(6), 956–964; doi:10.5455/OVJ.2022.v12.i6.23 Burns, K.A., Pearson, A.M., Slack, J.L., Por, E.D., Scribner, A.N., Eti, N.A. and Burney, R.O. 2022. Endometriosis in the mouse: challenges and progress toward a ‘best fit’ murine model. Front. Physiol. 12, 806574; doi:10.3389/fphys.2021.806574 Cano-Herrera, G., Salmun Nehmad, S., Ruiz De Chávez Gascón, J., Méndez Vionet, A., Van Tienhoven, X.A., Osorio Martínez, M.F., Muleiro Alvarez, M., Vasco Rivero, M.X., López Torres, M.F., Barroso Valverde, M.J., Noemi Torres, I., Cruz Olascoaga, A., Bautista Gonzalez, M.F., Sarkis Nehme, J.A., Vélez Rodríguez, I., Murguiondo Pérez, R., Salazar, F.E., Sierra Bronzon, A.G., Rivera Rosas, E.G., Carbajal Ocampo, D. and Cabrera Carranco, R. 2024. Endometriosis: a comprehensive analysis of the pathophysiology, treatment, and nutritional aspects, and its repercussions on the quality of life of patients. Biomedicines 12(7), 1476; doi:10.3390/biomedicines12071476 Carlberg, M., Nejaty, J., Fröysa, B., Guan, Y., Söder, O. and Bergqvist, A. 2000. Elevated expression of tumour necrosis factor alpha in cultured granulosa cells from women with endometriosis. Hum. Reprod. 15(6), 1250–1255; doi:10.1093/humrep/15.6.1250 Cavalcanti, G.S., Carvalho, K.C., Ferreira, C.D.S., Chedraui, P., Monteleone, P.A.A., Baracat, E.C. and Soares Júnior, J.M. 2023. Granulosa cells and follicular development: a brief review. Revista da. Associação. Médica . Brasileira. 69(6), doi:10.1590/1806-9282.20230175 Crowe, A.R. and Yue, W. 2019. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio. Protoc. 9(24), 3465; doi:10.21769/BioProtoc.3465 Didziokaite, G., Biliute, G., Gudaite, J. and Kvedariene, V. 2023. Oxidative stress as a potential underlying cause of minimal and mild endometriosis-related infertility. Int. J. Mol. Sci. 24(4), 3809; doi:10.3390/ijms24043809 Dwiningsih, S.R., Darmosoekarto, S., Hendarto, H., Dachlan, E.G., Rantam, F.A., Sunarjo, S., Wiyasa, I.W.A. and Widjiati, W. 2021. Effects of bone marrow mesenchymal stem cell transplantation on tumor necrosis factor-alpha receptor 1 expression, granulosa cell apoptosis, and folliculogenesis repair in endometriosis mouse models. Vet. World 14(7), 1788–1796; doi:10.14202/vetworld.2021.1788-1796 Dymanowska-Dyjak, I., Frankowska, K., Abramiuk, M. and Polak, G. 2024. Oxidative imbalance in endometriosis-related infertility-the therapeutic role of antioxidants. Int. J. Mol. Sci. 25, 6298; doi:10.3390/ijms25126298 El-Rahman, G.I.A., Behairy, A., Elseddawy, N.M., Batiha, G.E., Hozzein, W.N., Khodeer, D.M. and et.al.. 2020. Saussurea lappa ethanolic extract attenuates triamcinolone acetonide-induced pulmonary and splenic tissue damage in rats via modulation of oxidative stress, inflammation, and apoptosis. Antioxidants 8(5), 396; doi:10.3390/antiox9050396 Elshaer, S.E., Hamad, G.M., Sobhy, S.E., Darwish, A.M.G., Baghdadi, H.H., H. Abo Nahas, H., El-Demerdash, F.M., Kabeil, S.S., Altamimi, A.S., Al-Olayan, E. and Alsunbul, M. 2024. Supplementation of Saussurea costus root alleviates sodium nitrite-induced hepatorenal toxicity by modulating metabolic profile, inflammation, and apoptosis. Front. Pharmacol. 15, 1378249; doi:10.3389/fphar.2024.1378249 Esmaeilzadeh, S., Ghorbani, M., Abdolahzadeh, M., Chehrazi, M., Jorsaraei, S.G. and Mirabi, P. 2022. Stages of endometriosis: does it affect oocyte quality, embryo development and fertilization rate?. JBRA. Assist. Reprod. 26(4), 620–626; doi:10.5935/1518-0557.20220051 Fan, W., Yuan, Z., Li, M., Zhang, Y. and Nan, F. 2023. Decreased oocyte quality in patients with endometriosis is closely related to abnormal granulosa cells. Front. Endocrinol. Sec. Reprod. 14, 1226687; doi:10.3389/fendo.2023.1226687 Fitrianingsih, A.A., Santosaningsih, D., Djajalaksana, S., Muti’ah, R., Lusida, M.I., Karyono, S.S. and Prawiro, S.R. 2024. Network pharmacology and In Silico investigation on Saussurea lappa for viral respiratory diseases. TroJ. Nat. Prod. Res. 8(1), 5889–5896.; Galaris, D., Barbouti, A. and Pantopoulos, K. 2019. Iron homeostasis and oxidative stress: an intimate relationship. Biochim. Biophys. Acta Mol. Cell Res. 1866(12), 118535; doi:10.1016/j.bbamcr.2019.118535 Gensluckner, S., Wernly, B., Datz, C. and Aigner, E. 2024. Iron, oxidative stress, and metabolic dysfunction—associated steatotic liver disease. Antioxidants 13(2), 208; doi:10.3390/antiox13020208 Gu, X., Zhou, H., Miao, M., Hu, D., Wang, X., Zhou, J., Teichmann, A.T., Yang, Y. and Wang, C. 2024. Therapeutic potential of natural resources against endometriosis: current advances and future perspectives. Drug Des. Devel. Ther. 21, 3667–3696; doi:10.2147/DDDT.S464910 Guerrache, A. and Micheau, O. 2024. TNF-related apoptosis-inducing ligand: non-apoptotic signalling. Cells 13(6), 521; doi:10.3390/cells13060521 Harder, C., Velho, R.V., Brandes, I., Sehouli, J. and Mechsner, S. 2024. Assessing the true prevalence of endometriosis: a narrative review of literature data. Int. J. Gynecology &. Obstetrics . 167(3), 883–900; doi:10.1002/ijgo.15756 Hassan, R. and Masoodi, M.H. 2020. Saussurea lappa: a comprehensive review on its pharmacological activity and phytochemistry. Curr. Tradit. Med. 6(1), 13–23http://dx.doi.org/; doi: 10.2174/2215083805666190626144909 Jo, H.G., Lee, G.Y., Baek, C.Y., Song, H.S. and Lee, D. 2020. Analgesic and anti-inflammatory effects of Aucklandia lappa root extracts on acetic acid-induced writhing in mice and monosodium iodoacetate-induced osteoarthritis in rats. Plants 10(1), 42; doi:10.3390/plants10010042 Kacem-Berjeb, K., Braham, M., Massoud, C.B., Hannachi, H., Hamdoun, M., Chtourou, S., Debbabi, L., Bouyahia, M., Fadhlaoui, A., Zhioua, F. and Feki, A. 2023. Does endometriosis impact the composition of follicular fluid in IL6 and AMH? A case-control study. J Clin Med. 12(5), 1829; doi:10.3390/jcm12051829 Liu, S., Jia, Y., Meng, S., Luo, Y., Yang, Q. and Pan, Z. 2023. Mechanisms of and potential medications for oxidative stress in ovarian granulosa cells: a review. Int. J. Mol. Sci. 24(11), 9205; doi:10.3390/ijms24119205 Liu, X.N., Li, H.M., Wang, S.P., Zhang, J.Z. and Liu, D.L. 2021. Sesquiterpene lactones of Aucklandia lappa: pharmacology, pharmacokinetics, toxicity, and structure-activity relationship. Chin. Herb. Med. 13(2), 167–176; doi:10.1016/j.chmed.2020.11.005 Mammate, N., El Oumari, F.E., Imtara, H., Belchkar, S., Benjelloun Touimi, G., Al-Zharani, M., A. Rudayni, H., Ahmed Qurtam, A., S. Aleissa, M., A. Nasr, F., M. Noman, O. and Sqalli Houssaini, T. 2023. Anti-struvite, antimicrobial, and anti-inflammatory activities of aqueous and ethanolic extracts of Saussurea costus (Falc) Lipsch Asteraceae. Molecules 28(2), 667; doi:10.3390/molecules28020667 Mohsen, E., El-Far, A.H., Godugu, K., Elsayed, F., Mousa, S.A. and Younis, I.Y. 2022. SPME and solvent-based GC–MS metabolite profiling of Egyptian marketed Saussurea costus (Falc.) Lipsch. concerning its anticancer activity. Phytomed. Plus 2(1), 100209; doi:10.1016/j.phyplu.2021.100209 Monagas, M., Brendler, T., Brinckmann, J., Dentali, S., Gafner, S., Giancaspro, G., Johnson, H., Kababick, J., Ma, C., Oketch-Rabah, H., Pais, P., Sarma, N. and Marles, R. 2022. Understanding plant to extract ratios in botanical extracts. Front. Pharmacol. 13, 981978; doi:10.3389/fphar.2022.981978 Moradi, Y., Shams-Beyranvand, M., Khateri, S., Gharahjeh, S., Tehrani, S., Varse, F., Tiyuri, A. and Najmi, Z. 2021. A systematic review on the prevalence of endometriosis in women. Indian J. Med. Res. 154(3), 446–454. Moretti, E., Signorini, C., Menchiari, S., Liguori, L., Corsaro, R., Gambera, L. and Collodel, G. 2025 Are F2-isoprostanes a better marker of semen lipid peroxidation than MDA in reproductive pathologies with inflammatory basis? Cytokine 188, 156889; HYPERLINK “https://doi.org/10.1016/j.cyto.2025.156889” doi:10.1016/j.cyto.2025.156889 Mustafa, M., Ahmad, R., Tantry, I.Q., Ahmad, W., Siddiqui, S., Alam, M. and Abbas, K. 2024. Apoptosis: a comprehensive overview of signaling pathways, morphological changes, and physiological significance and therapeutic implications. Cells 13(22), 1838; doi:10.3390/cells13221838 Nadda, R.K., Ali, A., Goyal, R.C., Khosla, P.K. and Goyal, R. 2020. Aucklandia costus (Syn. Saussurea costus): ethnopharmacology of an endangered medicinal plant of the himalayan region. J. Ethnopharmacol. 5, 113199; doi:10.1016/j.jep.2020.113199 Oală, I.E., Mitranovici, M.I., Chiorean, D.M., Irimia, T., Crișan, A.I., Melinte, I.M. and et.al.. 2024. Endometriosis and the role of pro-inflammatory and anti-inflammatory cytokines in pathophysiology: a narrative review of the literature. Diagnostics 14(3), 312; doi:10.3390/diagnostics14030312 Okubo, S., Ohta, T., Fujita, H., Shoyama, Y. and Uto, T. 2021. Costunolide and dehydrocostuslactone from Saussurea lappa root inhibit autophagy in hepatocellular carcinoma cells. J. Nat. Med. 75(1), 240–245; doi:10.1007/s11418-020-01462-1 Omer, R.E.E., Koua, F.H.M., Abdelhag, I.M. and Ismail, A.M. 2019. Gas chromatography/mass spectrometry profiling of the costus plant Saussurea lappa (Decne.) C.B. Clarke root extracts and their anti-bacterial activity. J. Appl. Pharm. Sci. 9(05), 73–81; doi:10.7324/JAPS.2019.90509 Paço, A., Brás, T., Santos, J.O., Sampaio, P., Gomes, A.C. and Duarte, M.F. 2022. Anti-inflammatory and immunoregulatory action of sesquiterpene lactones. Molecules 27(3), 1142; doi:10.3390/molecules27031142 Prins, J.R., Marissen, L.M., Scherjon, S.A., Hoek, A. and Cantineau, A.E.P. 2020. Is there an immune modulating role for follicular fluid in endometriosis? a narrative review. Reproduction 159, R45–R54; doi:10.1530/REP-19-0050 Regan, S.L.P., Knight, P.G., Yovich, J.L., Leung, Y., Arfuso, F. and Dharmarajan, A. 2018. Granulosa cell apoptosis in the ovarian follicle-a changing view. Front. Endocrinol. (Lausanne). 9, 61; doi:10.3389/fendo.2018.00061 Saleem, M., Kastner, P., Mehr, P., Milne, G., Ishimwe, J., Park, J., Shibao, C. and Kirabo, A. 2023. Obesity is associated with increased F2-isoprostanes and IL-6 in black women. Endocrines 4(1), 38–54; doi:10.3390/endocrines4010003 Semwal, R.B., Joshi, K., Pandian, A., Badoni, P.P. and Semwal, D.K. 2020. Biological applications and secondary metabolites of Saussurea costus (Falc.) Lipsch. J. Conventional . Knowl. Holistic Health 4, 1–8; doi:10.53517/jckhh.2581-3331.412020201 Smolarz, B., Szyłło, K. and Romanowicz, H. 2021. Endometriosis: epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 22(19), 10554; doi:10.3390/ijms221910554 Sukmawati. and Muflihunna, A. 2024. GC-MS analysis of ethyl acetate fractions of Qust al Hindi (Saussurea lappa) root. Univ. J. Pharm. Res. 9(6), 35–38; doi:10.22270/ujpr.v9i6.1245 Taylor, C.R. and Levenson, R.M. 2006. Quantification of immunohistochemistry—issues concerning methods, utility and semiquantitative assessment II. J. Immunohistochem. 49(4), 411–424; doi:10.1111/j.1365-2559.2006.02513.x Tian, Z., Zhang, Y., Zhang, C., Wang, Y. and Zhu, H.L. 2021. Antral follicle count is reduced in the presence of endometriosis: a systematic review and meta-analysis. Reproductive. Biomed. Online 42(1), 237–247; doi:10.1016/j.rbmo.2020.09.014 Vachliotis, I.D. and Polyzos, S.A. 2023. The role of tumor necrosis factor-alpha in the pathogenesis and treatment of nonalcoholic fatty liver disease. Curr. Obesity. Rep. 12(3), 191–206; doi:10.1007/s13679-023-00519-y Vu, Q.V., Sayama, S., Ando, M. and Kataoka, T. 2024. Sesquiterpene lactones containing an α-methylene-γ-lactone moiety selectively down-regulate the expression of tumor necrosis factor receptor 1 by promoting its ectodomain shedding in human lung adenocarcinoma A549 cells. Molecules 29(8), 1866; doi:10.3390/molecules29081866 Webster, J.D. and Vucic, D. 2020. The balance of TNF mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues, review article. Front. Cell Dev. Biol. 8, 365; doi:10.3389/fcell.2020.00365 Xu, S., Zhang, F., Tao, L., Jiang, Y., Huang, T., Li, Y., Hu, Z., Yang, J., Hao, X. and Yuan, C. 2024. Three rare anti-inflammatory sesquiterpene lactones from Magnolia grandiflora. Chin. J. Nat. Med. 22(3), 265–272; doi:10.1016/S1875-5364(24)60601-1 Yan, D., Liu, X. and Guo, S.W. 2019. The establishment of a mouse model of deep endometriosis. Hum. Reprod. 34(2), 235–247; doi:10.1093/humrep/dey361 Yan, F., Li, K., Xing, W., Dong, M., Yi, M. and Zhang, H. 2022. Role of iron-related oxidative stress and mitochondrial dysfunction in cardiovascular diseases, role of iron-related oxidative stress and mitochondrial dysfunction in cardiovascular diseases. Oxid. Med. Cell. Longev. 2022, 5124553; doi:10.1155/2022/5124553 Yin, Y., Mao, Y., Liu, A., Shu, L., Yuan, C., Cui, Y., Hou, Z. and Liu, J. 2021. Insufficient cumulus expansion and poor oocyte retrieval in endometriosis-related infertile women. Reproductive. Sci. 28(5), 1412–1420; doi:10.1007/s43032-020-00410-4 | ||
| How to Cite this Article |
| Pubmed Style Ma'roef M, Hendarto H, Setiawan M, Mustika A. Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis mice. Open Vet. J.. 2025; 15(9): 4362-4374. doi:10.5455/OVJ.2025.v15.i9.43 Web Style Ma'roef M, Hendarto H, Setiawan M, Mustika A. Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis mice. https://www.openveterinaryjournal.com/?mno=253740 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.43 AMA (American Medical Association) Style Ma'roef M, Hendarto H, Setiawan M, Mustika A. Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis mice. Open Vet. J.. 2025; 15(9): 4362-4374. doi:10.5455/OVJ.2025.v15.i9.43 Vancouver/ICMJE Style Ma'roef M, Hendarto H, Setiawan M, Mustika A. Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis mice. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4362-4374. doi:10.5455/OVJ.2025.v15.i9.43 Harvard Style Ma'roef, M., Hendarto, . H., Setiawan, . M. & Mustika, . A. (2025) Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis mice. Open Vet. J., 15 (9), 4362-4374. doi:10.5455/OVJ.2025.v15.i9.43 Turabian Style Ma'roef, Moch., Hendy Hendarto, Meddy Setiawan, and Arifa Mustika. 2025. Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis mice. Open Veterinary Journal, 15 (9), 4362-4374. doi:10.5455/OVJ.2025.v15.i9.43 Chicago Style Ma'roef, Moch., Hendy Hendarto, Meddy Setiawan, and Arifa Mustika. "Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis mice." Open Veterinary Journal 15 (2025), 4362-4374. doi:10.5455/OVJ.2025.v15.i9.43 MLA (The Modern Language Association) Style Ma'roef, Moch., Hendy Hendarto, Meddy Setiawan, and Arifa Mustika. "Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis mice." Open Veterinary Journal 15.9 (2025), 4362-4374. Print. doi:10.5455/OVJ.2025.v15.i9.43 APA (American Psychological Association) Style Ma'roef, M., Hendarto, . H., Setiawan, . M. & Mustika, . A. (2025) Saussurea lappa enhances folliculogenesis by suppressing F2-isoprostane, TNF-α, and apoptosis in endometriosis mice. Open Veterinary Journal, 15 (9), 4362-4374. doi:10.5455/OVJ.2025.v15.i9.43 |