| Research Article | ||
Open Vet. J.. 2025; 15(9): 4403-4411
Open Veterinary Journal, (2025), Vol. 15(9): 4403-4411 Research Article Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet methodAhmad N. Abu-Awwad1*, Hasan Y. Muti2, Dima F. Khater3, Ahmad M. Khalaf4 and Tawfiq A. Arafat21Faculty of Pharmacy, Jerash University, Jerash, Jordan 2Jordan Center for Pharmaceutical Research, Amman, Jordan 3Department of Chemistry, Faculty of Arts and Science, Applied Science Private University, Amman, Jordan 4Faculty of Allied Medical Sciences, Al-Ahliyya Amman University, Amman, Jordan *Corresponding Author: Ahmad N. Abu-Awwad. Faculty of Pharmacy, Jerash University, Jerash, Jordan. Email: a.awwad [at] jpu.edu.jo Submitted: 18/04/2025 Revised: 29/07/2025 Accepted: 20/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
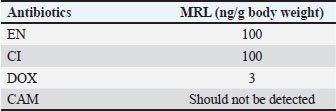
ABSTRACTBackground: Antibiotics are widely used in veterinary medicine; consequently, drug residues may remain in animal-based foods and cause adverse health effects in consumers. Thus, this study aimed to establish a screening method for residual investigation in different types of meat. Aim: This study aimed to develop and validate a high-performance liquid chromatography-ultraviolet method for the simultaneous determination of ciprofloxacin (CI), enrofloxacin (EN), doxycycline (DOX), and chloramphenicol (CAM) in poultry, red meat, and fish products and to investigate their residues in different meat samples. Methods: The targeted analytes with ondansetron as an internal standard were extracted from various meat matrices using acetonitrile in a single process step. Separation was achieved using an ACE®️ C18 column (10 cm, 4.6 mm i.d., 5 µm) with a mobile phase of 0.2% formic acid: acetonitrile (22:78, v/v) at a flow rate of 0.7 ml/minute. Detection was performed at a fixed wavelength of 280 nm with a runtime of 10 minutes. The developed method was validated for selectivity, linearity, sensitivity, accuracy, precision, and recovery following the European guideline EMA 2012. To evaluate the presence of the targeted antibiotic residues, 13 poultry, 25 red meat, and 3 fish samples were randomly collected from the local market. Results: The validation results for each analyte met the acceptable criteria. The targeted analytes exhibited a linear curve over the dynamic range of 10–1,000 ng/g for CI and EN, 80–8,000 ng/g for DOX, and 50–500 ng/g for CAM. Most of the randomly collected poultry and red meat samples contained CI residues. A few samples contained EN and DOX residues, whereas no CAM was detected in any of the collected samples. Conclusion: The developed method was successfully validated and applied to investigate antibiotic residues in various meat types. Keywords: Poultry, Red Meat, Fish, Antibiotic residues, Food safety. IntroductionAntibiotics are widely used in veterinary medicine; consequently, drug residues may remain in animal-based foods and may cause adverse health effects in consumers (Chanda et al., 2014). Human exposure to substantial antibiotic residues from animal products may exacerbate immune responses in susceptible individuals and negatively influence the intestinal microbiota (Normanno et al., 2007). Moreover, the irrational use of antibiotics is associated with the development of antibiotic-resistant strains, making antibiotics ineffective as therapeutic agents (Babapour et al., 2012). Antibiotics are essential in the poultry industry to prevent and reduce the incidence of infectious diseases. They are also used illegally as feed additives to promote animal growth and productivity (CAC, 2018). Some developed countries, including Sweden, Norway, Denmark, and the European Union, have already prohibited the use of antibiotics as growth promoters and for prophylaxis (Maron et al., 2013). Over the last few decades, antibiotics have been frequently used in animal husbandry for prophylactic and therapeutic purposes. Almost one-third of the antibiotics used in Europe are for veterinary applications, with the majority of antibiotics used for therapeutic purposes in poultry and pig livestock (Landers et al., 2012). Hence, the European Commission has established maximum residue limits (MRLs) in foodstuffs of animal origin and listed them in the Commission Regulation [European Union (EU)] No. 37/2010 (European Commission, 2010). MRLs have been set to avoid exposing end consumers to antibiotic residue risks. By regulation, the EU does not allow foodstuffs such as beef, milk, red meat, fish, and eggs to contain residual amounts of veterinary medicinal products or antibiotic products that are likely to expose public health to serious risk. The rules and procedures for setting up MRLs are described in Regulation (EC) No. 470/2009 of the European Parliament and the Council (European Union, 2009). This is not the case in developing or underdeveloped countries, where safety requirements and legislation are often inadequate or absent. Fluoroquinolones, such as ciprofloxacin (CI) and enrofloxacin (EN), have been licensed as veterinary drugs for three decades. They inhibit the catalytic activity of bacterial DNA gyrase with low toxicity and broad-spectrum activity. CI and EN are prescribed to treat or prevent infectious diseases in farm animals, including chickens, turkeys, and beef (Baiphethi and Jacobs, 2009; European Union Food and Veterinary Office, 2015). Tetracyclines are a large family of antibiotics with broad-spectrum activity that are naturally produced by Streptomyces species. Four tetracyclines are extensively used as veterinary antibiotics: tetracycline, chlortetracycline, oxytetracycline, and doxycycline (DOX) (Granados-Chinchilla and Rodríguez, 2017). The World Health Organization and the Food and Agriculture Organization recommend that tetracycline MRLs not exceed 200, 600, and 1,200 µg/kg in the liver, muscles, and kidneys, respectively (CAC, 2018). Chloramphenicol (CAM) is a broad-spectrum antibiotic with historical veterinary uses because of its low cost (Laxminarayan et al., 2013). The presence of CAM and its metabolites in foods, even at trace levels, represents a severe health risk. In addition to contributing to the development of resistant bacterial strains, CAM causes bone marrow depression and aplastic anemia (Shukla et al., 2011). Therefore, finding CAM or its metabolites in any food product is not acceptable. Competent authorities have not set MRLs for CAM in food products (Barton, 2000). Table 1 shows some accepted MRL values for meat residues of veterinary drugs. Table 1. Maximum residue limits (MRLs) for veterinary antibiotic residues.
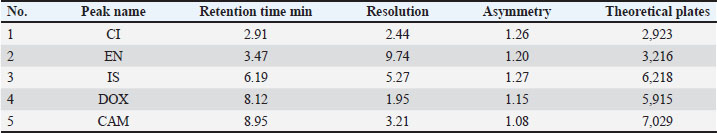
Meat consumption in South Africa was generally estimated to be 29.69 kg/person/year for white meat and 25.73 kg/person/year for red meat in 2007–2008 (European Commission, 2010; Government of Canada, 2015; Parmar et al., 2021). Consequently, investigating the presence of such antibiotic residues in different meat types available in the market is necessary to confirm the safety of consuming meat-based food for human health. Several methods have been reported that describe the determination of antibiotic residues in different meat matrices, either individually or in combination with other drugs, using different extraction methods, including solid-phase-diode array (Oyedeji et al., 2020), ELISA, TLC, and HPLC (Ramatla et al., 2017; Arslanbas, 2018), liquid chromatography photodiode array, and liquid chromatography mass spectrometry (LC-MS) (Won et al., 2011; Chan et al., 2022; Elsayed et al., 2023). To the best of our knowledge, methods for the simultaneous determination of such antibiotics in milk (Muti et al., 2021) but not in different meat matrices have been reported, and the most recently reported method included three antibiotics used in intensive chicken rearing systems (Kamouh et al., 2024). Nevertheless, methods that fit our demands in the current study are still lacking. Herein, we developed and validated a simple and cost-effective analytical method to simultaneously quantify the target analytes in different meat matrices using high-performance liquid chromatography (HPLC)-ultraviolet (UV), with a high-throughput single extraction step of direct protein precipitation and short runtime. Investigation methodologies for such antibiotic residues are still needed because veterinary antibiotic products are still widely used in animal farms. Materials and MethodsCAM palmitate (purity 99.6 %, working standard) was supplied by Mehta Api Private Limited (Mumbai, India). CI hydrochloride (purity >98%, working standard) was purchased from Shangyu Jingxin (Shangyu, China). EN base (99% purity, working standard) was provided by Zhejiang Guobang Pharmaceutical Co., Ltd. (Zhejiang, China). DOX hyclate was purchased from Wuhan Lipharma Chemicals (Wuhan, China), and Ondansetron was supplied by Virdev Intermediates Pvt (Palsana, India). Methanol, acetonitrile, water, and formic acid (99.0%) were obtained from Fisher Scientific (Loughborough, USA) and LC-MS grade. Blank samples of poultry, red meat, and fish used in the validation were obtained from green farms that do not use antibiotics for animal treatment. InstrumentationDionex Ultimate 3000 RS HPLC, attached to an UV detector [VWD-3x00(RS)] (Dionex Corporation, Sunnyvale, CA), controlled by Chameleon 6.8 software for data management. The elution system consisted of a mobile phase of 0.2% formic acid: acetonitrile (22:78, v/v) pumped isocratically at a constant flow rate of 0.7 ml/minute through the C18 ACE column, 100 × 4.6 mm, 5 µm, in a total liquid chromatography run time of 10 minutes. The injected volume and column temperature were 5 µl and 25 °C, respectively. All analytes with an internal standard (IS) were detected at a fixed wavelength of 280 nm. The multispeed homogenizer used for meat mincing was obtained from WORNER LAB in dimensions of (L*W*H): 380 × 80 × 50 mm Preparation of the stock and working solutionsStandard stock solutions of CI, EN, DOX, and CAM were prepared separately by weighing 100 mg of the active ingredient from each standard in a 10 ml volumetric flask and dissolving in methanol to obtain a final concentration of 10 mg/ml. A working mixed standard solution (working solution A) with a concentration of 10 µg/ml of CI, 10 µg/ml of EN, 80 µg/ml of DOX and 50 µg/ml of CAM was prepared by diluting 1, 1, 8, and 5 ml of each analyte’s stock solution, respectively, in 100 ml of 30% methanol. Then, the working mixed standard solution was serially diluted to prepare working serial solutions (working solution B) for the calibration curve (CC) and quality control (QC) samples. The serial dilution of working solution B was prepared by diluting a corresponding volume from the working solution A to 10 ml of 30% methanol in volumetric flask, whereas 0.1 ml from working solution A was diluted to prepare the first level calibration solution that contains 0.1 µg/ml of CI, 0.1 µg/ml of EN, 0.8 µg/ml of DOX and 0.5 µg/ml of CAM; 0.2 ml from working solution A was diluted to prepare the second level calibration solution that contains 0.2 µg/ml of CI, 0.2 µg/ml of EN, 1.6 µg/ml of DOX and 1.0 µg/ml of CAM; 0.5 ml from working solution A was diluted to prepare 3-level calibration solution that contains 0.5 µg/ml of CI, 0.5 µg/ml of EN, 4.0 µg/ml of DOX and 2.5 µg/ml of CAM; 0.4 ml from working solution A was diluted to prepare 4-level calibration solution that contains 0.4 µg/ml of CI, 0.4 µg/ml of EN, 3.2 µg/ml of DOX and 2.0 µg/ml of CAM; 1.2 ml from working solution A was diluted to prepare 5-level calibration solution that contains 1.2 µg/ml of CI, 1.2 µg/ml of EN, 9.6 µg/ml of DOX and 3.6 µg/ml of CAM; 2.4 ml from working solution A was diluted to prepare 6-level calibration solution that contains 2.4 µg/ml of CI, 2.4 µg/ml of EN, 19.2 µg/ml of DOX and 7.2 µg/ml of CAM; and 10.0 ml from working solution A was diluted to prepare 7-level calibration solution that contains 10.0 µg/ml of CI, 10.0 µg/ml of EN, 8.0 µg/ml of DOX and 5.0 µg/ml of CAM. Preparation of the ISOndansetron was used as the IS. A solution of 25.0 µg Ondansetron/ml was prepared in water-methanol (1:1 v/v) by diluting 0.25 ml of 1 mg/ml stock solution prepared in methanol in 10 ml. CCs and QC samplesA 50 µl of the appropriate corresponding calibration solution (working solution B) was spiked in 1 g of homogenized blank sample (separately, in each different meat matrix) and vortexed for 1 minutes to obtain the calibration levels of 10, 20, 50, 100, 300, 600, and 1,000 ng/g (30, 500, and 800 ng/g for QC low, mid, and high, respectively) for CI and EN, and 80, 160, 400, 800, 2,400, 4,800, and 8,000 ng/g (240, 4,000, and 6,400 ng/g for QC low, mid, and high, respectively) for DOX, and 50, 100, 250, 500, 1,500, 3,000, and 5,000 ng/g (150, 500, and 4,000 ng/g for QC low, mid, and high, respectively) for CAM. The established dynamic ranges for CI, EN, DOX, and CAM were selected based on the practical detection limits of the current method and the expected residue concentrations in the real samples, which were carefully designed to meet regulatory MRLs and anticipated field concentrations. ExtractionAnalytes with IS were extracted from tissues by a single extraction step of direct protein precipitation using acetonitrile as the precipitating reagent. A 4 ml acetonitrile solution was added to 1 g of homogenized meat blank in 10 polypropylene tubes after mixing for 1 minute with 200 µl of 0.05 M ammonium acetate buffer solution and 50 µl of IS solution. The mixture was vortexed for 1 minute and then centrifuged at 4,400 rpm under 5°C for 5 minutes. The supernatant layer was separated by direct decantation into a glass evaporation tube. The separated liquid layers were evaporated to dryness under compressed air at 50°C. The residues were reconstituted by 250 µl of 0.05 M ammonium acetate/methanol (90:10; v/v) and vortexed for 1 minute, followed by centrifugation to clear the injectable samples, which were then transferred into HPLC vials for analysis. Validation of the analytical methodThe developed method was partially validated according to the European guideline for bioanalytical methods validation (EMA, 2012), which is in agreement with the update revision of EU 2014, considering the US guidance for industry in bioanalytical method validation (FDA, 2001), which is also in agreement with the updated version of FDA 2018 guidance and the current ICH M10 guideline. Selectivity, recovery, linearity, sensitivity, accuracy, and precision were investigated in poultry meat, and sensitivity, accuracy, and precision were validated in fish and red meat. The validation runs were conducted on four separate days; each validation run consisted of blank, zero, and a set of freshly spiked standard calibrator samples of seven concentrations over the dynamic ranges of 10–1,000 ng/g for CI and EN, 80–8,000 ng/g for DOX and 50–5,000 ng/g for CAM, in addition to a set of freshly spiked QC samples that examining the validation sections. The first calibrator level in each analyte’s CC is considered the lower limit of quantitation (LLOQ) and treated as a QC sample to examine the sensitivity validation. This is based on EMA guideline criteria of acceptable accuracy and precision, rather than signal-to-noise ratios, which are not a mandatory requirement for UV-based bioanalytical methods. A single mixture of standard working solutions containing all the targeted analytes was spiked into the calibrators and QCs. All meat samples were homogenized before weighing 1 g for spiking to prepare calibrators and QC samples. Blank samples used for validation were subjected to antibiotic activity screening tests to confirm the absence of the targeted analytes used for spiking of QC and calibrator samples. SelectivitySix individual blanks from poultry, fish, and red meat were analyzed separately to confirm the absence of interfering peaks with the targeted analytes. The endogenous peaks were differentiated from the targeted peaks by comparing the LLOQ chromatogram with the blank screening results from different meat matrices. RecoveryThe recovery of CI, EN, DOX, and CAM was investigated in poultry meat by comparing the analytical peak areas of extracted QC samples with corresponding blank extracts spiked with the analytes at QC low, mid, and high post-extraction in triplicate analysis. LinearityThe linearity of calibrator levels against their corresponding response was investigated for CI, EN, DOX, and CAM in poultry meat. The established CC (freshly spiked) from blank, zero, and seven calibrators, including LLOQ, were analyzed in six replicate analyses within four validation days. Each CC was graphed by identifying the best fit of the peak area ratios (peak area analyte/peak area IS) versus the specified concentration and fitted to the equation y=mi + b by weighted least-squares regression (1/x). Sensitivity, accuracy, and precisionThe sensitivity, precision, and accuracy of CI, EN, DOX, and CAM in poultry meat were investigated at LLOQ and low, mid, and high QC. Six replicate analyses for each LLOQ and QC level were performed within a single analytical run with freshly spiked CC, and the analysis was repeated for all samples in another analytical run from freshly spiked samples three separate times within validation days for between-run evaluations. Each analytical run consisted of a blank, a zero sample, a CC, and QCs, including the LLOQ level. Application of the method to quantitate CI, EN, DOX, and CAM in poultry, fish, and red meatsA total of 41 samples were collected from different farms, representing approximately 80% of the farms in Jordan, where 13 samples of poultry, 25 samples of red meat, and 3 samples of fish were analyzed. Ethical approvalNot needed for this study. ResultsSeparation and chromatographyA system suitability test for HPLC was performed before executing the analysis for validation parameters. The responses were recorded for 10 replicate injections of the prepared standard at 10 µg/ml of CI, 10 µg/ml of EN, 80 µg/ml of DOX, and 50 µg/ml of CAM. The optimized HPLC method evaluated the capacity factor, peak resolution (K’), peak asymmetry, theoretical plate (N), selectivity factor, and RSD of the peak area (Table 2). For CI, EN, DOX, and CAM, the capacity factors were estimated as follows: Table 2. Summary of peak parameters for the highest calibration level spiked into poultry meat.
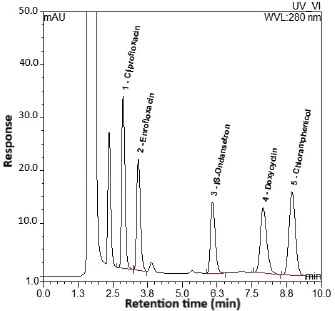
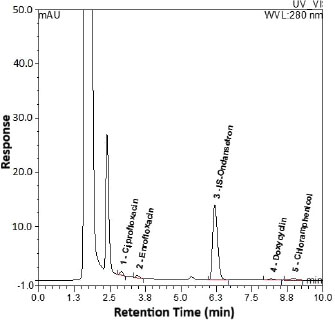
The selected wavelength of 280 nm was set upon inspection of the UV absorbance spectra for all analytes and the IS. Despite a slight difference in their maximum absorption, 280 nm provided a strong and consistent response for all targeted compounds without significant compromise in sensitivity or selectivity. The optimized chromatographic conditions achieved good resolution and symmetrical peaks for the analytes and IS, as well as a short run time of 10 minutes (Fig. 1) for the chromatogram of the highest level in CC spiked in a poultry blank. Table 2 summarizes the corresponding peak parameters.
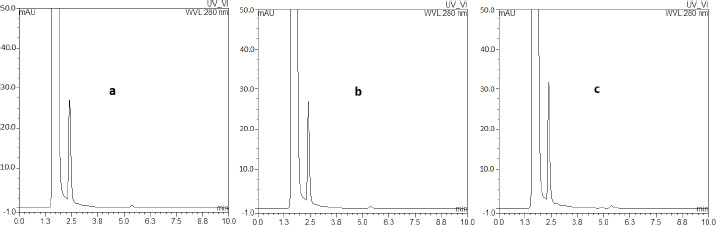
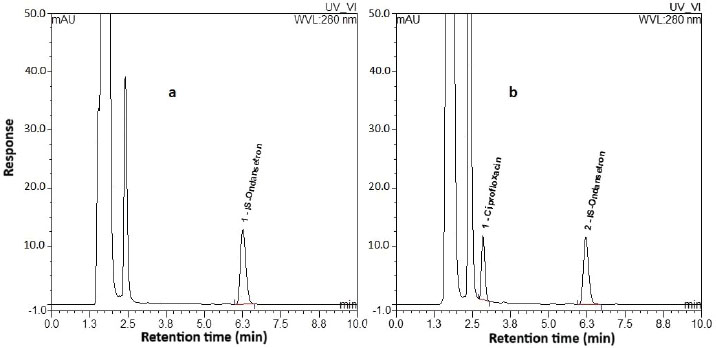
Fig. 1. The chromatogram representing the highest calibration level (calibrator 7). SelectivityAll analyzed blank samples for each of poultry, fish, and red meat showed identical chromatograms for good separation of endogenous compounds and absence of interferences at retention times of the targeted analytes, as shown in chromatograms of Figure 2a–c, respectively, compared to the chromatogram in Figure 3 for the LLOQ spiked in the corresponding poultry blank.
Fig. 2. Chromatograms of the blank samples extracted from poultry, fish, and red meat are shown in panels a, b, and c, respectively.
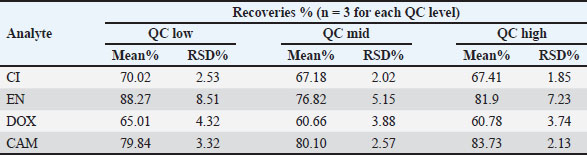
Fig. 3. Representative chromatogram of the LLOQ sample spiked in poultry meat. RecoveryThe extraction method was sufficiently efficient to successfully quantify the targeted analytes from poultry meat tissues. Table 3 summarizes the investigated recoveries for CI, EN, DOX, and CAM at low, mid, and high QCs. Table 3. Extraction recoveries and relative standard deviation (RSD) values for CI, EN, DOX, and CAM.
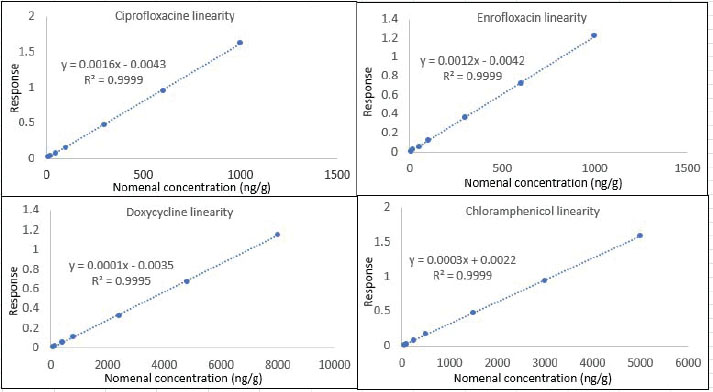
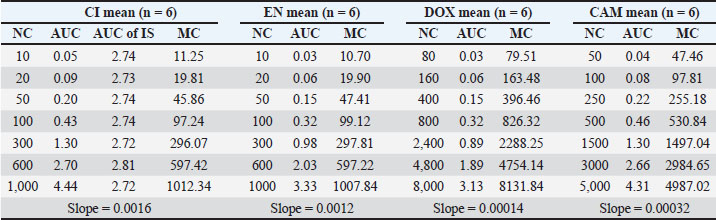
Calibration curveThe repeated analysis of CC for each of CI, EN, DOX, and CAM through validation days has exhibited a reproducible slope with a good regression value, as shown in Table 4 for the parameters summary of six replicate analyses for CC spiked in poultry blank meat, where all calibrators were within the acceptance criteria. The linear curve data were best-fit to a straight line with a weighting factor of 1/x regression function, and it was used to calculate the concentrations of all samples throughout the batch. Figure 4 shows how the relationship between the established dynamic range and the corresponding response for each analyte is perfect.
Fig. 4. CC for each analyte, derived from six replicate analyses of each level, spiked in the poultry matrix, n=1, n=1, n=2, n=1, Table 4. Summery for six replicate analyses of CC spiked in poultry blank matrix, including the nominal concentration (NC) and measured concentration (MC) in the unit of ng/g.
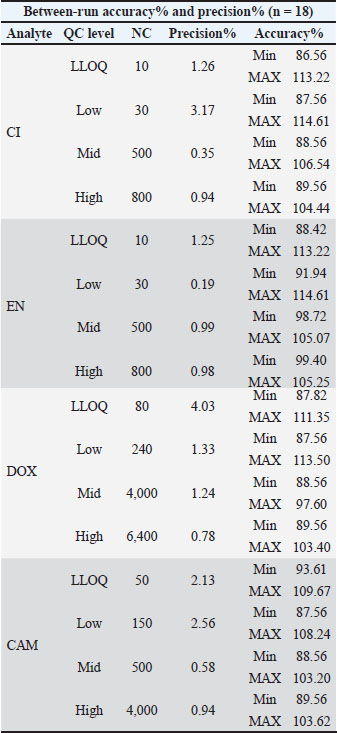
Sensitivity, accuracy, and precisionSensitivity, accuracy, and precision validation were investigated within- and between-runs for CI, EN, DOX, and CAM in poultry meat. All measurements obtained from the LLOQ and low mid and high QCs were within the acceptance criteria. Table 5 summarizes the mean measurements, including RDS% values. The measured accuracy and precision values for LLOQ represent the sensitivity of the applied method, where LLOQ is considered the QC level. Table 5. Summary of between-run accuracy and precision measurements including nominal concentration (NC) for each QC level of CI, EN, DOX, and CAM in poultry matrix.
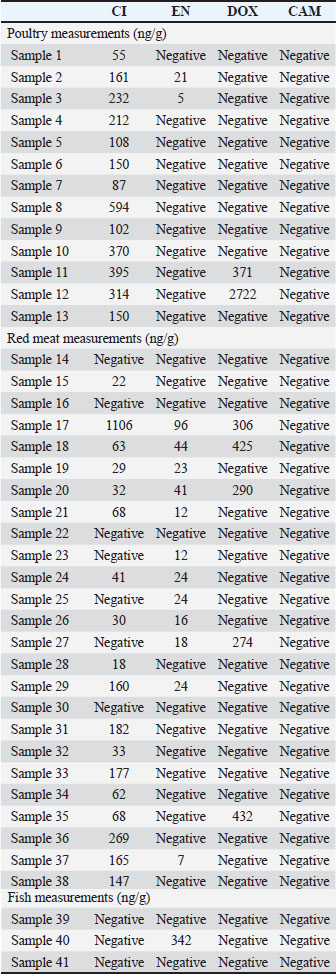
Quantification of CI, EN, DOX, and CAM in poultry, fish, and red meatAll 41 meat samples collected from various matrices were successfully analyzed using the proposed method. Table 6 presents all the measurements for these samples. Figure 4 shows the randomly selected chromatograms from the analysis of unknown samples, which represents the fish matrix analysis in Figure 5a, that does not detect any of the targeted analytes, while Figure 5b shows the chromatogram of a poultry matrix sample in which CI was detected at a concentration of 592.73 ng/g.
Fig. 5. Representative chromatograms for (a) a fish sample that did not contain any of the target analytes and (b) a poultry sample containing 592.73 ng/g CI. Table 6. Representative measurements of CI, EN, DOX, and CAM in poultry, fish, and red meat samples expected to contain antibiotic residues.
DiscussionAll the validation results for each analyte met the acceptable criteria of the guidelines. The method was applied to unfrozen meat samples that were freshly collected. Most of the randomly collected poultry and red meat samples contained CI residues. A few samples contained EN and DOX residues, whereas no CAM was detected in any of the collected samples. It was found that 100% of the poultry samples were contaminated with CI, 15% with EN, 15% with DOX, and 0% with CAM. Among the red meat samples, 80% were contaminated with CI, 48% with EN, 20% with DOX, and none with CAM. For the fish samples, 33% were contaminated with EN, while 0% were contaminated with CI, DOX, or CAM. Negative values in Table 6 indicate that the analyte was not detected, and no measurable peak corresponding to the analyte was observed at its retention time. These findings are consistent with those of previous studies, showing the common use of fluoroquinolones and tetracyclines in livestock production. The complete absence of CAM, a drug banned in food animals under EU law, may reflect some degree of regulatory enforcement. Compared with previous studies, including our earlier work on milk matrices (Muti et al., 2021), this study expands the application of HPLC-UV to solid meat tissues using a single, rapid, and cost-effective method. The novelty of the study lies in the simultaneous quantification of four antibiotics in three different meat matrices, which is not commonly addressed in a single UV-based assay. The method’s simplicity and adaptability make it suitable for routine use in laboratories with limited access to advanced equipment, such as liquid chromatography-tandem mass spectrometry (LC-MS/MS). This highlights the importance of monitoring veterinary drug residues in food products for public health and supports the need for tighter regulation and routine screening, especially in developing countries. ConclusionThis study clearly showed that poultry, red meat, and fish were not completely free from antibiotic residue. Some residue levels were above the MRL recommended limit according to the European Union EC. The developed method for the simultaneous determination of CI, EN, DOX, and CAM in different meat matrices using HPLC-UV was successfully validated following the European guidelines and then applied to investigate the residues of targeted antibiotics in poultry, red meat, and fish products. Some antibiotic residues were detected at higher concentrations than the accepted MRLs. This study has been conducted to increase the awareness and the risk of antibiotic residues on the health of people and will encourage the competent authorities to regulate and take the necessary measures to provide healthy meat free from antibiotic residues for humans. AcknowledgmentsThe authors acknowledge the Deanship of Scientific Research at Al-Ahliyya Amman University, Amman, Jordan, for their financial support and the support of the Jordan Center for Pharmaceutical Research (JCPR) for their facility. Conflict of interestThe authors declare no conflict of interest. FundingNo external funding was provided. Authors’ contributionsAll authors contributed to this study and contributed either by experimental application or manuscript writing and revision. Data availabilityAll data supporting this study’s findings are available upon reasonable request. ReferencesArslanbas, E. 2018. Determination of antibiotic residues in meat samples using high-performance liquid chromatography. Erciyes Univ. Vet. Fak. Derg. 13, 247–252. Babapour, A., Azami, L. and Fartashmehr, J. 2012. Overview of antibiotic residues in Iranian beef and mutton. World Appl. Sci. J. 19, 1495–1500. Baiphethi, M.N. and Jacobs, P.T. 2009. Subsistence farming’s contribution to food security in South Africa. Agrekon 48, 459–482. Barton, M. D. 2000. Antibiotic use in animal feed and its impact on human health. Nutr. Res. Rev. 13, 279–299. CAC. 2018. The codex alimentarius commission maximum residue limits for veterinary drugs in foods. Rome, Italy: Food and Agriculture Organization/WHO, Vol. 2. Chan, C.L., Wai, H.K.F., Wu, P., Lai, S.W., Chan, O.S.K. and Tun, H.M. 2022. Prevalence of antibiotic residues in meat: a systematic review. Antibiotics 11, 845. Chanda, R.R., Fincham, R.J. and Venter, P. 2014. Veterinary drug residues in animal products: health implications. Crit. Rev. Food Sci. Nutr. 54, 488–494. Elsayed, A.S., Abusham, A., Al-Touby, S.S.J., Al Rajhi, W.K.H. and Hossain, M.A. 2023. Antibiotic residue detection in meat using LC-MS. Food Anal. Methods 16, 1618–1626. European Commission. 2010. Commission Regulation (EU) No 37/2010 on pharmacologically active substances and their classification. Union L15, 1–72. European Medicines Agency (EMA). 2012. Framework for a correlation table (VNeeS:CTD) for ASMFs/Part 2 in CTD format for dossiers for veterinary medicinal products. Reference No. EMA/115282/2012. European Union Food and Veterinary Office. 2015. Final report of an audit on the control of residues. European Union Food Vet. Off. 1, 1–16. European Union. 2009. Regulation (EC) No 470/2009 of the European Parliament and of the Council. Off. J. Eur. Union L 152, 11–22. Government of Canada. 2015. Health Canada veterinary drugs directorate. Summary report. Drugs Direct 1, 1–101. Granados-Chinchilla, F. and Rodríguez, C. 2017. Tetracyclines in food and feedingstuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications. J Anal Methods Chem. 2017, 1315497. Kamouh, A., Al-Kaf, A.G., Al-Kaf, M.A., Baqir, S.J., Al-Sharafi, W.A. and Al-Mansoury, R.M. 2024. Detection of antibiotic residues in broiler meat marketed in Dhamar city, Yemen, and the effect of boiling on residue levels. Open Vet. J. 14, 438–448. Landers, T.F., Cohen, B., Wittum, T.E. and Larson, E.L. 2012. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep®. 127(4), 4–22. Laxminarayan, R., Duse, A., Wattal, C., Zaidi, A.K.M., Wertheim, H.F.L., Sumpradit, N., Vlieghe, E., Hara, G.L., Gould, I.M., Goossens, H. and Greko, C. 2013. Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 13, 1057–1098. Maron, D.F., Smith, T.J.S. and Nachman, K.E. 2013. Restrictions on antimicrobial use in animal food production: an international regulatory and economic perspective. Global Health 9, 48. Muti, H.Y., Khater, D.F., Khalaf, A.M., Abu-awwad, A.N. and Arafat, T.A. 2021. Simultaneous determination of antibiotic residues (EN, CI, DOX, and CAM) in Jordan market milk. Acta Pol. Pharm. Drug Res. 78, 11–19. Normanno, G., La Salandra, G., Dambrosio, A., Quaglia, N.C., Corrente, M., Parisi, A., Santagada, G., Firinu, A., Crisetti, E. and Celano, G.V. 2007. Occurrence, characterization, and antimicrobial resistance of enterotoxigenic Staphylococcus aureus in meat products. Int. J. Food Microbiol. 115, 290–296. Oyedeji, A.O., Msagati, T.A.M., Williams, A.B. and Benson, N.U. 2020. Simultaneous determination of antibiotics in meat samples using high-performance liquid chromatography-diode array. Chem. Data Collect. 25, 100313. Parmar, J.K., Chaubey, K.K., Gupta, V. and Bharath, M.N. 2021. Prevalence and public health implications of antibiotic residues in food animals. World 14, 1650–1664. Ramatla, T., Ngoma, L., Adetunji, M. and Mwanza, M. 2017. Evaluation of antibiotic residues in meat samples using microbial inhibition tests. Antibiotics 6, 1–17. Shukla, P., Bansode, F.W. and Singh, R.K. 2011. Antibiotic resistance and food safety. J. Med. Med. Sci. 2, 1313–1316. U.S. Food and Drug Administration (FDA). 2001. Bioanalytical method validation: guidance for industry. Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), and Center for Biologics Evaluation and Research (CBER). U.S. Department of Health and Human Services. Won, S.Y., Lee, C.H., Chang, H.S., Kim, S.O., Lee, S.H. and Kim, D.S. 2011. Simultaneous detection of multi-class antibiotic residues in meat using LC-MS/MS. Food Control 22, 1101–1107. | ||
| How to Cite this Article |
| Pubmed Style Abu-awwad AN, Muti HY, Khater DF, Khalaf AM, Arafat TA. Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet method. Open Vet. J.. 2025; 15(9): 4403-4411. doi:10.5455/OVJ.2025.v15.i9.47 Web Style Abu-awwad AN, Muti HY, Khater DF, Khalaf AM, Arafat TA. Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet method. https://www.openveterinaryjournal.com/?mno=253345 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.47 AMA (American Medical Association) Style Abu-awwad AN, Muti HY, Khater DF, Khalaf AM, Arafat TA. Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet method. Open Vet. J.. 2025; 15(9): 4403-4411. doi:10.5455/OVJ.2025.v15.i9.47 Vancouver/ICMJE Style Abu-awwad AN, Muti HY, Khater DF, Khalaf AM, Arafat TA. Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet method. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4403-4411. doi:10.5455/OVJ.2025.v15.i9.47 Harvard Style Abu-awwad, A. N., Muti, . H. Y., Khater, . D. F., Khalaf, . A. M. & Arafat, . T. A. (2025) Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet method. Open Vet. J., 15 (9), 4403-4411. doi:10.5455/OVJ.2025.v15.i9.47 Turabian Style Abu-awwad, Ahmad N., Hasan Y. Muti, Dima F. Khater, Ahmad M. Khalaf, and Tawfiq A. Arafat. 2025. Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet method. Open Veterinary Journal, 15 (9), 4403-4411. doi:10.5455/OVJ.2025.v15.i9.47 Chicago Style Abu-awwad, Ahmad N., Hasan Y. Muti, Dima F. Khater, Ahmad M. Khalaf, and Tawfiq A. Arafat. "Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet method." Open Veterinary Journal 15 (2025), 4403-4411. doi:10.5455/OVJ.2025.v15.i9.47 MLA (The Modern Language Association) Style Abu-awwad, Ahmad N., Hasan Y. Muti, Dima F. Khater, Ahmad M. Khalaf, and Tawfiq A. Arafat. "Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet method." Open Veterinary Journal 15.9 (2025), 4403-4411. Print. doi:10.5455/OVJ.2025.v15.i9.47 APA (American Psychological Association) Style Abu-awwad, A. N., Muti, . H. Y., Khater, . D. F., Khalaf, . A. M. & Arafat, . T. A. (2025) Simultaneous determination of ciprofloxacin, enrofloxacin, doxycycline, and chloramphenicol residues in poultry, red meat, and fish by high-performance liquid chromatography ultraviolet method. Open Veterinary Journal, 15 (9), 4403-4411. doi:10.5455/OVJ.2025.v15.i9.47 |