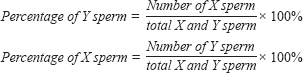| Research Article | ||
Open Vet. J.. 2025; 15(8): 3727-3736 Open Veterinary Journal, (2025), Vol. 15(8): 3727-3736 Research Article Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiencyAthhar Manabi Diansyah1, Aeni Nurlatifah2*, Crista Damaris3, Rahmat Rahmat4, Takdir Saili5, Muhammad Fajar Amrullah1, Andi Muhammad Alfian1, Ahmad Alfaruqi Syahrandi Adam1, Ekayanti Mulyawati Kaiin6 and Erni Damaynti11Faculty of Animal Husbandry, Hasanuddin University, Makassar, Indonesia 2Faculty of Animal Science, Gadjah Mada University, Yogyakarta, Indonesia 3Department of Biology Reproduction, Faculty of Veterinary Science, Gadjah Mada University, Yogyakarta, Indonesia 4Faculty of Agriculture, Lambung Mangkurat University, Banjarbaru, Indonesia 5Department of Animal Husbandry, Faculty of Animal Husbandry, Halu Oleo University, Kendari, Indonesia 6Research Center for Applied Zoology, National Research and Innovation Agency, Cibinong Science Center, Cibinong, Indonesia *Corresponding Author: Aeni Nurlatifah. Faculty of Animal Science, Gadjah Mada University, Depok, Sleman Regency, Special Region of Yogyakarta, Indonesia, 55281. Email: aeninurlatifah [at] ugm.ac.id Submitted: 30/03/2025 Revised: 29/06/2025 Accepted: 09/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Sperm sexing techniques face challenges such as damage to sperm, lower fertility rates, high costs, and technical limitations, which hinder their widespread application... This study evaluates the effectiveness of BSA and albumin column sperm sexing techniques in Garut rams, focusing on separation efficiency, sperm quality, and fertility post-AI. Evaluating alternative methods like the BSA column medium in Garut rams could offers critical insights into the practical application of sexing methods, potentially improving the viability and practicality of sperm sexing in local Indonesian livestock.. Aim: This study aims to evaluated two sperm sexing techniques—Bovine Serum Albumin (BSA) column and albumin column—in Garut rams (Ovis aries), an economically important breed in Indonesia. The research focused on sperm separation efficiency, sperm quality, and fertility performance following artificial insemination (AI). Methods: This study compared BSA and albumin column techniques for sperm sexing in Garut rams. Sperm quality, kinematics, and sexing validation were analyzed using CASA and morphometric analysis. Frozen sexed semen was used for artificial insemination, and pregnancy rates were recorded. Data were analyzed using the Mann-Whitney U test and Pearson correlation to assess differences and relationships among all parameters, with significance set at p < 0.05. Results: BSA medium outperforms Albumin in motility, kinematic stability (Y-sperm), and fertility, though abnormalities and movement patterns (e.g., WOB) require optimization. Pre-sexing Garut ram sperm averaged 70.4% motility, 78.4% viability, and 7.8% abnormalities. Y-sexed sperm in BSA medium showed higher motility than in Albumin, while viability remained stable (74–75%); X-sexed sperm in Albumin had the highest abnormalities (11.2%). Post-sexing, VSL and DCL were significantly higher for Y-sperm in BSA (*p* < 0.05), but other parameters (VCL, VAP) showed no difference. Sperm post-sexing exhibited STR (90.06%) > WOB (81.88%) > LIN (72.72%), with WOB differing significantly for X-sperm between media (p < 0.05). BSA yielded higher fractions of both X- (65.4% vs. 62.8%) and Y-sperm (66.4% vs. 61.6%) than Albumin (p< 0.05). Fertility rates were higher in BSA for X- (62.8% vs. 56.6%) and Y-sperm (63.4% vs. 57.8%) (p< 0.05). Conclusion: The BSA column method is a cost-effective alternative to flow cytometry for sperm sexing in Garut rams, preserving sperm quality and improving fertility. The BSA column method provides small-scale breeders with an affordable and practical approach to sperm sexing, offering reasonable gender accuracy. However, its adoption requires careful consideration of potential trade-offs in sperm quality and fertility rates. While the technique’s simplicity is advantageous, optimal outcomes depend on rigorous sperm quality management and possible supplementation with alternative albumin sources. Keywords: Albumin, BSA, Garut rams, Reproductive efficiency, Sperm sexing IntroductionGarut rams (Ovis aries) is a breed of significant economic value in Indonesia, renowned for its high-quality meat production and cultural importance (Athifa et al. 2022). As one of the key breeds in Indonesia’s livestock sector, Garut rams plays an essential role in meeting both local food security and the cultural heritage of the region (Ibrahim et al. 2019; Sujarwnta et al. 2024). To enhance the productivity and efficiency of Garut sheep breeding, the development and application of advanced reproductive technologies are crucial (Gebreselassi et al. 2020). Among these, sperm sexing has emerged as a pivotal tool in livestock management, offering breeders the ability to control the sex of offspring, which is particularly advantageous for optimizing meat production and milk yields (Yotov et al. 2021). Sperm sexing refers to the process of separating sperm based on their X- or Y-chromosome content, allowing for the production of offspring with the desired sex. In industries like dairy farming, where female offspring are preferred for milk production, and meat production, where male offspring are often more desirable for their superior growth rates, sperm sexing provides a means to optimize herd management and production efficiency (Yotov et al. 2021). Several researchers have performed sperm sexing with various methods and results. Hayakawa et al. (2012), in his study using flow cytometry commonly used by industry for sperm sexing, found that although highly accurate, this method can cause physical and physiological damage to sperm, resulting in reduced fertility compared to conventional methods. Asma-ul-Husna et al. (2017), in her study using Nili-Ravi buffalo bull sperm by swim-up sexing method found that higher sperm recovery rate and better post-thaw quality in sperm fraction containing X chromosome compared to sperm fraction containing Y chromosome. Paitoon et al. (2024), found that magnetic-activated cell sorting (MACS) was effective in producing high quality semen with a high percentage of desirable sex chromosomes with the implication that the Y-enriched fraction showed lower quality than X-enriched semen and conventional semen. Utami et al. (2025) in their research using percoll density gradient centrifugation (PGDC for sexing sperm of Holstein-Friesian bulls found that 20%-65% gradient was more effective in maintaining sperm quality than the 20%-60% gradient, post-sexing motility and kinetic parameters were reduced compared to fresh semen, but still above the threshold for artificial insemination (AI). By utilizing sperm from the sex of choice, breeders can reduce the costs associated with raising less profitable animals, enhancing overall productivity (Quelhas et al. 2023). The process of sperm sexing is influenced by various factors, including technological, biological, and environmental conditions. Among the different methods available, density gradient separation, particularly using Bovine Serum Albumin (BSA) and egg white albumin columns, is one of the most commonly used techniques in livestock breeding. BSA, in particular, is favored for its ability to help separate sperm populations while preserving motility, viability, and overall sperm quality, even though sperm quality may be compromised during the sexing process (Teken et al. 2020; Priyanto et al. 2023). This method exploits the physical differences between X- and Y-chromosome-bearing sperm, particularly in their size and DNA content, with X-bearing sperm typically being larger due to their higher DNA mass (Pozdyshev et al. 2023; Maulana et al. 2022). These differences allow for effective separation using albumin gradients, but questions remain regarding the long-term impact of sperm sexing on fertility outcomes and the functionality of sperm post-separation. Despite being widely used in breeding programs, the efficiency of these methods in maintaining sperm quality and their impact on fertility rates require further investigation. This study aims to directly compare the separation efficiency of BSA and albumin column methods for sperm sexing in Garut rams by quantifying differences in X- and Y-sperm recovery rates and purity, evaluate the impact of each technique on critical sperm quality parameters (motility, viability, abnormalities, and kinematics) to determine optimal sperm integrity preservation, validate post-artificial insemination fertility outcomes of sexed sperm - the first such assessment for this economically important Indonesian breed, develop tailored, cost-effective protocols addressing the specific needs of small-scale Indonesian breeders, and provide evidence-based recommendations to minimize sperm quality trade-offs while maximizing fertility outcomes, ultimately advancing practical reproductive technologies for local livestock improvement.. Matherials and MethodsExperimental DesignThis study was conducted to evaluate sexing sperm techniques using Bovine Serum Albumin (BSA) columns and Albumin columns for sperm separation. Semen samples were collected from 10 Garut rams aged 2-4 years with an average body weight of 40-60 kg, which were kept under standard ram management procedures to ensure the health of the rams.. The animals were sourced from local farmers in the Bogor region, West Java. Semen collection was performed twice a week using an artificial vagina. Only semen samples that met the quality standards specified in the Indonesian National Standard (SNI) 4869-3:2024 were included in this study. Assessments of Sperm Quality and KinematicsSperm quality and kinematics were evaluated based on motility, viability, morphological abnormalities, and kinematic parameters. Sperm motility and kinematics were analyzed using a computer-assisted sperm analyzer (CASA) equipped with the Sperm Vision™ 3.7.8 Program (Minitube®, Tiefenbach, Germany). A total of 10 μL of semen sample was placed on a glass slide, covered with a cover slip, and examined under a microscope at 200× magnification. The assessment included kinematic motility parameters, including total motility percentage (MOT, %), Velocity Curveline (VCL, μm/s), Velocity Average Path (VAP, μm/s), Velocity Straight Line (VSL, μm/s), Distance Curve Line (DCL, μm), Distance Average Path (DAP, μm), Distance Straight Line (DSL, μm), Linearity (LIN, %), Straightness (STR, %), and Wobble (WOB, %) (Diansyah et al. 2025). Sperm viability and abnormalities were assessed using the eosin-nigrosin staining method. A 2 µL semen sample was mixed with 10 µL of eosin-nigrosin stain, homogenized, and smeared onto a glass slide. The smear was dried on a heating plate and examined under a microscope at 400× magnification across 10 fields of view, with a minimum evaluation of 200 spermatozoa cells. Viability assessment was based on membrane integrity, where spermatozoa with color-absorbed heads were classified as non-viable (Nurgina et al. 2024). Semen Sexing MethodsBSA ColumnsThe sexing of semen using Bovine Serum Albumin (BSA) columns is a technique that has been effectively demonstrated by Maulana et al. (2021). Semen samples were diluted in Brackett-Oliphant (BO) medium to achieve a final concentration of 300 × 106 sperm/mL. The modified BSA column method was then applied for sperm separation. BSA columns were prepared in graduated glass tubes, with the bottom layer containing 1 mL of 10% BSA and the upper layer containing 1 mL of 5% BSA in BO medium. Subsequently, 1 mL of diluted semen from each ejaculate was carefully added to the BSA column. The sexing medium was then incubated for 60 minutes in a water bath at 37°C. After incubation, each sperm fraction was collected and centrifuged at 1800 rpm for 10 minutes. The sexed sperm was then loaded into 0.25 mL mini straws, followed by equilibration at 4°C for 2 to 4 hours, pre-freezing for 15 minutes, and storage in liquid nitrogen at -196°C. Albumin ColumnsThe sexing of semen using albumin (egg white albumin) columns follows a modified method based on the studies of Sahiruddin et al. (2020) and Rahmat et al. (2024). Semen was diluted in BO medium at a 1:1 ratio, with a final concentration of 300 × 106 sperm/mL. The separation medium consisted of upper and lower albumin fractions, which were prepared by mixing albumin diluted with BO medium. A gradient was formed by layering 2 mL of 30% albumin in BO medium (bottom layer) and 2 mL of 10% albumin in BO medium (upper layer) in a tube. Subsequently, 1 mL of diluted semen was carefully added to the top of the gradient. The mixture was then incubated for 30 minutes in a water bath at 37°C. After incubation, the upper and bottom fractions were separated and centrifuged at 1800 rpm for 10 minutes. The sexed sperm was then loaded into 0.25 mL mini straws, followed by equilibration at 4°C for 2 to 4 hours, pre-freezing for 15 minutes, and storage in liquid nitrogen at -196°C. Validation of Sperm Sexing Results for X and Y Chromosome SeparationThe validation of X and Y sperm separation was conducted following the method described by Yekti et al. (2024). Morphometric analysis was used to determine the proportion of X- and Y-chromosome-bearing sperm from sexed samples obtained using Bovine Serum Albumin (BSA) columns and Albumin columns. The morphometric test involved measuring the sperm head length and width using Olympus LC Micro software. While morphometric analysis offers several advantages for X and Y sperm separation, including safety and potential accuracy, it faces limitations such as sample variability and lower sorting efficiency compared to flow cytometry. Potential sources of error include sample handling, measurement variability, and biological differences. Further validation and optimization are needed to enhance the reliability of this method (Calvalho et al. 2013; Santolaria et al. 2016; Sharma et al. 2016). Observations were performed on 1,000 spermatozoa from fresh semen samples to establish the natural proportion of X and Y sperm (expected 50:50 ratio). The mean sperm head area ± standard error (SE) was calculated to account for variation within the sample population. Spermatozoa with a head size equal to or larger than the mean head size plus SE were classified as X-chromosome-bearing sperm, whereas those with a head size smaller than the mean head size minus SE were designated as Y-chromosome-bearing sperm.
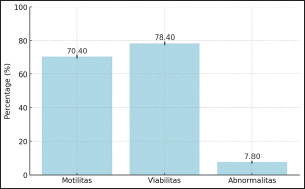
Fertility PerformanceFrozen semen produced from sexed sperm using Bovine Serum Albumin (BSA) and Albumin media was utilized for artificial insemination (AI). The semen freezing process followed the protocols described by Diansyah et al. (2023), while the AI procedure adhered to the guidelines outlined by Prasad et al. (2021). The fertility performance of sexed sperm was evaluated based on the pregnancy rate, defined as the proportion of sheep that became pregnant out of a total of 300 sheep inseminated during each estrous cycle (Mohar et al. 2022). Statistical AnalysisThe observed parameters included sperm quality, sperm kinematics, validation of sperm sexing, and pregnancy rate. Data were reported as mean ± standard deviation (Mean ± SD). Before statistical analysis, all parameter values were tested for normality and homogeneity of variances using the Kolmogorov-Smirnov test. Since the data did not follow a normal distribution, comparisons between sperm sexing methods (BSA and Albumin) were performed using the non-parametric Mann-Whitney U test. Differences were considered statistically significant at p < 0.05. Additionally, Pearson correlation analysis was conducted to evaluate the relationships among all observed parameters, including sperm quality, sperm kinematics, validation of sperm sexing, and pregnancy rate. All statistical analyses were performed using SPSS version 25 for Windows (IBM Corp., Chicago, IL, USA). Ethical ApprovalEthical approval for this research was obtained from the Animal Care and Use Committee of the National Research and Innovation Agency (Reference No. 204/KE.02/SK/11/2023). ResultsSperms Quality and Kinematic SeparationThe sperm quality before the sexing of Garut rams, including motility, viability, and sperm abnormality were presented in Figure 1. The average sperm motility, sperm viability, and sperm abnormality were 70.40%, 78.40%, and 7.80%, respectively. All sperm quality is an initial test before further semen processing treatment is carried out. After the sexing of Garut rams, the sperm quality was presented in Figure 2..The average sperm motility of Garut rams after sexing is higher in Y-sexed sperm on the BSA medium than in Y-sexed sperm on the Albumin medium. Furthermore, the viability of Garut rams sperm after sexing in X-sexed and Y-sexed sperm in each medium has an average of 74-75%. The abnormalities of Garut rams sperm after sexing have the highest average in the abnormality of X-sexed sperm on albumin media (11.2%) and the lowest in Y-sexed sperm on albumin media (6.28%). However, sperm motility, sperm viability, and sperm abnormality of X-sexed and Y-sexed between BSA and Albumin medium showed no significant difference (p>0.05).
Figure 1: Sperms Quality Before Sexing of Garut Rams
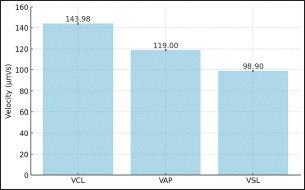
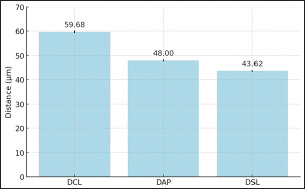
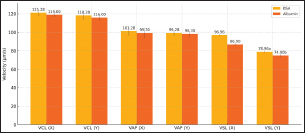
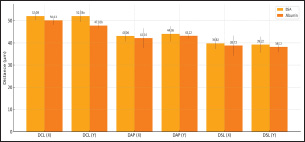
Figure 2: Sperms Quality After Sexing of Garut Rams The sperm kinematics of Garut rams before sexing, including velocity Figure 3. and distance Figure 4. has been presented. These parameters are often measured using computer-assisted sperm analysis (CASA) systems, which provide detailed insights into sperm movement characteristics. Regarding the velocity and distance of Garut rams sperm before sexing, it is higher because there is no effect of using the sexing medium on the sperm. After sexing, the sperm velocity and distance of Garut rams decreased. The mean percentage values of Velocity Curveline (VCL), Velocity Average Path (VAP) of X-sexed and Y-sexed sperm (Figure 5) showed no significant difference (p>0.05) between BSA medium and Albumin medium. On the other, the mean percentage value of Velocity Straight Line (VSL) of X-sexed sperm between BSA medium and Albumin medium (Figure 5). showed no significant difference (p>0.05), while VSL of Y-sexed sperm showed significant (p<0.05). The average percentage value of Distance Curve Line (DCL) (Figure 6) of X-sexed sperm showed no significant difference (p>0.05), while for Y-sexed sperm showed a significant difference (p<0.05) between BSA medium and Albumin medium. The average value of the percentage of Distance Average Path (DAP), Distance Straight Line (DSL) of X-sexed and Y-sexed sperm between BSA medium and Albumin medium (Figure 6). showed no significant difference (p>0.05).
Figure 3: Sperms Velocity Before Sexing of Garut Rams
Figure 4: Sperms Distance Before Sexing of Garut Rams
Figure 5. Sperms Velocity After Sexing of Garut Rams
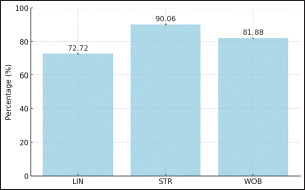
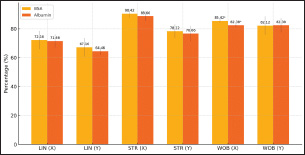
Figure 6. Sperms Distance After Sexing of Garut Rams Furthermore, the sperm movement pattern data of Garut rams before and after sexing are shown in Figure 7 and Figur 8, respectively. Sperm movement pattern data including linearity (LIN), Straightness (STR), and Wobble (WOB). Linearity is calculated as the ratio of straight-line velocity (VSL) to curvilinear velocity (VCL), it indicates how straight the sperm’s path is, with higher values representing more linear movement. Straightness is the ratio of VSL to average path velocity (VAP), it measures the directness of sperm’s trajectory, with higher percentages indicating a more direct path, Wooble is calculated as the ratio of VAP to VCL, it reflects the consistency of the sperm’s movement, with higher values indicating less deviation from the average path. Referring to the sperm movement pattern data, the highest average value is STR (90.06%), followed by the lowest is LIN (72.72%) and WOB (81.88%).
Figure 7: Sperms Motion Pattern Before Sexing of Garut Rams
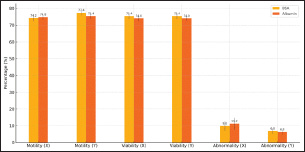
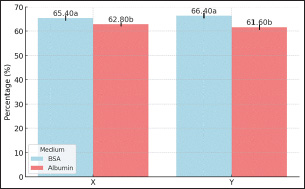
Figure 8: Sperms Motion Pattern After Sexing of Garut Rams The results of statistical analysis of the mean value of sperm movement patterns in WOB sexing results (Figure 8) showed significant differences between sperm sexed on BSA medium and albumin medium in X-sexed sperm (p<0.05). At the same time, statistical analysis of the mean value of sperm movement patterns of LIN, STR in sexed-X and sexed-Y sperm, and WOB of sexed-Y sperm between BSA and Albumin medium did not show significant differences (p>0.05). Validation of X and Y Sperms FractionsMorphometric measurements show that X-sperm are generally larger than Y-sperm, differences in individual morphometric parameters are not always significant in different bulls, but morphometric measurements are still used as a reference in determining the proportion of X-sperm and Y-sperm. Validation of X-sexed sperm and Y-sexed sperm fractions showed (shown in Fig. 9) significant differences (p<0.05) in each sexing medium. X-sexed fraction sperm was higher in BSA medium compared to Albumin medium, it was also the same for Y-sexed fraction sperm. The results indicated that the mean percentage of sexed-X sperm in the treatments ranged between 65.40% and 62.80%, while the percentage of sexed-Y sperm varied between 66.40% and 61.60% in BSA medium and Albumin medium, respectively. X-sexed sperm have larger head morphometry than do Y-sexed sperm.
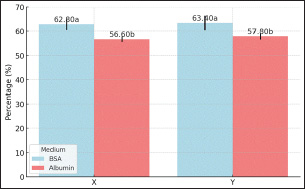
Figure 9: Validation of X and Y Sperms Fraction of Garut Rams; Means with different superscripts differ on the same sperm sexed significantly at p < 0.05. Fertility Performance Post-Artificial InseminationThe timing of AI, reproductive health, semen quality, and environmental factors are critical determinants of pregnancy rates in cows. Optimizing these factors through careful management and monitoring can significantly enhance reproductive success. Fertility performance after artificial insemination resulted (shown in Fig. 10) in varying mean percentage values and showed significant differences (p<0.05). The percentage of fertility performance of sexed-X sperm was higher in BSA medium compared to Albumin medium, 62.80% and 56.60% respectively, the same thing happened to the fertility performance of sexed-Y sperm 63.40% and 57.80% respectively. Knowing the fertility performance of X and Y sperm will provide many benefits that can improve the selection of the right method of sperm sexing. DiscussionGarut rams is highly valued in Indonesia, not only for their exceptional meat production but also for its cultural significance (Rusdiana et al. 2021; Sujarwanta et al. 2024). As an integral part of both the economy and traditional practices, Garut rams plays a vital role in Indonesia’s agriculture and livestock industry (Maulana et al. 2022). Given the growing demand for efficient breeding techniques, sperm sexing technology has become essential in enhancing meat production and milk yields in Garut rams by enabling controlled selection of offspring gender (Rusdiana et al. 2021; Dovenski et al. 2022). This study assessed the efficacy of BSA column and Albumin column methods for sperm sexing in Garut rams, providing valuable insights into their potential for improving the efficiency and productivity of this breed. The quality of sperm is a crucial determinant of successful artificial insemination (AI), as it directly impacts the ability of sperm to reach and fertilize the oocyte. This study found that BSA column was more effective in preserving motility than Albumin column, which is essential for successful fertilization. Although viability did not differ significantly between the two methods (p>0.05), the BSA column better maintained membrane integrity, which is crucial for sperm capacitation and acrosomal reaction during fertilization (Maulana et al. 2022). Utami et al. (2025), concluded that 20%-65% gradient was more effective in maintaining sperm quality, further more Post-sexing motility and kinetic parameters were reduced compared to fresh semen Holstein-Friesian bulls. Nurcholis et al. (2024), has found that 2%:4% Ophiocephalus striatus fish albumin extract (OSAE) concentration produced the highest sperm motility and viability post-thawing, while the 4%:6% concentration was effective for separating X and Y sperm of Brahman cross bulls. In the meantime, Yendraliza et al. (2023), founded that adding up to 0.4% sucrose to Tris-Egg Yolk Diluent maintained motility, viability, and integrity of cell membranes and acrosomes in sexed sperm. Furthermore, kinematics, including progressive velocity (VSL) and curvilinear distance (DCL), were significantly improved in sperm separated using BSA column. This enhanced kinematic performance suggests that sperm separated by BSA column are more efficient at navigating toward and interacting with the oocyte, thereby increasing the likelihood of successful fertilization (Sahiruddin et al. 2021). In contrast, sperm separated by the Albumin column exhibited reduced kinematic performance, likely due to the viscosity of the medium, which increases osmotic stress on sperm, thereby impairing their motility and movement efficiency (Afriani et al. 2022).
Figure 10: Fertility Performance Post-Artificial Insemination Garut Rams; Means with different Sperm morphology also plays a significant role in fertilization success. In this study, BSA column yielded sperm with fewer abnormalities than Albumin column, which is critical for improving fertilization potential. Abnormal sperm morphology can hinder the sperm’s ability to penetrate the oocyte, and previous studies have shown that sperm with morphological defects are less effective at achieving fertilization (Yotov et al., 2021). The lower abnormality rate observed with BSA column can be attributed to its ability to maintain membrane fluidity, which helps prevent morphological damage during the sperm separation process (Ratnawati et al., 2020). BSA protects sperm motility and reduces abnormalities during the sexing process through its roles in energy production protein modifications, oxidative stress reduction, and membrane integrity maintenance (Naijian et al. 2013; Fu et al. 2017; Yeni et al. 2019),. Optimal BSA concentrations and conditions are crucial for maximizing these protective effects. In contrast, Albumin column resulted in higher sperm abnormalities, likely due to the increased osmotic stress associated with the higher viscosity of the separation medium. In terms of reproductive performance, the study showed that sperm separated by BSA column resulted in a higher pregnancy rate post-insemination compared to sperm separated by Albumin column. This finding is consistent with the superior motility and lower abnormalities in sperm from BSA column, which contribute directly to higher fertilization success. The higher pregnancy rates observed with BSA column demonstrate that maintaining high-quality sperm, with better motility, kinematics, and morphology, is essential for improving artificial insemination success in Garut rams (Sahiruddin et al. 2021). While sperm sexing technologies like flow cytometry have been widely adopted in commercial livestock breeding, their high costs—driven by specialized equipment, expensive media, and reduced fertility rates—often render them economically unviable for small-scale farmers, as evidenced by higher per-straw prices, negative economic values due to increased insemination needs, and delayed returns on investment (Seidel. 2014; Jabarzareh et al. 2018;. However, the BSA column method presents a promising alternative, offering lower operational costs and comparable sperm viability (74–75%) and fertility rates (62–63%) for Garut rams, potentially bridging the gap between advanced reproductive technologies and smallholder accessibility. Despite these advantages, challenges remain, including the need for infrastructure improvements, farmer training in semen handling, and financial mechanisms (e.g., subsidies) to offset initial adoption costs—particularly critical given smallholders’ limited willingness to pay (Verma et al. 2020; Gallardo et al. 2024; Fue et al. 2025). Strategic interventions, such as integrating BSA-based sexing into government-supported breeding programs and piloting cost-sharing models, could enhance feasibility, leveraging the high demand for sexed semen (especially female calves in dairy systems) while addressing economic pressures through optimized conception rates and improved herd management practice (Barrientos-Blanco et al. 2018; Pahmeyer and Britz. 2020; Fue et al. 2025). Future efforts should focus on scaling BSA protocols for tropical smallholder conditions, conducting rigorous cost-benefit analyses against flow cytometry, and exploring hybrid models that combine affordability with precision breeding. This study has significant implications for the Indonesian livestock industry, particularly in the breeding of Garut rams, by providing a cost-effective and efficient method for sperm sexing. With the BSA column method, farmers can more easily select offspring gender, whether for more productive ewes for milk production or superior rams for meat production. This technology offers a practical solution for improving reproductive efficiency and productivity in Garut rams, leading to economic benefits and contributing to national food security. The lower cost and ease of use of the BSA column make it an ideal option for small- to medium-scale farmers, especially in regions with limited access to more expensive technologies like flow cytometry (Maia et al. 2020). Furthermore, this study paves the way for further research incorporating omics technologies to deepen the understanding of molecular changes in sperm during the sexing process, enhancing the application of sperm sexing technologies in livestock breeding. Despite the promising results, several limitations of this study should be acknowledged. First, while the BSA column was effective in maintaining sperm quality, it showed slightly lower accuracy in sperm separation compared to flow cytometry, which may be a limitation when high precision in sperm sexing is required. Additionally, the study did not assess the impact of cryopreservation and thawing on the quality of sperm separated by BSA column, which is a critical aspect in long-term storage and successful artificial insemination. Further research is necessary to compare BSA column directly with flow cytometry in terms of fertilization success and to incorporate comprehensive molecular analyses using omics approaches (such as proteomics or genomics) to explore the molecular mechanisms underlying the changes in sperm during the sexing process (Brito et al. 2021). This would provide a more complete understanding of the factors affecting sperm quality and fertility potential ConclusionIn conclusion, this study demonstrates that the BSA column method serves as a practical and cost-effective sperm sexing technique for Garut rams, effectively maintaining sperm motility (>70%), viability (74-75%), and kinematic function while minimizing abnormalities (<8%) and achieving promising fertility rates (62.8-63.4%). The method’s superior performance compared to albumin-based separation and its accessibility for small-scale farmers positions it as a valuable tool for improving breeding efficiency, meat/milk production, and economic returns in Indonesia’s livestock sector. Future research should focus on direct comparisons with flow cytometry for fertilization success rates, incorporation of omics technologies to elucidate molecular changes during sperm sexing, and field trials to optimize practical implementation – advances that could further enhance breeding outcomes and support sustainable agriculture in tropical smallholder systems.. AcknowledgementsThe authors would like to express their appreciation and gratitude to Lembaga Pengelolaan Dana Pendidikan (LPDP) under the Ministry of Finance of the Republic of Indonesia for their financial support of this publication and collaboration. FundingThe authors did not receive support from any organization for the submitted work. Authors ContributionThe study was carried out by AMD, AN, CD, TS, MFA, RR, AMA, AASA, EMK, and ED and all authors contributed equally. In addition, all authors above consented to be held responsible for all parts of the work and partic-ipated in its preparation, drafting, and revision. Conflict of InterestThe authors declared that there is no conflict of interests. Data availabilityAll data are provided in the manuscript. AbbreviationsAI, Artificial Insemination; BO, Brackett-Oliphant; BSA, Bovine Serum Albumin; CASA, Computer-assisted sperm analyzer; DAP, Distance Average Path; DCL, Distance Curve Line; DNA, Deoxyribonucleic Acid; DSL, Distance Straight Line; LIN, Linearity; MOT, Motility; SE, Standard Error; STR, Straightness; VAP, Velocity Average Path; VCL, Velocity Curveline; VSL, Velocity Straight Line; WOB, Wobble. ReferencesAfriani, T., Udin, Z., Hellyward, J., Purwati, E., Rastosari, A., and Wahyudi, D. 2022. Separation of bull spermatozoa bearing x-and y-chromosome by using albumin gradient and swim-up technique in Pesisir cattle. J. Anim. Health Prod. 10(3):337-343. http://dx.doi.org/10.17582/journal.jahp/2022/10.3.337.343. Asma-ul-Husna, Awan, M.A., Mehmood, A., Sultana, T., Shahzad, Q., Ansari, M.S., Rakha, B.A., Saqlan, N.S.M., and Akhter, S. 2017. Sperm sexing in Nili-Ravi buffalo through modified swim up: Validation using SYBR® green real-time PCR. Anim. Repro. Sci. 182:69-76. https://doi.org/10.1016/j.anireprosci.2017.04.011. Athifa, I.R., Sari, A.P.Z.N.L., Maharani, D., Budisatria, I.G.S., Bintara, S., Noorm Y.A.N.G., Hidayat, R., and Panjono, P. 2022. The pre-weaning growth of lambs from crossbreeding between Garut ewes and Dorper rams. Biodiversitas 23(11):5738-5743. http://dx.doi.org/10.13057/biodiv/d231125. Barrientos-Blanco, J.A., Thompson, N.M., Widmar, N.J.O., Wolf, C.A., and Unruh, S.L. 2018. Expected value of crossbred dairy cattle artificial insemination breeding strategies in virgin heifers and lactating cows. Livestoc. Sci. 211:66-74. https//doi.org/10.1016/j.livsci.2018.03.005. Brito, B.F., Santos, B.M.B., Cabral, L.A.R., Maia, L.C.P., Negreiros, N.A.B., Silva, H.V.R., de Resende Matta, M.F., de Mello Salgueiro, C.C., and Nunes, J.F. 2021. Ram and goat semen immunosexed and diluted in powdered coconut water-based preservation medium (ACP101/102c). Acta Sci. Vet. 49:1820. http://dx.doi.org/10.22456/1679-9216.114066. Calvalho, J.O., Silva, L.P., Sartori, R., and Dode, M.A.N. 2013. Nanoscale Differences in the Shape and Size of X and Y Chromosome-Bearing Bovine Sperm Heads Assessed by Atomic Force Microscopy. Plos. One. 8(3): e59387. https://doi.org/10.1371/journal.pone.0059387. Diansyah, A.M., Santoso, S., Herdis, H., Yusuf, M., Priyatno, T.P., Maulana, T., Toleng, A.L., Dagong, M.I.A., Said, S., Iskandar, H., Nurlatifah, A., Lestari, P., Affandy, L., and Baharun, A. 2025 Identification of reproductive performance in Bali-polled bulls using computer-assisted semen analysis and plasma seminal proteomics. Vet. World. 18(1):102-109. http://dx.doi.org/10.14202/vetworld.2025.102-109. Diansyah, A.M., Yusuf, M., Toleng, A.L., Dagong, M.I.A., Maulana, T., Hasrin, H., and Baharun, A. 2023. The sperms post-thawing quality and proteomic seminal plasma on fertility performance of Bali-polled bull. Adv. Anim. Vet. Sci. 11(4):517-525. http://dx.doi.org/10.17582/journal.aavs/2023/11.4.517.525. Dovenski, T., Trojacanec, P., Atanasov, B., Nikolovski, M., Petkov, V., Popovska-Percinic, F., Dovenska, M., Grizelj, J., and Vince, S. 2022. Novelties in ovine assisted reproductive technologies – a review. Maced. Vet. Rev. 45(2):109-125. http://dx.doi.org/10.2478/macvetrev-2022-0018. Fu, J., Li, Y., Wang, L., Zhen, L., Yang, Q., Li, P., and Li, X. 2017. Bovine serum albumin and skim-milk improve boar sperm motility by enhancing energy metabolism and protein modifications during liquid storage at 17 °C. Theriogenology. 102:87-97. https://doi.org/10.1016/j.theriogenology.2017.07.020. Fue, K.G., Baitu, G.P., Jokonya, O., Banwart, S., and Korsten, L. 2025. Digitalization of precision fertilization in East Africa: adoption, benefits and losses. Front. Sust. Food. Sys. 9:1497577. https://doi.org/10.3389/fsufs.2025.1497577. Gallardo, H.Á., Duarte, D.U.A., and Roque, A.V. 2024. Use and evolution of sperm sexing in cattle. Review. Revis. Mexicana. Cien. Pecu. 2024;15(3):641-668. https://doi.org/10.22319/rmcp.v15i3.6372. Gebreselassie, G., Berihulay, H., Jian, L., and Ma, Y. 2020. Review on genomic regions and candidate genes associated with economically important production and reproduction traits in sheep (Ovies aries). Animals 10(1):33. http://dx.doi.org/10.3390/ani10010033. Hayakawa, H. 2012. Sperm sexing in the cattle industry. J. Mamm. Ova. Res. 29(3):119-123. https://doi.org/10.1274/jmor.29.119 Ibrahim, A., Budisatria, I.G.S., Widayanti, R., and Artama, W.T. 2019. Consumer’’s preferences for sheep attributes for Eid al-Adha celebration in Yogyakarta, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 387(1):012001. http://dx.doi.org/10.1088/1755-1315/387/1/012001. Jabarzareh, A., Sadeghi-Sefidmazgi, A., Ghorbani, G., and Cabrera, V. 2018. Economic evaluation of sexed semen use in Iranian dairy farms according to field data. Repro. Dom. Anim. 53(6):1271-1278. https://doi.org/10.1111/rda.13247. Maia. L.C.P., Brito, B.F., Santos, B.M.B., Cabral, L.A.R., and De Mello Salgueiro, C.C. 2020. Economic aspects and evaluation of the viability period of immunosexed and diluted ram sperm in powdered coconut water conservation extender (ACP-102c). Acta Sci. Vet. 48:1740. http://dx.doi.org/10.22456/1679-9216.101058. Maulana, T., Afiati, F., Gunawan, M., and Kaiin, E.M. 2021. Kinematics motility of friesian-holstein sperm sexing in l-ascorbic acid treatments. IOP Conf. Ser. Earth Environ. Sci. 762(1):012081. http://dx.doi.org/0.1088/1755-1315/762/1/012081. Maulana, T., Riwan, R., Gunawan, M., Agung, P.P., Afiati, F., Kaiin, E.M., and Said, S. 2022. Successful separation of X and Y-spermatozoa Ongole Crossbreed using a nano-albumen gradient column. Trop. Anim. Sci. J. 45(4):397-404. http://dx.doi.org10.5398/tasj.2022.45.4.397. Maulana, Y.P., Ramdani, D., Indrijani, H., Yunasaf, U., and Mayasari, N. 2022. Physiological responses, performance, behaviour, and welfare of Garut sheep raised in semi-intensive system in Indonesia. J. Ilmu Ternak Vet. 27(3):130-141. http://dx.doi.org/10.14334/jitv.v27i3.3068. Mohar, J.A.R., Díaz, C.A.L., Cerón, J.H., and Santiago, R.T. 2022. Economic analysis of different pregnancy rates in dairy herds under intensive management. Vet. Méx. OA. 9:1-11. http://dx.doi.org/10.22201/fmvz.24486760e.2022.631. Naijian, H.R., Kohram, H., Shahneh, A.Z., and Sharafi, M. 2013. Effects of various concentrations of BSA on microscopic and oxidative parameters of Mahabadi goat semen following the freeze-thaw process. Small. Rum. Res. 113(2-3):371-375. https://doi.org/10.1016/j.smallrumres.2013.03.015. Nurcholis, N., Salamony, S.M., and Baharun, A. 2024. Quality of frozen semen sexing Brahman cross bovine using Ophiocephalus striatus albumin extract. Tech. Inn. Trop. Livestock. Dev. Env. Sust. Food. Sec. Publisher: CRC Press. 67-74. https://doi.org/10.1201/9781003468943-12 Nurgina, A.T., Kaiin, E.M., Damayanti, E., Baco, S., Herry, S., Gustina, S., and Hasbi H. 2024. Quality of polled Bali bull spermatozoa results from sexing using the bovine serum albumin (BSA) column method. Int. J. Chem. Biochem. Sci. 25(19):990-995. https://doi.org/10.62877/119-IJCBS-24-25-19-119. Pahmeyer, C. and Britz, W. 2020. Economic opportunities of using crossbreeding and sexing in Holstein dairy herds. J. Dairy. Sci. 103(9):8218-8230. https://doi.org/10.3168/jds.2019-17354. Paitoon, P., Sartsook, A., Thongkham, M., Chot, O., Sathanawongs, A., Pattanawong, W., and Sringarm, K. 2024. Evaluation of quality and sex ratio of sperm in post-thaw sexed Brahman semen using magnetic-activated cell sorting conjugated with Y-scFv antibodies (M-Zlex). Vet. Int. Sci. 22(3):1089-1102. https://doi.org/10.12982/VIS.2024.073. Pozdyshev, D.V., Kombarova, N.A., and Muronetz, V.I. 2023. Biochemical features of X or Y chromosome-bearing spermatozoa for sperm sexing. Biochemistry (Moscow). 88(5):655-666. http://dx.doi.org/10.1134/S0006297923050085. Prasad, C.B., Naidu, V.G., Srinivas, M., Raghunath, M., and Kumar, A. 2024. Studies on progesterone concentration and fertility response in postpartum subestrus buffaloes during breeding and low breeding seasons. Indian J. Anim. Res. 58(12):2070-2073. http://dx.doi.org/10.18805/IJAR.B-4702. Priyanto, L., Herdis, H., Santoso, S., Anwar, R.I., Priyatno, T.A.P.I., Sitaresmi, P.I., Azhari, F., Gunawan, M., Putranti, O.D. 2023. The reproductive success of Simmental bovine after sex-sorting under various incubation and centrifugation protocols. Vet. World. 16(3):631-637. http://dx.doi.org/10.14202/vetworld.2023.631-637. Quelhas, J., Pinto-Pinho, P., Lopes, G., Rocha, A., Pinto-Leite, R., Fardilha, M., and Colaco, B. 2023. Sustainable animal production: exploring the benefits of sperm sexing technologies in addressing critical industry challenges. Front. Vet. Sci. 10:1181659. https://doi.org/10.3389/fvets.2023.1181659. Rahmat, R., Yusuf, M., Toleng, A.L., Herdis, H., Diansyah, A.M., and Hasrin, H. 2024. The quality of bali bull sexed sperms using freeze dry albumin at different concentrations of sexing medium. Adv. Anim. Vet. Sci. 12(2):249-258. http://dx.doi.org/10.17582/JOURNAL.AAVS/2024/12.2.249.258. Ratnawati, D., Luthfi, M., Pamungkas, D., and Affandhy, L. 2020. Motility characterization of albumin sexed spermatozoa in two different diluents and additional antioxidant. J. Indonesia Trop. Anim. Agric. 45(4):277-286. http://dx.doi.org/10.14710/jitaa.45.4.277-286. Rusdiana, S., Talib, C., Adiati, U., Kusumaningrum, D.A., Sutedi, E., and Fanindi, A. 2021. The role of Garut agricultural science and technology park in Garut, sheep business supporting community economic development. IOP Conf. Ser. Earth Environ. Sci. 788(1):169971. http://dx.doi.org/10.1088/1755-1315/788/1/012195. Sahiruddin, S., Widjiati, W., Madyawati, S.P., Toleng, A.L., Yusuf, M., and Amrullah, M.F. 2021. The quality of Bali bull sexed sperms at different incubation time using egg white sedimentation method. IOP Conf. Ser. Earth Environ. Sci. 788(1):012142. http://dx.doi.org/10.1088/1755-1315/788/1/012142. Saidel, G.E. 2014. Update on sexed semen technology in cattle. Animal. 8(1):160-164. https://doi.org/10.1017/S1751731114000202. Santolaria, P., Pauciullo, A., Silvestre, M., Vicente-Fiel, S., Villanova, L., Pinton, A., Viruel, J., Sales, E., and Yániz, J. 2016. Computer-assisted sperm morphometry fluorescence-based analysis has potential to determine progeny sex. Asian. J. Andro. 18(6):858-862. https://doi.org/10.4103/1008-682X.187578. Sharma, M. and Sharma, N. 2016. Sperm sexing in animal. Adv. Anim. Vet. Sci. 4(10):543-549. https://doi.org/10.14737/JOURNAL.AAVS/2016/4.10.543.549. Sujarwanta, R.O., Afidah, U., Suryanto, E., Rusman, R., Triyannanto, E., Hoffman, L.C. 2024. Review: Goat and sheep meat production in Indonesia. Sustainability (Switzerland) 16(11):4448. http://dx.doi.org/10.3390/su16114448. Teken, G.E., Yusuf, M., Said, S., and Toleng, A.L. 2020. The quality of sexed sperm separated using bovine serum albumin column and extended using tris aminomethane at different temperatures. IOP Conf. Ser. Earth Environ. Sci. 492(1):012067. http://dx.doi.org/10.1088/1755-1315/492/1/012067. Utami, P., Yekti, A.P.A., Simbolon, C.N.A., Syah, H.A., Amaliya, A., Siswoyo, T.A., Isnaini, N., and Susilawati, T. 2025. Analysis of kinetic parameters of sexed Holstein-Friesian bull spermatozoa using Percoll density gradient centrifugation with computer-assisted sperm analysis. Vet. World. 18(2):287-295. https://doi.org/10.14202/vetworld.2025.287-295. Verma, K.V.S., Garai, S., Maiti, S., Meena, B.S., Bhakat, M., and Kadian, K.S. 2020. Indian dairy farmers’ willingness to pay for sexed semen. J. Dairy. Res. 87(4):406-409. https://doi.org/10.1017/S0022029920001065. Yekti, A.P.A., Arif, A.A., Utami, P., Syah, H.A., Pramudhita, A.D., Rizqianti, R.A., and Susilawati, T. 2024. Quality and proportion of x and y sperm after sexing process using percoll density gradient centrifugation method on different gradients and diluents in belgian blue cross bull. Adv. Anim. Vet. Sci. 12(11):2211-2220. http://dx.doi.org/10.17582/JOURNAL.AAVS/2024/12.11.2211.2220. Yendraliza, Y., Bukhori, A., Erwan, E., Sianturi, R.S.G., and Kusumaningrum, D.A. 2023. The Effectiveness of Adding Different Sucrose in Tris-Egg Yolk Diluent on the Sperm Fertility of Sexing Swamp Buffalo (Bubalus bubalis). J. Trop. Life. Sci. 13(2):383-392. https://doi.org/10.11594/jtls.13.02.17. Yeni, D., Avdatek, F., and Gündoğan, M. 2019. The effect of different concentrations of bovine serum albumin on sperm quality and DNA damage in short term stored ram semen. Ataturk. Univ. Vet. Bilimleri. Dergisi. 14(3):299-306. https://doi.org/10.17094/ataunivbd.594130. Yotov, S., Kistanova, E., Abadjieva, D., Karadaev, M., Ivanova, B., Boney, G., Yarkov, D., and Sinapov, B. 2021. Comparative analysis of motility characteristics and kinematic parameters of fresh, chilled and sexed ram semen – preliminary study. Vet. Zootech. 79(2):37-42. http://dx.doi.org/vetzoo.lsmuni.lt/data/vols/2021/7902/pdf/yotov.pdf. | ||
| How to Cite this Article |
| Pubmed Style Diansyah AM, Nurlatifah A, Damaris C, Rahmat R, Saili T, Amrullah MF, Alfian AM, Adam AAS, Kaiin EM, Damaynti E. Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiency. Open Vet. J.. 2025; 15(8): 3727-3736. doi:10.5455/OVJ.2025.v15.i8.37 Web Style Diansyah AM, Nurlatifah A, Damaris C, Rahmat R, Saili T, Amrullah MF, Alfian AM, Adam AAS, Kaiin EM, Damaynti E. Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiency. https://www.openveterinaryjournal.com/?mno=253682 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.37 AMA (American Medical Association) Style Diansyah AM, Nurlatifah A, Damaris C, Rahmat R, Saili T, Amrullah MF, Alfian AM, Adam AAS, Kaiin EM, Damaynti E. Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiency. Open Vet. J.. 2025; 15(8): 3727-3736. doi:10.5455/OVJ.2025.v15.i8.37 Vancouver/ICMJE Style Diansyah AM, Nurlatifah A, Damaris C, Rahmat R, Saili T, Amrullah MF, Alfian AM, Adam AAS, Kaiin EM, Damaynti E. Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiency. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3727-3736. doi:10.5455/OVJ.2025.v15.i8.37 Harvard Style Diansyah, A. M., Nurlatifah, . A., Damaris, . C., Rahmat, . R., Saili, . T., Amrullah, . M. F., Alfian, . A. M., Adam, . A. A. S., Kaiin, . E. M. & Damaynti, . E. (2025) Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiency. Open Vet. J., 15 (8), 3727-3736. doi:10.5455/OVJ.2025.v15.i8.37 Turabian Style Diansyah, Athhar Manabi, Aeni Nurlatifah, Crista Damaris, Rahmat Rahmat, Takdir Saili, Muhammad Fajar Amrullah, Andi Muhammad Alfian, Ahmad Alfaruqi Syahrandi Adam, Ekayanti Mulyawati Kaiin, and Erni Damaynti. 2025. Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiency. Open Veterinary Journal, 15 (8), 3727-3736. doi:10.5455/OVJ.2025.v15.i8.37 Chicago Style Diansyah, Athhar Manabi, Aeni Nurlatifah, Crista Damaris, Rahmat Rahmat, Takdir Saili, Muhammad Fajar Amrullah, Andi Muhammad Alfian, Ahmad Alfaruqi Syahrandi Adam, Ekayanti Mulyawati Kaiin, and Erni Damaynti. "Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiency." Open Veterinary Journal 15 (2025), 3727-3736. doi:10.5455/OVJ.2025.v15.i8.37 MLA (The Modern Language Association) Style Diansyah, Athhar Manabi, Aeni Nurlatifah, Crista Damaris, Rahmat Rahmat, Takdir Saili, Muhammad Fajar Amrullah, Andi Muhammad Alfian, Ahmad Alfaruqi Syahrandi Adam, Ekayanti Mulyawati Kaiin, and Erni Damaynti. "Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiency." Open Veterinary Journal 15.8 (2025), 3727-3736. Print. doi:10.5455/OVJ.2025.v15.i8.37 APA (American Psychological Association) Style Diansyah, A. M., Nurlatifah, . A., Damaris, . C., Rahmat, . R., Saili, . T., Amrullah, . M. F., Alfian, . A. M., Adam, . A. A. S., Kaiin, . E. M. & Damaynti, . E. (2025) Evaluation of sperm sexing technique using BSA column medium in Garut rams: Enhancing sperm quality, kinematics, and fertility performance for improved breeding efficiency. Open Veterinary Journal, 15 (8), 3727-3736. doi:10.5455/OVJ.2025.v15.i8.37 |