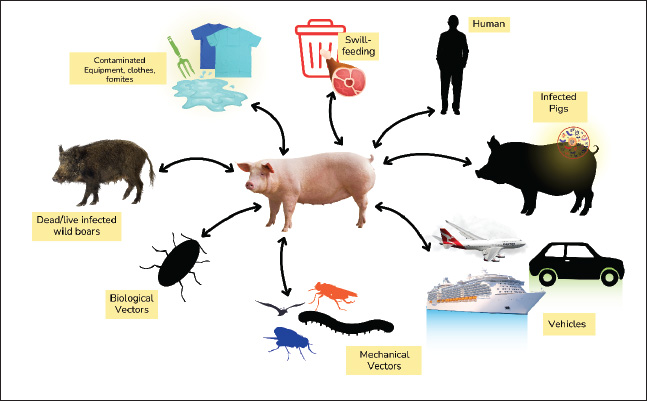
| Review Article | ||
Open Vet. J.. 2025; 15(8): 3399-3418 Open Veterinary Journal, (2025), Vol. 15(8): 3399-3418 Review Article African swine fever: A highly fatal disease that is spreading globallyMohammad Sukmanadi1, Aswin Rafif Khairullah2, Imam Mustofa3*, Agus Wiyono2, Bantari Wisynu Kusuma Wardhani4, Muharam Saepulloh2, Annise Proboningrat5, Wasito Wasito2, Alifiani Kartika Putri6, Riza Zainuddin Ahmad2, Andi Thafida Khalisa7, Muhammad Khaliim Jati Kusala2, Adeyinka Oye Akintunde8, Ima Fauziah2, Ikechukwu Benjamin Moses9, Dea Anita Ariani Kurniasih10 and Syahputra Wibowo111Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Research Center for Pharmaceutical Ingredients and Traditional Medicine, National Research and Innovation Agency (BRIN), Bogor, Indonesia 5Division of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 6Muhammadiyah Hospital Tuban, Tuban, Indonesia 7Faculty of Military Pharmacy, Universitas Pertahanan, Bogor, Indonesia 8Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria 9Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 10Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 11Eijkman Research Center for Molecular Biology, National Research and Innovation Agency (BRIN), Bogor, Indonesia *Corresponding Author: Imam Mustofa, Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: imam.mustofa [at] fkh.unair.ac.id Submitted: 20/04/2025 Revised: 18/07/2025 Accepted: 29/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
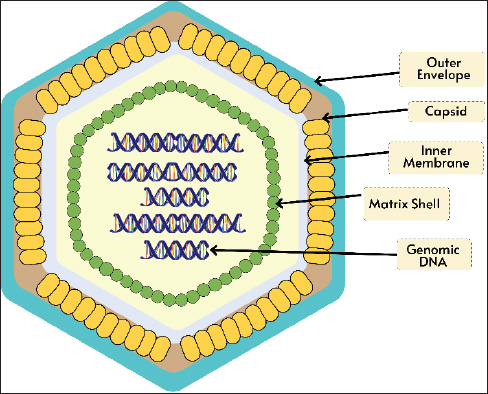
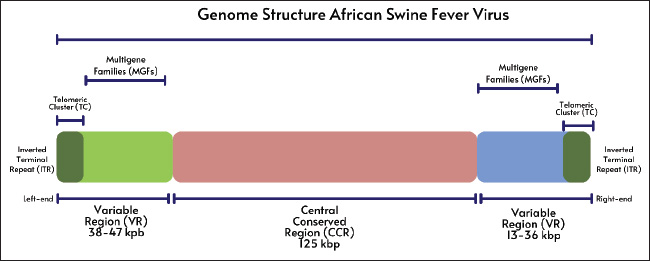
ABSTRACTAfrican swine fever (ASF) is caused by the ASF virus (ASFV), a double-stranded DNA virus classified under the family Asfarviridae and genus Asfivirus. Although outbreaks have been occurring since 1909, ASF was first documented in Kenya in 1921. The three primary epidemiological cycles that contribute to the spread of ASF include ticks, wild boars, and domestic pigs. Numerous studies on acute ASF infection in pigs have demonstrated that the virus typically enters the body through the tonsils or dorsal pharyngeal mucosa and travels to the mandibular or retropharyngeal lymph nodes, where it spreads through viremia. Serious immunosuppression and apoptosis are hallmarks of ASFV infection, which mainly multiplies in monocytes and macrophages and enters cells via receptor-mediated endocytosis. The most distinctive ASF lesion found at autopsy is severe hemorrhagic splenomegaly, which is seen when the abdominal cavity of an animal with ASF is opened. ASF illness takes 3–15 days to incubate. ASF comes in four clinical forms: acute, subacute, chronic, and peracute. ASF can be diagnosed through laboratory analysis using techniques such as polymerase chain reaction, virus isolation, and serology assays. Domestic pigs may contract ASF disease directly from pigs or the body fluids of diseased pigs or indirectly through contact with virus-containing objects and exposure to virus-contaminated settings. Continued research and surveillance of ASF vaccine development are essential to support more effective prevention and control efforts, as well as to minimize the global economic and health impacts of this disease. Keywords: African swine fever virus, Boars, Pigs, Ticks, Virus. IntroductionAfrican swine fever (ASF) is a viral hemorrhagic disease that has a nearly 100% fatality rate and affects both domestic pigs and wild boars (Li et al., 2022). The ASF virus (ASFV), a double-stranded DNA virus belonging to the Asfarviridae family and genus Asfivirus, causes this illness (Njau et al., 2021). Although ASF outbreaks have been documented since 1909, the disease was originally discovered in Kenya, East Africa, in 1921 (Mulumba-Mfumu et al., 2019). The World Organization for Animal Health WOAH has classified ASF disease as one of the most dangerous animal pathogenic diseases with the capacity to spread quickly and across national borders (Juszkiewicz et al., 2023). Since its initial emergence in Georgia in 2007, the disease has spread almost unchecked throughout the world (Sánchez-Cordón et al., 2018). The illness lasted until 2015 and quickly spread throughout Eastern and Central Europe (Cwynar et al., 2019). The illness then made its way to Western Europe before reaching China in 2018 (Ding and Wang, 2020). In the years since its initial discovery in China, the virus has rapidly spread throughout Asia, leaving only a few nations free. The outbreak continues to spread over Europe, East Asia, and Southeast Asia and has not slowed down in recent years (Ceruti et al., 2025). In 2021, ASF traveled to Haiti and the Dominican Republic (Schambow et al., 2023). The battle against this illness is currently in a state of urgency and emergency. Since its initial identification in Indonesia in 2019, the ASF outbreak has expanded to practically every region in the country (Tenaya et al., 2023). ASFV is naturally found in soft ticks of the genus Ornithodoros and wild boars (De Oliveira et al., 2020a). In Africa, warthog infections can persist for extended periods without causing any symptoms (Ståhl et al., 2019). The complex genome and particle structure of ASFV allow it to live in the natural environment for extended periods (Li and Zheng, 2025). ASF disease can spread in domestic pigs either directly through contact with other pigs or the body fluids of infected pigs or indirectly through contact with virus-containing objects and exposure to virus-contaminated environments (Guinat et al., 2016). Additionally, the bite of the soft tick Ornithodoros might result in indirect transmission (Lv et al., 2022). The ASF disease incubation period lasts 3–15 days. The health status of the infected pigs and the virulence of the virus strain determine the severity of ASF. This illness generally comes in acute, subacute, chronic, and peracute forms (Li et al., 2022). Pigs with ASF may exhibit high temperature, decreased appetite, lethargy, vomiting, diarrhea, skin redness or bleeding, and even death without any outward signs (Nguyen et al., 2023). Laboratory procedures include virus isolation (VI), polymerase chain reaction (PCR), and serology testing (Penrith et al., 2024). The high contagiousness and lethality of ASF necessitate international cooperation for its control and prevention (Chuong et al., 2025). This review aims to provide a comprehensive understanding of the disease, including its characteristics, spread, health impacts, and control strategies. EtiologyVirologyThe genome of the big enveloped virus known as ASFV is made up of linear double-stranded DNA and is approximately 190 kbp long (Gaudreault et al., 2020). ASFV is a member of the family Asfarviridae and genus Asfivirus (Keita et al., 2010). The ASFV virion is 200 nm in diameter and has a complex icosahedral structure encased in a membrane layer (Andrés et al., 2020). Figure 1 shows the structure of the ASFV virion. A nucleoprotein encased in a matrix protein constitutes the virus’s core. An inner membrane capsid layer envelopes the core and matrix (Hawes et al., 2008). The icosahedral symmetric capsid layer, which is made up of the capsid protein (p72) (Venkateswaran et al., 2024), is covered by an outer membrane made from the infected cell’s plasma membrane. ASFV is contagious even in the absence of its outer membrane. The DNA virus known as ASFV multiplies within the cytoplasm of infected cells (Avagyan et al., 2022). RNA polymerase, nucleoside triphosphate phosphohydrolase, topoisomerase, mRNA-binding enzymes, and protein kinases are among the enzymes that may be present in virions and are necessary for the initial stages of cytoplasmic replication (Karger et al., 2019). Because ASFV virions are frequently tainted with a variety of biological constituents, they share immunoreactivity with tubulin and actin, among other cellular proteins (Alonso et al., 2013). Genome organization and multigene familiesOver a dozen full genomic sequencing of different ASFV isolates are already available. The strain BA71 was used in the first ASFV genome investigation. It was obtained during a Spanish outbreak in 1971 and subsequently adapted to Vero cells (Rodríguez et al., 2015). The two ends of the 170 kbp BA71 genome are covalently joined to create ITRs (Portugal et al., 2015). According to other genomic analyses employing distinct isolates, the ASFV genome ranges in length, reaching a maximum of approximately 190 kbp (Mazloum et al., 2023). As shown in Figure 2, the ASFV genome includes ITRs, terminal cross-links, central conversion regions (CCRs), and variable areas that surround the CCRs. MGF has been found to be present in the variable area by sequence analysis, and the sections flanking the CCR, which is around 125 kbp long, are where MGF insertions and deletions mostly cause genome length variation in ASFV (Tamás et al., 2023). The right variable area is 13–16 kbp long, whereas the left variable region is 38–47 kbp long (Yoo et al., 2020). The total size of the viral genome is also impacted by slight changes in the CCR. Large-scale changes are limited to the left and right terminal regions of the CCR, and major insertions and deletions are uncommon (Chen et al., 2024). The tandemly organized gene sequence arrangement known as MGF is similar to that of the ASFV isolates under investigation, albeit with varying copy counts (Torma et al., 2021). MGF encodes full-length or specific domain proteins with comparable sequences. MGF may have been inherited by duplication or variation in a common ancestral gene, which might have developed via sequence transposition, homologous recombination, or recurrent deletion (Zheng et al., 2025). The central variable region, which is frequently employed as an epidemiological marker in genotype distinction, exhibits small-scale variation as tandem repetitions of varied lengths across closely related African ASFV isolates (Sanna et al., 2017).
Fig. 1. Structure of the ASFV virion.
Fig. 2. Genome structure of the ASFV. Five distinct MGF members are present in the ASFV genome. The average length of the anticipated gene products is reflected in the MGF members’ nomenclature. For instance, MGF110 and MGF360 may encode proteins with 110 and 360 amino acids, respectively (Ramirez-Medina et al., 2023). The left terminal genomic area, which varies greatly across BA71V isolates and other European isolates, contains the MGF100 and MGF360 genes. The right-terminal genomic region also contains these genes (Liu et al., 2021a). MGF110, MGF300, and MGF505/530 are more common MGF members (Tamás et al., 2023). The left terminal region contains a highly variable noncoding repeat next to MGF300 (Petrini et al., 2023). In the isolate Lil20/1 from Malawi, MGF505 is known as MGF530 (O’Donnell et al., 2015). The sequence analysis of the terminal section of isolate BA71 revealed a preponderance of MGF members, including the MGF100, MGF110, MGF300, MGF360, and MGF505/530 genes (Wang et al., 2023). The central core of the ASFV genome encodes a number of proteins that are comparatively conserved between isolates, such as membrane proteins, additional structural proteins, and elements needed for virion production and morphogenesis (Yang et al., 2023). Other ASFV proteins are involved in DNA replication and repair, transcription, nucleotide metabolism, and protein modification (Alejo et al., 2018). Entry to the cellReceptor-dependent entry types, such as micropinocytosis or clathrin-mediated dynamics-dependent endocytosis, have been proposed for ASFV entry into cells (Hernaez and Alonso, 2010). Actin-dependent micropinocytosis is linked to dynamic plasma membrane activity (Venkateswaran et al., 2024). Viral ligand–cellular receptor interaction, along with attachment factors, activates and stimulates the phosphoinositide 3-kinase, Rho GTPase, epidermal growth factor receptor, and Pak-1 signaling cascade, which control actin dynamics and create ruffles for ASFV internalization in Vero cells (Zhang et al., 2021). Clathrin-mediated endocytosis allows ASFV to enter pig macrophage cells (Hernaez and Alonso, 2010). After internalization, particles are taken to late endosomes, where pH-dependent shedding occurs, after being endocytosed in early endosomes and macropinosomes (Hernáez et al., 2016). The viral core is then delivered to the cytosol by disintegration of the outer envelope and fusion of the inner envelope with the endosomal membrane (Zhang et al., 2021). Both Vero cells and macrophages employ this entrance method. The precise cellular receptors that allow ASFV to enter cells remain unknown. Prior research found a link between monocyte/macrophage surface CD163 and ASFV infection susceptibility, and anti-CD163 antibody-induced infection inhibition suggested a function for CD163 in ASFV infection (Han et al., 2025). Nevertheless, a recent investigation revealed that pigs with complete CD163 knockout were equally vulnerable to the Georgia 2007/1 ASFV strain; both wild-type and CD163 knockout penmate were infected and did not exhibit any appreciable variations in pathology, viremia, mortality, or clinical symptoms (Popescu et al., 2017). Moreover, no alterations were found following in vitro infection with CD163-negative macrophages. These findings rule out a function for CD163 in ASFV infection, at least for ASFV genotype 2, as CD163 is not necessary for ASFV infection. The ASFV proteins p12, p30 (p32), and p54 attach to the cell surface (Jia et al., 2017). The transmembrane p12 protein, which is found outside the capsid coat, can bind vulnerable cells, such as macrophages (Li and Zheng, 2025). Although antibodies against p12 are unable to neutralize the infectious agent and do not shield pigs from ASF, recombinant p12 prevents the specific binding of ASFV to cells (Zhu, 2022). Additionally, p30 and p54 proteins attach to macrophages. p54 antibodies inhibit ASFV-specific attachment to macrophages, while p30 antibodies block viral internalization (Gómez-Puertas et al., 1998). Neutralizing antibodies are produced by the p54 protein and the main capsid protein p72, and they prevent the adhesion of viruses to macrophages (Neilan et al., 2004). Even after the virus has adhered to these cells, p30 antibodies neutralize it and prevent infection (Wang et al., 2022a). Therefore, it appears that p30 is active in virus internalization, whereas p72 and p54 are involved in virus attachment (Gómez-Puertas et al., 1998). Nevertheless, neutralizing antibodies to this protein alone cannot protect pigs against ASFV infection. Viral DNA replication and mRNA transcriptionAfter being released into the cytoplasm, the virion travels to its replication site in the perinuclear area for further gene expression (Aicher et al., 2021). ASFV virions can produce viral mRNA inside cells and contain DNA-dependent RNA polymerase (Ran et al., 2022). ASFV replication does not require cellular RNA polymerase II activity. The expression of the ASFV gene follows transcriptional kinetics and starts early in the post-infection phase (p.i.) (Cackett et al., 2020). A cascade model may explain ASFV gene expression, as four types of viral mRNAs with different synthesis kinetics have been identified: immediate-early, early, intermediate, and late mRNAs (Galindo and Alonso, 2017). Early and immediate-early genes are expressed before DNA replication onset, whereas intermediate and late genes are expressed after the onset of DNA replication (Rodríguez and Salas, 2013). The virus uses the enzymes contained in the virion particle for replication, and the enzymes necessary for DNA replication are produced shortly after the virus enters the cytoplasm (Alonso et al., 2013). The ASFV mRNA is polyadenylated and has a methylation cap structure. Early ASFV gene expression can be detected as early as 2 hours after infection, whereas late gene expression peaks 12–16 hours after infection and depends on viral DNA replication (Duan et al., 2022). Transcription of intermediate and late genes begins after DNA replication. Although ASFV-infected cells exhibit nuclear stages in DNA replication, the precise function of the nucleus in ASFV DNA replication remains unclear. Larger DNA molecules are created later in the cytoplasm, whereas tiny DNA fragments are found in the nucleus during the early stages of DNA replication (Wang et al., 2021). The cytoplasm and the nucleus produce replication intermediates, which are head-to-head concatemer (Dixon et al., 2013). The nucleus might supply certain elements that are crucial for the initial phases of ASFV DNA replication (Simões et al., 2015). Protein synthesis and viral maturationCells infected with the virus can synthesize over 150 ASFV-specific proteins. The virus particle comprises approximately 50 structural proteins (Huang et al., 2024). Most viral proteins, including the primary capsid protein p72, are found in the cell’s viral factories, although they are also present in the cell membrane, cytoplasm, and nucleus (Suárez et al., 2010). Proteolysis processes the two major polyproteins encoded by ASFV, pp220 and pp62, to produce mature structural proteins (Andrés et al., 2002). The 2,475 amino acid pp220 protein can be broken down to produce p150, p37, p34, and p14, whereas the 530 amino acid pp62 protein can be broken down to produce p35, p15, and p8 (Andrés et al., 2001). The major components of the virion core envelope are cleavage products of the pp220 and pp62 polyproteins, both of which are produced throughout the post-infection period (Alejo et al., 2003). Mature intracellular virions are carried to the plasma membrane, where they emerge as extracellular virions (Salas and Andrés, 2013). The p14.5 protein helps encapsidate the viral genome by binding to the viral DNA and interacting with p72 (Martin et al., 2024). Furthermore, p14.5 contributes to the transfer of intracellular virions from the viral factory to the plasma membrane (Rodríguez et al., 2009). On the cell surface, ASFV virions can trigger actin nucleation, which helps the virus propagate from one cell to another (Jouvenet et al., 2006). HistoryASF has existed for almost a hundred years. ASF is a fatal and extremely contagious illness that affects both domestic and wild pigs, severely harming the global pork sector (Li et al., 2022). Although outbreaks have been occurring since 1909, ASF was first documented in Kenya in 1921 (Mulumba-Mfumu et al., 2019). The disease remained restricted to Africa until 1957, although it spread to some sub-Saharan African nations in the ensuing decades (Costard et al., 2009). In 1957, ASF made its way to Europe after being discovered on a Portuguese pig farm (Cwynar et al., 2019). Authorities swiftly contained the infection by drastically reducing the pig population by over 10,000 animals. In 1960, the illness reemerged in Portugal and rapidly spread to Spain and France (Sánchez-Cordón et al., 2018). ASF first visited the Dominican Republic in 1978 (Schambow et al., 2023). The epidemic spread to Haiti within a year, killing approximately half of the nation’s pig population (Ruiz-Saenz et al., 2022). In the Dominican Republic in 1980 and Haiti in 1984, these governments collaborated with the United States Department of Agriculture (USDA) to eradicate ASF (Jean-Pierre et al., 2022). ASF was still present in Europe in 1995 (Cwynar et al., 2019). EpidemiologyThe three primary epidemiological cycles that contribute to the spread of ASF are ticks, wild boars, and domestic pigs (Chenais et al., 2018). Controlling all three is challenging. Ticks have not yet been implicated in the current pandemic. However, this potential cannot be ruled out very soon because ASF is found in nations with rich biodiversity and conducive tick involvement settings (Martínez-Avilés, 2023). Only Belgium, where only wild boars are impacted, has successfully eradicated ASF during the present pandemic (Sauter-Louis et al., 2021). ASFV spreads more slowly than other viral diseases because of its hazardous nature and capacity to endure in the environment as well as in frozen and refrigerated meat (Petrini et al., 2019). Backyard or subsistence pig rearing makes control measures more difficult. Additional elements, including social influences and underestimating of risk, drive transmission and perpetuation (Fasina et al., 2020). The global spread of ASF and the difficulty of controlling it in wild boars have prompted more research on a vaccine to prevent the disease, which was previously unattainable (Turlewicz-Podbielska et al., 2021). Less than five ASF outbreaks were documented annually prior to 2007 (when ASF first appeared in Georgia, Europe); all of these outbreaks occurred in Africa (Malakauskas et al., 2022). ASF illness has spread largely unchecked since it first surfaced in Georgia. It first spread to Eastern Europe, affecting the Russian Federation, Armenia, and Azerbaijan, before spreading to Belarus and Ukraine (Cwynar et al., 2019). Between 2007 and 2014, ASF incidences rose to an average of 77 per year (Bosch et al., 2020). ASF entered the European Union (EU) in 2014 after passing through Lithuania, Poland, Latvia, and Estonia, and then nine additional European nations (EFSA et al., 2018). Between 2014 and 2018, ASFV spread throughout the Baltic region, causing approximately 2000 cases in wild boars and nearly 300 outbreaks in domestic pigs annually on average (230,000 pigs impacted) (Martínez-Avilés, 2023). In 2020, the two EU nations that produce the most pigs, France and Germany, are now more vulnerable to the threat of ASF (de la Torre et al., 2022). Since then, Germany and Northern Italy have been found to have ASF (Iscaro et al., 2022). Both wild boars and domestic pigs have contracted the disease throughout Europe. Wild boars significantly aid the spread of the virus across the continent (Sauter-Louis et al., 2021). While ASF in Europe endured years of suffering, it was unknown in Asia until it surfaced in China in 2018 (Salling, 2025). It has impacted more than a dozen other Asian nations and is spreading more quickly in China than in other areas. In 2019, the disease was discovered in North Korea, Laos, Myanmar, the Philippines, Timor Leste, Mongolia, Vietnam, Cambodia, and Hong Kong (Ito et al., 2023). The first formal announcement of the spread of ASF in Indonesia was made in December 2019 in the province of North Sumatra (Tenaya et al., 2023). As of December 2024, ASF has spread throughout 32 Indonesian provinces, including Papua, Central Papua, and East Nusa Tenggara, since its initial discovery in Indonesia (Dharmayanti et al., 2021). Between 2018 and 2022, the average number of ASF-infected domestic pigs rose to 1,800 and wild boars to 4,500 annually (Shaw et al., 2024). For domestic pigs, 2019 was the worst year, with 8.5 million pigs infected in Asia alone (Martínez-Avilés, 2023). For wild boars, 2020 was the worst year, with 16,715 infected and 5,795 cases (Sehl-Ewert et al., 2022). ASF still spreads and affects swine sector livelihoods and health globally. Although the US is still free of ASF today, the threat still exists (Sánchez-Cordón et al., 2018). In 2021, the detection of ASF in Haiti and the Dominican Republic led to ASF outbreaks on all five continents (Schambow et al., 2023). Pig health is at increased risk because of this closeness. History has demonstrated that ASF is deadly and difficult to control. This disease is causing millions of pigs worldwide to go extinct (Costard et al., 2009). PathogenesisNumerous studies on acute ASFV infection in pigs have demonstrated that the virus typically enters the body through the tonsils or dorsal pharyngeal mucosa and travels to the mandibular or retropharyngeal lymph nodes, where it spreads through viremia (de Marco et al., 2007; Ko et al., 2023). Once exposure occurs naturally or through the air, the first site of infection is the bronchial, gastrohepatic, or mesenteric lymph nodes (Sánchez-Cordón et al., 2019). The virus spreads through the blood after first infecting and replicating in lymphoid tissues, and in severe instances, viremia can exceed 108 HAD50/ml (Guinat et al., 2016). Erythrocytes, which contain over 90% of the circulating virus, are linked to the virus in the blood in diseases caused by hemadsorbent isolates; however, lymphocytes and neutrophils are also linked to the virus (Franzoni et al., 2023). According to early research on ASFV pathogenesis in neonatal pigs, primary viremia was detected as early as 8 hours after infection, while secondary viremia was found between 15 and 24 hours after infection (Droesbeke et al., 2024). Secondary viral growth occurs in the lungs, liver, lymph nodes, and spleen (Fan et al., 2022). The virus was present in all neonatal pig tissues by 30 hours, and peak titers were attained as early as 72 hours following inoculation (Ambagala et al., 2024). ASFV mostly replicates in the monocytes and macrophages of the mononuclear phagocyte system, which are the main targets of viral replication in vivo (Dixon et al., 2019). Although viral replication has not been proven in these cases, various researchers have demonstrated that ASFV also infects megakaryocytes, thymic reticulum epithelial cells, endothelial cells, glomerular mesangial cells, renal collecting duct epithelial cells, hepatocytes, neutrophils, and lymphocytes (Gómez-Villamandos et al., 1995; Vallée et al., 2001). Many researchers do not accept the infection of such a wide variety of host cells, and in some of these cases, the cells only became infected at a late stage of pig infection, indicating that they became susceptible during the course of the infection for unknown reasons and thus relied on early macrophage infection. Researchers have hypothesized that the bleeding is caused by viral multiplication in interstitial capillary endothelial cells (Oh et al., 2021). However, others have disproved this theory by demonstrating renal and lymph node bleeding before viral multiplication in these cells (Li and Zheng, 2025). In addition to bleeding, endothelial damage, which includes proliferation of lysosomes and phagocytosed cell debris, an increase in fenestrations within the area, and even endothelial cell necrosis and loss, has been reported, which exposes the screen’s basement membrane, to which platelets adhere (Blome et al., 2013). This could be one of the reasons for the disseminated intravascular coagulation (DIC) that is typical of ASF. Other researchers have connected this phenomenon to the release of cytokines by infected macrophages, specifically IL-1 and TNF-α, and the effects of mediators, such as prostaglandin E2, secreted by infected macrophages on endothelial cells, which activate the coagulation cascade and cause DIC (Franzoni et al., 2023). Because the affected animal rapidly deteriorates, thrombocytopenia is typically seen in the late stages of the acute form of the disease, after bleeding has been detected (Sánchez-Vizcaíno et al., 2015). It has been linked to platelet consumption from coagulopathy, direct viral effects on megakaryocytes, and a number of immune-mediated processes that cause platelet aggregation involving immune complexes of ASFV antigens and antibodies (Pérez et al., 1997). Nowadays, it is widely believed that hemostasis abnormalities are largely caused by the huge death of macrophages, which release active chemicals such as complement factors, cytokines, and metabolites of AABA (Salguero et al., 2002). Pigs infected with ASFV typically experience acute, severe lymphopenia in the early-middle stages of the disease (Salguero, 2020). Several authors first proposed lymphopenia brought on by lymphocyte apoptosis after describing the morphological characteristics of this mechanism of death in the spleens of pigs suffering from acute ASF (Ruedas-Torres et al., 2024). According to electron microscopy (EM) examinations of tissues from pigs infected with several virulent isolates, uninfected lymphocytes in the lymph nodes and in the kidney and liver’s interstitial tissue underwent apoptosis (Oura et al., 1998). Numerous pathogenic mechanisms have been proposed to explain why lymphocytes undergo programmed cell death during infection. ASFV replication could not trigger apoptosis in lymphocytes because it could infect them but not replicate, as others have noted. Infected macrophages release pro-inflammatory cytokines that induce apoptosis in lymphocyte populations (Zhang et al., 2024a). It is unclear what causes persistent ASFV infection. Various researchers have proposed that certain variants of the disease include an autoimmune component and that the lesions may be related to the deposition of immune complexes in tissues such as the kidneys, lungs, and skin with subsequent binding to complement (Fan et al., 2022). Immune responseSerious immunosuppression and apoptosis are hallmarks of ASFV infection, which mainly multiplies in monocytes and macrophages and enters cells via receptor-mediated endocytosis (Han et al., 2025). The acute phase reaction, inflammation, endothelial cell activation, and apoptosis are all influenced by the IL-1, IL-6, and TNFα released by activated macrophages (Franzoni et al., 2023). ASFV strains with higher virulence have been linked to more severe tissue damage; however, all strains have been found to have similar cell tropism and organ dispersion (Droesbeke et al., 2024). The host immunological response to ASFV is thought to be significantly influenced by neutralizing antibodies, CD8+ T cells, and natural killer cells (Zhang et al., 2024b). The majority of the cellular activities that are changed after infection are still unknown, despite in vitro investigations demonstrating that ASFV regulates a number of cellular systems by encoding particular regulatory genes and interacting with viral and cellular proteins (Sánchez et al., 2013). According to proteomic analysis, ASFV affects >65% of cellular proteins and suppresses most protein production (Fenster et al., 2024). The majority of the cellular proteins overexpressed after ASFV infection are involved in coagulation, programmed cell death, and redox homeostasis (Li et al., 2024). Studies on neutralizing antibody function have produced a range of findings. Onisk et al. (1994) investigated passive transfer in farm pigs and discovered that 85% of pigs receiving anti-ASFV IgG survived the attack, whereas 0% of unimmunized controls did not. Animals receiving treatment experienced a brief fever, but otherwise appeared clinically normal. Pigs undergoing antibody transfer showed decreased and delayed viremia. After identifying virus-neutralizing epitopes on three viral capsid proteins (p30, p54, and p72), domestic pigs were immunized with baculoviruses expressing each of these proteins before they were challenged with homologous viruses. Immunized animals showed decreased viremia and a 2-day delay in the onset of clinical illness, but no change in disease course, progression, or outcome. The authors concluded that antibody-mediated protection requires more than just neutralizing antibodies to specific ASFV proteins. The results of Neilan et al. (2004) and Onisk et al. (1994) seem to be very different from one another, and this is thought to be partly due to differences in challenge dose and virus strain (and thus virulence). The virulence of the tested ASFV isolate may determine the relative importance of neutralizing antibodies, with neutralizing antibodies offering a higher protective response against less virulent strains. Onisk et al. (1994) used passive transfer, which is a mixture of numerous antibodies, whereas Neilan et al. (2004) inoculated pigs with specific epitopes. However, these significant changes in the study design make comparison extremely challenging. Further characterization of the function of antibodies is necessary. Interestingly, domestic pig populations in northern Mozambique, an area where ASF is endemic, have significant levels of antibodies against ASFV (Penrith et al., 2004). A subset of pigs from this population was gathered, and the experimental ASFV challenge was used to assess the heredity of this ASF resistance in their offspring. Resistance to ASFV in the parental population was not heritable, as evidenced by the high susceptibility of the offspring to challenge with a virulent strain of the virus. The authors speculate that maternal antibody resistance, exposure to a small number of infections that may result in sublethal infections that confer immunity to subsequent challenges, and prior exposure to a less virulent but antigenically similar field virus before exposure to the virulent strain are the causes of this observed resistance. PathologyThe most distinctive ASF lesion found at autopsy is severe hemorrhagic splenomegaly, which is seen when the abdominal cavity of an animal with ASF is opened (Salguero, 2020). The liver is heavily obstructed. In addition to multifocal hemorrhages in the lymph nodes that appear marbled, severe hemorrhagic lymphadenopathy in the renal lymph nodes, severe hemorrhagic lymphadenopathy in the ileocecal lymph nodes, severe hemorrhagic lymphadenopathy in the gastrohepatic lymph nodes, and moderate hemorrhagic lymphadenopathy in the mesenteric lymph nodes, a very large, dark-colored spleen with rounded edges (hemorrhagic splenomegaly) are observed, taking up a significant portion of the abdominal cavity (Nga et al., 2020). Animals with post-mortem examinations usually exhibit multifocal edema, ascites, and hydropericardium in the perirenal fat or gallbladder wall (Gómez-Villamandos et al., 2003). The acute form of the disease may cause hemorrhagic splenomegaly in certain animals, but many animals may only have partial splenomegaly, with some spleen patches afflicted and others untouched (Yoo et al., 2020). A condition known as multifocal hemorrhagic lymphadenitis can also be seen when several lymph nodes throughout the body exhibit bleeding and “marbling” (Salguero et al., 2002). Clinical symptomsASF illness takes 3–15 days to incubate. ASF comes in four clinical forms: acute, subacute, chronic, and peracute (Li et al., 2022). The severity of each form varies according to a number of criteria, such as the infectious dose, the exposure route, the kind of pig, the virulence of the virus, the endemic status of the area, and related illnesses (Niederwerder et al., 2019). Domestic pigs typically exhibit a peracute or acute presentation of clinical symptoms, but African Warthogs (Sus scrofa algira) typically exhibit subclinical disease carriage (Ramirez-Medina et al., 2022). Peracute presentation symptoms include lethargy, appetite loss, and a high fever of 105°F (40.5°C) (Sánchez-Vizcaíno et al., 2015). Sudden death typically occurs within 1–3 days, and it might occur before other symptoms appear (Chenais et al., 2019). Similar clinical signs to the peracute presentation are present in the acute presentation, but additional symptoms, such as an elevated respiratory rate, eye and nose discharge, bloody foam from the mouth or nose, bleeding (spot or widespread) in the ears, stomach, and/or hind legs, redness of the skin on the chest, belly, perineum, tail, and legs, constipation or diarrhea, which can range from mucoid to bloody, and vomiting, are frequently observed. Additionally, abortion in sows throughout their entire pregnancy is observed (Li et al., 2022). Depending on the aggressiveness of the virus, death might happen anywhere from 6 to 15 days. The mortality rate in domestic pigs is 90%–100% (Sánchez-Vizcaíno et al., 2015). The clinical indicators of subacute presentation are similar to those of acute presentation; however, they are typically milder (Ruedas-Torres et al., 2024). Vascular abnormalities, such as hemorrhage and edema, are an exception and are more severe in subacute ASF presentations. Edema may cause serous pericarditis, enlarged joints, and consequent walking pain (Sánchez-Vizcaíno et al., 2015). Fever, sadness, and appetite loss also frequently change as the illness progresses (Fernandez-Colorado et al., 2024). Death typically occurs 7–20 days after the onset of clinical symptoms, and the survivors recover after a month. The mortality rate for subacute ASF presentation is 30%–70% (Ståhl et al., 2019). Areas where ASF is endemic are typically where chronic presentation occurs (Ceruti et al., 2025). Mortality is usually 30%, and clinical indications occur 14–21 days following exposure (Olesen et al., 2024). Most infected pigs recover from the disease, and the clinical indications are typically milder, such as lower fever, less depression, and decreased appetite rather than anorexia (Nguyen et al., 2023). DiagnosisTesting for ASFV can be divided into two categories. The first category is the direct detection of viral antigens, which includes VI, real-time PCR, direct fluorescence antibody testing, and sequencing; the second is serological testing for antibody detection, which includes enzyme-linked immunosorbent assay (ELISA), immunoperoxidase test (IPT), and indirect fluorescence antibody (IFA) (Penrith et al., 2024). Since individual animal sampling is the only verified technique for detecting ASFV, current viral antigen testing is based on this technique (Elnagar et al., 2022). Nevertheless, in April 2020, the National Veterinary Services Laboratory Foreign Animal Disease Diagnostic Laboratory (NVSL FADDL) was working to validate aggregate samples, including cord sampling for oral fluid testing (Goonewardene et al., 2021). The only sample types authorized for testing by the NVSL FADL are whole blood and fresh tissue (spleen, lymph nodes, and tonsils), blood spots, blood smears, and spleen swabs, although lung and bone marrow tissue samples are recommended for testing by the World Organization for Animal Health (Hu et al., 2023). As there is no vaccination for ASFV in the United States, the presence of antibodies is a sign of prior infection or chronic or subacute infection (Zhang et al., 2023). In Vietnam, where an ASF vaccine is currently under field evaluation, a companion enzyme-linked immunosorbent assay that separates Differentiating Infected from Vaccinated Animals (DIVA) is needed (Wang et al., 2022b). Complementary DIVA antibody ELISAs are anticipated to be available following the release of other EU-developed vaccines for domestic pigs and wild boar, although no release date has been set (González-García et al., 2025). For samples that test positive for antibodies by enzyme-linked immunosorbent assay, immunoblotting, IFA, or IPT should be used for confirmatory testing (Nah et al., 2022). Differential diagnosisThe most crucial differential diagnosis for ASF is hog cholera or classical swine fever (Schulz et al., 2017). Both illnesses are communicable and share postmortem lesions and clinical symptoms. The virus must be identified to accurately distinguish between ASF and CSF (Khairullah et al., 2024). Collecting blood, spleen, kidney, lymph node, and tonsil samples is necessary for laboratory confirmation (Walczak et al., 2022). Distinguishing between ASF virus (ASFV) and CSF virus (CSFV) is possible using the real-time reverse transcription PCR (rRT-PCR) multiplex assay (Nishi et al., 2022). Additional differential diagnoses include PDNS, Aujeszky’s illness (pseudorabies), acute salmonellosis, erysipelas, and HP-PRRS (Sánchez-Vizcaíno et al., 2015). TransmissionAs depicted in Figure 3, ASFV can infect domestic pigs directly and indirectly, in multiple ways. Such an intricate network of complexity makes implementing farm biosecurity policies and effective prevention of ASF particularly challenging. There are two infection cycles in the epidemiology of ASF: recent (domestic) and ancient (sylvatic). The sylvatic cycle includes warthogs and wild African pigs. In the domestic cycle, infection occurs solely in the domestic pig population, which remains permanently infected and becomes a carrier of the virus (Patil et al., 2020). ASFV has been identified in bodily fluids, feces, and secretions (Davies et al., 2017). Southern African nations frequently harbor asymptomatic ASFV carriers. These include the red river pig (Potamochoerus porcus), sometimes referred to as the river pig, and the warthog (Phacochoerus africanus) (Gallardo et al., 2015). The soft tick, Ornithodoros moubata, is a tick species belonging to the genus Ornithodoros and family Argasidae that is the vector of ASFV in this geographic area (Anholt et al., 2023). Ornithodoros erraticus, another soft tick species that contributes to the sylvatic cycle of transmission, has been identified as a significant vector of ASF transmission throughout southern Europe, specifically in Spain and Portugal (Boinas et al., 2014). It is important to note that infected ticks can live for up to 5 years and spread the virus either transovarially (from mother to child) or transstadially (from immature to adult tick) (Malakauskas et al., 2022). ASFV replication does not occur in pasture ticks (Dermacentor reticulatus) or the most prevalent European ticks (Ixodes ricinus), nor does it contribute to ASFV transmission (Lv et al., 2022). Nonetheless, since the virus can live in these hard ticks for six to eight weeks, they could be useful mechanical vectors (Ferreira et al., 2014).
Fig. 3. Transmission routes of the ASFV. In Europe, the primary source of the virus and a significant direct or indirect vector of the disease are sick and dead wild pigs (Sauter-Louis et al., 2021). These animals are extremely vulnerable to ASFV isolates. The huge populations and high densities of wild boars, particularly in ASF-affected regions of Europe, significantly contribute to the disease’s onset and spread (Gervasi and Guberti, 2021). ASFV infection can spread among wild boars and pigs (Guinat et al., 2016). Contact between domestic pigs and infected wild boars, particularly in Eastern Europe (Caucasus, Russian Federation), where biosecurity is insufficient, is a major contributing element to the disease’s transmission (Brookes et al., 2021). Potential attempts to eradicate and control ASF are severely hampered by the widespread wild boar population in affected areas (Jo and Gortázar, 2021). Additionally, people help diseases spread to far-off places through a variety of actions. The illicit transportation of sick pigs or pork products is an important factor that leads to the spread of ASFV in Africa, Europe, and Asia (Cheng and Ward, 2022). ASFV is disseminated by tick bites and direct transmission (contact with sick pigs, wild boars, tissues, or carcasses), but it can also spread through other species (Brown et al., 2024). The ability of ASFV to be conveyed by flies could lead to the unanticipated spread of this virus to farms with high biosecurity standards (Fila and Woźniakowski, 2020). Numerous studies have demonstrated that the oral ingestion of fed stable flies (Stomoxys calcitrans) can result in ASFV transmission under experimental conditions (Baldacchino et al., 2013; Olesen et al., 2025). According to recent research on Hirudo medicinalis (e.g., pigs and ticks of the species Ornithodoros) (Karalyan et al., 2019), leeches may act as an ASFV reservoir in the absence of conspecific hosts. Attention should be paid to potential mechanical vectors, such as domestic or wild animals, that may spread virus-contaminated plant tissue or even pieces of carcass (Liu et al., 2021b). In studies on dead wild boar carcasses, three predatory mammal species and six bird species, including the most prevalent crows and eagles, ate on corpses in different states of decomposition (Probst et al., 2019). This thereby validates the virus’s potential for mechanical transmission, even across extended distances. The importation of tainted pork products, automobiles, clothing, feed, and equipment mostly disseminates ASFV (Mazur-Panasiuk et al., 2019). Transatlantic shipping routes are among the many environmental variables in which ASFV can thrive in animal feed (Dee et al., 2019). The movement of tainted pork products and the habit of feeding domestic pigs swill, which is prevalent in China, the Russian Federation, and the Caucasus, are significant epidemiological contributors to ASFV epidemics (Gogin et al., 2013). ASFV exhibits a high degree of resistance to environmental conditions, including temperature and pH, which is crucial for transmission to remote locations (Zheng et al., 2023). Because of its extremely low susceptibility to drying, rotting, freezing, and thawing, the virus remains stable in the environment for extended periods of time (Petrini et al., 2019). The virus may infect frozen meat for over a thousand days, refrigerated meat for over 500 days, spleens buried in the ground for 280 days, corpses decaying at room temperature for up to 18 months, and bone marrow for more than six months (Fischer et al., 2020). According to reports, ASFV can live for 140, 112, and 399 days in Iberian and white ham, 112 days in Iberian loin, and 399 days in Parma ham (Turlewicz-Podbielska et al., 2021). Additionally, ASFV was found in Italian salami, pig belly, and loin after 18, 60, and 83 days of curing, respectively (Olesen et al., 2020). Therefore, meat products may continue to be a significant source of ASFV. These viruses can spread to previously undiscovered locations, as can these items, which are frequently carried across great distances (Ratnawati et al., 2025). The ASF pandemic is expected to continue to expand due to the virus’s biological nature and its strong resilience to physical and chemical elements. The only way to stop the spread of ASFV is to introduce a safe and effective vaccine (Turlewicz-Podbielska et al., 2021). The nations that appear to be most at risk are those in Asia and Europe, which are near the ASF locations (Ito et al., 2023). However, other nations and continents that engage in a heavy trade in pigs and pork products, such as the United States and Canada, may also experience the disease (Sánchez-Cordón et al., 2018). BioterrorismBioterrorism is defined as the intentional release or dissemination of germs, viruses, or poisons that infect humans, other animals, or plants and cause illness or death (Pereira De Oliveira et al., 2020). ASFV may be released in a bioterrorism incident (Pavone et al., 2024a). This is due to the high rates of infection-related morbidity and mortality, the virus’s endemic spread throughout many nations, its stability in frozen and refrigerated foods, the fact that the pathogen is not zoonotic, the safety of those involved in the release, and the devastating economic effects that accompany its introduction (Li et al., 2022). Since it is difficult to stop this introduction route, it is important to have a strong surveillance system in place for both domestic and feral pigs to guarantee quick detection and differential diagnosis (Pepin et al., 2022). Economic impactThe livestock industry, particularly the pig farming sector, has been severely impacted by ASF disease, where losses occur from upstream to downstream in the form of trade restrictions, declining market value, food insecurity, environmental effects, and animal disease control efforts. These factors have a substantial financial impact on the public and private sectors (Piao et al., 2024). According to the Food and Agriculture Organization (FAO) reports, ASF disease has resulted in significant financial losses in a number of nations, particularly those in Southeast Asia (FAO, 2010). The disease has killed at least 5 million pigs in Southeast Asia (Thailand, Vietnam, and the Philippines), with billions of dollars in economic losses resulting from the disease’s spread throughout the pig population (Fernandez-Colorado et al., 2024). Pig stocks in China fell sharply from 31.3 million to 19.0 million, or 39.3%, in August 2019 and from 320.8 million to 190.9 million, or 40.5%, in August 2019 (Ma et al., 2021). Data from the East Nusa Tenggara Provincial Animal Husbandry Service in Indonesia indicate that by mid-2020, the ASF outbreak that affected 12 districts and cities in 2020 had killed over 24,000 pigs, with total losses estimated at 168 billion rupiahs at an average price of 5 million rupiahs per head. The losses from pig deaths alone, excluding indirect losses from the ASF outbreak, such as barriers to trade in pigs and their products, decreased market value, food insecurity, environmental effects, and expenses for disease management, are undoubtedly even greater, reaching trillions of rupiah as of January 2022. These data were only recorded in 2020; however, ASF disease was still present in 2022 (Ciputra et al., 2023). VaccinationCreating a vaccination against ASFV that works is difficult. Currently, vaccination is not available for several reasons, such as the inability to identify protective antigens, the lack of knowledge about the diversity of virus strains that circulate in natural reservoirs, and the imperfect understanding of virus–host cell interactions (Arias et al., 2017). Several vaccination options, such as DNA vaccines, subunit vaccines based on recombinant proteins, and vaccines with naturally or artificially deleted genes, have been explored with differing degrees of success (Revilla et al., 2018). Nevertheless, none offers total security. Several low-virulence strains that produced chronic forms of ASF disease during the 1960–1995 pandemic are thought to have originated from the use of live attenuated vaccine strains in the Iberian Peninsula, despite the fact that these strains have been established and demonstrated to offer protection against homologous strain challenge (van den Born et al., 2025). Despite these setbacks, live attenuated vaccines are still being tested for their ability to provide protection. Although the effectiveness of ASFV knockout mutants has been assessed, the results have been mixed. Afonso et al. (1998) identified a highly conserved gene known as NL and discovered that the virus was completely attenuated in domestic pigs when the gene from European pathogenic strains was deleted. Two extremely virulent African ASFV strains were given the NL deletion mutants, and when these strains were injected into domestic pigs, they remained virulent even without NL. These results imply that the function of the NL gene is not necessary for this ASFV strain and that a live attenuated ASFV vaccine cannot be created by merely deleting the NL gene. In vitro analysis of the highly conserved 9GL gene revealed that its protein affects virus proliferation and virion maturation in macrophage cells (Vu and McVey, 2024). Deletion of 9GL resulted in a growth-deficient mutant in culture, which was significantly attenuated in domestic pigs (O’Donnell et al., 2016). This mutant is being further tested as a potential ASFV vaccine candidate. Complete protection was achieved by immunization with the 9GL knockout virus and then challenge with the wild-type ASFV strain (Yang et al., 2025). Additionally, the ASFV immunomodulatory protein A238L prevents the activation of the NFAT and NF-B pathways, which control the production of pro-inflammatory cytokines (Niu et al., 2023). This protein may help the virus evade the host immune response because it is believed to be a strong immunosuppressor. Pigs inoculated with the A238L mutant virus had higher levels of TNFα, a strong pro-inflammatory cytokine (Zheng et al., 2022). The use of viruses with modified immunomodulatory proteins to boost the host immune response against virulent threats requires further investigation. Several viral proteins have also been used to describe recombinant protein vaccines. The externally situated proteins p30 and p54 are implicated in the attachment and internalization of viruses, respectively (Gómez-Puertas et al., 1998). Although domestic pigs are immunized with recombinant p54 or p30 proteins, neutralizing antibodies are produced, the illness course remains unaltered, and they are not protected against lethal challenge (Neilan et al., 2004). The p54 and p30 vaccinations work together to develop neutralizing antibodies and alter disease progression, providing a broad spectrum of protection (Orosco, 2023). Simbulan et al. (2024) assessed the capacity of 46 peptides resembling viral proteins to trigger a protective immune response. Vaccinating domestic pigs with certain combinations of these peptides delayed mortality, which calls for more research. All pigs survived a challenge with a virulent virus following vaccination with a baculovirus vector expressing ASFV hemagglutinin (Lacasta et al., 2014). ASFV has a great deal of genetic and antigenic heterogeneity, and the virus uses a variety of tactics to avoid the host immune response, making vaccine development difficult and continuous. More research is necessary to create a vaccine that offers a high level of protection against aggressive ASFV strains and is physiologically safe. According to subject matter experts, live attenuated vaccines are the most promising options for the near future due to their successful trials (Deutschmann et al., 2022). Nevertheless, additional studies are required to guarantee vaccination safety, long-term effectiveness, and the capacity to differentiate between naturally infected and vaccinated animals (Cadenas-Fernández et al., 2024). ControlBecause ASF is extremely contagious and transcends national boundaries, international cooperation is essential to managing the outbreak (Ratnawati et al., 2025). Contaminated pork products, live pig commerce, and the movement of feral pigs are all ways that ASF can spread (Ceruti et al., 2025). It is challenging to stop the spread of the virus without international cooperation, particularly in regions next to afflicted nations (Pavone et al., 2024b). ASF control depends on preventive measures, including biosecurity, quarantine, and trade restrictions, because no proven vaccine or therapy has been established (Kim et al., 2021). International agencies such as the Food and Agriculture Organization (FAO) and the WOAH collaborate with nations to establish control guidelines, exchange data, and create plans for preventing and detecting outbreaks (FAO of the United Nations, 2010). Monitoring the trade and movement of animals is another crucial aspect of international collaboration to stop the unlawful spread of ASF (Ceruti et al., 2025). Furthermore, international technology and information exchange can hasten the creation of long-term fixes, such as successful vaccination (Wu et al., 2020). ASF outbreaks can be better managed with international cooperation, which will lessen their harmful effects on the global economy and livestock sector (Costard et al., 2009). Education is also crucial for farmers and the community to identify ASF signs and promptly report suspected cases (Moskalenko et al., 2024). Strict control measures and strong collaboration can stop the spread of ASF, thereby reducing its effects on the pig farming sector and the entire economy. In addition to implementing conventional biosecurity measures, unique vector control tactics are needed when soft ticks from the genus Ornithodoros are used as vectors (Gaudreault et al., 2020). Management of tick populations surrounding farms should be part of control strategies because Ornithodoros ticks can be persistent carriers and spreaders of viruses (Makwarela et al., 2025). Maintaining the cleanliness of the cage environment, eliminating locations that can become flea habitats, and administering safe insecticides to minimize the vector population are successful techniques (Liu et al., 2021b). Furthermore, producers must ensure that pigs do not come into direct contact with tick-infected places, such as dirty pens or tainted feed troughs (De Oliveira et al., 2020b). The primary control measure is biosecurity, which includes restricting vehicle and human access to the farm, cleaning equipment, and avoiding feed that contains tainted pork products (Juszkiewicz et al., 2023). To prevent the virus from spreading to uninfected areas, it is also crucial to monitor the movement of pigs and pork products (Penrith et al., 2023). In the event of an outbreak, the transmission chain must be broken immediately by implementing emergency measures, including rigorous quarantine and culling of affected pigs (Wang et al., 2018). Strict surveillance, biosecurity procedures, and vector management will reduce the risk of ASF transmission, thereby protecting the pig population and the livestock sector. LimitationsAlthough various scientific databases have been used to search for ASF literature, this review still has some limitations. The selection of keywords and time limits may result in some relevant articles not being accommodated. Not all available studies are fully accessible, which may lead to selection bias. Nevertheless, this step was taken to ensure the quality and relevance of the literature used and to provide a comprehensive and up-to-date overview of ASF. ConclusionIn conclusion, ASF disease poses a significant threat to global pig farming due to its high mortality rate and rapid spread across continents, necessitating urgent international cooperation and stringent biosecurity measures for effective control and prevention. As no treatment or vaccine is available, the focus remains on rapid diagnosis and sanitation to reduce the impact of this deadly disease. AcknowledgmentsThe authors thank Universitas Airlangga and the National Research and Innovation Agency (BRIN) for their support. Author’s contributionsMS, ARK, AW, and MS drafted the manuscript. WW, BWKW, RZA, and AOA revised and edited the manuscript. IM, IF, SW, IBM, and DAAK prepared and critically checked this manuscript. AP, AKP, ATK, and MKJK edited the references. All authors have read and approved the final version of the manuscript. Conflict of interestThe authors declare no conflict of interest. FundingThe authors would like to thank Universitas Airlangga for managerial support and Salma Firdausya Qurrotunnada Noor, Eunice Wong Hui Wen, Joo Jia Yin, and Rahma Novhira for technical support. This research was funded by the Directorate of Research and Community Service, Deputy for Strengthening Research and Technology, Ministry of Research and Technology/National Research and Innovation Agency for the fiscal year 2022 (Chancellor’s Decree number: 770/UN3.14/PT/2022). Data availabilityThe literature used in this study was collected from scientific databases, such as PubMed, Web of Science, and Scopus, using the keywords “ASF” and “African Swine Fever.” A search was conducted for articles published between 2000 and 2025 to obtain the latest and relevant information on ASF development, epidemiology, and control. All relevant articles were then selected based on the availability of the title, abstract, and full text. ReferencesAfonso, C.L., Carrillo, C., Zsak, L., Rock, D.L. and Borca, M.V. 1998. African swine fever virus NL gene is not required for virus virulence. J. Gen. Virol. 79(Pt 10), 2543–2547. Aicher, S.M., Monaghan, P., Netherton, C.L. and Hawes, P.C. 2021. Unpicking the secrets of African swine fever viral replication sites. Viruses 13(1), 77. Alejo, A., Andrés, G. and Salas, M.L. 2003. African Swine Fever virus protease is essential for core maturation and infectivity. J. Virol. 77(10), 5571–5577. Alejo, A., Matamoros, T., Guerra, M. and Andrés, G. 2018. A proteomic atlas of the African swine fever virus particle. J. Virol. 92(23), e01293. Alonso, C., Galindo, I., Cuesta-Geijo, M.A., Cabezas, M., Hernaez, B. and Muñoz-Moreno, R. 2013. African swine fever virus-cell interactions: from virus entry to cell survival. Virus Res. 173(1), 42–57. Ambagala, A., Goonewardene, K., Kanoa, I.E., Than, T.T., Nguyen, V.T., Lai, T.N.H., Nguyen, T.L., Erdelyan, C.N.G., Robert, E., Tailor, N., Onyilagha, C., Lamboo, L., Handel, K., Nebroski, M., Vernygora, O., Lung, O. and Le, V.P. 2024. Characterization of an African swine fever virus field isolate from Vietnam with deletions in the left variable multigene family region. Viruses 16(4), 571. Andrés, G., Alejo, A., Salas, J. and Salas, M.L. 2002. African swine fever virus polyproteins pp220 and pp62 assemble into the core shell. J. Virol. 76(24), 12473–12482. Andrés, G., Alejo, A., Simón-Mateo, C. and Salas, M.L. 2001. African swine fever virus protease, a new viral member of the SUMO-1-specific protease family. J. Biol. Chem. 276(1), 780–787. Andrés, G., Charro, D., Matamoros, T., Dillard, R.S. and Abrescia, N.G.A. 2020. The cryo-EM structure of African swine fever virus unravels a unique architecture comprising two icosahedral protein capsids and two lipoprotein membranes. J. Biol. Chem. 295(1), 1–12. Anholt, H., Hillman, V., Vaughan, J. and Smyth, N. 2023. The soft tick Ornithodoros moubata and ts role in the epidemiology of African swine fever in Central Malawi. J. Wildl. Dis. 59(3), 465–471. Arias, M., De La Torre, A., Dixon, L., Gallardo, C., Jori, F., Laddomada, A., Martins, C., Parkhouse, R.M., Revilla, Y., Rodriguez, F. and Sanchez-Vizcaino, J.M. 2017. Approaches and perspectives for development of African swine fever virus vaccines. Vaccines 5(4), 35. Avagyan, H.R., Hakobyan, S.A., Poghosyan, A.A., Bayramyan, N.V., Arzumanyan, H.H., Abroyan, L.O., Avetisyan, A.S., Hakobyan, L.A., Karalova, E.M. and Karalyan, Z.A. 2022. African Swine fever virus manipulates the cell cycle of G0-infected cells to access cellular nucleotides. Viruses 14(8), 1593. Baldacchino, F., Muenworn, V., Desquesnes, M., Desoli, F., Charoenviriyaphap, T. and Duvallet, G. 2013. Transmission of pathogens by Stomoxysflies (Diptera, Muscidae): a review. Parasite 20(1), 26. Blome, S., Gabriel, C. and Beer, M. 2013. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 173(1), 122–130. Boinas, F., Ribeiro, R., Madeira, S., Palma, M., De Carvalho, I.L., Núncio, S. and Wilson, A.J. 2014. The medical and veterinary role of Ornithodoros erraticuscomplex ticks (Acari: ixodida) on the Iberian Peninsula. J. Vector Ecol. 39(2), 238–248. Bosch, J., Barasona, J.A., Cadenas-Fernández, E., Jurado, C., Pintore, A., Denurra, D., Cherchi, M., Vicente, J. and Sánchez-Vizcaíno, J.M. 2020. Retrospective spatial analysis for African swine fever in endemic areas to assess interactions between susceptible host populations. PLoS. One 15(5), e0233473. Brookes, V.J., Barrett, T.E., Ward, M.P., Roby, J.A., Hernandez-Jover, M., Cross, E.M., Donnelly, C.M., Barnes, T.S., Wilson, C.S. and Khalfan, S. 2021. A scoping review of African swine fever virus spread between domestic and free‐living pigs. Transboundary Emerg. Dis. 68(5), 2643–2656. Brown, V.R., Miller, R.S., Pepin, K.M., Carlisle, K.M., Cook, M.A., Vanicek, C.F., Holmstrom, L.K., Rochette, L.T. and Smyser, T.J. 2024. African swine fever at the wildlife-livestock interface: challenges for management and outbreak response within invasive wild pigs in the United States. Front. Vet. Sci. 11(1), 1348123. Cackett, G., Sýkora, M. and Werner, F. 2020. Transcriptome view of a killer: african swine fever virus. Biochem. Soc. Trans. 48(4), 1569–1581. Cadenas-Fernández, E., Barroso-Arévalo, S., Kosowska, A., Díaz-Frutos, M., Gallardo, C., Rodríguez-Bertos, A., Bosch, J., Sánchez-Vizcaíno, J.M. and Barasona, J.A. 2024. Challenging boundaries: is cross-protection evaluation necessary for African swine fever vaccine development? A case of oral vaccination in wild boar. Front. Immunol. 15(1), 1388812. Ceruti, A., Kobialka, R.M., Abd El Wahed, A. and Truyen, U. 2025. African swine fever: a one health perspective and global challenges. Animals 15(7), 928. Chen, S., Wang, T., Luo, R., Lu, Z., Lan, J., Sun, Y., Fu, Q. and Qiu, H.J. 2024. Genetic variations of African swine fever virus: major challenges and prospects. Viruses 16(6), 913. Chenais, E., Depner, K., Guberti, V., Dietze, K., Viltrop, A. and Ståhl, K. 2019. Epidemiological considerations on African swine fever in Europe 2014-2018. Porcine Health Manag. 5(1), 6. Chenais, E., Ståhl, K., Guberti, V. and Depner, K. 2018. Identification of wild boar-habitat epidemiologic cycle in African swine fever epizootic. Emerg. Infect. Dis. 24(4), 810–812. Cheng, J. and Ward, M.P. 2022. Risk factors for the spread of African Swine Fever in China: a systematic review of Chinese‐language literature. Transbound. Emerg. Dis. 69(5), e1289–e1298. Chuong, V.D., Schambow, R.A., Diep, N.T., Minh, P.Q., Long, N.V., To Nga, B.T. and Perez, A.M. 2025. Epidemiology and control of African swine fever in Vietnam: a scoping review. Pathogens 14(4), 329. Ciputra, L.A., Rahman, A.S. and Nurfadhillah, B. 2023. African swine fever and its socio-economic impacts in Indonesia. Media Kedokteran Hewan 34(3), 171–182. Costard, S., Wieland, B., De Glanville, W., Jori, F., Rowlands, R., Vosloo, W., Roger, F., Pfeiffer, D.U. and Dixon, L.K. 2009. African swine fever: how can global spread be prevented?. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 364(1530), 2683–2696. Cwynar, P., Stojkov, J. and Wlazlak, K. 2019. African swine fever status in Europe. Viruses 11(4), 310. Davies, K., Goatley, L.C., Guinat, C., Netherton, C.L., Gubbins, S., Dixon, L.K. and Reis, A.L. 2017. Survival of African swine fever virus in excretions from pigs experimentally infected with the Georgia 2007/1 isolate. Transbound. Emerg. Dis. 64(2), 425–431. De La Torre, A., Bosch, J., Sánchez-Vizcaíno, J.M., Ito, S., Muñoz, C., Iglesias, I. and Martínez-Avilés, M. 2022. African swine fever survey in a European context. Pathogens 11(2), 137. De Oliveira, R.P., Hutet, E., Lancelot, R., Paboeuf, F., Duhayon, M., Boinas, F., De León, A.A.L., Filatov, S., Le Potier, M.F. and Vial, L. 2020a. Differential vector competence of Ornithodoros soft ticks for African swine fever virus: what if it involves more than just crossing organic barriers in ticks?. Parasites Vectors 13(1), 618. De Oliveira, M., Mason-Buck, G., Ballard, D., Branicki, W. and Amorim, A. 2020b. Biowarfare, bioterrorism and biocrime: a historical overview on microbial harmful applications. Forensic Sci. Int. 314(1), 110366. Dee, S.A., Bauermann, F.V., Niederwerder, M.C., Singrey, A., Clement, T., De Lima, M., Long, C., Patterson, G., Sheahan, M.A., Stoian, A.M.M., Petrovan, V., Jones, C.K., Jong, J., Ji, J., Spronk, G.D., Minion, L., Christopher-Hennings, J., Zimmerman, J.J., Rowland, R.R.R., Nelson, E., Sundberg, P. and Diel, D.G. 2019. Correction: survival of viral pathogens in animal feed ingredients under transboundary shipping models. PLoS. One 14(3), e0214529. Deutschmann, P., Carrau, T., Sehl-Ewert, J., Forth, J.H., Viaplana, E., Mancera, J.C., Urniza, A., Beer, M. and Blome, S. 2022. Taking a promising vaccine candidate further: efficacy of ASFV-G-ΔMGF after intramuscular vaccination of domestic pigs and oral vaccination of wild boar. Pathogens 11(9), 996. Dharmayanti, N.I., Sendow, I., Ratnawati, A., Settypalli, T.B.K., Saepulloh, M., Dundon, W.G., Nuradji, H., Naletoski, I., Cattoli, G. and Lamien, C.E. 2021. African swine fever in North Sumatra and West Java provinces in 2019 and 2020, Indonesia. Transbound. Emerg. Dis. 68(5), 2890–2896. Ding, Y. and Wang, Y. 2020. Big government: the fight against the African Swine Fever in China. J. Biosaf. Biosecur. 2(1), 44–49. Dixon, L.K., Chapman, D.A., Netherton, C.L. and Upton, C. 2013. African swine fever virus replication and genomics. Virus Res. 173(1), 3–14. Dixon, L.K., Islam, M., Nash, R. and Reis, A.L. 2019. African swine fever virus evasion of host defences. Virus. Res. 266(1), 25–33. Droesbeke, B., Balmelle, N., Cay, A.B., Han, S., Oh, D., Nauwynck, H.J. and Tignon, M. 2024. Replication kinetics and infectivity of African swine fever virus (ASFV) variants with different genotypes or levels of virulence in cell culture models of primary porcine macrophages. Microbiol. Res. 15(3), 1690–1708. Duan, X., Ru, Y., Yang, W., Ren, J., Hao, R., Qin, X., Li, D. and Zheng, H. 2022. Research progress on the proteins involved in African swine fever virus infection and replication. Front. Immunol. 13(1), 947180. EFSA (European Food Safety Authority)., Boklund, A., Cay, B., Depner, K., Földi, Z., Guberti, V., Masiulis, M., Miteva, A., More, S., Olsevskis, E., Šatrán, P., Spiridon, M., Stahl, K., Thulke, H.H., Viltrop, A., Wozniakowski, G., Broglia, A., Abrahantes, J.C., Dhollander, S., Gogin, A., Verdonck, F., Amato, L., Papanikolaou, A. and Gortázar, C. 2018. Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018). EFSA. J. 16(11), 5494. Elnagar, A., Blome, S., Beer, M. and Hoffmann, B. 2022. Point-of-care testing for sensitive detection of the African swine fever virus genome. Viruses 14(12), 2827. Fan, W., Cao, Y., Jiao, P., Yu, P., Zhang, H., Chen, T., Zhou, X., Qi, Y., Sun, L., Liu, D., Zhu, H., Liu, W., Hu, R. and Li, J. 2022. Synergistic effect of the responses of different tissues against African swine fever virus. Transbound. Emerg. Dis. 69(4), e204–e215. Fasina, F.O., Kissinga, H., Mlowe, F., Mshang’A, S., Matogo, B., Mrema, A., Mhagama, A., Makungu, S., Mtui-Malamsha, N., Sallu, R., Misinzo, G., Magidanga, B., Kivaria, F., Bebay, C., Nong’Ona, S., Kafeero, F. and Nonga, H. 2020. Drivers, risk factors and dynamics of African swine fever outbreaks, Southern Highlands, Tanzania. Pathogens 9(3), 155. Fenster, J.A., Azzinaro, P.A., Dinhobl, M., Borca, M.V., Spinard, E. and Gladue, D.P. 2024. African swine fever virus protein-protein interaction prediction. Viruses 16(7), 1170. Fernández De Marco, M., Salguero, F.J., Bautista, M.J., Núñez, A., Sánchez-Cordón, P.J. and Gómez-Villamandos, J.C. 2007. An immunohistochemical study of the tonsils in pigs with acute African swine fever virus infection. Res. Vet. Sci. 83(2), 198–203. Fernandez-Colorado, C.P., Kim, W.H., Flores, R.A. and Min, W. 2024. African swine fever in the Philippines: a review on surveillance, prevention, and control strategies. Animals 14(12), 1816. Ferreira, H.C., Zúquete, S.T., Wijnveld, M., Weesendorp, E., Jongejan, F., Stegeman, A. and Loeffen, W.L. 2014. No evidence of African swine fever virus replication in hard ticks. Ticks Tick. Borne. Dis. 5(5), 582–589. Fila, M. and Woźniakowski, G. 2020. African swine fever virus - the possible role of flies and other insects in virus transmission. J. Vet. Res. 64(1), 1–7. Fischer, M., Hühr, J., Blome, S., Conraths, F.J. and Probst, C. 2020. Stability of African swine fever virus in carcasses of domestic pigs and wild boar experimentally infected with the ASFV “Estonia 2014” isolate. Viruses 12(10), 1118. Food and Agriculture Organization of the United Nations/World Organisation for Animal Health/World Bank. 2010. Good practices for biosecurity in the pig sector – issues and options in developing and transition countries. In: FAO Animal Production and Health Paper. Vol. 169. Rome: FAO. Franzoni, G., Pedrera, M. and Sánchez-Cordón, P.J. 2023. African swine fever virus infection and cytokine response in vivo: an update. Viruses 15(1), 233. Galindo, I. and Alonso, C. 2017. African swine fever virus: a review. Viruses 9(5), 103. Gallardo, M.C., Reoyo, A.T., Fernández-Pinero, J., Iglesias, I., Muñoz, M.J. and Arias, M.L. 2015. African swine fever: a global view of the current challenge. Porcine Health Manag. 1(1), 21. Gaudreault, N.N., Madden, D.W., Wilson, W.C., Trujillo, J.D. and Richt, J.A. 2020. African swine fever virus: an emerging DNA arbovirus. Front. Vet. Sci. 7(1), 215. Gervasi, V. and Guberti, V. 2021. African swine fever endemic persistence in wild boar populations: key mechanisms explored through modelling. Transbound. Emerg. Dis. 68(5), 2812–2825. Gogin, A., Gerasimov, V., Malogolovkin, A. and Kolbasov, D. 2013. African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus. Res. 173(1), 198–203. Gómez-Puertas, P., Rodríguez, F., Oviedo, J.M., Brun, A., Alonso, C. and Escribano, J.M. 1998. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology 243(2), 461–471. Gómez-Villamandos, J.C., Carrasco, L., Bautista, M.J., Sierra, M.A., Quezada, M., Hervas, J., Mde, L.C., Ruiz-Villamor, E., Salguero, F.J., Sónchez-Cordón, P.J., Romanini, S., Núñez, A., Mekonen, T., Méndez, A. and Jover, A. 2003. African swine fever and classical swine fever: a review of the pathogenesis. Dtsch. Tierarztl. Wochenschr. 110(4), 165–169. Gómez-Villamandos, J.C., Hervás, J., Méndez, A., Carrasco, L., Villeda, C.J., Wilkinson, P.J. and Sierra, M.A. 1995. Ultrastructural study of the renal tubular system in acute experimental African swine fever: virus replication in glomerular mesangial cells and in the collecting ducts. Arch. Virol. 140(3), 581–589. González-García, G., Gallardo, C., Montón, M., Barroso-Arévalo, S., Casado, N., Barasona, J.A., Sánchez-Vizcaíno, J.M., Venteo, A., Sastre, P. and Rueda, P. 2025. A novel prototype African swine fever virus DIVA (differentiation between infected and vaccinated animals) serological assay based on the detection of antibodies against the pEP153R, eGFP, and p72 proteins. Vaccines 13(3), 211. Goonewardene, K.B., Chung, C.J., Goolia, M., Blakemore, L., Fabian, A., Mohamed, F., Nfon, C., Clavijo, A., Dodd, K.A. and Ambagala, A. 2021. Evaluation of oral fluid as an aggregate sample for early detection of African swine fever virus using four independent pen‐based experimental studies. Transbound. Emerg. Dis. 68(5), 2867–2877. Guinat, C., Gogin, A., Blome, S., Keil, G., Pollin, R., Pfeiffer, D.U. and Dixon, L. 2016. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Vet. Rec. 178(11), 262–267. Han, S., Oh, D., Vanderheijden, N., Xie, J., Balmelle, N., Tignon, M. and Nauwynck, H.J. 2025. Monoclonal antibodies targeting porcine macrophages are able to inhibit the cell entry of macrophage-tropic viruses (PRRSV and ASFV). Viruses 17(2), 167. Hawes, P.C., Netherton, C.L., Wileman, T.E. and Monaghan, P. 2008. The envelope of intracellular African swine fever virus is composed of a single lipid bilayer. J. Virol. 82(16), 7905–7912. Hernaez, B. and Alonso, C. 2010. Dynamin- and clathrin-dependent endocytosis in African swine fever virus entry. J. Virol. 84(4), 2100–2109. Hernáez, B., Guerra, M., Salas, M.L. and Andrés, G. 2016. African swine fever virus undergoes outer envelope disruption, capsid disassembly and inner envelope fusion before core release from multivesicular endosomes. PLoS Pathog. 12(4), e1005595. Hu, Z., Tian, X., Lai, R., Wang, X. and Li, X. 2023. Current detection methods of African swine fever virus. Front. Vet. Sci. 10(1), 1289676. Huang, T., Li, F., Xia, Y., Zhao, J., Zhu, Y., Liu, Y., Qian, Y. and Zou, X. 2024. African swine fever virus immunosuppression and virulence-related gene. Curr. Issues Mol. Biol. 46(8), 8268–8281. Iscaro, C., Dondo, A., Ruocco, L., Masoero, L., Giammarioli, M., Zoppi, S., Guberti, V. and Feliziani, F. 2022. January 2022: index case of new African swine fever incursion in mainland Italy. Transbound. Emerg. Dis. 69(4), 1707–1711. Ito, S., Kawaguchi, N., Bosch, J., Aguilar-Vega, C. and Sánchez-Vizcaíno, J.M. 2023. What can we learn from the five-year African swine fever epidemic in Asia?. Front. Vet. Sci. 10(1), 1273417. Jean-Pierre, R.P., Hagerman, A.D. and Rich, K.M. 2022. An analysis of African Swine Fever consequences on rural economies and smallholder swine producers in Haiti. Front. Vet. Sci. 9(1), 960344. Jia, N., Ou, Y., Pejsak, Z., Zhang, Y. and Zhang, J. 2017. Roles of African swine fever virus structural proteins in viral infection. J. Vet. Res. 61(2), 135–143. Jo, Y.S. and Gortázar, C. 2021. African swine fever in wild boar: assessing interventions in South Korea. Transbound. Emerg. Dis. 68(5), 2878–2889. Jouvenet, N., Windsor, M., Rietdorf, J., Hawes, P., Monaghan, P., Way, M. and Wileman, T. 2006. African swine fever virus induces filopodia-like projections at the plasma membrane. Cell Microbiol. 8(11), 1803–1811. Juszkiewicz, M., Walczak, M., Woźniakowski, G. and Podgórska, K. 2023. African swine fever: transmission, spread, and control through biosecurity and disinfection, including polish trends. Viruses 15(11), 2275. Karalyan, Z., Avetisyan, A., Avagyan, H., Ghazaryan, H., Vardanyan, T., Manukyan, A., Semerjyan, A. and Voskanyan, H. 2019. Presence and survival of African swine fever virus in leeches. Vet. Microbiol. 237(1), 108421. Karger, A., Pérez-Núñez, D., Urquiza, J., Hinojar, P., Alonso, C., Freitas, F., Revilla, Y., Le Potier, M.F. and Montoya, M. 2019. An update on African swine fever virology. Viruses 11(9), 864. Keita, D., Heath, L. and Albina, E. 2010. Control of African swine fever virus replication by small interfering RNA targeting the A151R and VP72 genes. Antivir. Ther. 15(5), 727–736. Khairullah, A.R., Effendi, M.H., Moses, I.B., Fauzia, K.A., Puspitasari, Y., Riwu, K.H.P., Fauziah, I., Raissa, R., Silaen, O.S.M., Wibowo, S., Yanestria, S.M., Kusala, M.K.J., Abdila, S.R., Pratama, B.P. and Hasib, A. 2024. Classical swine fever: unveiling the complexity through a multifaceted approach. Open Vet. J. 14(10), 2497–2508. Kim, Y.J., Park, B. and Kang, H.E. 2021. Control measures to African swine fever outbreak: active response in South Korea, preparation for the future, and cooperation. J. Vet. Sci. 22(1), e13. Ko, Y.S., Tark, D., Moon, S.H., Kim, D.M., Lee, T.G., Bae, D.Y., Sunwoo, S.Y., Oh, Y. and Cho, H.S. 2023. Alteration of the gut microbiota in pigs infected with African swine fever virus. Vet. Sci. 10(5), 360. Lacasta, A., Ballester, M., Monteagudo, P.L., Rodríguez, J.M., Salas, M.L., Accensi, F., Pina-Pedrero, S., Bensaid, A., Argilaguet, J., López-Soria, S., Hutet, E., Le Potier, M.F. and Rodríguez, F. 2014. Expression library immunization can confer protection against lethal challenge with African swine fever virus. J. Virol. 88(22), 13322–13332. Li, M. and Zheng, H. 2025. Insights and progress on epidemic characteristics, pathogenesis, and preventive measures of African swine fever virus: a review. Virulence 16(1), 2457949. Li, S., Song, J., Liu, J., Zhou, S., Zhao, G., Li, T., Huang, L., Li, J. and Weng, C. 2024. African swine fever virus infection regulates pyroptosis by cleaving gasdermin A via active caspase-3 and caspase-4. J. Biol. Chem. 300(6), 107307. Li, Z., Chen, W., Qiu, Z., Li, Y., Fan, J., Wu, K., Li, X., Zhao, M., Ding, H., Fan, S. and Chen, J. 2022. African swine fever virus: a review. Life 12(8), 1255. Liu, Y., Li, Y., Xie, Z., Ao, Q., Di, D., Yu, W., Lv, L., Zhong, Q., Song, Y., Liao, X., Song, Q., Wang, H. and Chen, H. 2021a. Development and in vivo evaluation of MGF100-1R deletion mutant in an African swine fever virus Chinese strain. Vet. Microbiol. 261(1), 109208. Liu, Y., Zhang, X., Qi, W., Yang, Y., Liu, Z., An, T., Wu, X. and Chen, J. 2021b. Prevention and control strategies of African swine fever and progress on pig farm repopulation in China. Viruses 13(12), 2552. Lv, T., Xie, X., Song, N., Zhang, S., Ding, Y., Liu, K., Diao, L., Chen, X., Jiang, S., Li, T., Zhang, W. and Cao, Y. 2022. Expounding the role of tick in Africa swine fever virus transmission and seeking effective prevention measures: a review. Front. Immunol. 13(1), 1093599. Ma, M., Wang, H.H., Hua, Y., Qin, F. and Yang, J. 2021. African swine fever in China: impacts, responses, and policy implications. Food Policy 102(1), 102065. Makwarela, T.G., Seoraj-Pillai, N. and Nangammbi, T.C. 2025. Tick control strategies: critical insights into chemical, biological, physical, and integrated approaches for effective hard tick management. Vet. Sci. 12(2), 114. Malakauskas, A., Schulz, K., Kukanauskaitė, I., Masiulis, M., Conraths, F.J. and Sauter-Louis, C. 2022. African swine fever outbreaks in lithuanian domestic pigs in 2019. Animals 12(1), 115. Martin, V., Guerra, B., Hernaez, B., Kappler-Gratias, S., Gallardo, F., Guerra, M., Andres, G. and Alejo, A. 2024. A novel live DNA tagging system for African swine fever virus shows that bisbenzimide Hoechst 33342 can effectively block its replication. Antiviral Res. 230(1), 105973. Martínez-Avilés, M. 2023. African swine fever: epidemiology, the design of new diagnostic methods, and vaccine development. Pathogens 12(8), 1042. Mazloum, A., Van Schalkwyk, A., Shotin, A., Zinyakov, N., Igolkin, A., Chernishev, R., Debeljak, Z., Korennoy, F. and Sprygin, A.V. 2023. Whole-genome sequencing of African swine fever virus from wild boars in the Kaliningrad region reveals unique and distinguishing genomic mutations. Front. Vet. Sci. 9(1), 1019808. Mazur-Panasiuk, N., Żmudzki, J. and Woźniakowski, G. 2019. African swine fever virus – persistence in different environmental conditions and the possibility of its indirect transmission. J. Vet. Res. 63(3), 303–310. Moskalenko, L., Schulz, K., Nedosekov, V., Mõtus, K. and Viltrop, A. 2024. Understanding smallholder pigkeepers’ awareness and perceptions of African swine fever and its control measures in Ukraine. Pathogens 13(2), 139. Mulumba-Mfumu, L.K., Saegerman, C., Dixon, L.K., Madimba, K.C., Kazadi, E., Mukalakata, N.T., Oura, C.A.L., Chenais, E., Masembe, C., Ståhl, K., Thiry, E. and Penrith, M.L. 2019. African swine fever: update on Eastern, Central and Southern Africa. Transbound. Emerg. Dis. 66(4), 1462–1480. Nah, J.J., Kwon, O.K., Choi, J.D., Jang, S.H., Lee, H.J., Ahn, D.G., Lee, K., Kang, B., Kang, H.E. and Shin, Y.K. 2022. Development of an indirect ELISA against African swine fever virus using two recombinant antigens, partial p22 and p30. J. Virol. Methods 309(1), 114611. Neilan, J.G., Zsak, L., Lu, Z., Burrage, T.G., Kutish, G.F. and Rock, D.L. 2004. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 319(2), 337–342. Nga, B.T.T., Tran Anh Dao, B., Nguyen Thi, L., Osaki, M., Kawashima, K., Song, D., Salguero, F.J. and Le, V.P. 2020. Clinical and pathological study of the first outbreak cases of African swine fever in Vietnam, 2019. Front. Vet. Sci. 7(1), 392. Nguyen, T.T.H., Nguyen, V.T., Le, P.N., Mai, N.T.A., Dong, V.H., Bui, T.A.D., Nguyen, T.L., Ambagala, A. and Le, V.P. 2023. Pathological characteristics of domestic pigs orally infected with the virus strain causing the first reported African swine fever outbreaks in Vietnam. Pathogens 12(3), 393. Niederwerder, M.C., Stoian, A.M.M., Rowland, R.R.R., Dritz, S.S., Petrovan, V., Constance, L.A., Gebhardt, J.T., Olcha, M., Jones, C.K., Woodworth, J.C., Fang, Y., Liang, J. and Hefley, T.J. 2019. Infectious dose of African swine fever virus when consumed naturally in liquid or feed. Emerg. Infect. Dis. 25(5), 891–897. Nishi, T., Okadera, K., Fukai, K., Yoshizaki, M., Nakasuji, A., Yoneyama, S. and Kokuho, T. 2022. Establishment of a direct PCR assay for simultaneous differential diagnosis of African swine fever and classical swine fever using crude tissue samples. Viruses 14(3), 498. Niu, S., Guo, Y., Wang, X., Wang, Z., Sun, L., Dai, H. and Peng, G. 2023. Innate immune escape and adaptive immune evasion of African swine fever virus: a review. Virology 587(1), 109878. Njau, E.P., Machuka, E.M., Cleaveland, S., Shirima, G.M., Kusiluka, L.J., Okoth, E.A. and Pelle, R. 2021. African swine fever virus (ASFV): biology, genomics and genotypes circulating in Sub-Saharan Africa. Viruses 13(11), 2285. O’Donnell, V., Holinka, L.G., Gladue, D.P., Sanford, B., Krug, P.W., Lu, X., Arzt, J., Reese, B., Carrillo, C., Risatti, G.R. and Borca, M.V. 2015. African swine fever virus Georgia isolate harboring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus. J. Virol. 89(11), 6048–6056. O’Donnell, V., Risatti, G.R., Holinka, L.G., Krug, P.W., Carlson, J., Velazquez-Salinas, L., Azzinaro, P.A., Gladue, D.P. and Borca, M.V. 2016. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. J. Virol. 91(1), e01760–16. Oh, T., Do, D.T., Vo, H.V., Kwon, H.I., Lee, S.C., Kim, M.H., Nguyen, D.T.T., Le, Q.T.V., Tran, T.M., Nguyen, T.T., Lee, J.Y. and Chae, C. 2021. The isolation and replication of African swine fever virus in primary renal-derived swine macrophages. Front. Vet. Sci. 8(1), 645456. Olesen, A.S., Belsham, G.J., Bruun Rasmussen, T., Lohse, L., Bødker, R., Halasa, T., Boklund, A. and Bøtner, A. 2020. Potential routes for indirect transmission of African swine fever virus into domestic pig herds. Transbound. Emerg. Dis. 67(4), 1472–1484. Olesen, A.S., Lazov, C.M., Accensi, F., Johnston, C.M., Rasmussen, T.B., Bøtner, A., Lohse, L. and Belsham, G.J. 2025. Evaluation of the dose of African swine fever virus required to establish infection in pigs following oral uptake. Pathogens 14(2), 119. Olesen, A.S., Lohse, L., Accensi, F., Goldswain, H., Belsham, G.J., Bøtner, A., Netherton, C.L., Dixon, L.K. and Portugal, R. 2024. Inefficient transmission of African swine fever virus to sentinel pigs from an environment contaminated by ASFV-infected pigs under experimental conditions. Transbound. Emerg. Dis. 1(1), 863641. Onisk, D.V., Borca, M.V., Kutish, G., Kramer, E., Irusta, P. and Rock, D.L. 1994. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 198(1), 350–354. Orosco, F. and L. 2023. Current progress in diagnostics, therapeutics, and vaccines for African swine fever virus. Vet. Integr. Sci. 21(3), 751–781. Oura, C.A., Powell, P.P. and Parkhouse, R.M. 1998. African swine fever: a disease characterized by apoptosis. J. Gen. Virol. 79(Pt 6), 1427–1438. Patil, S.S., Suresh, K.P., Vashist, V., Prajapati, A., Pattnaik, B. and Roy, P. 2020. African swine fever: a permanent threat to Indian pigs. Vet. World 13(10), 2275–2285. Pavone, S., Bellini, S., Iscaro, C., Farioli, M., Chiari, M., Lavazza, A., Ruocco, L., Lelli, D., Pintus, G., Prati, P. and Feliziani, F. 2024a. Strategic challenges to the eradication of African swine fever genotype II in domestic pigs in North Italy. Animals 14(9), 1295. Pavone, S., Iscaro, C., Giammarioli, M., Beato, M.S., Righi, C., Petrini, S., Costarelli, S. and Feliziani, F. 2024b. Biological containment for African swine fever (ASF) laboratories and animal facilities: the Italian challenge in bridging the present regulatory gap and enhancing biosafety and biosecurity measures. Animals 14(3), 454. Penrith, M.L., Thomson, G.R., Bastos, A.D., Phiri, O.C., Lubisi, B.A., Du Plessis, E.C., Macome, F., Pinto, F., Botha, B. and Esterhuysen, J. 2004. An investigation into natural resistance to African swine fever in domestic pigs from an endemic area in southern Africa. Rev. Sci. Tech. 23(3), 965–977. Penrith, M.L., Van Emmenes, J., Hakizimana, J.N., Heath, L., Kabuuka, T., Misinzo, G., Odoom, T., Wade, A., Zerbo, H.L. and Luka, P.D. 2024. African swine fever diagnosis in Africa: challenges and opportunities. Pathogens 13(4), 296. Penrith, M.L., Van Heerden, J., Pfeiffer, D.U., Oļševskis, E., Depner, K. and Chenais, E. 2023. Innovative research offers new hope for managing African swine fever better in resource-limited smallholder farming settings: a timely update. Pathogens 12(2), 355. Pepin, K.M., Brown, V.R., Yang, A., Beasley, J.C., Boughton, R., Vercauteren, K.C., Miller, R.S. and Bevins, S.N. 2022. Optimising response to an introduction of African swine fever in wild pigs. Transbound. Emerg. Dis. 69(5), e3111–e3127. Pereira De Oliveira, R., Hutet, E., Duhayon, M., Guionnet, J.M., Paboeuf, F., Vial, L. and Le Potier, M.F. 2020. Successful infection of domestic pigs by ingestion of the European soft tick O. erraticus that fed on African swine fever virus infected pig. Viruses 12(3), 300. Pérez, J., Bautista, M.J., Rodríguez, F., Wilkinson, P.J., Sierra, M.A. and Martin De Las Mulas, J. 1997. Double‐labelling immunohistochemical study of megakaryocytes in African swine fever. Vet. Rec. 141(15), 386–390. Petrini, S., Feliziani, F., Casciari, C., Giammarioli, M., Torresi, C. and De Mia, G.M. 2019. Survival of African swine fever virus (ASFV) in various traditional Italian dry-cured meat products. Prev. Vet. Med. 162(1), 126–130. Petrini, S., Righi, C., Mészáros, I., D’Errico, F., Tamás, V., Pela, M., Olasz, F., Gallardo, C., Fernandez-Pinero, J., Göltl, E., Magyar, T., Feliziani, F. and Zádori, Z. 2023. The production of recombinant African swine fever virus Lv17/WB/Rie1 strains and their in vitro and in vivo characterizations. Vaccines 11(12), 1860. Piao, S., Jin, X., Hu, S. and Lee, J.Y. 2024. The impact of African swine fever on the efficiency of China’s pig farming industry. Sustainability 16(17), 7819. Popescu, L., Gaudreault, N.N., Whitworth, K.M., Murgia, M.V., Nietfeld, J.C., Mileham, A., Samuel, M., Wells, K.D., Prather, R.S. and Rowland, R.R.R. 2017. Genetically edited pigs lacking CD163 show no resistance following infection with the African swine fever virus isolate, Georgia 2007/1. Virology 501(1), 102–106. Portugal, R., Coelho, J., Höper, D., Little, N.S., Smithson, C., Upton, C., Martins, C., Leitão, A. and Keil, G.M. 2015. Related strains of African swine fever virus with different virulence: genome comparison and analysis. J. Gen. Virol. 96(Pt 2), 408–419. Probst, C., Gethmann, J., Amler, S., Globig, A., Knoll, B. and Conraths, F.J. 2019. The potential role of scavengers in spreading African swine fever among wild boar. Sci. Rep. 9(1), 11450. Ramirez-Medina, E., O’Donnell, V., Silva, E., Espinoza, N., Velazquez-Salinas, L., Moran, K., Daite, D.A., Barrette, R., Faburay, B., Holland, R., Gladue, D.P. and Borca, M.V. 2022. Experimental infection of domestic pigs with an African swine fever virus field strain isolated in 2021 from the Dominican Republic. Viruses 14(5), 1090. Ramirez-Medina, E., Vuono, E.A., Rai, A., Espinoza, N., Valladares, A., Spinard, E., Velazquez-Salinas, L., Gladue, D.P. and Borca, M.V. 2023. Evaluation of the function of ASFV gene E66L in the process of virus replication and virulence in swine. Viruses 15(2), 566. Ran, Y., Li, D., Xiong, M.G., Liu, H.N., Feng, T., Shi, Z.W., Li, Y.H., Wu, H.N., Wang, S.Y., Zheng, H.X. and Wang, Y.Y. 2022. African swine fever virus I267L acts as an important virulence factor by inhibiting RNA polymerase III-RIG-I-mediated innate immunity. PLoS Pathog. 18(1), e1010270. Ratnawati, A., Hartawan, R., Sendow, I., Saepulloh, M., Sumarningsih, U., Hewajuli, D.A., Zainuddin, N., Dharmayanti, N.L.P.I., Wibawan, I.W.T. and Mayasari, N.L.P.I. 2025. Transboundary risk of African swine fever (ASF): detection of ASF virus genotype II in pork products carried by international travelers to Indonesia. Vet. World 18(2), 280–286. Revilla, Y., Pérez-Núñez, D. and Richt, J.A. 2018. African swine fever virus biology and vaccine approaches. Adv. Virus Res. 100(1), 41–74. Rodríguez, I., Nogal, M.L., Redrejo-Rodríguez, M., Bustos, M.J. and Salas, M.L. 2009. The African swine fever virus virion membrane protein pE248R is required for virus infectivity and an early postentry event. J. Virol. 83(23), 12290–12300. Rodríguez, J.M. and Salas, M.L. 2013. African swine fever virus transcription. Virus. Res. 173(1), 15–28. Rodríguez, J.M., Moreno, L.T., Alejo, A., Lacasta, A., Rodríguez, F. and Salas, M.L. 2015. Genome sequence of African swine fever virus BA71, the virulent parental strain of the nonpathogenic and tissue-culture adapted BA71V. PLoS One 10(11), e0142889. Ruedas-Torres, I., Thi To Nga, B. and Salguero, F.J. 2024. Pathogenicity and virulence of African swine fever virus. Virulence 15(1), 2375550. Ruiz-Saenz, J., Diaz, A., Bonilla-Aldana, D.K., Rodríguez-Morales, A.J., Martinez-Gutierrez, M. and Aguilar, P.V. 2022. African swine fever virus: a re-emerging threat to the swine industry and food security in the Americas. Front. Microbiol. 13(1), 1011891. Salas, M.L. and Andrés, G. 2013. African swine fever virus morphogenesis. Virus. Res. 173(1), 29–41. Salguero, F.J. 2020. Comparative pathology and pathogenesis of African swine fever infection in swine. Front. Vet. Sci. 7(1), 282. Salguero, F.J., Ruiz-Villamor, E., Bautista, M.J., Sánchez-Cordón, P.J., Carrasco, L. and Gómez-Villamandos, J.C. 2002. Changes in macrophages in spleen and lymph nodes during acute African swine fever: expression of cytokines. Vet. Immunol. Immunopathol. 90(1–2), 11–22. Salling, M. 2025. Assessing the price effects of African swine fever in the China market. Explor. Foods Foodomics 3(1), 101064. Sánchez, E.G., Quintas, A., Nogal, M., Castelló, A. and Revilla, Y. 2013. African swine fever virus controls the host transcription and cellular machinery of protein synthesis. Virus Res. 173(1), 58–75. Sánchez-Cordón, P.J., Montoya, M., Reis, A.L. and Dixon, L.K. 2018. African swine fever: a re-emerging viral disease threatening the global pig industry. Vet. J. 233(1), 41–48. Sánchez-Cordón, P.J., Nunez, A., Neimanis, A., Wikström-Lassa, E., Montoya, M., Crooke, H. and Gavier-Widén, D. 2019. African swine fever: disease dynamics in wild boar experimentally infected with ASFV isolates belonging to genotype I and II. Viruses 11(9), 852. Sánchez-Vizcaíno, J.M., Mur, L., Gomez-Villamandos, J.C. and Carrasco, L. 2015. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 152(1), 9–21. Sanna, G., Dei Giudici, S., Bacciu, D., Angioi, P.P., Giammarioli, M., De Mia, G.M. and Oggiano, A. 2017. Improved strategy for molecular characterization of African swine fever viruses from sardinia, based on analysis of p30, CD2V and I73R/I329L variable regions. Transbound. Emerg. Dis. 64(4), 1280–1286. Sauter-Louis, C., Conraths, F.J., Probst, C., Blohm, U., Schulz, K., Sehl, J., Fischer, M., Forth, J.H., Zani, L., Depner, K., Mettenleiter, T.C., Beer, M. and Blome, S. 2021. African swine fever in wild boar in Europe-a review. Viruses 13(9), 1717. Schambow, R.A., Hussain, S., Antognoli, M.C., Kreindel, S., Reyes, R. and Perez, A.M. 2023. Epidemiological assessment of African swine fever spread in the dominican republic. Pathogens 12(12), 1414. Schulz, K., Staubach, C. and Blome, S. 2017. African and classical swine fever: similarities, differences and epidemiological consequences. Vet. Res. 48(1), 84. Sehl-Ewert, J., Deutschmann, P., Breithaupt, A. and Blome, S. 2022. Pathology of African swine fever in wild boar carcasses naturally infected with German virus variants. Pathogens 11(11), 1386. Shaw, C., Mclure, A. and Glass, K. 2024. African swine fever in wild boar: investigating model assumptions and structure. R. Soc. Open Sci. 11(5), 231319. Simbulan, A.M., Banico, E.C., Sira, E.M.J.S., Odchimar, N.M.O. and Orosco, F.L. 2024. Immunoinformatics-guided approach for designing a pan-proteome multi-epitope subunit vaccine against African swine fever virus. Sci. Rep. 14(1), 1354. Simões, M., Martins, C. and Ferreira, F. 2015. Early intranuclear replication of African swine fever virus genome modifies the landscape of the host cell nucleus. Virus. Res. 210(1), 1–7. Ståhl, K., Sternberg-Lewerin, S., Blome, S., Viltrop, A., Penrith, M.L. and Chenais, E. 2019. Lack of evidence for long term carriers of African swine fever virusa systematic review. Virus. Res. 272(1), 197725. Suárez, C., Gutiérrez-Berzal, J., Andrés, G., Salas, M.L. and Rodríguez, J.M. 2010. African swine fever virus protein p17 is essential for the progression of viral membrane precursors toward icosahedral intermediates. J. Virol. 84(15), 7484–7499. Tamás, V., Righi, C., Mészáros, I., D’Errico, F., Olasz, F., Casciari, C., Zádori, Z., Magyar, T., Petrini, S. and Feliziani, F. 2023. Involvement of the MGF 110-11L gene in the African swine fever replication and virulence. Vaccines 11(4), 846. Tenaya, W.M., Swacita, I.B.N., Wirata, K., Damriyasa, M., Besung, N.K., Suarsana, N., Sari, T.K. and Agustina, K.K. 2023. A study of African swine fever virus in regional VI of the disease investigation center of Denpasar Bali in Indonesia. Vet. World 16(4), 844–850. Torma, G., Tombácz, D., Csabai, Z., Moldován, N., Mészáros, I., Zádori, Z. and Boldogkői, Z. 2021. Combined short and long-read sequencing reveals a complex transcriptomic architecture of African swine fever virus. Viruses 13(4), 579. Turlewicz-Podbielska, H., Kuriga, A., Niemyjski, R., Tarasiuk, G. and Pomorska-Mól, M. 2021. African swine fever virus as a difficult opponent in the fight for a vaccine-current data. Viruses 13(7), 1212. Vallée, I., Tait, S.W. and Powell, P.P. 2001. African swine fever virus infection of porcine aortic endothelial cells leads to inhibition of inflammatory responses, activation of the thrombotic state, and apoptosis. J. Virol. 75(21), 10372–10382. Van Den Born, E., Olasz, F., Mészáros, I., Göltl, E., Oláh, B., Joshi, J., Van Kilsdonk, E., Segers, R. and Zádori, Z. 2025. African swine fever virus vaccine strain Asfv-G-∆I177l reverts to virulence and negatively affects reproductive performance. NPJ Vaccines 10(1), 46. Venkateswaran, D., Prakash, A., Nguyen, Q.A., Salman, M., Suntisukwattana, R., Atthaapa, W., Tantituvanont, A., Lin, H., Songkasupa, T. and Nilubol, D. 2024. Comprehensive characterization of the genetic landscape of African swine fever virus: insights into infection dynamics, immunomodulation, virulence and genes with unknown function. Animals 14(15), 2187. Vu, H.L.X. and McVey, D.S. 2024. Recent progress on gene-deleted live-attenuated African swine fever virus vaccines. NPJ Vaccines 9(1), 60. Walczak, M., Szczotka-Bochniarz, A., Żmudzki, J., Juszkiewicz, M., Szymankiewicz, K., Niemczuk, K., Pérez-Núñez, D., Liu, L. and Revilla, Y. 2022. Non-invasive sampling in the aspect of African swine fever detection-a risk to accurate diagnosis. Viruses 14(8), 1756. Wang, L., Fu, D., Tesfagaber, W., Li, F., Chen, W., Zhu, Y., Sun, E., Wang, W., He, X., Guo, Y., Bu, Z. and Zhao, D. 2022a. Development of an ELISA method to differentiate animals infected with wild-type African swine fever viruses and attenuated HLJ/18-7GD vaccine candidate. Viruses 14(8), 1731. Wang, T., Luo, R., Zhang, J., Lu, Z., Li, L.F., Zheng, Y.H., Pan, L., Lan, J., Zhai, H., Huang, S., Sun, Y. and Qiu, H.J. 2023. The MGF300-2R protein of African swine fever virus is associated with viral pathogenicity by promoting the autophagic degradation of IKKα and IKKβ through the recruitment of TOLLIP. PLoS Pathogens 19(8), e1011580. Wang, T., Sun, Y. and Qiu, H.J. 2018. African swine fever: an unprecedented disaster and challenge to China. Infect. Dis. Poverty 7(1), 111. Wang, Y., Kang, W., Yang, W., Zhang, J., Li, D. and Zheng, H. 2021. Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: a review. Front. Immunol. 12(1), 715582. Wang, Z., Ai, Q., Huang, S., Ou, Y., Gao, Y., Tong, T. and Fan, H. 2022b. Immune escape mechanism and vaccine research progress of African swine fever virus. Vaccines (Basel). 10(3), 344. Wu, K., Liu, J., Wang, L., Fan, S., Li, Z., Li, Y., Yi, L., Ding, H., Zhao, M. and Chen, J. 2020. Current state of global African swine fever vaccine development under the prevalence and transmission of ASF in China. Vaccines 8(3), 531. Yang, S., Miao, C., Liu, W., Zhang, G., Shao, J. and Chang, H. 2023. Structure and function of African swine fever virus proteins: current understanding. Front. Microbiol. 14(1), 1043129. Yang, Y., Yuan, H., Zhang, Y., Luan, J. and Wang, H. 2025. Progress in African swine fever vector vaccine sevelopment. Int. J. Mol. Sci. 26(3), 921. Yoo, D., Kim, H., Lee, J.Y. and Yoo, H.S. 2020. African swine fever: etiology, epidemiological status in Korea, and perspective on control. J. Vet. Sci. 21(2), 38. Zhang, D., Hao, Y., Yang, X., Shi, X., Zhao, D., Chen, L., Liu, H., Zhu, Z. and Zheng, H. 2024. ASFV infection induces macrophage necroptosis and releases proinflammatory cytokine by ZBP1-RIPK3-MLKL necrosome activation. Front. Microbiol. 15(1), 1419615. Zhang, H., Zhao, S., Zhang, H., Qin, Z., Shan, H. and Cai, X. 2023. Vaccines for African swine fever: an update. Front. Microbiol. 14(1), 1139494. Zhang, K., Li, S., Liu, S., Li, S., Qu, L., Gao, G.F. and Qiu, H.J. 2021. Spatiotemporally orchestrated interactions between viral and cellular proteins involved in the entry of African swine fever virus. Viruses 13(12), 2495. Zhang, T., Lu, Z., Liu, J., Tao, Y., Si, Y., Ye, J., Cao, S. and Zhu, B. 2024. Host innate and adaptive immunity against African swine fever virus infection. Vaccines 12(11), 1278. Zheng, L., Yan, Z., Qi, X., Ren, J., Ma, Z., Liu, H., Zhang, Z., Li, D., Pei, J., Xiao, S., Feng, T., Wang, X. and Zheng, H. 2025. The deletion of the MGF360-10L/505-7R genes of African swine fever virus results in high attenuation but no protection against homologous challenge in pigs. Viruses 17(2), 283. Zheng, W., Xi, J., Zi, Y., Wang, J., Chi, Y., Chen, M., Zou, Q., Tang, C. and Zhou, X. 2023. Stability of African swine fever virus genome under different environmental conditions. Vet. World 16(11), 2374–2381. Zheng, X., Nie, S. and Feng, W.H. 2022. Regulation of antiviral immune response by African swine fever virus (ASFV). Virol. Sin. 37(2), 157–167. Zhu, J.J. 2022. African swine fever vaccinology: the biological challenges from immunological perspectives. Viruses 14(9), 2021. | ||
| How to Cite this Article |
| Pubmed Style Sukmanadi M, Khairullah AR, Mustofa I, Wiyono A, Wardhani BWK, Saepulloh M, Proboningrat A, Wasito W, Putri AK, Ahmad RZ, Khalisa AT, Kusala MKJ, Akintunde AO, Fauziah I, Moses IB, Kurniasih DAA, Wibowo S. African swine fever: A highly fatal disease that is spreading globally. Open Vet. J.. 2025; 15(8): 3399-3418. doi:10.5455/OVJ.2025.v15.i8.4 Web Style Sukmanadi M, Khairullah AR, Mustofa I, Wiyono A, Wardhani BWK, Saepulloh M, Proboningrat A, Wasito W, Putri AK, Ahmad RZ, Khalisa AT, Kusala MKJ, Akintunde AO, Fauziah I, Moses IB, Kurniasih DAA, Wibowo S. African swine fever: A highly fatal disease that is spreading globally. https://www.openveterinaryjournal.com/?mno=253548 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.4 AMA (American Medical Association) Style Sukmanadi M, Khairullah AR, Mustofa I, Wiyono A, Wardhani BWK, Saepulloh M, Proboningrat A, Wasito W, Putri AK, Ahmad RZ, Khalisa AT, Kusala MKJ, Akintunde AO, Fauziah I, Moses IB, Kurniasih DAA, Wibowo S. African swine fever: A highly fatal disease that is spreading globally. Open Vet. J.. 2025; 15(8): 3399-3418. doi:10.5455/OVJ.2025.v15.i8.4 Vancouver/ICMJE Style Sukmanadi M, Khairullah AR, Mustofa I, Wiyono A, Wardhani BWK, Saepulloh M, Proboningrat A, Wasito W, Putri AK, Ahmad RZ, Khalisa AT, Kusala MKJ, Akintunde AO, Fauziah I, Moses IB, Kurniasih DAA, Wibowo S. African swine fever: A highly fatal disease that is spreading globally. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3399-3418. doi:10.5455/OVJ.2025.v15.i8.4 Harvard Style Sukmanadi, M., Khairullah, . A. R., Mustofa, . I., Wiyono, . A., Wardhani, . B. W. K., Saepulloh, . M., Proboningrat, . A., Wasito, . W., Putri, . A. K., Ahmad, . R. Z., Khalisa, . A. T., Kusala, . M. K. J., Akintunde, . A. O., Fauziah, . I., Moses, . I. B., Kurniasih, . D. A. A. & Wibowo, . S. (2025) African swine fever: A highly fatal disease that is spreading globally. Open Vet. J., 15 (8), 3399-3418. doi:10.5455/OVJ.2025.v15.i8.4 Turabian Style Sukmanadi, Muhammad, Aswin Rafif Khairullah, Imam Mustofa, Agus Wiyono, Bantari Wisynu Kusuma Wardhani, Muharam Saepulloh, Annise Proboningrat, Wasito Wasito, Alifiani Kartika Putri, Riza Zainuddin Ahmad, Andi Thafida Khalisa, Muhammad Khaliim Jati Kusala, Adeyinka Oye Akintunde, Ima Fauziah, Ikechukwu Benjamin Moses, Dea Anita Ariani Kurniasih, and Syahputra Wibowo. 2025. African swine fever: A highly fatal disease that is spreading globally. Open Veterinary Journal, 15 (8), 3399-3418. doi:10.5455/OVJ.2025.v15.i8.4 Chicago Style Sukmanadi, Muhammad, Aswin Rafif Khairullah, Imam Mustofa, Agus Wiyono, Bantari Wisynu Kusuma Wardhani, Muharam Saepulloh, Annise Proboningrat, Wasito Wasito, Alifiani Kartika Putri, Riza Zainuddin Ahmad, Andi Thafida Khalisa, Muhammad Khaliim Jati Kusala, Adeyinka Oye Akintunde, Ima Fauziah, Ikechukwu Benjamin Moses, Dea Anita Ariani Kurniasih, and Syahputra Wibowo. "African swine fever: A highly fatal disease that is spreading globally." Open Veterinary Journal 15 (2025), 3399-3418. doi:10.5455/OVJ.2025.v15.i8.4 MLA (The Modern Language Association) Style Sukmanadi, Muhammad, Aswin Rafif Khairullah, Imam Mustofa, Agus Wiyono, Bantari Wisynu Kusuma Wardhani, Muharam Saepulloh, Annise Proboningrat, Wasito Wasito, Alifiani Kartika Putri, Riza Zainuddin Ahmad, Andi Thafida Khalisa, Muhammad Khaliim Jati Kusala, Adeyinka Oye Akintunde, Ima Fauziah, Ikechukwu Benjamin Moses, Dea Anita Ariani Kurniasih, and Syahputra Wibowo. "African swine fever: A highly fatal disease that is spreading globally." Open Veterinary Journal 15.8 (2025), 3399-3418. Print. doi:10.5455/OVJ.2025.v15.i8.4 APA (American Psychological Association) Style Sukmanadi, M., Khairullah, . A. R., Mustofa, . I., Wiyono, . A., Wardhani, . B. W. K., Saepulloh, . M., Proboningrat, . A., Wasito, . W., Putri, . A. K., Ahmad, . R. Z., Khalisa, . A. T., Kusala, . M. K. J., Akintunde, . A. O., Fauziah, . I., Moses, . I. B., Kurniasih, . D. A. A. & Wibowo, . S. (2025) African swine fever: A highly fatal disease that is spreading globally. Open Veterinary Journal, 15 (8), 3399-3418. doi:10.5455/OVJ.2025.v15.i8.4 |