| Research Article | ||
Open Vet. J.. 2025; 15(9): 4375-4381
Open Veterinary Journal, (2025), Vol. 15(9): 4375-4381 Research Article Indication and identification of Helicobacter spp. in the cat stomachFarit M. Nurgaliev1, Oscar K. Pozdeev2, Khamid Khalimovich Gilmanov3, Azat R. Gatiyatullin4, Albina V. Moskvicheva4, Liana R. Nagumanova1, Gueriche Achouak1* and Leysan Shagiakhmatovna Galyavieva11Federal State Budgetary Educational Institution of Higher Education, Kazan State Agrarian University, Kazan, Russia 2Kazan State Medical Academy - Branch Campus of the Federal State Budgetary Educational Institution of Further Professional Education, Russian Medical Academy of Continuous Professional Education, Ministry of Healthcare of the Russian Federation, Kazan, Russia 3Federal State Budget Scientific Institution, Federal Scientific Centre VIEV, Moscow, Russia 4LLC “Zoocity-Life”, Veterinary Clinic, Kazan, Russia *Corresponding Author: Gueriche Achouak. Department of Microbiology, Virology and Immunology, Candidate of Veterinary Sciences, Federal State Budgetary Educational Establishment of Higher Education, “Kazan State Agrarian University”, Kazan, Russia. Email: guericheachouak92 [at] gmail.com Submitted: 21/04/2025 Revised: 30/07/2025 Accepted: 17/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
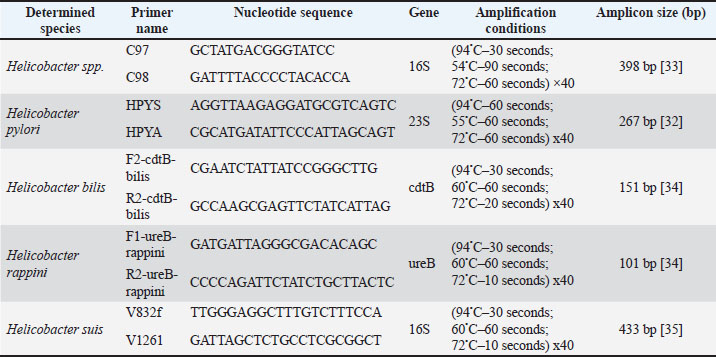
AbstractBackground: Helicobacter species are spiral-shaped gram-negative bacteria that colonize the gastrointestinal tract of humans and animals. Helicobacter pylori are most famous for their role in gastritis, peptic ulcers, and gastric cancer. Their unique adaptation to the harsh acidic environment of the stomach is a key aspect of pathogenicity. Aim: This study aimed to investigate the prevalence of Helicobacter spp. in cats in Kazan and identify the species of these bacteria using molecular genetic methods. Methods: The research is based on endoscopic, microscopic, and molecular genetic methods of investigation. Cats with pronounced clinical signs of gastrointestinal disease were examined. Results: Endoscopic examination revealed ulcers, erosions, and hyperplasia in all cases. Microscopic examination of biopsy samples from these animals identified characteristic spirally shaped Helicobacter spp. in 70% of cases. A noninvasive method for the laboratory diagnosis of Helicobacter infection was used to identify Helicobacter spp. in cats. Fecal samples from cats with gastrointestinal clinical signs and from asymptomatic cats were investigated using polymerase chain reaction method. The deoxyribonucleic acid of Helicobacter spp was present in 61.7% of cases, with the species Helicobacter rappini being the most frequently isolated (51.35%). Additionally, H. pylori was detected in 18.92% of cases and Helicobacter bilis in 45.95%, highlighting the variety of bacterial combinations in the stomachs of cats. Conclusion: Based on the obtained data, this study concluded that there exists an important relationship between Helicobacter spp. and gastrointestinal diseases in cats. Furthermore, this widespread occurrence indicated a high degree of adaptation of these bacteria to this animal species. Keywords: Helicobacter, Cats, Gastritis, Stomach ulcer, PCR. IntroductionResearch on the stomach microflora of domestic animals is still developing at a slow pace, mainly due to the ingrained idea of the stomach as a sterile organ. For a long time, it was believed that the stomach was devoid of microorganisms due to the high concentration of hydrochloric acid, the reflux of bile acids, mucus, and peristalsis, which were assumed to prevent bacterial colonization of this organ (Sheh and Fox, 2013; Teixeira et al., 2022). In recent years, studies have presented evidence that acid-resistant strains of bacteria inhabit the stomachs of cats. Among these bacteria, the most frequently detected bacteria include Streptococcus, Bifidobacterium, Neisseria, and Lactobacillus according to various studies (Alessandri et al., 2020; Wernimant et al., 2020; Makino et al., 2021; Kulikov et al., 2023). Research on the gastric microbiota began following the discovery of Helicobacter pylori and the establishment of its role in the etiology and pathogenesis of gastritis in humans by scientists B. Marshall and R. Warren in 1981 (Bordin et al., 2022; Taillieu et al., 2022b). Helicobacter spp. belong to the proteobacteria class and inhabit the gastrointestinal tract of various mammals, including humans. Helicobacter spp. currently includes 52 officially recognized species, and 16 species that have yet to receive official confirmation, highlighting the diversity of this group of bacteria (Taillieu et al., 2023a, 2023b). These bacteria possess a unique structure that enables them to endure unfavorable living conditions. For instance, in cats, the stomach contains elevated levels of hydrochloric acid (pH=1); however, these acidic conditions do not hinder Helicobacter colonization, indicating that these bacteria have developed adaptations to their surrounding environment (Neiger and Sampson, 2000; Sapashnikov et al., 2023). A number of factors contributed to the colonization and adaptation of Helicobacter spp. in the gastrointestinal environment. Upon entering an environment with low pH, to survive in an acidic environment, these bacteria upregulate the production of enzymes, including urease, formamidase, arginase, and others, which decompose substrates to produce NH4+ and CO2. This process facilitates the formation of an “ammonia cloud” around the microbe with an alkaline pH, neutralizing the acidic environment of the stomach (Vermoote, 2011; Jakhir, 2024). The adhesion of Helicobacter to the epithelial cells of the stomach facilitates the bacterial access to nutrients and secretion of effector molecules, a crucial factor in pathogenicity (Bansil et al., 2023). The motility of bacteria plays a crucial role in the colonization of the gastric mucosa. These bacteria possess a significant advantage in moving through viscous environments like mucus with their flagella and a spiral shape (Zakarenko, 2013; Belyaeva, 2016). Many species of the Helicobacter spp. can cause inflammatory diseases of the gastrointestinal tract in cats, such as chronic gastritis, gastroduodenitis, ulcerative colitis, and other serious diseases (Pozdeev, 2018; Ménard and Smet, 2019; Hlaoperm et al., 2021). These diseases may manifest through clinical signs such as vomiting, diarrhea, altered appetite, anorexia, and weight loss (Rossi et al., 2008; Mladenova et al., 2017; Ochoa and Collado, 2021). A number of researchers report the presence of the following species in cats: H. hepaticus, (Taillieu et al., 2023a, 2023b), H. bilis (Isaeva, 2012), H. rappini (Nurgaliyev, 2012), H. salomonis (Isaeva and Pozdeev, 2007; Taillieu et al., 2022a), H. cinaedi (Fox et al., 2002), H. canis (Erginsoy, 2006), H. bizzozeronii, H. heilmanii, H. felis (Shodieva, 2021), and others (Menard et al., 2002). Helicobacter in cats may pose a health risk to humans, particularly for individuals with weakened immune systems (Taillieu et al., 2022b). These bacterial species can be transmitted from cats to humans, emphasizing the importance of controlling and treating this infection (Fox et al., 1998). This study aimed to investigate the prevalence of Helicobacter spp. in cats in Kazan and identify species of these bacteria using molecular genetic methods. Materials and MethodsIn this study, the operations of a private veterinary clinic in Kazan were investigated. The nosological profile of diseases in cats referred to the clinic for treatment was analyzed. During the clinical examination of the animals admitted to the clinic, 10 cats with pathologies of the digestive system were examined, and gastroscopy was prescribed for diagnosis. Gastrointestinal endoscopy was performed in accordance with scheduled examinations, either in the morning on an empty stomach or no earlier than 6–8 hours after feeding. The procedure was performed by an endoscopist, who performed the manipulation and collected samples. The AOHUA VME-5B gastroscope was equipped with a light source, an instrument channel, and an air supply, along with the necessary manipulators. During the procedure, air was insufflated to enhance gastrointestinal wall visualization and facilitate necessary manipulations. Upon completion of all procedures, air was removed from the lumens of the organs, the gastroscope was withdrawn, and the patient was awoken under the supervision of an anesthetist. During gastroscopy, biopsy samples were taken from the gastric mucosa to assess the degree of gastric mucosal colonization by Helicobacter bacteria in cats. For this purpose, 10 imprint smears were prepared and stained using the Romanowsky method. Four degrees of Helicobacter colonization of the mucosa were distinguished: mild degree (+) – up to 1–2 microbial cells per field of view, moderate degree (++) – from 4 to 10 microbial cells, pronounced degree (+++) – from 10 to 20 microbial cells, and high degree (++++) – from 20 to 30 microbial cells per field of view. Furthermore, a non-invasive method of laboratory diagnosis of helicobacteriosis was used to study and determine the bacteria of the genus Helicobacter in cats, fecal samples were taken. To account for data on the indication and identification of Helicobacter spp., 60 cats were examined and divided into two groups: the first group included 30 cats exhibiting signs of gastrointestinal pathology, whereas the second group consisted of 30 cats without any symptoms of digestive tract diseases. A non-invasive method of laboratory diagnosis of helicobacteriosis was employed for the investigation and identification of Helicobacter bacteria in animals. Molecular genetic indication and identification of Helicobacter species were conducted using the PCR method was applied to fecal samples. DNA extraction from the samples was carried out using a commercial reagent kit produced by “Ampliprime DNA-sorb-B.” Table 1 presents the nucleotide sequences of the primers used, the PCR conditions, and the amplified fragment. Table 1. Primers and PCR conditions used for amplification.
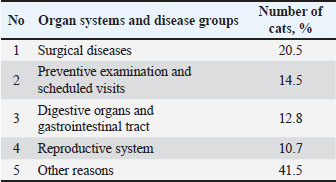
Detection was performed using electrophoresis in a 2% agarose gel containing ethidium bromide. The amplification results were visualized under ultraviolet light. The obtained data were subjected to statistical analysis using Fisher’s method. Ethical approvalThis study was approved by the Local Ethics Committee of the federal state Budgetary Scientific Institution “Federal Center for Toxicological, Radiation and Biological Safety”. The owners of the animals provided their voluntary informed consent to participate in the experiment. ResultsAccording to the patient records, the average number of visits by cat owners per day in 2022 was 18 cases. Subsequently, an analysis of the nosological picture of the diseases occurring in cats was conducted (Table 2). Table 2. Nosological profile of feline morbidity based on veterinary clinic.
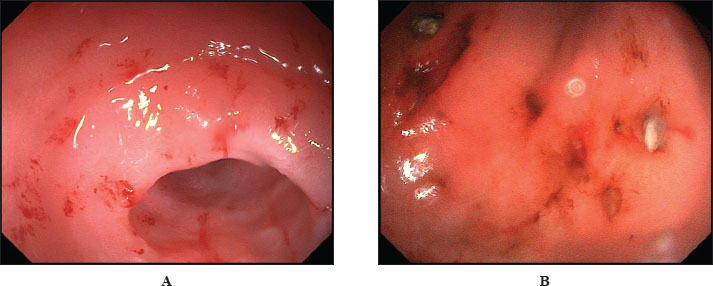
As shown in Table 2, surgical diseases were the most prevalent, affecting 20.5% of the cats. Preventive examinations ranked second among the reasons for visits (14.5%). Digestive organ diseases occupied the third position (12.8%). Gastroscopy allowed for a visual assessment of the endoscopic appearance of the gastric mucosa in cats. A total of 10 cats exhibiting pronounced clinical symptoms of gastrointestinal disease underwent gastroscopy. As a result, it was established that all examined cats displayed characteristic signs of gastric mucosa inflammation. These signs included the presence of ulcers, erosions, and hyperplasia (Fig. 1).
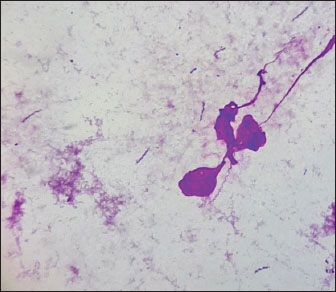
Fig. 1. Gastroscopy. Inflammatory changes in the gastric mucosa in cats. (A) Erosive gastric mucosa lesions. (B) Erosive-ulcerative gastric mucosa lesions. Furthermore, we analyzed imprint smears derived from biopsy samples collected during endoscopic procedures of the gastric mucosa in cats to indicate the presence of Helicobacter species. As a result of the microscopic examination of the imprint smears, spiral bacteria were observed in three cases (30%) with low colonization density, and in 4 cases (40%) with moderate colonization density (Fig. 2).
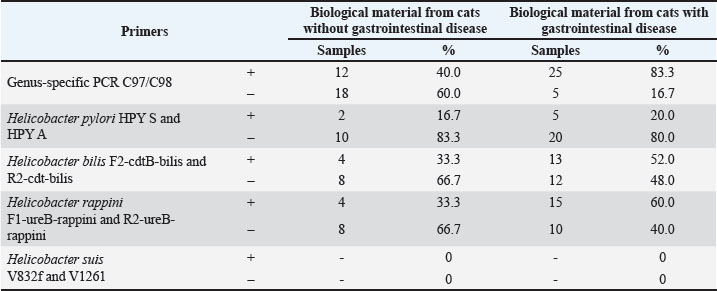
Fig. 2. Imprint smear of the gastric mucosa in a cat (moderate colonization density by helicobacter). Staining according to Romanowsky’s method. Original magnification ×900. Fecal samples were collected from privately owned cats presenting at the clinic for further investigation. Samples were obtained from 60 animals, of which 30 exhibited clinical signs of gastrointestinal diseases, and 30 showed no such signs. The age of the animals ranged from 1 to 12 years. Subsequently, species-specific and genus-specific amplifications were conducted using various primers (Figs. 3–6). The results are presented in Table 3.
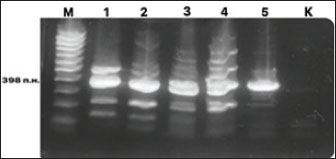
Fig. 3. Electrophoresis gel of the PCR amplification results for the detection of DNA from Helicobacter species. Primers C97 and C98 initiate amplification of a 398-bp fragment of the 16S rRNA gene of Helicobacter spp. Designations: M, DNA marker; 1–5 – PCR samples; K, negative control.
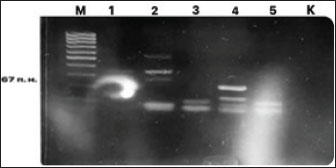
Fig. 4. Electrophoresis gel of the PCR amplification results for H. pylori DNA detection. Primers HPY S and HPY A initiate amplification of a 267-bp fragment of the 23S rRNA gene of H. pylori. Designations: M, DNA marker; 1-5 – PCR samples; K, negative control.
Fig. 5. Electrophoresis gel of the PCR amplification results for H. bilis DNA detection. Primers F2-cdtB-bilis and R2-cdtB-bilis initiate amplification of a 151-bp fragment of the cdtB gene of H. bilis. Designations: M, DNA marker; 1–5 – PCR samples; K, negative control.
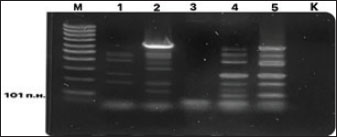
Fig. 6. Electrophoresis gel of the PCR amplification results for detecting H. rappini DNA. Primers F1-ureB-rappini and R2-ureB-rappini initiate amplification of a 101 bp fragment of the ureB gene of H. rappini. Designations: M, DNA marker; 1–5 – PCR samples; K, negative control. Table 3. Results of detection and identification of Helicobacter spp. and species DNA in biological material from cats using PCR method.
From Table 3, it is evident that the genus-specific PCR C97/C98 was present in 12 samples (40.0%) from cats without gastrointestinal disease symptoms and in 25 samples (83.3%) from cats with clinical signs of gastrointestinal tract lesions. Helicobacter pylori was detected in two samples (16.7%) from cats without gastrointestinal disease symptoms and in five samples (20.0%) from cats with clinical signs of gastrointestinal disease. Helicobacter bilis was present in four samples (33.3%) from cats without gastrointestinal disease symptoms and in 13 samples (52.0%) from cats with clinical signs of gastrointestinal disease. Helicobacter rappini was found in four samples (33.3%) from cats without gastrointestinal disease and in 15 samples (60.0%) from cats with clinical signs of gastrointestinal disease. Helicobacter suis was not detected in any samples. For the statistical analysis of the obtained data. The relationship between the presence and absence of genus-specific Helicobacter DNA in biological material from groups of animals with and without clinical signs of gastrointestinal disease was investigated. The calculations revealed a p-value of p=0.0012 (p < 0.05). This indicates a significant association between Helicobacter spp. colonization of the gastrointestinal tract in cats and the likelihood of developing gastrointestinal disorders. DiscussionThe results of the present study demonstrated a significant prevalence of Helicobacter spp. among cats in Kazan, which is consistent with the data of other authors who studied the gastric microbiota of these pets (Ménard and Smet, 2019; Ochoa and Collado, 2021). In our studies, the most frequently detected DNA belonged Helicobacter rappini (51.35%), followed closely by Helicobacter bilis (45.95%). Fox et al. (2002, 1998) describe H. bilis and H. hepaticus, isolated in cats Fox et al., 2002). Rossi et al. (2008) indicate that H. felis, H. bizzozeronii is most frequently isolated in cats with gastroenteritis (Rossi et al., 2008). The following species were most frequently detected in cats: H. felis, H. heilmannii, H. bizzozeronii, H. bilis, H. rappini, and H. salomonis. In some studies, H. pylori-like microorganisms were detected among other species (Mladenova et al., 2017; Taillieu et al., 2022a). H. pylori-like, known for its pathogenic potential in humans, was detected in 18.92% of cases in our study, which may indicate the species specificity of this microorganism. However, the detection of this microorganism in cats raises concerns about potential zoonotic transmission, particularly among immunocompromised individuals. As noted by Mladenova et al. (2017), further research, including genomic analysis of trains, is necessary to clarify their origin. The efficiency of the PCR method used in this work for non-invasive diagnostics, which is especially important in veterinary practice, was confirmed. However, our data on the species composition of Helicobacter spp. in the samples, in contrast to data of other authors, may reflect regional differences in the prevalence of species or limitations of the primers chosen. The data obtained from the isolation of Helicobacter spp. confirm that cats are natural hosts for multiple Helicobacter species. The presence of Helicobacter DNA in 40% of clinically healthy cats indicates possible carriage or commensalism, which requires investigation of specific strains’ virulence factors. ConclusionGastrointestinal diseases account for one-third of all cases presented by cats in veterinary clinics, highlighting the relevance of the issue and the necessity for its investigation. In the initial phase of our study, gastroscopy was performed on the stomachs of cats with clinical signs of digestive system disorders, and the endoscopic appearance of the gastric mucosa was all cases; the presence of ulcers, erosions, and hyperplasia was identified. Microscopic examination revealed spiral-shaped bacteria, which were found in 40% of cases with moderate colonization of the mucosal surface in cats. The identification and detection of Helicobacter species in cats were performed using molecular genetic methods. DNA from these microorganisms was present in 61.7% of cases, H. rappini being the most frequently isolated species (51.35%). Helicobacter pylori was detected in 18.92% of cases and H. bilis in 45.95% of cases, emphasizing the diversity of bacterial combinations present in the stomachs of cats. Based on the obtained data, we can conclude that a significant relationship between Helicobacter species and digestive system diseases in cats. Furthermore, the widespread prevalence of these bacteria indicates a high level of adaptation to this specific animal species. AcknowledgmentsThis work was supported by a grant from the Academy of Sciences of the Republic of Tatarstan, provided to young PhD candidates (postdoctoral researchers) for the purpose of defending their doctoral dissertations, conducting research activities, and fulfilling professional functions in scientific and educational organizations of the Republic of Tatarstan within the framework of the State Program of the Republic of Tatarstan for “Scientific and Technological Development of the Republic of Tatarstan”. The team of authors expresses their gratitude to Zoocity - Life LLC for providing data and materials for the study. Conflict of interestThe authors declare no conflict of interest. FundingThis research was supported by a grant from the state program of the Republic of Tatastan for “Scientific and Technological Development of the Republic of Tatarstan.” Authors’ contributionsNurgaliev F.M.: data curation, original draft writing, proofreading and editing, funding acquisition. Pozdeev O.K.: conceptualization, writing – proofreading; editing. Gilmanov Kh. Kh.: investigation, methodology, writing – proofreading editing.Gatiyatullin A.R.: investigation, resources. Moskvicheva A.V.: investigation, resources.Nagumanova L.R.: investigation, project administration. Gueriche Achouak: investigation and manuscript editing. Galyavieva L. Sh.: formal analysis, writing – original draft Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAlessandri, G., Christian, M., Leonardo, M., Giulia, L., Rosaria, A., Gabriele, A.L., Sabrina, D., Francesca, T., Maria, C.O., Douwe, V.S. and Marco, V. 2020. Deciphering the bifidobacteria populations within the canine and feline gut microbiota. AEM 86(7), e02875-19; doi:10.1128/AEM.02875-19 Bansil, R., Constantino, M.A., Su-Arcaro, C., Liao, W., Shen, Z. and Fox, J.G. 2023. Motility of different gastric Helicobacter spp. Microorganisms 11(3), 634; doi:10.3390/microorganisms11030634 Belyaeva, Y.A. 2016. Frequency of gastrointestinal tract pathologies in dogs and cats in the city of Minusinsk and Minusinsk District. FGBU 20(2), 80. Bordin, D.S., Shengelia, M.I., Ivanova, V.A. and Voynovan, I.N. 2022. [The history of the discovery of the Helicobacter pylori]. Ter. Arkh. 94(2), 283–288; doi:10.26442/00403660.2022.02.201377 Erginsoy, S.D., Sözmen, M. and Özcan, K. 2006. Gastric Helicobacter-like organisms in stray cats. Acta Vet. Brno. 75(75), 91–98; doi:10.2754/avb200675010091 Fox, J.G., Dewhirst, F.E., Shen, Z., Feng, Y., Taylor, N.S., Paster, B.J., Ericson, R.L., Lau, C.N., Correa, P., Araya, J.C. and Roa, I. 1998. Hepatic Helicobacter species identified in bile and gallbladder tissue from chileans with chronic cholecystitis. Gastroenterology 114(4), 755–763; doi:10.1016/s0016-5085(98)70589-x Fox, J.G., Shen, Z., Xu, S., Feng, Y., Dangler, C.A., Dewhirst, F.E., Paster, B.J. and Cullen, J.M. 2002. Helicobacter marmotae sp. nov. isolated from livers of woodchucks and intestines of cats. J. Clin. Microbiol. 40(7), 2513–2519; doi:10.1128/JCM.40.7.2513-2519.2002 Hlaoperm, C., Choowongkomon, K., Pruksakorn, C. and Rattanasrisomporn, J. 2021. Development of an easy-to-use urease kit for detecting Helicobacter pylori in canine gastric mucosa. Vet. World 14(7), 1977–1987. Isaeva, G.S. 2012. Molecular detection of helicobacter species in gastrointestinal tract diseases. Mod. Prob. Sci. Educ. 1, 41. Isaeva, G.S. and Pozdeev, O.K. 2007. The role of Helicobacter species in human pathology. Kazan Med. J. 88(1), 905–912. Jakhir, HK. 2024. The pathogenicity of the gastric bacteria and its potential threat to humans. In SHIFAA Peninsula Publishing L.L.C, Dubai, UAE; 2024, pp 18–28. Kulikov, E.V., Nikolai, V.B., Alena, I.T., Nikolai, S.B. and Pavel, A. 2023. Analysis of pathogenetic manifestation of decompensated intestinal dysbacteriosis in c cats. RUDN 18(1), 124–134; doi:10.22363/2312-797X-2023-18-1-124-134 Makino, H., De Sousa, A.T.H.I., Pavelegini, L.A.D., Trevisan, Y.P.A., Colodel, E.M., Sousa, V.R.F., Dutra, V. and Nakazato, L. 2021. Pneumonia in Cats associated with Neisseria sp. Acta Veterinaria 71(2), 211–218. Ménard, A. and Smet, A. 2019. Other Helicobacter species. Helicobacter 24, e12744. doi: 10.1111/hel.12645 Ménard, A., Santos, A., Mégraud, F. and Oleastro, M. 2002. PCR-restriction fragment length polymorphism can also detect point mutation A2142C in the 23S rRNA gene, associated with Helicobacter pylori resistance to clarithromycin. Antimicrob. Agents Chemother. 46(4), 1156–1157; doi:10.1128/AAC.46.4.1156-1157.2002 Mladenova, H.I., Grekova, O. and Patel, A. 2017. Zoonotic potential of Helicobacter spp. J. Microbiol. Immunol. Infect. 50(3), 265–269. Neiger, R. and Simpson, K.W. 2000. Helicobacter infection in dogs and cats. Facts and Fiction. JVIM 14(2), 125–133. Nurgaliyev, F.M. 2012. Helicobacter species in animals. KABM 211(3), 121–125. Ochoa, S. and Collado, L. 2021. Enterohepatic Helicobacter specie clinical importance, host range, and zoonotic potential. Crit. Rev. Microbiol. 47(6), 728–761. Pozdeev, O.K. and 2018. Mechanisms of Helicobacter pylori interaction with the gastric mucosal epithelium. I. Pathogenic factors that facilitate successful colonization. Infect. Immun. 8(3), 273–283. Rossi, M., Hänninen, M.L., Revez, J., Hannula, M. and Zanoni, R.G. 2008. Occurrence and species level diagnostics of Campylobacter spp., enteric Helicobacter spp. and Anaerobiospirillum spp. in healthy and diarrheic dogs and cats. Vet. Microbiol. 129(3-4), 304–314; doi:10.1016/j.vetmic.2007.11.014 Sapozhnikov, V.G., Tarasova, O.V. and Kharitonov, D.V. 2023. Chapters on pediatric gastroenterology. Monograph. Tula: Publishing House of Tula State University, 210. Sheh, A. and Fox, J.G. 2013. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes 4(6), 505–531; doi:10.4161/gmic.26205 Shodieva, M. and S. 2021. Characteristics of Helicobacter pylori and issues of infection epidemiology (Literature Review). OMICS 5(52), 42–57. Taillieu, E., Chiers, K., Amorim, I., Gärtner, F., Maes, D., Van Steenkiste, C. and Haesebrouck, F. 2022a. Gastric Helicobacter species associated with dogs, cats and pigs: significance for public and animal health. Vet. Res. 53(1), 42; doi:10.1186/s13567-022-01059-4 Taillieu, E., Chiers, K., Amorim, I., Gärtner, F., Maes, D., Van Steenkiste, C. and Haesebrouck, F. 2022b. Gastric Helicobacter species associated with dogs, cats and pigs: significance for public and animal health. Vet. Res. 53(42), 42. Taillieu, E., De Bruyckere, S., Van Steenkiste, C., Chiers, K. and Haesebrouck, F. 2023a. Presence of potentially novel Helicobacter pylori-like organisms in gastric samples from cats and dogs. Vet. Res. 54(1), 93. Taillieu, E., Haesebrouck, F. and Van, S.C. 2023b. Gastric and enterohepatic Helicobacters other than Helicobacter pylori–the evolving importance of non-helicobacter pylori helicobacter species for strain. Microb. Helth. Dis. 5, doi: 10.26355/mhd_202310_926 Teixeira, S., Filipe, D., Cerqueira, M., Barradas, P., Cortez Nunes, F., Faria, F., Haesebrouck, F., Mesquita, J.R., Gärtner, F. and Amorim, I. 2022. Helicobacter spp. in the stomach of cats: successful colonization and absence of relevant histopathological alterations reveals high adaptation to the host gastric niche. Vet. Sci. 9(5), 228; doi:10.3390/vetsci9050228 Vermoote, M., Vandekerckhove, T.T., Flahou, B., Pasmans, F., Smet, A., De Groote, D., Van Criekinge, W., Ducatelle, R. and Haesebrouck, F. 2011. Genome sequence of Helicobacter suis supports its role in gastric pathology. Vet. Res. 42, 51. Wernimont, M.S., Jennifer, R., Matthew, I.J., Eden, E., Dayakar, V.B., Jennifer, M.M., Dennis, E.J. and Jan, S.S. 2020. The effects of nutrition on the gastrointestinal microbiome of cats and dogs. impact on health and disease. Front. Microbiol. 11(11), 1266; doi:10.3389/fmicb.2020.01266 Zakarenko, S.M. and 2013. Innovative approaches to the treatment of acid-dependent diseases effective pharmacotherapy. Gastroenterology 17(2), 32–38. | ||
| How to Cite this Article |
| Pubmed Style Nurgaliev FM, Pozdeev OK, Gilmanov KK, Gatiyatullin AR, Moskvicheva AV, Nagumanova LR, Achouak G, Galyavieva LS. Indication and identification of Helicobacter spp. in the cat stomach. Open Vet. J.. 2025; 15(9): 4375-4381. doi:10.5455/OVJ.2025.v15.i9.44 Web Style Nurgaliev FM, Pozdeev OK, Gilmanov KK, Gatiyatullin AR, Moskvicheva AV, Nagumanova LR, Achouak G, Galyavieva LS. Indication and identification of Helicobacter spp. in the cat stomach. https://www.openveterinaryjournal.com/?mno=253652 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i9.44 AMA (American Medical Association) Style Nurgaliev FM, Pozdeev OK, Gilmanov KK, Gatiyatullin AR, Moskvicheva AV, Nagumanova LR, Achouak G, Galyavieva LS. Indication and identification of Helicobacter spp. in the cat stomach. Open Vet. J.. 2025; 15(9): 4375-4381. doi:10.5455/OVJ.2025.v15.i9.44 Vancouver/ICMJE Style Nurgaliev FM, Pozdeev OK, Gilmanov KK, Gatiyatullin AR, Moskvicheva AV, Nagumanova LR, Achouak G, Galyavieva LS. Indication and identification of Helicobacter spp. in the cat stomach. Open Vet. J.. (2025), [cited January 24, 2026]; 15(9): 4375-4381. doi:10.5455/OVJ.2025.v15.i9.44 Harvard Style Nurgaliev, F. M., Pozdeev, . O. K., Gilmanov, . K. K., Gatiyatullin, . A. R., Moskvicheva, . A. V., Nagumanova, . L. R., Achouak, . G. & Galyavieva, . L. S. (2025) Indication and identification of Helicobacter spp. in the cat stomach. Open Vet. J., 15 (9), 4375-4381. doi:10.5455/OVJ.2025.v15.i9.44 Turabian Style Nurgaliev, Farit M., Oscar K. Pozdeev, Khamid Khalimovich Gilmanov, Azat R. Gatiyatullin, Albina V. Moskvicheva, Liana R. Nagumanova, Gueriche Achouak, and Leysan Shagiakhmatovna Galyavieva. 2025. Indication and identification of Helicobacter spp. in the cat stomach. Open Veterinary Journal, 15 (9), 4375-4381. doi:10.5455/OVJ.2025.v15.i9.44 Chicago Style Nurgaliev, Farit M., Oscar K. Pozdeev, Khamid Khalimovich Gilmanov, Azat R. Gatiyatullin, Albina V. Moskvicheva, Liana R. Nagumanova, Gueriche Achouak, and Leysan Shagiakhmatovna Galyavieva. "Indication and identification of Helicobacter spp. in the cat stomach." Open Veterinary Journal 15 (2025), 4375-4381. doi:10.5455/OVJ.2025.v15.i9.44 MLA (The Modern Language Association) Style Nurgaliev, Farit M., Oscar K. Pozdeev, Khamid Khalimovich Gilmanov, Azat R. Gatiyatullin, Albina V. Moskvicheva, Liana R. Nagumanova, Gueriche Achouak, and Leysan Shagiakhmatovna Galyavieva. "Indication and identification of Helicobacter spp. in the cat stomach." Open Veterinary Journal 15.9 (2025), 4375-4381. Print. doi:10.5455/OVJ.2025.v15.i9.44 APA (American Psychological Association) Style Nurgaliev, F. M., Pozdeev, . O. K., Gilmanov, . K. K., Gatiyatullin, . A. R., Moskvicheva, . A. V., Nagumanova, . L. R., Achouak, . G. & Galyavieva, . L. S. (2025) Indication and identification of Helicobacter spp. in the cat stomach. Open Veterinary Journal, 15 (9), 4375-4381. doi:10.5455/OVJ.2025.v15.i9.44 |