| Research Article | ||
Open Vet. J.. 2025; 15(9): 4432-4440
Open Veterinary Journal, (2025), Vol. 15(9): 4432-4440 Research Article A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus)Dhyaa Ab. Abood* and Mohammed Sulaiman DawoodDepartment of Anatomy and Histology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Dhyaa Ab. Abood. Department of Anatomy and Histology, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Email: dhyaa.ab [at] covm.uobaghdad.edu.iq Submitted: 16/05/2025 Revised: 25/07/2025 Accepted: 18/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
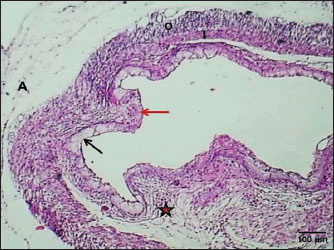
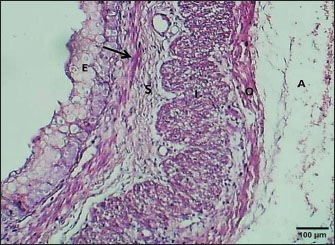
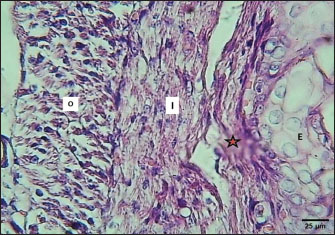
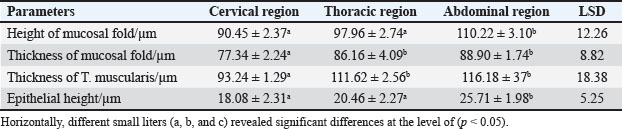
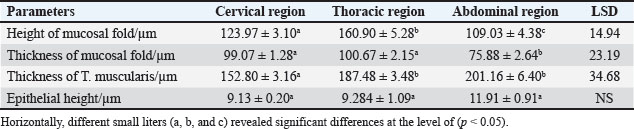
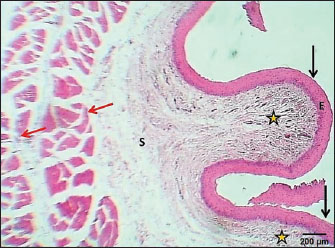
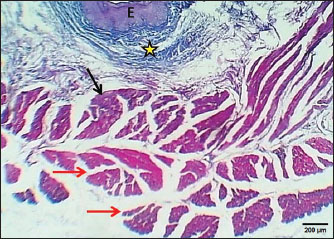
ABSTRACTBackground: The esophagus is vital for the efficient transport of food from the mouth to the stomach. It features mucous membrane and muscle fiber layers that are essential for generating powerful peristaltic movements to propel food forward. Additionally, the inner lining of the esophagus comprises specialized epithelial cells specifically designed to handle the passage of both rough and smooth food types with ease. Aim: The current study was aimed as a comparative model for investigating the changes that occur in the esophagus cytoarchitecture of the same animal species. Methods: Tissue specimens were processed according to the protocol of the paraffin embedding technique and stained with hematoxylin and eosin and Masson’s streptomycin (Masson’s trichrome). Results: In this study, the esophagi of lactating, weaned, and adult cats were used to investigate histological and histochemical features. The esophagus wall of lactating kittens revealed immature cytoarchitecture of all tunicae with little mucosal folds covered by proliferating epithelial cells, the muscularis mucosa was absent, and the muscularis externa comprised a bilayer of newly differentiating smooth myofibers. In weaned kittens, the esophagus had a slightly thickened wall, the mucosa of the cervical and thoracic segments had long folds, and the thoracic and abdominal segments revealed differentiation of the muscularis mucosa and skeletal muscle fibers, which differentiated in the cervical and thoracic segments, while the muscularis revealed smooth muscle bundles in the abdominal segments. In mature cats, the mucosa of the entire length of the esophagus comprised enlarged mucosal folds that were covered by very thick stratified squamous cells. The lamina propria submucosa had no esophageal glands. Muscular mucosa was absent in the cervical segment, whereas it was observed in the thoracic and abdominal segments. The muscularis externa at the neck and chest segments of the esophagus is constructed of inner circular and outer longitudinal bundles of skeletal muscle, whereas it turns into smooth muscle fibers in the abdominal segment. The statistical analysis of the data revealed significant differences in fold height and epithelium, fold thickness, and tunica muscularis among segments in weaned, lactating, and adult cats. Conclusion: The current study concluded that the cat esophagus was not a glandular part of the gastrointestinal tract compared with other mammals, which makes this species a reliable experimental model of digestive tissue, and explained the role of the pharyngeal or oral part of the digestive system in the lubrication of the upper digestive tract. Keywords: Esophagus, Histochemical, Cats, Kitten. IntroductionCats (Filus domesticus) are domesticated animals belonging to the carnivore species that have some physiological characteristics common with humans than laboratory (James, 1995). Cats are neglected in anatomical and histological studies, and most studies are mainly focused on surgical aspects and other sectors of veterinary medicine, eg, stricture, foreign body (Birchard and Sherding, 2006; Samadi et al., 2010). The esophagus can be vulnerable to a wide array of diseases, each presenting its unique challenges and implications for health (Nelson and Couto, 2019) and esophageal disorder (Gelber, 2014), foreign body obstructions represent the most prevalent clinical challenges associated with the esophagus, while instances of act masses also emerge in these cases (Allouch and Kaddi, 2018). The esophagus passes food from the oral cavity to the next organ of the gut. This function is important to make an esophagus with several unique layers of mucous membrane and myofibers that are essential to generate peristaltic movement for moving food. The esophagus lumen requires a specialized epithelial type for sustaining the passage of rough or smooth raw food (Zhang et al., 2017). Many recent studies have investigated the cytoarchitecture in addition to the histochemical structure of this part of the gut in two animal species that have different types of food and behavior as that of canine (Dawood et al., 2022) and donkey as a species of equine (Abood et al., 2023). A previous study was limited to the structural development of the esophagus in the embryo of a one-humped camel (Bello et al., 2012) and in the adult camel (Hussein et al., 2016), whereas other studies dealt with the esophagus of wild ruminants such as wild Camelidae (Sukon et al., 2009) and in lab animals (Mahmood et al., 2017). The current study was designed as a comparative model to investigate the changes that occur in the cytoarchitecture of the esophagus of the same animal species (Files catus). Materials and MethodsAnimals’ preparationTwelve samples of esophagus were used in the current study involving 4 breast lactation kittens, age 1–2 weeks; 4 weaned kittens, age 4–5 weeks; and 4 mature cats, age 1–2 years. This study was conducted in the Department of Anatomy and Histology, College of Veterinary Medicine, University of Baghdad. The animals were obtained from hopeless, cureless cases of car accidents from many veterinary clinics in Baghdad provenance, and all animals were euthanized according to the protocol of Al-Falahi (2017) and Al-Saffar and Ab Abood (2015). The esophagus samples were immediately collected from the bodies of the animals and then washed with 0.9% normal saline. Esophageal tissue specimens (1 cm) were collected at the levels of the neck, chest, and abdominal regions of animals where the esophagus passes throughout the body. Tissue specimens were fixed in 10% buffered formalin saline for 72 hours. Tissue specimens were treated according to the paraffin embedding histological method at 58°C–60°C. The tissue sections were meticulously sliced into delicate ribbons measuring 5 to 6 micrometers in thickness using a precision rotary microtome. Tissue sections were stained with H&E and Masson’s stain (Bancroft and Marilyn, 2008; Abood et al., 2023; Yousif et al., 2023; Ab Abood et al., 2024). Sections have been investigated using an Olympus microscope camera. The histometrical data of each esophageal segment were estimated and analyzed according to Suad et al. (2018). Statistical analysesAll numerical data were displayed as mean ± SE, and one-way ANOVA was used to detect a significant difference at p < 0.05. Ethical approvalAll study methods and preparations were approved (Approval No. PG/2334) by the Animal Care and Use Committee (ACUC) of the Faculty of Veterinary Medicine, University of Baghdad, Iraq, on June 28, 2022. ResultsLactating kittenThe esophagus wall of breast lactating kittens revealed four immature tunicae cytoarchitectures. The tumor mucosa was very thin and displayed few mucosal folds (Fig. 1). The mucosal folds were elegantly adorned with immature, proliferating epithelial cells, showcasing striking mitotic figures that vividly attest to their dynamic and vigorous growth. The mucosal folds were elegantly adorned with immature, proliferating epithelial cells, showcasing striking mitotic figures that vividly attest to their dynamic and vigorous growth. Muscularis mucosa was absent along the entire length of the esophagus, so the lamina propria merged with the tunica submucosa, which comprised immature fibrous tissue rich with fibroblasts (Figs. 2 and 3). Muscularis externa comprised a bilayer (inner circular followed by outer longitudinal oriented layers) that displayed newly differentiated smooth muscle cells (Fig. 3). Analysis of the histometric data revealed an important difference at (p < 0.05) in the height of the folds and epithelia at the level of the abdominal segment, whereas the thickness of the folds and externa muscularis thickness displayed significant increase (p < 0.05) in the chest and abdominal segments (Table 1).
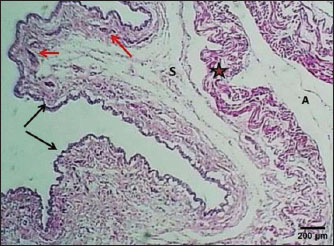
Fig. 1. Section of esophagus at cervical region (lactating kitten) shows: (red arrow) low fold, (black arrow) no differentiated epithelium, (asterisk) lamina propria submucosa, (I) inner circular smooth muscle, (O) outer longitudinal smooth muscle layer and tunica adventitia (A). H&E stain.
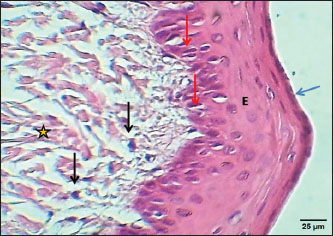
Fig. 2. Section of the esophagus at the thoracic region (lactating kitten) shows: (E) no differentiated epithelium, (black arrow) lamina propria, (S) submucosa, (I) inner circular smooth muscle, (O) outer longitudinal smooth muscle layer and tunica adventitia (A). H&E stain.
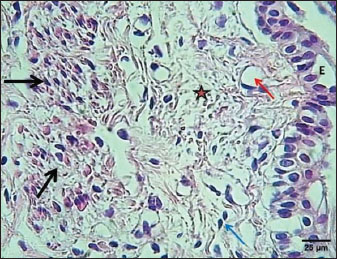
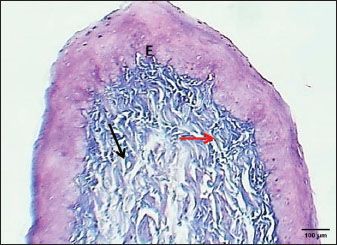
Fig. 3. Section of mucosa of the esophagus at abdominal region (lactating kitten) shows (E) immature proliferating stratified epithelium, (asterisk) immature collagen fiber of lamina propria with active fibroblasts, (I) inner layer, and (O) outer layer of smooth muscle cells. H&E stain. Table 1. The height of mucosal folds and epithelium and thickness of mucosal folds and muscularis in the esophagus of a lactating kitten (n=4).
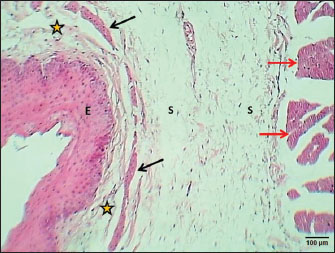
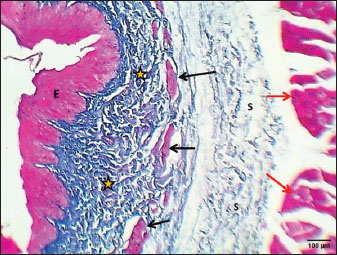
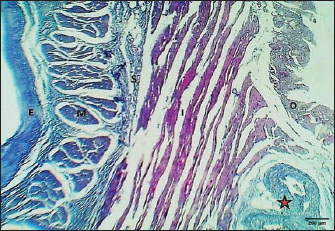
Weaned kittensThe esophagus of weaned kittens displayed a slightly thickened wall and had well-distincted tunicae (Fig. 4). The mucosa of the cervical and thoracic segments displayed many long mucosal folds (Fig. 4). These folds were covered by three layers of prismatic-shaped cells: basal, epibasal basal, and superficial epithelial cells (Figs. 5 and 6). The lamina propria of the chest and abdominal segments of the esophagus revealed patches of newly differentiated interrupted myofibers of the muscularis mucosa (Figs. 4 and 5). Lamina propria-tunica submucosa comprises immature fibrous tissue that is rich in fibroblasts and newly produced immature collagen fibrils (Figs. 4–6). Skeletal muscle fibers were differentiated and organized into a circularly oriented inner bundle and a longitudinally oriented outer bundle in the neck and chest regions of the esophagus (Fig. 4). Meanwhile, in the abdominal region, the muscularis of the esophagus turned into non-striated myofibers (smooth type) (Fig. 5). Important differences were recorded in the height of mucosal folds among all segments of the esophagus; meanwhile, fold thickness was significantly increased in the abdominal region. The thickness of the tunica muscularis revealed a significant increase in the thoracic and abdominal segments (Table 2).
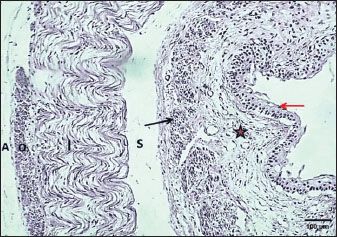
Fig. 4. Cross-section of esophagus at thoracic region (weaning cat) shows: (black arrows) long folds with thin epithelium, (red arrow) muscularis mucosa, (S) submucosa, (asterisk) inner circular and outer longitudinal skeletal muscle layer, and tunica adventitia (A). H&E stain.
Fig. 5. Section of esophagus: abdominal region (weaning cat) shows: (red arrow) epithelium, (asterisk) lamina propria, (black arrow) muscularis mucosa, (S) submucosa, (I) inner circular skeletal muscle, (O) outer longitudinal smooth muscle layer, and tunica adventitia (A). H&E stain.
Fig. 6. Section of mucosa of esophagus at abdominal region (weaning cat) shows (E) immature proliferating cells composed of basal, epibasal, and superficial epithelial cells, (asterisk) immature collagen fiber of lamina propria with active furthermore active fibroblasts (blue arrow) with well angiogenesis (red arrow), and differentiating myofibers of muscularis mucosa (black arrows). H&E stain. Table 2. The height of mucosal folds and epithelium with thickness of mucosal folds and muscularis in esophagus of weaned kitten (n=4).
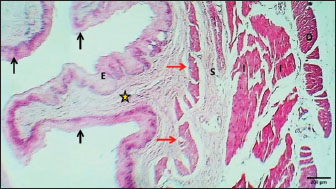
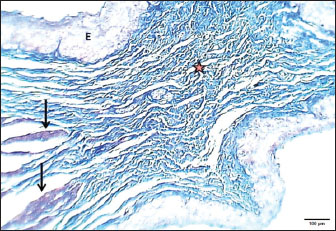
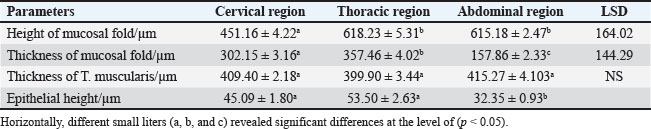
Mature catThe results revealed that the cervical segment’s mucosa was very thick, with many mucosal folds covered by slightly thick non-keratinized stratified squamous epithelium with marked parakeratosis (Figs. 7 and 8). Lamina propria submucosa revealed the absence of the muscularis mucosa, which comprised dense, irregular, collagenous connective tissue rich with blood vessels (Figs. 8 and 9). Muscularis externa of the esophagus at the chest segment is composed of thickened circularly oriented bundles and longitudinally oriented bundles of skeletal muscle fibers (Fig. 10). At the chest segment, the esophagus also showed many huge folds, and the epithelium was similar to that of the cervical segment (Fig. 11). The lamina propria comprised slightly thick fibrous tissue separated from the tunica submucosa by 3–4 layers of interrupted circular smooth muscle fibers of the muscularis mucosa (Fig. 12). The tumor submucosa consisted of thick layers of non-glandular highly vascular fibrous connective tissue (Fig. 12). Tunica muscularis of the thoracic region was continuing that in the cervical segment. The esophagus in the abdominal region was similar to that observed in the thoracic segment (Fig. 13). The lamina propria was built up by dense collagenous connective tissue separated by very thick longitudinal and circular layers of smooth myofibers (muscularis mucosa) (Figs. 14 and 15). The muscularis externa comprised a very thick circular bundle and a thin longitudinal bundle of smooth myofibers (Fig. 15). The histometric results revealed significant differences in the height of mucosal folds in the chest and abdominal segments, whereas the fold thickness was significantly increased in the abdominal region. The thickness of the muscularis externa revealed no significant differences in the chest and abdominal regions. The epithelium height was significantly reduced in the abdominal region (Table 3).
Figure 7. Longitudinal section of esophagus- cervical region (mature cat) shows (black arrows) mucosal folds, (E) mature epithelium, (asterisk) mature fibrous tissue, (S) non-glandular submucosa, (red arrows) thick inner longitudinal layer of tunica muscularis. H&E stain.
Fig. 8. Section of mucosa of esophagus at cervical region (mature cat) shows (E) non-keratinize stratified squamous epithelium, (blue arrow) marked parakeratosis, (red arrows) marked mitotic figures of stratum basalis cells, (asterisk) irregular collagen fiber of lamina propria and fibroblasts (black arrows) esophageal mucous glands. H&E stain.
Fig. 9. Section of mucosal folds of esophagus at cervical region (mature cat) shows (E) stratified squamous epithelialis, (red arrow) mature collagen bundles of lamina propria, (black arrow) capillaries. Masson’s trichrome stain.
Fig. 10. Cross-section of esophageal thoracic region (mature cat) shows (E) epithelium, (asterisk) lamina propria submucosa, and (black arrow) inner circular bundles of skeletal muscle, (red arrows) outer longitudinal bundles of skeletal muscle. Masson’s trichrome stain.
Fig. 11. Longitudinal section of esophagus-thoracic region (mature cat) shows (E) very thick epithelium, (asterisks) thin lamina propria, (black arrows) thin muscularis mucosa, (S) very thick loose connective tissue of submucosa, (red arrow) thick inner circular bundles of skeletal muscle of tunica muscularis. H&E stain.
Fig. 12. Cross-section of esophagus: thoracic region (mature cat) shows (E) very thick epithelium, (asterisks) thick dense lamina propria, (black arrows) thin interrupted smooth muscle of muscularis mucosa, (S) thick fibrous connective tissue of the submucosa, and (red arrow) inner circular bundles of skeletal muscle of tunica muscularis. H&E stain.
Fig. 13. Cross section of esophagus: abdominal region (mature cat) shows: (black arrows) huge mucosal folds (E) very thick epithelium, (asterisk) dense fibrous tissue of lamina propria, (red arrows) thick muscularis mucosa (I) thick inner circular smooth muscle, (O) thin outer longitudinal muscle layer. H&E stain.
Fig. 14. Cross section of esophageal fold (mature cat) shows (E) very thick epithelium, (asterisks) dense fibrous lamina propria, (arrows) smooth muscle of muscularis mucosa. Masson’s trichrome stain.
Fig. 15. Cross-section of esophagus: abdominal region (mature cat) shows (E) very thick epithelium, (M) thick muscularis mucosa, (S) submucosa, (I) inner circular layer, and (O) outer layer of smooth muscle of tunica muscularis. Masson’s trichrome stain. Table 3. The height of mucosal folds and epithelium with a thickness of mucosal folds and muscularis in the esophagus of adult cats (n=4).
DiscussionThis study investigated the histogenesis of the esophagus during three periods of postnatal life in cats. During lactation, the esophageal mucosa revealed still differentiations of all tunicae, and the epithelium showed proliferating cells as observed in neonate mice by (Daiko et al., 2006) who mentioned that the tunicae of the esophagus revealed not well-differentiated endoderm lining cells, differentiated fibroblasts, and myoblast cells. The histogenesis of the epithelium of mucosa started as a simple columnar and then turned into a stratified squamous type during development (Yu et al., 2005; Rodriguez et al., 2010; Zhang et al., 2017). A few layers of stratified epithelium remained low until the postnatal period in mice. Meanwhile, the suprabasal cells also started developing and gradually lost their proliferating capability (Yu et al., 2005). This finding revealed that in the entire length of the esophagus, the muscle during the lactation period revealed differentiated smooth muscle cells. McHugh (1996, 1995) reported that the mesenchymal cells turned into smooth muscle at approximately 11.0E. Kablar et al. (2000) reported that the primordium of striated muscle is formed in the cervical region of the esophagus at 13.E, and smooth myofibers are changed by striated myofibers in a descending manner from anterior to posterior manner after 3 weeks until adulthood. This result in differentiation is regulated by gene expression (Romer et al., 2013), who noted that several genes have been identified as responsible for the growth of esophageal musculature. In weaned cats, the epithelioid mucosa comprises three layers of prismatic-shaped cells: basal cells, epibasal cells, and superficial cells. This result agrees with the results of Daiko et al. (2006), who reported that the basal cell layer contains stem cells. In weaned kittens, the mucosal muscularis layer started at the esophageal neck and abdominal regions. This result is in accordance with the results of Meyer et al. (1986), who stated that the generation of muscular contractions to pass food along the entire esophageal length requires coordinated action of multiple layers of muscles during the weaning period. Furthermore, cells differentiate to build up the top layers. The weaning period was the time at which complete differentiation of the muscularis externa was observed, similar to that observed in mice in which the neck and chest segments are composed of skeletal-type myofibers, whereas the lower chest and abdominal segments consist of smooth myofibers (Kablar, 2000; Jacobs et al., 2012). The statistical analysis revealed a significant difference in the growth of the folds among all segments of the esophagus. The thickness of the tunica muscularis also revealed a significant increase in the thoracic and abdominal regions. These results suggest that the developmental processes involved both different ions and growth. The present study revealed a thick wall with numerous mucosal folds covered by stratified squamous cells in an adult cat. This result is in agreement with the results of Abood et al. (2023) in donkeys, Dawood et al. (2022) in dogs, Berghes et al. (2011) in guinea pigs, Devi (2021) in humans and dogs, Kumar et al. (2009) in small ruminants, and Allouch and Kaddi (2018) in cats and rabbits. The current study did not agree with the result observed in camels by Hussein et al. (2016), who reported a smooth mucosal surface, meaning no folds were present. The esophageal folds are present in carnivores and are related to protection from damage by hard food. This fold allows for quite expansion of the esophageal lumen, allowing food to pass easily to the stomach (Samadi et al., 2010; Dawood et al., 2022; Fails and Magee, 2018). The epithelial mucosa of the esophagus in adult mice loses nuclei at the same time as keratinization, similar to that observed in skin morphogenesis (Fuchs and Raghavan, 2002; Fuchs, 2007). Our statistical analysis showed a significant increase in the height of the folds in the thoracic and abdominal segments of the esophagus. This suggests that these folds facilitate swallowing due to the absence of esophageal glands. On the other hand, the study showed that the height of the epithelium mucosa was higher at the upper two-thirds of the cat esophagus, similar to that recorded by Abood et al. (2023) in donkey, Dawood et al. (2022) in dog, and Devi (2021) who mentioned that the neck segment of the esophagus revealed the thickest epithelium, while the thoracic segment of the esophagus was slightly thick and the abdominal segment was very thin epithelium. In Agouti (Garcia et al., 2000), in goat (Kumar et al., 2009), the epithelium was thicker in the chest region than in the neck and abdominal regions. In humans, a high thickness of epithelium is observed in the neck region of the esophagus, which is manifested by the presence of many fibrous protrusions within the connective tissue of the lamina propria (Kumar et al., 2009). The variation in mucosal thickness is related to the natural hard diet of carnivores (Dawood et al., 2022; Abood et al., 2023). Our study showed that the lamina propria of the esophageal neck region revealed the absence of muscularis mucosa, whereas the muscular mucosa appeared in the thoracic and abdominal regions, similar to that observed in Gray Mongoose esophagus which showed thin interrupted myofibers that thickened in the chest region of the esophagus (Kadhim, 2019), in goats (Kumar et al., 2009) and in buffalo by Goetsch (1910). The muscular mucosa is markedly developed in the entire esophageal length in agouti (Garcia et al., 2000) and camels (Bello et al., 2012). In dogs, the smooth-type muscularis mucosa was observed in the posterior region of the esophagus (Jamdar and Ema, 1982; Ali et al., 2008), indicating the presence of muscularis mucosae in the caudal part of the camel. Dissimilarly those mentioned by most authors in (one humped camel, dogs, gray mongoose, guinea pig, goat, sheep, and ox) the current study revealed no glandular tissue was present in the lamina propria or submucosa of esophagus of cat, this make a feline has a unique feature in esophageal architecture, this result suggests that the absent of esophageal glands is related with short length of feline esophagus and with amount of saliva produced by salivary glands that suitable for allow swallowing. Current results revealed well-musculature composite was build up the tunica muscularis of adult cat, this was similar for that observed in other mammal, in humans, the upper third of esophagus is building up by bilayer of striated myofibers, while in the middle third is building up a mixture of striated and smooth myofibers, then completely turning into smooth myofibers at the lower third (Jacobs et al., 2012; Vegesna et al., 2012; Yazaki and Sifrim, 2012), also the present result similar that observed in gray mongoose by Kadhim (2019) which is composed of circularly oriented inner layer and longitudinal oriented outer layers of striated myofibers, but this thickness of cat muscularis was dissimilar (170 and 200 μm) respectively, and turned into smooth muscle fibers in caudal region (315 μm). This result is also similar to the tunica muscular of the esophagus in dogs (Dawood et al., 2022), donkeys (Abood et al., 2023), and humans (Devi, 2021). The current result is dissimilar to that seen in the esophagus of a one-humped camel (Bello et al., 2012; Naghani and Andi, 2012), who recorded that striated myofibers completely build up the esophageal muscularis externa. The agouti tunica muscularis consists of circular and longitudinal smooth myofibers (Garcia et al., 2000). In addition, in horses and pigs, the externa muscularis consists of oblique striated myofibers in the neck, which are spirally oriented in the chest region and then organized in the form of circular inner and longitudinal outer layers of smooth myofibers in the abdominal region. In ruminants, the muscularis is striated with myofibers along the esophageal length (Fails and Magee, 2018). In goats, the muscularis externa is thickened and has inner circular orientation and much thinner longitudinally oriented bundles of striated myofibers (Kumar et al., 2009). ConclusionThe current study investigated a series of postnatal life periods from neonate (lactating) to weaning and adulthood to show the main and textural changes for each period and whether the type of food has an effect. This study considered that the cat esophagus was not a glandular part of the GI tract compared with other mammals, making this species reliable as an experimental model of digestive tissue and explaining the role of the pharyngeal or oral part of the digestive system in lubricating the upper digestive tract. AcknowledgmentThe authors would like to acknowledge the assistance of Mr. Muoaid Fadil and staff in the laboratory of histological techniques at the Department of Anatomy, Histology and Embryology, College of Veterinary Medicine, University of Baghdad. FundingNone. Authors’ contributionsDhyaa Ab. Abood: original draft writing; review and editing; conceptualization; data curation; investigation; methodology; project administration; resources; software. Mohammed Sulaiman Dawood: supervision, validation, writing, review, and editing. Conflict of interestThe authors declare no conflicts of interest regarding the publication of this manuscript. Data availabilityData are available within the article. ReferencesAb Abood, D., Dawood, M.S., Yousif, N.H. and Karim, A.J. 2024. Histological and histochemical features of the mature female reproductive tract of local breed dog (Canis familiaris). J. Adv. Vet. Anim. Res. 11(4), 835. Abood, D.A., Dawood, M.S., Mohammed, L.E. and Karim, A.J. 2023. Histological and histochemical characteristics of the esophagus in local breed donkey (Equus asinus). J. Adv. Vet. Anim. Res. 10(1), 14–20; doi:10.5455/javar.2023.j647 Al-Falahi, N.H. 2018. Comparative evaluation of bovine pericardial membrane and amniotic membrane in wounds skin healing in rabbits. Iraqi J. Vet. Med. 41(2), 137–145. Ali, M.N., Byanet, O., Salami, S.O., Imam, J., Maidawa, S., Umosen, A.D. and Nzalak, J.O. 2008. Gross anatomical aspect of gastrointestinal tract of the wild African giant rat (Cricetomys gambianus). J. Sci. Res. 3(10), 518–520. Allouch, M. and Kaddi, M. 2018. Surgical Anatomy of the esophagus in cats and removal of esophageal foreign bodies (sneeze spine) using laryngoscope technique. Dairy Vet. Sci. J. 5(5), 1–4. Al-Saffar, F.J. and Ab Abood, D. 2015. The post hatching development of the female genital system in indigenous mallard duck (Anas platyrhynchos). Iraqi J. Vet. Med. 39(2), 17–25; doi:10.30539/iraqijvm.v39i2.172 Bancroft, J.D. and Marilyn, G. 2008. Theory and practice of histological techniques. 1st ed. London: Elsevier Limited, p: 168–73. Bello, A., Onyeanusi, B.I., Sonfada, M.L., Adeyanju, J.B., Umar, A.A., Umaru, M.A., Shehu, S.A. and Hena, S.A. 2012. Histomorphological studies of the prenatal development of oesophagus of one humped camel (camelus dromedarius). Sci. J. Agric. 1(4), 100–104. Berghes, C., Tanase, P., Parvu, M., Dinu, C. and Cuca, D. 2011. Contributions to the study of the esophagus and stomach morphology in guinea pig. Sci. Anim. Sci. Biotechnolo. 44(2), 150–154. Birchard, S.J. and Sherding, R.G. 2006. Esophageal diseases and swallowing disorders. In: Saunders manual of small animal practice, 3rd ed. Columbus, OH: The Ohio State University, pp: 636–5. Daiko, H., Isohata, N., Sano, M., Aoyagi, K., Ogawa, K., Kameoka, S., Yoshida, T. and Sasaki, H. 2006. Molecular profiles of the mouse postnatal development of the esophageal epithelium showing delayed growth start. Int. J. Mol. Med. 18(6), 1057–1066. Dawood, M.S., Abood, D.A. and Hameza, A.Y. 2022. The histological and histochemical features of the esophagus in local breed dogs (Canis familiaris). Iraqi J. Vet. Sci. 36(4), 1069–1074; doi:10.33899/ijvs.2022.133034.2164 Devi, Y.D. 2021. Comparative histological study of the esophagus of mammals. J. Res. Med. Den. Sci. 9(7), 397–399. Fails, A.D. and Magee, C. 2018. Anatomy and physiology of farm animals. Hoboken, NJ: John Wiley & Sons, p: 380. Fuchs, E. 2007. Scratching the surface of skin development. Nature 445(7130), 834–842. Fuchs, E. and Raghavan, S. 2002. Getting under the skin of epidermal morphogenesis. Nat. Rev. Genet. 3(3), 199–209. Garcia, G.W., Baptiste, Q.S., Adogwa, A.O., Kakuni, M., Arishima, K. and Makita, T. 2000. The digestive system of the agouti (Dasyprocta leporina)-gross anatomy and histology. J. Biol. Sci. U. S. A. Japanese J. Zoo. Wildlife Med. 5(1), 55–66; doi:10.5686/jjzwm.5.55 Gelberg, H.B. 2014. Comparative anatomy, physiology, and mechanisms of disease production of the esophagus, stomach, and small intestine. Toxicol. Pathol. 42, 54–66; doi:10.1177/0192623313518113 Goetsch, E. 1910. The structure of the mammalian esophagus. Am. J. Anat. 10, 1–40. Hussein, A.J., Cani, M.M. and Hussein, D.M. 2016. Anatomical and histological studies of esophagus of one-humped camel (Camelus dromedarius). MRVSA 5, 11–18. Jacobs, I.J., Ku, W.Y. and Que, J. 2012. Genetic and cellular mechanisms regulating anterior foregut and esophageal development. Dev. Biol. 369(1), 54–64. Jamdar, M.N. and Ema, A.N. 1982. The submucosal glands and the orientation of the musculature in the esophagus of the camel. J. Anat. 135(1), 165–171. James, A.E. 1995. The laboratory cats. ANZCCART News 8(1), 1–2. Kablar, B., Tajbakhsh, S. and Rudnicki, M.A. 2000. Transdifferentiation of esophageal smooth to skeletal muscle is myogenic bHLH factor-dependent. Development 127(8), 1627–1639. Kadhim, K.K. 2019. Histomorphology and histochemical study of the esophagus and stomach in gray mongoose (Herpestes edwardsii) in Iraq. Indian J. Nat. Sci. 9(52), 16458–16475. Kumar, P., Mahesh, R. and Kumar, P. 2009. Histological architecture of esophagus of goat (Capra hircus). Haryana Vet. 48, 29–32. Mahmood, H.B., Al-Aameli, M.H. and Obead, W.F. 2017. Histological study of esophagus in dogs and rabbits. J. Kerbala Univ. 15(3), 55–62. Mchugh, K.M. 1995. Molecular analysis of smooth muscle development in the mouse. Develop. Dyn. 204(3), 278–290. McHugh, K.M. 2019. Molecular analysis of gastrointestinal smooth muscle development. J. Pediatr. Gastroenterol. Nutr. 23(4), 379–394. Meyer, G.W., Austin, R.M., Brady, C.E. and Castell, D.O. 1986. Muscle anatomy of the human esophagus. J. Clin. Gastroenterol. 8(82), 131–134. Naghani, E.S. and Andi, M.A. 2012. Some histological and histochemical study of the esophagus in one-humped camel (Camelus dromedaries). Global Vet. 8(2), 124–127. Nelson, R.W. and Couto, C.G. 2019. Small animal internal medicine-E-book. Elsevier Health Sciences, Inc. Rodriguez, P., Da Silva, S., Oxburgh, L., Wang, F., Hogan, B.L. and Que, J. 2010. Signaling in the development of the mouse esophagus and forestomach. Development 137(24), 4171. Romer, A.I., Singh, J., Rattan, S. and Krauss, R.S. 2013. Smooth muscle fascicular reorientation is required for esophageal morphogenesis and is Cdo-dependent. J. Cell Biol. 201(2), 309–323. Samadi, F., Levine, M.S., Rubesin, S.E., Katzka, D.A. and Laufer, I. 2010. Feline esophagus and gastroesophageal reflux. AJR. Am. J. Roentgenol. 194(4), 972–976. Suad, K.A., Al-Shamire, J.S.H. and Dhyaa, A.A. 2018. Histological and biochemical evaluation of supplementing broiler diet with β-hydroxy-methyl butyrate calcium (β-HMB-Ca). Iran. J. Vet. Res. 19, 27–34. Sukon, P., Timm, K.I. and Valentine, B.A. 2009. Esophageal anatomy of the Llama (Lama glama). Int. J. Morphol. 27(3), 811–817; doi:10.4067/S0717-95022009000300028 Vegesna, A.K., Chuang, K.Y., Besetty, R., Phillips, S.J., Braverman, A.S., Barbe, M.F., Ruggieri, M.R. and Miller, L.S. 2012. Circular smooth muscle contributes to esophageal shortening during peristalsis. World J. Gastroenterol. 18, 4317–4322. Yazaki, E. and Sifrim, D. 2012. Anatomy and physiology of the esophageal body. Dis. Esophagus 25(4), 292–298. Yousif, N.H., Hadi, H.D. and Jihad, H.M. 2023. Histological study of the liver of guinea pig Cavia porcellus (Linnaeus, 1758) in Iraq. Revis. Bionat. J. Vet. Sci. 8(3), 80. Yu, W.Y., Slack, J.M. and Tosh, D. 2005. Conversion of columnar to stratified squamous epithelium in the developing mouse esophagus. Dev. Biol. 284(1), 157–170. Zhang, Y., Jiang, M., Kim, E., Lin, S., Liu, K., Lan, X. and Que, J. 2017. Development and stem cells of the esophagus. Semin. Cell Dev. Biol. 66, 25–35; doi:10.1016/j.semcdb.2016.12.008 | ||
| How to Cite this Article |
| Pubmed Style Abood DA, Dawood MS. A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus). Open Vet. J.. 2025; 15(9): 4432-4440. doi:10.5455/OVJ.2025.v15.i9.50 Web Style Abood DA, Dawood MS. A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus). https://www.openveterinaryjournal.com/?mno=253179 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.50 AMA (American Medical Association) Style Abood DA, Dawood MS. A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus). Open Vet. J.. 2025; 15(9): 4432-4440. doi:10.5455/OVJ.2025.v15.i9.50 Vancouver/ICMJE Style Abood DA, Dawood MS. A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus). Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4432-4440. doi:10.5455/OVJ.2025.v15.i9.50 Harvard Style Abood, D. A. & Dawood, . M. S. (2025) A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus). Open Vet. J., 15 (9), 4432-4440. doi:10.5455/OVJ.2025.v15.i9.50 Turabian Style Abood, Dhyaa Ab., and Mohammed Sulaiman Dawood. 2025. A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus). Open Veterinary Journal, 15 (9), 4432-4440. doi:10.5455/OVJ.2025.v15.i9.50 Chicago Style Abood, Dhyaa Ab., and Mohammed Sulaiman Dawood. "A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus)." Open Veterinary Journal 15 (2025), 4432-4440. doi:10.5455/OVJ.2025.v15.i9.50 MLA (The Modern Language Association) Style Abood, Dhyaa Ab., and Mohammed Sulaiman Dawood. "A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus)." Open Veterinary Journal 15.9 (2025), 4432-4440. Print. doi:10.5455/OVJ.2025.v15.i9.50 APA (American Psychological Association) Style Abood, D. A. & Dawood, . M. S. (2025) A comparative histological and histochemical features of esophagus at weaning and mature local breed cats (Felis catus). Open Veterinary Journal, 15 (9), 4432-4440. doi:10.5455/OVJ.2025.v15.i9.50 |