| Research Article | ||
Open Vet. J.. 2025; 15(8): 3746-3758 Open Veterinary Journal, (2025), Vol. 15(8): 3746-3758 Research Article Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal diseaseMohammed T. Jaafar1, Ahmed S. Abukhomra2, Ahmed Jassim Almialy3, Isra’a M. Essa4 and Hasanain A.J. Gharban5*1Department of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, University of Wasit, Kut, Iraq 2Department of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, Al-Qasim Green University, Al Qasim, Iraq 3Department of Veterinary Clinical Sciences, Faculty of Veterinary Medicine, University of Kufa, Najaf, Iraq 4Department of Public Health, College of Veterinary Medicine, University of Basrah, Basra, Iraq 5Department of Internal and Preventive Veterinary Medicine, College of Veterinary Medicine, University of Wasit, Kut, Iraq *Corresponding Author: Hasanain A.J. Gharban. Department of Internal and Preventive Veterinary Medicine, College of Veterinary Medicine, University of Wasit, Kut, Iraq. Email: hghirban [at] uowasit.edu.iq Submitted: 16/04/2024 Revised: 15/07/2025 Accepted: 31/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
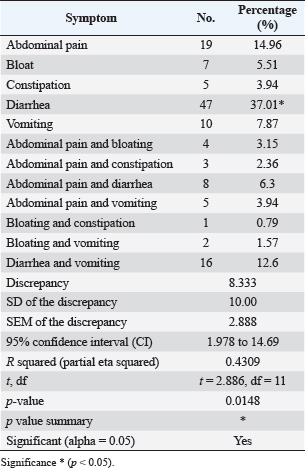
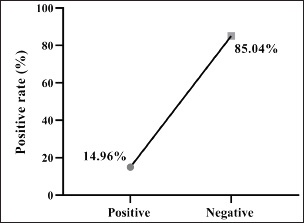
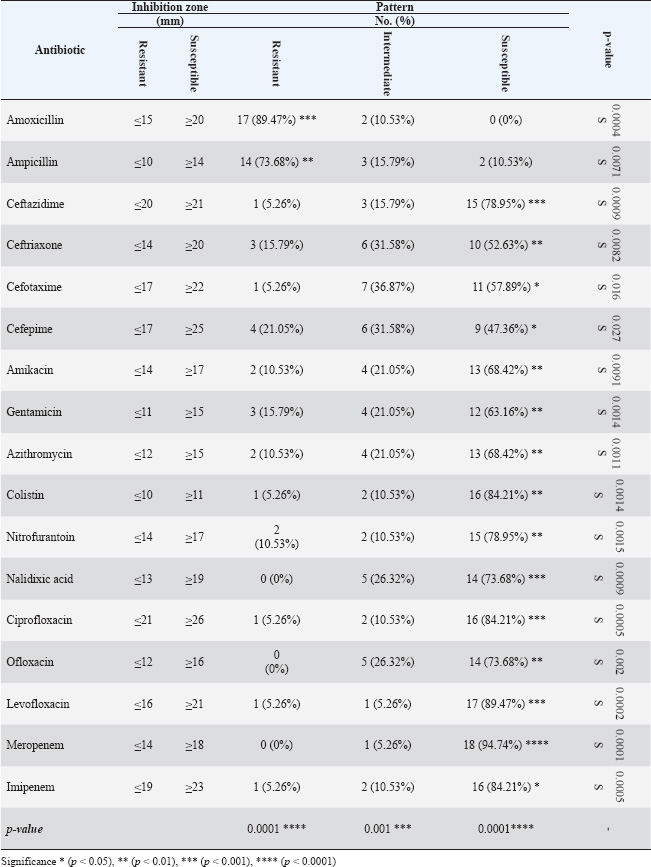
ABSTRACTBackground: Gastrointestinal disorders in dogs are important clinical conditions in veterinary medicine, which have multifactorial non-infectious and infectious etiologies. Colibacillosis, caused by Escherichia coli, is an infectious bacterial disease that contributes to host health and pathogenic variants that are responsible for a wide spectrum of diseases. Aim: This study aims to investigate the prevalence rate of gastrointestinal signs in diseased pet dogs, phylogenetic analysis of colibacillosis, measurement of antibiotic sensitivity, and levels of immune and antioxidant markers in dog populations. Methods: A total of 127 pet dogs admitted with various gastrointestinal signs were examined clinically. Fresh fecal samples were traditionally and molecularly analyzed to identify E. coli isolates for phylogenetic analysis and antibiotic susceptibility. The cephalic venous blood samples were examined quantitatively by enzyme-linked immunosorbent assay to measure immune markers such as interleukins (IL-1, IL-4, IL-6, and IL-8) and interferon-alpha (INF-α), as well as antioxidants such as catalase (CAT), glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA). Results: Clinically, diarrhea was the most identified gastrointestinal sign in the study dogs. Traditional findings revealed that 14.96% of fecal samples were positive to E. coli isolates that showed a high sensitivity to antibiotics such as meropenem, levofloxacin, and ciprofloxacin with a significant resistance to amoxicillin and ampicillin. Molecularly, 78.95% of study isolates were positive to E. coli strains, which were found to be phylogenetically (NCBI-BLAST) identical to the Iraqi strain (LC844819.1). Serologically, elevated levels of IL-1, IL-6, IL-8, and MDA with reduced levels of IL-2, CAT, GSH, and SOD were observed in E. coli-positive dogs. However, no significant alteration was seen in values of INF-α among the dogs with and without colibacillosis. Conclusion: The present study represents a multifaceted analysis of the incidence rate of gastrointestinal disorders in pet dogs, prevalence rate of E. coli using traditional and molecular phylogenetic diagnostics, and the association of colibacillosis to immune and antioxidant markers in Iraq. Keywords: Canine gastrointestinal infections, Escherichia coli, Interferon, Interleukin, Oxidative stress, Iraq. IntroductionGastrointestinal disorders in dogs encompass a wide range of etiologies, ranging from acute self-limiting to chronic debilitating diseases requiring intensive management. Therefore, understanding the multifaceted nature of canine gastrointestinal health is crucial for effective diagnosis and therapeutic intervention (Isidori et al., 2022; Kollannur et al., 2024). The prevalence of infectious diseases poses a substantial threat to both animals and humans, with an estimated 60% of known pathogens and up to 75% of new or re-emerging diseases resulting in severe economic losses and global health impacts (Marks et al., 2011; Bloom and Cadarette, 2019). The emergence of antibiotic resistance in many bacterial species further compounds this challenge, threatening the efficacy of conventional treatments and necessitating innovative therapeutic strategies (Stachelek et al., 2021). However, gastrointestinal disorders in dogs frequently occur as a result of dysbiosis of gut microbiota, particularly alterations in the population dynamics of Enterobacteriaceae, including alterations in microbial diversity, reduction in beneficial organisms, and increase in potentially harmful ones (Pilla and Suchodolski, 2020; Ziese and Suchodolski, 2021). Colibacillosis is an important disease for humans and animals, as it is caused by Escherichia coli, a typical pathogenic bacterium (Poirel et al., 2018; Fairbrother and Nadeau, 2019). Escherichia coli is a highly diverse and adaptable Gram-negative bacterium, which was first identified in the 1885s and further characterized in the 1886s due to its dual nature, encompassing both commensal strains that contribute to host health and pathogenic variants that are responsible for a wide spectrum of diseases (Riley, 2020; Ruiz and Silhavy, 2022). Commensal strains play beneficial roles in nutrient processing, immune protection, and vitamin K production; however, some strains are pathogenic and can induce various intestinal and extraintestinal diseases (Namazi et al., 2019; Ramos et al., 2020; Riley, 2020). In recent decades, the remarkable adaptability of E. coli has emerged due to its capacity to rapidly evolve and acquire new traits through horizontal gene transfer, mutation, and recombination, leading to the emergence of novel pathotypes and antimicrobial resistance (Nasrollahian et al., 2024; Saini et al., 2024). Diagnostic scrutiny often necessitates a multifaceted approach, integrating historical data, physical examination findings, and laboratory analyses to pinpoint the causative factors and initiate targeted treatment intervention (Riaz et al., 2023; Singh et al., 2024). In Iraq, few studies have been performed in dogs to determine the virulence genes of the pathogenic E. coli or its antimicrobial resistance (Yousif et al., 2016; Al-Kubaisi et al., 2020; Kadhim and Alrammah, 2024; Taalab et al., 2024). Hence, this study aimed to investigate the prevalence rate of colibacillosis in dogs with gastrointestinal infections based on clinical, traditional, and molecular data and to measure the levels of immunity and antioxidants in the dogs. This study also determined the incidence of antimicrobial resistance in the E. coli isolates. Materials and MethodsAnimal data and samplesA total of 127 diseased dogs with different clinical gastrointestinal signs were admitted to the private clinics located in Wasit province (Iraq) from October 2024 to March 2025. Initially, fresh fecal samples were collected from each animal into labeled plastic containers. Then, 2.5 ml of cephalic vein blood was collected from each animal and placed in an anticoagulant-free gel tube. Both fecal and blood samples were transported to the Laboratory of Microbiology (College of Veterinary Medicine) under aseptic cooled conditions. Freshly collected fecal samples were immediately subjected to culture, while blood samples were centrifuged (5,000 rpm, 5 minutes) to collect sera that were stored frozen for analysis of immune and antioxidant markers (Gharban and Yousif, 2020; Razooqi et al., 2022). Isolation and biochemical analysisApproximately 1 ml/g of feces was suspended in modified peptone water, and 50 µl of the mixture was streaked onto MacConkey agar medium. Then, the single suspected colonies were selected and cultured on eosin-methylene blue agar and then on Sorbitol MacConkey agar. For identification, the purified isolates were biochemically tested using the following enzymes: catalase (CAT), citrate, coagulase, gas production, gelatin hydrolysis, lactose, H2S production, indole, sorbitol, urease, and Voges–Proskauer tests. Antibiotic susceptibilityThe Kirby–Bauer method was used to assess antibiotic susceptibility, as described by Bouacha et al. (2023). The tested antibiotics discs included amikacin (30 µg), amoxicillin (20 µg), ampicillin (20 µg), azithromycin (30 µg), cefepime (30 µg), cefotaxime (30 µg), ceftazidime (30 µg), ceftriaxone (30 µg), ciprofloxacin (10 µg), colistin (10 µg), gentamicin (10 µg), imipenem (10 µg), levofloxacin (5 µg), meropenem (10 µg), nalidixic acid (30 µg), nitrofurantoin (30 µg), and ofloxacin (10 µg). The plate was then incubated at 37°C for 24 hours. The results were interpreted according to the guidelines of the Clinical and Laboratory Standards Institute (Gaur et al., 2023). Molecular analysisFollowing the manufacturer’s instructions for the PrestoTM Mini gDNA Bacteria Kit (Geneaid, Taiwan), genomic DNA was extracted from the suspected E. coli isolates using one set of primers: (F: 5′-TCT GAG AGG ATG ACC AGC CA-3′) and (R: 5′-TTT AAC CTT GCG GCC GTA CT-3′) by targeting the 16S rRNA gene (GenBank ID: PP809701.1) to prepare the MasterMix tubes at 25 µl final volume. For DNA amplification, the following thermocycler conditions were used: 1 cycle for initial denaturation (95°C, 7 minutes); 34 cycles for denaturation (95°C, 40 seconds), annealing (58°C, 40 seconds), and extension (72°C, 40 seconds); and 1 cycle for final extension (72°C, 10 minutes). The amplified polymerase chain reaction (PCR) products were electrophoresed in an agarose gel (1.5%) and stained with ethidium bromide at 100 V and 80 mA for 90 minutes. Finally, the positive PCR products were visualized under the UV-transilluminator at approximately product size of approximately 615 bp. PhylogenyThe amplified positive products were sequenced by the Macrogen Company (Korea), and the data were submitted to the National Center for Biotechnology Information-GenBank database. Phylogenetic analysis was performed using multiple sequence alignment analysis and phylogenetic tree analysis in the MEGA-11 software and MSA Viewer in the NCBI to identify its identity to the NCBI-BLAST E. coli strains. Serological measurement of immune and antioxidant markersEnzyme-linked immunosorbent assay kits for the quantitative estimation of IL-1, IL-4, IL-6, IL-8, interferon-alpha (INF-α), CAT, glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA) levels were purchased from SunLong Biotech Company (China). The above protocol was followed to obtain the optical density of the samples and standards to obtain the standard curve. The concentration was calculated from the standard curve. Statistical analysisThe study data were analyzed by one-way analysis of variance and t-test in GraphPad Prism Software. Differences between values were considered significant at p < 0.05 (Ibraheim et al., 2023). Ethical approvalThis study was conducted under the license (CVM-WU, 28/09/2024) provided by the Scientific Committee of the College of Veterinary Medicine at the University of Wasit. ResultsClinically, a significant prevalence (p < 0.0148) was observed in the case of diarrhea (37.01%) among the gastrointestinal disorders when compared to other identified clinical signs, including abdominal pain (14.96%), diarrhea and vomiting (12.6%), vomiting (7.87%), abdominal pain and diarrhea (6.3%), bloating (5.51%), constipation (3.94%), abdominal pain and vomiting (3.94%), abdominal pain and bloating (3.15%), abdominal pain and constipation (2.36%), bloating and vomiting (1.57%), and bloating and constipation (0.79%) (Table 1). Culture tests revealed that 14.96% (19/127) of the fecal samples of dogs with gastrointestinal disease were positive for E. coli isolates (Fig. 1). On MacConkey agar, the suspected colonies exhibited a pink color, whereas on eosin-methylene blue agar, the colonies appeared as a distinctive metallic sheen. The suspected purified isolates were observed as colorless colonies on Sorbitol MacConkey agar (Fig. 2). Biochemically, the suspected E. coli isolates showed a positive reaction to CAT, gas production, indole, lactose, and sorbitol but a negative reaction to citrate, coagulase, gelatin hydrolysis, Gram staining, H2S production, urease, and Voges–Proskauer tests. The antibiotic susceptibility testing revealed that E. coli strains were sensitive to meropenem (94.74%), levofloxacin (89.47%), ciprofloxacin (84.21%), colistin (84.21%), imipenem (84.21%), ceftazidime (78.95%), nitrofurantoin (78.95%), nalidixic acid (73.68%), ofloxacin (73.68%), amikacin (68.42%), azithromycin (68.42%), gentamicin (63.16%), cefotaxime (57.89%), ceftriaxone (52.63%), and cefepime (47.36%); however, they were resistant to amoxicillin (89.47%) and ampicillin (73.68%) (Table 2). Table 1. Total results of clinical gastrointestinal signs identified in the study dogs (total number: 127).
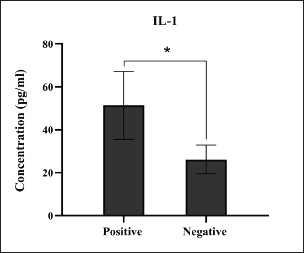
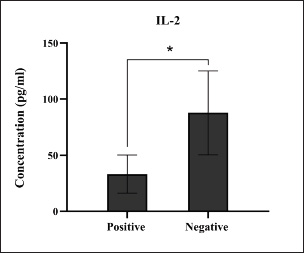
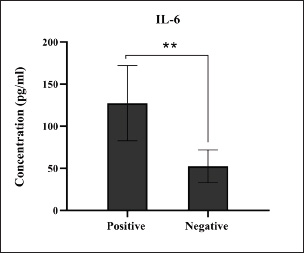
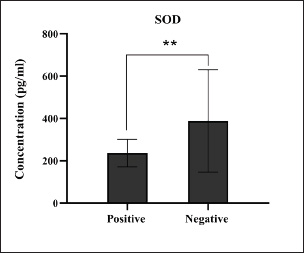
Fig. 1. Prevalence rate of colibacillosis using traditional diagnostics (total number of examined fecal samples=127).
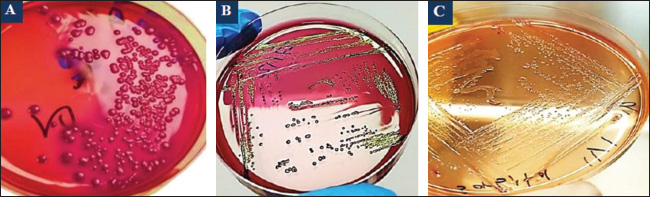
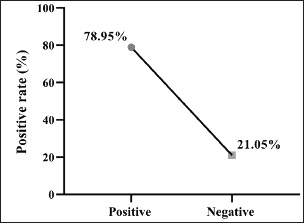
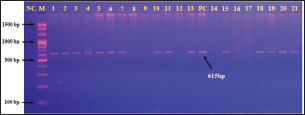
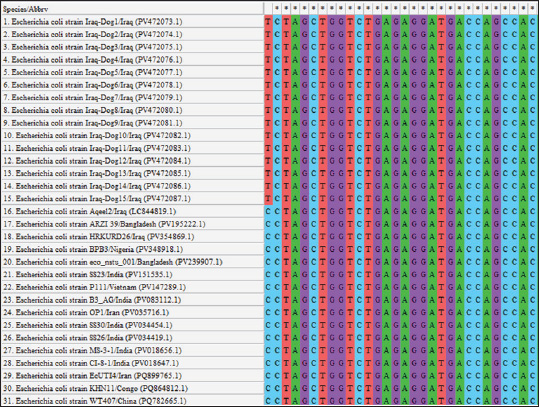
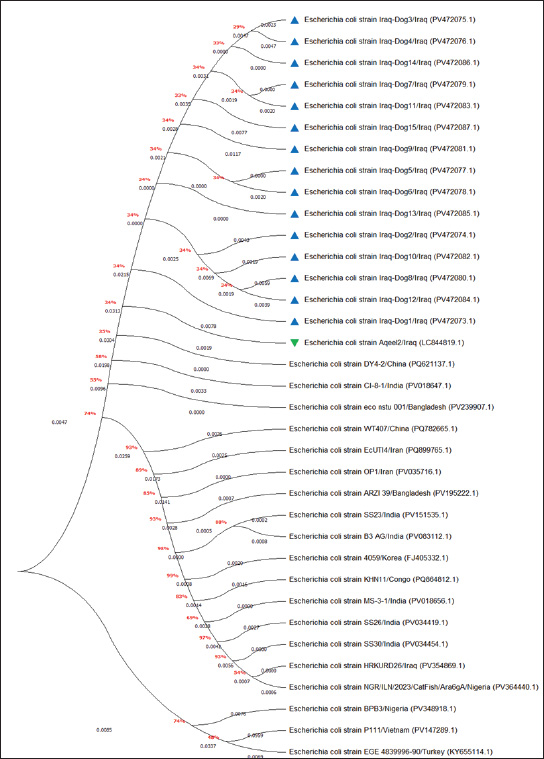
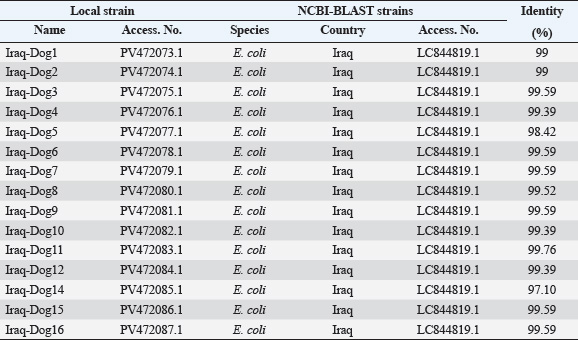
Fig. 2. Colonies of E. coli isolates on MacConkey agar (A), eosin-methylene blue agar (B), and sorbitol MacConkey agar (C). Targeting the 16S rRNA gene, 78.95% (15/19) of suspected E. coli isolates were positive by conventional PCR assay (Figs. 3 and 4). Sequencing data of all positive E. coli strains were named (Iraq-Dog1-Iraq-Dog15) and submitted to the NCBI-GenBank under specific accession numbers (PV472073.1-PV472087.1). Phylogenetic analysis of the E. coli strains was performed using the MEGA software tools, which detected the presence of nucleotide similarity (*) and substitution/mutation at 98.42%–99.76% and 0.0047%–0.0085%, respectively, with the NCBI-BLAST Iraqi strain (LC844819.1) (Figs. 5 and 6, Table 3). Regarding the serological investigation of immune markers and antioxidants, significant variation was identified among the positive and negative dogs for E. coli infection. Significantly (p < 0.05), the findings of IL-1 (51.358 ± 3.623 pg/ml), IL-6 (127.263 ± 10.249 pg/ml), IL-8 (828.105 ± 71.352 pg/ml), and MDA (304.842 ± 7.897 ng/ml) were elevated in positive dogs to E. coli infection when compared to those of negative infections (26.126 ± 1.206, 52.415 ± 3.036, 358.075 ± 23.678, and 180.268 ± 10.531 ng/ml, respectively); however, significant reduction was observed in values of IL-2 (33.195 ± 3.915 pg/ml), CAT (0.585 ± 0.048 ng/ml), GSH (3.524 ± 0.415 µg/ml), and SOD (236.526 ± 14.907 pg/ml) in comparison with those of negative infections (87.788 ± 5.833, 0.654 ± 0.034, 5.65 ± 0.324, and 388.39 ± 37.71 pg/ml, respectively). No significant differences (p > 0.05) were seen in values of INF-α among the positive (31.547 ± 2.247 pg/ml) and negative E. coli infections (31.31 ± 2.66 pg/ml), (Figs. 7– DiscussionDogs are the most successful canines that were kept as pets by many people all over the world irrespective of their social status, and most of these dogs were kept as watch, companion animals, and as a guide for the handicapped forces (Rakha et al., 2015). In the present study, diarrhea, abdominal pain, and vomiting were the most identified gastrointestinal disorders, and these findings are in agreement with those of other studies (Volkmann et al., 2017; Furukawa et al., 2022; Skotnitzki et al., 2022). However, multiple differential diagnostic approaches must be considered in patients with gastrointestinal signs and other systemic metabolic or toxic disorders (Holzmann et al., 2023). The prevalence observed in this study (14.96%) falls within the range reported in other regions: 8.9% in Germany (Werhahn Beining et al., 2023), 11.54% in the UAE (Habib et al., 2023), 22.2% in Turkey (Aslantaş and Yilmaz, 2017), 24.2% in Ethiopia (Gebremedhin et al., 2021), 24.3% in India (Mustapha and Goel, 2020), 31.3%–43.1% in Brazil (Carvalho et al., 2016), 44.8% in the UK (Wedley et al., 2017), 47.1% in Iran (Torkan et al., 2016), and 63.75% in China (Zhou et al., 2022). This variation might be attributed to the animal (age, sex, and health status), pathogen (strain and prevalence rate of resistance genes), and environmental (contamination level) factors in addition to the diagnosis protocol. Concerning the antibiotic susceptibility testing, our findings recorded the higher sensitivity of E. coli strains to meropenem and levofloxacin than to ciprofloxacin, colistin, imipenem, ceftazidime, nitrofurantoin, nalidixic acid, ofloxacin, amikacin, azithromycin, gentamicin, cefotaxime, ceftriaxone, and cefepime. However, remarkable resistance was detected to amoxicillin and ampicillin. In Iraq, Yousif et al. (2017) found that E. coli had a high sensitivity to ciprofloxacin (80.76%) and the highest resistance to cephalexin (100%), ampicillin and erythromycin (96.14%), and chloramphenicol (88.46%). Another recent study recorded the significantly higher sensitivity of E. coli isolates to ciprofloxacin (80%), gentamycin, and levofloxacin (60%), whereas the highest resistance rates were shown to cefotaxime and tetracycline (60%) (Kadhim and Alrammah, 2024). Internationally, Naziri et al. (2015) reported the highest resistance to ampicillin (100%), followed by amoxicillin (96.6%), trimethoprim-sulfamethoxazole (57.1%), and imipenem (5.5%) and meropenem (2.3%). However, the difference in sensitivity to antimicrobial agents might be attributed to variation in the prevalence of resistance genes encoded by the chromosomes and plasmids harbored by bacterial organisms, as detected by Torkan et al. (2016), who molecularly demonstrated the variable distribution of antimicrobial genes in pathogenic E. coli isolates [64.3% to ampicillin, 42.9% to streptomycin and cephalothin, 35.7% to tetracycline and trimethoprim, 21.4% to sulfonamide and chloramphenicol, 14.3% to fluoroquinolone, and 7.1% to erythromycin]. Aslantaş and Yilmaz (2017) confirmed the occurrence of various β-lactamase genes in E. coli isolated from dogs alone or in combination. Erzaim and Ikiz (2021) detected a lack of plasmid-mediated mcr-1 gene, though resistance to colistin has been found, indicating the presence of many genes for resistance or that the chromosome is responsible for resistance. However, routine antibiotic therapy in dogs might increase the number of E. coli-resistant strains (Schmidt et al., 2018). Close contact between dogs and humans might play a role in the transmission of antimicrobial resistance bacteria. Additionally, pet and stray dogs may act as antimicrobial-resistant E. coli reservoirs (Marchetti et al., 2021). Table 2. Antibiotic susceptibility of 19 positive E. coli isolates as determined by culture and biochemical tests.
Fig. 3. Prevalence rate of colibacillosis using molecular PCR assay (total number of E. coli isolates examined=19).
Fig. 4. Agarose gel electrophoresis of polymerase chain reaction products at 100 V and 80 mA for 90 minutes. Lane (M): ladder marker. Lane (NC): negative control. Lane (PC): positive control. Lanes 8, 9, 12, 14, 16, and 17: negative samples. Lanes 1, 2, 3, 4, 5, 6, 7, 10, 11, 13, 15, 18, 19, 20, and 21: positive samples at 615 bp.
Fig. 5. Multiple sequence alignment of local and global NCBI-GenBank E. coli strains using MEGA-11 software.
Fig. 6. Phylogenetic tree analysis of local and global NCBI-GenBank E. coli strains using MEGA-11 software. In this study, molecular testing of E. coli isolates revealed that only 78.95% were positive by conventional PCR, which was phylogenetically identical to the NCBI-BLAST Iraqi strain (LC844819.1) isolated from chicken. These findings confirm the hypothesis that E. coli could be transmitted directly or indirectly from animal to animal or from animal to human and vice versa, indicating zoonotic importance (Bélanger et al., 2011; Etcheverría et al., 2016; Merkeviciene et al., 2018). Table 3. Homology sequence identification for local and NCBI-GenBank BLAST E. coli isolates/strains.
Fig. 7. Levels of immune IL-1 marker among the positively (total number=19) and negatively (total number=108) infected dogs with colibacillosis; significance * (p < 0.0241).
Fig. 8. Levels of immune IL-2 marker among the positively (total number=19) and negatively (total number=108) infected dogs with colibacillosis; significance * (p < 0.0116). Serological quantification of immune and antioxidant markers in positively infected dogs with colibacillosis revealed a significant variation in their values. For immune markers, the findings of dogs positive for colibacillosis described significant elevation in IL-1, IL-6, and IL-8, with significant reduction in IL-2; however, no significant changes were shown in INF-α. Worldwide, various data were obtained during E. coli infection in both animals and humans. In a previous study, Wakabayashi et al. (1991) reported that a specific receptor antagonist for IL-1 prevents E. coli-induced shock in rabbits. Van Heeckeren et al. (1993) reported that TNF-α, IL-1α, IL-6, and prostaglandin E2 were produced in murine peritoneal macrophages infected with E. coli. In 2001, Brauner et al. (2001) identified that E. coli can induce the expression of IL-1 (α, β), IL6, and IL-8 in normal human thymic epithelial cells. In dogs, Karlsson et al. (2015) reported that pathogenic E. coli enhances the expression of IL-8 in endometrial stromal cells, while other researchers observed that E. coli can stimulate the production of TNF-α, IL-6, and IL-10 (Hoffman et al., 2018; Merrill et al., 2021). Recently, Nagao et al. (2024) reported that pathogenic E. coli can induce acute colitis in dogs, leading to signs similar to those observed in humans, disrupting intestinal barrier integrity, and increasing the production of IL-8 and TNF-α.
Fig. 9. Levels of immune IL-6 marker among the positively (total number=19) and negatively (total number=108) infected dogs with colibacillosis; significance ** (p < 0.0093).
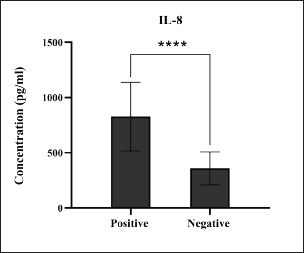
Fig. 10. Levels of immune IL-8 marker among the positively (total number=19) and negatively (total number=108) infected dogs with colibacillosis. ***** (p < 0.0001).
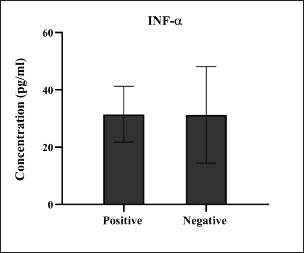
Fig. 11. Levels of immune INF-α marker among the positively (total number=19) and negatively (total number=108) infected dogs with colibacillosis; non-significance (p < 0.0935).
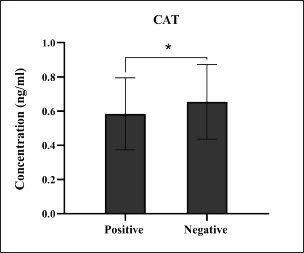
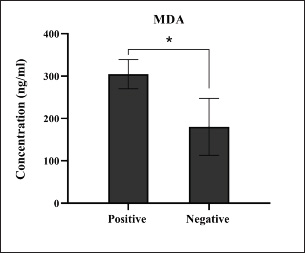
Fig. 12. Levels of antioxidant CAT marker among the positively (total number=19) and negatively (total number=108) infected dogs with colibacillosis; significance * (p < 0.0461). Among the molecularly positive dogs to colibacillosis, the findings of antioxidants were reported a significant reduction in values of CAT, GSH, and SOD but a significant increase in values of MDA as reported by Yazlık et al. (2023) Worldwide, several studies have demonstrated that damage developed during various infections is attributable to oxidative stress due to endogenous and exogenous production of reactive oxygen species (ROS) and elaborating of antioxidants to limit the damage developed by bacterial infections (Pohanka, 2013; Paiva and Bozza, 2014; Sarkar and Sil, 2019). In addition to the direct antimicrobial effects of ROS, these molecules can act as signaling molecules, modulating the inflammatory response and influencing the recruitment of immune cells to the site of infection (Li et al., 2017; Memar et al., 2018; Banerjee et al., 2020). The interplay between bacterial virulence factors, host immune responses, and oxidative stress determines bacterial infection severity and progression (Chamoun et al., 2018). However, bacterial pathogens employ diverse strategies to manipulate the host’s oxidative stress response to their advantage by either directly inducing ROS production, thereby causing oxidative damage to host tissues and promoting bacterial dissemination, or suppressing the host’s antioxidant defenses (Kim et al., 2019; Sahu et al., 2022).
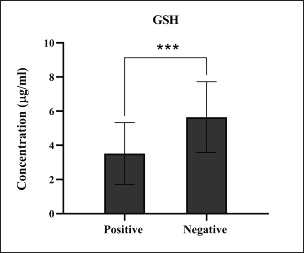
Fig. 13. Levels of antioxidant GSH marker among the positively (total number=19) and negatively (total number=108) infected dogs with colibacillosis; significance *** (p < 0.0006).
Fig. 14. Levels of antioxidant SOD markers among the positively (total number=19) and negatively (total number=108) infected dogs with colibacillosis; significance ** (p < 0.0081).
Fig. 15. Levels of lipid peroxidation MDA marker among the positively (total number=19) and negatively (total number=108) infected dogs with colibacillosis; significance * (p < 0.0105). ConclusionTo the best of our knowledge, this is the first study in Iraq to detect the prevalence rate of colibacillosis in pet dogs using traditional and molecular phylogenetic assays and demonstrate the susceptibility of E. coli strains to various antibiotics. This study also demonstrated that E. coli infection was associated with immune markers and antioxidants, which might indicate that a delicate balance between various infections and immune/antioxidant defense is crucial in determining disease outcome. However, regular surveys are needed to determine the effect of colibacillosis on other body enzymes, hormones, and tissues, as well as to investigate the effect of other pathogens on the health status of dogs. AcknowledgmentsThe authors thank all private clinic veterinarians who supported the clinical examination and collection of study samples. Conflict of interestThere are no conflicts of interest. FundingThe authors declare that no external funds (private funding) were received. Authors’ contributionMTJ: Measurement of immune markers; ASA: Measurement of antioxidant markers; AJA: Clinical examination and collection of fecal and blood samples; IME: Culture of fecal samples and biochemical testing of suspected E. coli isolates; HAJG: Molecular examination, phylogenetic and statistical analyses of study data. All authors equally contributed to the study design and approved the final copy of the manuscript. Data availabilityAll data were included in the study text. ReferencesAl-Kubaisi, S.M.A., Saed, O.A.S., Suleiman, J.M., Hasan, M.S., Owain, M.S. and Jarad, A.S. 2020. Isolation and identification of facultative anaerobic bacteria from pet dog feces. Med. Leg. Update 20(1), 785–798. Aslantaş, Ö. and Yilmaz, E.Ş. 2017. Prevalence and molecular characterization of extended-spectrum β-lactamase (ESBL) and plasmidic AmpC β-lactamase (pAmpC) producing Escherichia coli in dogs. J. Vet. Med. Sci. 79(6), 1024–1030. Banerjee, S., Ghosh, S., Mandal, A., Ghosh, N. and Sil, P.C. 2020. ROS-associated immune response and metabolism: a mechanistic approach with implication of various diseases. Arch. Toxicol. 94, 2293–2317. Bélanger, L., Garenaux, A., Harel, J., Boulianne, M., Nadeau, E. and Dozois, C.M. 2011. Escherichia coli from animal reservoirs as a potential source of human extraintestinal pathogenic E. coli. FEMS Immunol. Med. Microbiol. 62(1), 1–10. Bloom, D.E. and Cadarette, D. 2019. Infectious disease threats in the twenty-first century: strengthening the global response to the disease Front. Immunol. 10, 549–561. Bouacha, M., Besnaci, S. and Boudiar, I. 2023. An overview of the most commonly used methods for determining the in vitro antibacterial activity of honey. Acta Microbiol. Hell. 39(1), 23–30. Brauner, A., Söderhäll, M., Jacobson, S.H., Lundahl, J., Andersson, U. and Andersson, J. 2001. Escherichia coli-induced expression of IL-1 α, IL-1 β, IL-6 and IL-8 in normal human renal tubular epithelial cells. Clin. Exp. Immunol. 124(3), 423–428. Carvalho, A.C., Barbosa, A.V., Arais, L.R., Ribeiro, P.F., Carneiro, V.C. and Cerqueira, A.M.F. 2016. Resistance patterns, ESBL genes, and genetic relatedness of dogs and their owners to Escherichia coli. Braz. J. Microbiol. 47(1), 150–158. Chamoun, M.N., Blumenthal, A., Sullivan, M.J., Schembri, M.A. and Ulett, G.C. 2018. Bacterial pathogenesis and interleukin-17: interconnecting mechanisms of immune regulation, host genetics, and microbial virulence influence infection severity. Crit. Rev. Microbiol. 44(4), 465–486. Erzaim, N. and Ikiz, S. 2021. Phenotypic and mcr-1Mediated colistin resistance in Escherichia coli. Acta Vet. Eurasia 47(2), 82–87. Etcheverría, A.I., Lucchesi, P.M.A., Krüger, A., Bentancor, A.B. and Padola, N.L. 2016. “Escherichia coli in animals,” in Escherichia coli in the Americas. Ed., Torres, A.G. Cham, Switzerland: Springer International Publishing, pp: 149–172. Fairbrother, J.M. and Nadeau, É. 2019. Colibacillosis. In Diseases of the swine. Eds., Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W. and Zhang, J. Hoboken, NJ: John Wiley & Sons, Inc., pp: 807–834. Furukawa, R., Takahashi, K., Hara, Y., Nishimura, R., Furuya, K., Shingaki, T. and Ohmori, K. 2022. Clinical characteristics of dogs with chronic enteropathy presenting with vomiting as a gastrointestinal sign. Vet. Anim. Sci. 17, 10–25. Gaur, P., Hada, V., Rath, R.S., Mohanty, A., Singh, P. and Rukadikar, A. 2023. Interpretation of antimicrobial susceptibility testing using breakpoints from the European Committee on Antimicrobial Susceptibility Testing and Clinical and Laboratory Standards Institute: analysis of agreement. Cureus 15(3), 113–215. Gebremedhin, E.Z., Merga, D., Sarba, E.J., Marami, L.M., Tola, G.K. and Endale, S.S. 2021. Prevalence, risk factors and antibiogram of Escherichia coli isolated from dogs in Ambo, Gojo and Bako towns of Oromia region, Ethiopia. Ethiop. Vet. J. 25(1), 1–22. Gharban, H.A. and Yousif, A.A. 2020. Serological and molecular phylogenetic detection of Coxiella burnetii in Iraqi lactating cows. Iraqi J. Vet. Med. 44(E0), 42–50. Habib, I., Mohteshamuddin, K., Mohamed, M.Y.I., Lakshmi, G.B., Abdalla, A. and Bakhit Ali Alkaabi, A. 2023. Domestic pets in the United Arab Emirates as reservoirs for antibiotic-resistant bacteria: a comprehensive analysis of extended-spectrum beta-lactamase producing Escherichia coli prevalence and risk factors. Animals 13(10), 1587–1599. Hoffman, D., Amorim, J. and DeClue, A. 2018. Immune function in dogs with critical illness. J. Vet. Int. Med. 32(1), 208–216. Holzmann, B., Werner, M., Unterer, S. and Dörfelt, R. 2023. Utility of diagnostic tests in dogs with vomiting who presented to an internal medicine emergency service. Front. Vet. Sci. 10, 10–20. Ibraheim, H.K., Madhi, K.S., Baqer, G.K. and Gharban, H.A. 2023. Effectiveness of raw bacteriocin produced from lactic acid bacteria on methicillin-resistant Staphylococcus aureus biofilm. Vet. World 16(3), 491–499. Isidori, M., Corbee, R.J. and Trabalza-Marinucci, M. 2022. Nonpharmacological treatment strategies for managing canine chronic inflammatory enteropathy-a narrative review. Vet. Sci. 9(2), 37–52. Kadhim, R.J. and Alrammah, H.S.A. 2024. Molecular detection of MDR-E. coli isolated from infected ears of owners of pet-cats in Baghdad. Afr. J. Biomed. Res. 27(3s), 3719–3727. Karlsson, I., Hagman, R., Guo, Y., Humblot, P., Wang, L. and Wernersson, S. 2015. Pathogenic Escherichia coli and lipopolysaccharide enhance IL-8, CXCL5, and CXCL10 expression in canine endometrial stromal cells. Theriogenology 84(1), 34–42. Kim, S.Y., Park, C., Jang, H.J., Kim, B.O., Bae, H.W., Chung, I.Y. and Cho, Y.H. 2019. Antibacterial strategies inspired by oxidative stress and response networks. J. Microbiol. 57, 203–212. Kollannur, J.D., Jameel, A.J. and Choudhary, S. 2024. Gastrointestinal disorders in dogs and cats introduction to diseases, diagnosis, and management of dogs and cats. Cambridge, MA: Academic Press, pp: 271–287. Li, Z., Xu, X., Leng, X., He, M., Wang, J., Cheng, S. and Wu, H. 2017. Roles of reactive oxygen species in cell signaling pathways and immune responses to viral infections. Arch. Virol. 162, 603–610. Marchetti, L., Buldain, D., Gortari Castillo, L., Buchamer, A., Chirino-Trejo, M. and Mestorino, N. 2021. Pet and stray dogs as reservoirs of antimicrobial-resistant Escherichia coli. Int. J. Microbiol. 2021(1), 6664557. Marks, S.L., Rankin, S.C., Byrne, B.A. and Weese, J.S., 2011. Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control. J. Vet. Int. Med. 25(6), 1195–1208. Memar, M.Y., Ghotaslou, R., Samiei, M. and Adibkia, K. 2018. Antimicrobial use of reactive oxygen therapy: current insights. Infect. Drug Resist. 2018(11), 567–576. Merkeviciene, L., Klimiene, I., Siugzdiniene, R., Virgailis, M., Mockeliunas, R. and Ruzauskas, M. 2018. Prevalence and molecular characteristics of multi-resistant Escherichia coli infection in wild birds. Acta Vet. Brno 87(1), 9–17. Merrill, K.M., Hull, M.B., Stoker, A. and DeClue, A.E. 2021. In vitro effects of epinephrine, norepinephrine, and dobutamine on lipopolysaccharide-stimulated production of tumor necrosis factor-α, interleukin-6, and interleukin-10 in blood from healthy dogs. Am. J. Vet. Res. 82(5), 374–380. Mustapha, M. and Goel, P. 2020. Isolation and prevalence of uropathogenic E. coli in dogs of different breeds, sex, and age. Rev. Vét. Clin. 55(2), 49–54. Nagao, I., Kawasaki, M., Goyama, T., Kim, H.J., Call, D.R. and Ambrosini, Y.M. 2024. Enterohemorrhagic Escherichia coli (EHEC) disrupts intestinal barrier integrity in monolayers derived from translational canine stem cells. Microbiol. Spect. 12(10), 1–24. Namazi, N., Larijani, B. and Azadbakht, L. 2019. Vitamin K and the immune system. In Nutrition and immunity. Eds., Mahmoudi, M., Rezaei, N. Cham Switzerland: Springer; doi:10.1007/978-3-030-16073-9_4 Nasrollahian, S., Graham, J.P. and Halaji, M. 2024. A review of the mechanisms that confer antibiotic resistance in E. coli pathotypes. Front. Cell. Infect. Microbiol. 14, 1–22. Naziri, Z., Firouzi, R., Derakhshandeh, A. and Tabrizi, A.S. 2015. Comparative analysis of the phylogenetic group and antimicrobial resistance pattern of fecal Escherichia coli isolates between healthy dogs and their owners. Comp. Clin. Pathol. 24, 1211–1220. Paiva, C.N. and Bozza, M.T. 2014. Are reactive oxygen species always harmful to pathogens? Antioxid. Redox Signal. 20(6), 1000–1037. Pilla, R. and Suchodolski, J.S. 2020. Role of the canine gut microbiome and metabolome in health and gastrointestinal disease. Front. Vet. Sci. 6, 498–513. Pohanka, M. 2013. Role of oxidative stress in infectious diseases: a review. Folia Microbiol. 58, 503–513. Poirel, L., Madec, J.Y., Lupo, A., Schink, A.K., Kieffer, N., Nordmann, P. and Schwarz, S. 2018. Antimicrobial resistance in E. coli. Microbiol. Spect. 6(4), 1110–1128. Rakha, G.M., Abdl-Haleem, M.M., Farghali, H.A. and Abdel-Saeed, H. 2015. Prevalence of common canine digestive problems compared with other health problems in a teaching veterinary hospital, Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt. Vet. World 8(3), 403–414. Ramos, S., Silva, V., Dapkevicius, M.L.E., Caniça, M., Tejedor-Junco, M.T., Igrejas, G. and Poeta, P. 2020. Escherichia coli as commensal and pathogenic bacteria among food-producing animals: health implications of extended spectrum β-lactamase (ESBL) production. Animals 10(12), 2239–2252. Razooqi, M.A., Gharban, H.A.J. and Al-Kaabi, M.A.F. 2022. Molecular and seroprevalence of toxoplasmosis in goat blood and milk in Iraq. Arch. Razi Instit. 77(5), 1749–1755. Riaz, A., Mohiuddin, A., Erum, R. and Bano, Q. 2023. E. coli in the era of antimicrobial resistance: a multidisciplinary approach to understanding and mitigating its impact in healthcare and community setups. Front. Microbiol. Biotechnol. 1, 1–13. Riley, L.W. (2020). Distinguishing pathovars from nonpathovars: E. coli. Microbiol. Spect. 8(4), 1110–1128. Ruiz, N. and Silhavy, T.J. 2022. How Escherichia coli became the molecular biology’s flagship bacterium. J. Bacteriol. 204(9), 230–242. Sahu, P.K., Jayalakshmi, K., Tilgam, J., Gupta, A., Nagaraju, Y., Kumar, A. and Rajawat, M.V.S. 2022. ROS generated from biotic stress: effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 13, 1–16. Saini, P., Bandsode, V., Singh, A., Mendem, S.K., Semmler, T., Alam, M. and Ahmed, N. 2024. Genomic insights into virulence, antimicrobial resistance, and adaptation acumen of Escherichia coli isolated from an urban environment. mBio 15(3), 1–23. Sarkar, K. and Sil, P.C. 2019. Infectious lung diseases and endogenous oxidative stress. In Oxidative stress in lung diseases: volume 1. Eds., Chakraborti, S., Chakraborti, T., Das, S. and Chattopadhyay, D Singapore: Springer Singapore, pp: 125–148. Schmidt, V.M., Pinchbeck, G., McIntyre, K.M., Nuttall, T., McEwan, N., Dawson, S. and Williams, N.J. 2018. Routine antibiotic therapy increases the detection of antimicrobial-resistant fecal Escherichia coli in dogs. J. Antimicrob. Chemother. 73(12), 3305–3316. Singh, S., Sharma, P., Pal, N., Sarma, D.K., Tiwari, R. and Kumar, M. 2024. A holistic health surveillance framework: synergizing environmental, animal, and human determinants for enhanced infectious disease management. ACS Infect. Dis. 10(3), 808–826. Skotnitzki, E., Suchodolski, J.S., Busch, K., Werner, M., Zablotski, Y., Ballhausen, B.D. and Unterer, S. 2022. Frequency of signs of chronic gastrointestinal disease in dogs after acute hemorrhagic diarrhea. J. Vet. Int. Med. 36(1), 59–65. Stachelek, M., Zalewska, M., Kawecka-Grochocka, E., Sakowski, T. and Bagnicka, E. 2021. Overcoming bacterial resistance to antibiotics: a review of the urgent need. Ann. Anim. Sci. 21(1), 63–87. Taalab, R.H., Hamouda, R.H., Abdallah, M.S. and Elkhayat, M.E. 2024. Determination of pathogenic E. coli and antimicrobial resistance genes in dog and human semen: evidence of multidrug resistance and antimicrobial resistance gene profiles. Benha Vet. Med. J. 47(1), 135–142. Torkan, S., Bahadoranian, M.A., Khamesipour, F. and Anyanwu, M.U. 2016. Detection of virulence and antimicrobial resistance genes in Escherichia coli isolates from dogs with diarrhea in Iran. Arch. Med. Vet. 48(2), 181–190. Van Heeckeren, A.M., Rikihisa, Y., Park, J. and Fertel, R. 1993. Production of tumor necrosis factor alpha, interleukin-1 alpha, interleukin-6, and prostaglandin E2 in murine peritoneal macrophages infected with Ehrlichia risticii. Infect. Immun. 61(10), 4333–4337. Volkmann, M., Steiner, J.M., Fosgate, G.T., Zentek, J., Hartmann, S. and Kohn, B. 2017. Chronic diarrhea in dogs: a retrospective study of 136 cases. J. Vet. Int. Med. 31(4), 1043–1055. Wakabayashi, G.O., Gelfand, J.A., Burke, J.F., Thompson, R.C. and Dinarello, C.A. 1991. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 5(3), 338–343. Wedley, A.L., Dawson, S., Maddox, T.W., Coyne, K.P., Pinchbeck, G.L., Clegg, P. and Williams, N.J. 2017. Carriage of antimicrobial-resistant Escherichia coli in dogs: prevalence, associated risk factors, and molecular characteristics. Vet. Microbiol. 199, 23–30. Werhahn Beining, M., Hartmann, M., Luebke-Becker, A., Guenther, S., Schaufler, K., Hille, K. and Kreienbrock, L. 2023. Carriage of extended-spectrum beta-lactamase-producing Escherichia coli: prevalence and factors associated with fecal dog colonization from a pet clinic in Lower Saxony, Germany. Animals 13(4), 584–604. Yazlık, M.O., Mutluer, İ., Kaya, U., Özkan, H., Müştak, İ.B., Çolakoğlu, H.E. and Vural, M.R. 2023. The role of nutritional-immunological indices in estimating serum lipopolysaccharide (LPS) and antioxidant enzyme activity and sepsis status in female dogs with E. coli-induced pyometra. Anim. Reprod. Sci. 255, 1–18. Yousif, A.A., Hasan, M.S. and Alwan, M.J. 2016. Clinical and molecular study of E. coli O157: H7 isolated from Diarrheic and non-diarrheic dogs. Mirror Res. Vet. Sci. Anim. 5(2), 1–10. Yousif, A.A., Hasan, M.S., Najim, T.M. and Hussein, M.A. 2017. Detection of virulence and antimicrobial susceptibility testing of Escherichia coli O157: H7 isolated from pets and stray dogs. J. Entomol. Zool. Stud. 5(4), 573–578. Zhou, Y., Ji, X., Liang, B., Jiang, B., Li, Y., Yuan, T. and Sun, Y. 2022. Antimicrobial resistance and prevalence of extended spectrum β-lactamase-producing Escherichia coli in dogs and cats in Northeastern China from 2012 to 2021. Antibiotics 11(11), 1506–1522. Ziese, A.L. and Suchodolski, J.S. 2021. Impact of changes in the gastrointestinal microbiota in canine and feline digestive diseases. Vet. Clin. North Am. Small Anim. Pract. 51(1), 155–169. | ||
| How to Cite this Article |
| Pubmed Style Jaafar MT, Abukhomra AS, Almialy AJ, Essa IM, Gharban HA. Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal disease. Open Vet. J.. 2025; 15(8): 3746-3758. doi:10.5455/OVJ.2025.v15.i8.39 Web Style Jaafar MT, Abukhomra AS, Almialy AJ, Essa IM, Gharban HA. Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal disease. https://www.openveterinaryjournal.com/?mno=253027 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.39 AMA (American Medical Association) Style Jaafar MT, Abukhomra AS, Almialy AJ, Essa IM, Gharban HA. Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal disease. Open Vet. J.. 2025; 15(8): 3746-3758. doi:10.5455/OVJ.2025.v15.i8.39 Vancouver/ICMJE Style Jaafar MT, Abukhomra AS, Almialy AJ, Essa IM, Gharban HA. Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal disease. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3746-3758. doi:10.5455/OVJ.2025.v15.i8.39 Harvard Style Jaafar, M. T., Abukhomra, . A. S., Almialy, . A. J., Essa, . I. M. & Gharban, . H. A. (2025) Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal disease. Open Vet. J., 15 (8), 3746-3758. doi:10.5455/OVJ.2025.v15.i8.39 Turabian Style Jaafar, Mohammed T., Ahmed S. Abukhomra, Ahmed Jassim Almialy, Isra'a M. Essa, and Hasanain A.j. Gharban. 2025. Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal disease. Open Veterinary Journal, 15 (8), 3746-3758. doi:10.5455/OVJ.2025.v15.i8.39 Chicago Style Jaafar, Mohammed T., Ahmed S. Abukhomra, Ahmed Jassim Almialy, Isra'a M. Essa, and Hasanain A.j. Gharban. "Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal disease." Open Veterinary Journal 15 (2025), 3746-3758. doi:10.5455/OVJ.2025.v15.i8.39 MLA (The Modern Language Association) Style Jaafar, Mohammed T., Ahmed S. Abukhomra, Ahmed Jassim Almialy, Isra'a M. Essa, and Hasanain A.j. Gharban. "Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal disease." Open Veterinary Journal 15.8 (2025), 3746-3758. Print. doi:10.5455/OVJ.2025.v15.i8.39 APA (American Psychological Association) Style Jaafar, M. T., Abukhomra, . A. S., Almialy, . A. J., Essa, . I. M. & Gharban, . H. A. (2025) Immunity, antioxidant activity, and antibiotic susceptibility of clinically and phylogenetically confirmed colibacillosis in dogs with gastrointestinal disease. Open Veterinary Journal, 15 (8), 3746-3758. doi:10.5455/OVJ.2025.v15.i8.39 |