| Research Article | ||
Open Vet. J.. 2025; 15(8): 3780-3786 Open Veterinary Journal, (2025), Vol. 15(8): 3780-3786 Research Article Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemiaZanan Mohamed-Ameen Taha*Department of Pathology and Microbiology, College of Veterinary Medicine, University of Duhok, Duhok, Iraq *Corresponding Author: Zanan Mohamed-Ameen Taha. Department of Pathology and Microbiology, College of Veterinary Medicine, University of Duhok, Duhok, Iraq. Email: zanan.taha [at] uod.ac Submitted: 16/04/2025 Revised: 19/07/2025 Accepted: 29/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
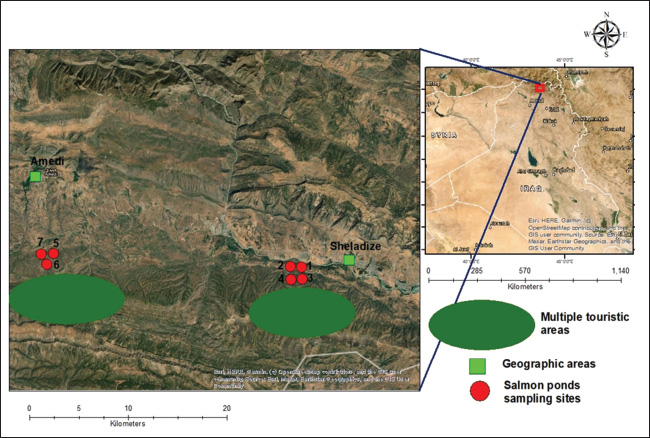
ABSTRACTBackground: Pseudomonas aeruginosa is an opportunistic pathogen in aquaculture that can cause significant infections and spread through genetically diverse strains. Aim: This study investigated the clonal relatedness and genotyping analysis of P. aeruginosa isolates from infected salmon trout in various trout farms using random amplified polymorphic DNA (RAPD) polymerase chain reaction (PCR) fingerprinting. Methods: A total of 35 P. aeruginosa isolates were collected from lesions in different organs of trout from seven farms across Sheladize and Amedi districts in Duhok province, Iraq. RAPD-PCR was performed using specific primers, and amplified DNA fragments were analyzed using GelJ software to generate a dendrogram based on Unweighted Pair Group Method with Arithmetic Mean clustering. Results: The results showed a genetic similarity range of 50.00%–100.0%, identifying 33 unique genotypes. Notably, two isolates from different farms and geographic locations shared the same genotype, indicating the potential for bacterial transmission. However, the remaining strains were grouped into a single genotype due to the significant genetic diversity seen in their DNA. Conclusion: The findings emphasize the role of bacterial clonal differentiation in the spread of P. aeruginosa infections in fish farms, driven by multiple transmission sources. This genetic variation highlights the need for enhanced biosecurity measures to mitigate P. aeruginosa spread in aquaculture settings. Keywords: Pseudomonas aeruginosa, Salmon trout, RAPD-PCR, Genetic diversity. IntroductionPseudomonas aeruginosa is a Gram-negative, rod-shaped bacterium ubiquitously found in soil, water, and living hosts (Ambreetha and Singh, 2023). Although it is often considered an opportunistic pathogen in humans, it has been increasingly recognized as a significant pathogen in aquaculture, particularly affecting species such as trout, salmon, and common carp (Duman et al., 2023). Hemorrhagic septicemia, characterized by internal and external bleeding, exophthalmia, and ascites; ulcerative dermatitis, presenting as open skin lesions and necrosis; and fin and tail rot, leading to frayed, inflamed fins and impaired swimming, are the common diseases. In some cases, gill disease may also occur, causing respiratory distress. In aquaculture operations, these infections result in significant health deterioration, increased mortality, reduced growth performance, and substantial economic losses (Sadiq, 2024). In fish, P. aeruginosa is associated with diseases such as hemorrhagic septicemia, which can result in hemorrhagic spots, scale loss, and fin erosions, resulting in substantial economic losses in aquaculture due to high mortality rates (Elham et al., 2017). Bacterial genetic diversity and clonal relatedness are crucial for fish farms and pond management. They aid in disease surveillance by identifying different strains and tracing infection sources, which enhances biosecurity by pinpointing pathogen sources and transmission routes (Khor et al., 2015). Antimicrobial resistance monitoring helps track resistance gene spread and potential horizontal gene transfer (Baron et al., 2017; Fu et al., 2024). Additionally, these analyses reveal how environmental factors affect bacterial evolution and indicate poor management when identical clones appear across sites (Muthu et al., 2020). Genetic diversity and clonal relationships among P. aeruginosa strains in aquaculture settings are not well understood. Random amplified polymorphic DNA (RAPD) analysis is a valuable tool for assessing genetic variability and establishing clonal relationships among bacterial isolates. RAPD-PCR offers several advantages over other genotyping methods. It is rapid, simple, and cost-effective, and requires no prior sequence information or specialized reagents, making it ideal for preliminary screening and outbreak investigations. Additionally, it can generate strain-specific DNA profiles within a few hours and enable the detection of genetic polymorphisms across the entire genome, making it well-suited for identifying genetic diversity, clonal relationships, and potential transmission routes among bacterial pathogens (Stefańska et al., 2022). Therefore, this technique has been effectively used to analyze the genetic diversity of bacterial pathogens within the fish farming sector (Sadok et al., 2013; Hematzadeh and Haghkhah, 2021). Understanding the clonal structure of P. aeruginosa populations in aquaculture environments is crucial for developing effective management strategies to control the spread of this pathogen (Hematzadeh and Haghkhah, 2021). This study aimed to perform a clonal analysis of P. aeruginosa isolates obtained from trout salmon farms affected by hemorrhagic septicemia using RAPD polymerase chain reaction. The findings of this study are expected to provide insights into the genetic diversity of P. aeruginosa in these settings and inform future disease control measures. Materials and MethodsBacterial strains and description of the study areaFrom September 2021 to August 2022, clinical isolates of P. aeruginosa used in this study were gathered at the “Duhok Research Center, College of Veterinary Medicine, University of Duhok,” by Dr. Shaaban Tayar Sadiq as part of his PhD research. This study employed RAPD- polymerase chain reaction (PCR) fingerprinting to examine the clonal relationship (cluster evaluation) of 35 P. aeruginosa isolates. These strains have previously been identified among salmon trout that had hemorrhagic septicemia symptoms from lesions in various organs (liver, kidney, heart, and skin) at different trout farms (including four farms in the Sheladize area (farms 1, 2, 3, and 4) and three farms in the Amedi region (farms 5, 6, and 7) (Sadiq, 2024). Each farm was situated in a different area of Duhok to provide a broad sampling distribution (Fig. 1). Materials from organs were collected using sterile cotton swabs, with each swab immediately placed into a tube containing 10 ml of BHI broth (Lab M, UK). The researcher pre-enriched the samples by incubating them at 37°C for 20 hours. Following enrichment, one to two loops of the broth culture were inoculated onto nutrient agar plates (Lab M, UK) and incubated at 37°C for 24 hours. Colonies suspected to be P. aeruginosa were identified by their soluble pigments and characteristic grape-like odor and were subjected to further testing. These included a positive oxidase test and a negative indole test. Final confirmation of P. aeruginosa was performed using the VITEK 2 identification system and PCR amplification targeting the PA431C gene (Sadiq, 2024).
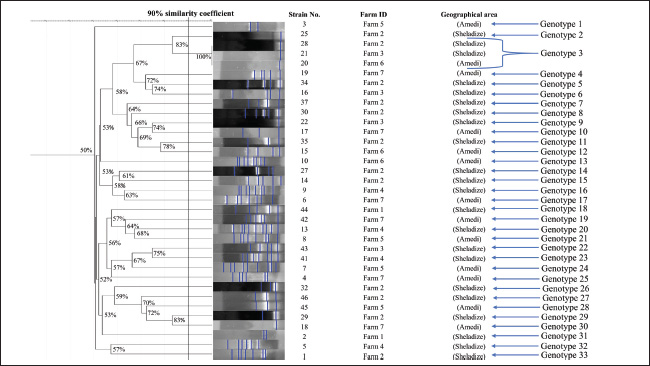
Fig. 1. Map of the sampling sites of the study area showing the Rainbow Trout (O. mykiss) farms in the Amedi and Sheladize/Duhok/Kurdistan regions of Iraq. The map was constructed using “ArcMap (version 10.2)” (Sadiq, 2024). Unfortunately, some risk factors were observed in the study area during the sample collection; one of these is the presence of touristic areas (anthropogenic activities) around the water sources in these rainbow trout farms. Another key observation during sampling was the mixing of various fish from the nearby river with salmon as a trash fish meal, and there were no restricted biosecurity zones preventing the circulation of livestock animals and birds. Furthermore, there is typically a significant chance of severe rainfall in our area (>50 mm in a 24-hour period during certain seasons). Genomic DNA extractionA single colony was selected from each positive sample for DNA extraction. Genomic DNA was obtained using the freeze-thaw extraction method, following the procedure described by Bose et al. (2025). RAPD-PCRKadhim et al. (2012) recently described the primer sequences (RAPD 1: 5′-GGTGCGGGAA-3′ and RAPD 2: 5′-AACGCGCAAC-3′). Total PCR reaction components (25 μl) were prepared by mixing 12.5 μl of “hot start master mix” (AddBio, Korea), 2 μl of each primer (10 pmol/μl), 2 μl of DNA at an average concentration of 50–100 ng/μl, and 6.5 μl of “nuclease-free water” (Qiagen, Germany).” The “PCR setting” was as follows: 95°C for 5 minutes and 45 cycles consisting of 95°C for 1 minute, 36°C for 1 minute, and 72°C for 2 minutes. The final extension was performed for 10 minutes at 72°C. The amplified DNA fragments were electrophoresed in “1.5% agarose gel and stained by “red safe DNA staining solution (GeNetBio, Korea).” A “100-bp DNA ladder (Genedirex, Taiwan)” was used to determine the band sizes. Diversity assessmentThe GelJ software version 2.0 “(available at https://sourceforge.net/projects/gelj/)” was used to create a dendrogram after an image comprising 35 wells corresponding to all P. aeruginosa isolates was separately observed for the existence or absence of DNA bands in the gel obtained by “RAPD-PCR” (Heras et al., 2015). All strains were categorized using the “Unweighted Pair Group Method with Arithmetic Mean” analysis, combined with the “Dice similarity coefficient, allowing a 1% tolerance.” Strains with a similarity level of 90% were classified as a single genotype (Sadiq et al., 2024; Taha et al., 2024). Isolates were clustered according to the farm source and geographic location. Ethical approvalNot needed for this study. ResultsAccording to the results of the “RAPD-PCR” fingerprinting investigation, the 35 P. aeruginosa isolates exhibited genetic similarities ranging from 50% to 100%. Of the 35 strains, 33 distinct individual genotypes were identified. However, two strains (strains 20 and 21) isolated from distinct farms (3 and 6), and geographical areas were found to have an identical genotype (genotype 3). Despite coming from the same farm and geographic area, the other strains (n=31; 88.6%) were categorized as a single genotype (genotypes 1, 2, 4–33) because of the significant genetic heterogeneity found within the strains (Fig. 2). DiscussionBacterial clonal differentiation in fish farms is important for learning more about strain variation and infection spread. The information gained from this study will help us develop better biosecurity measures, early detection protocols, and effective control strategies to lessen the effects of P. aeruginosa infections in fish farms. Regarding strain diversity and similarity, most isolates were grouped into a single genotype, irrespective of their geographic origin or farm location. The high level of genetic variation indicates that multiple clones of a single P. aeruginosa strain are present in these fish farms, potentially introduced from different geographic sources, such as human activities and wastewater contamination (Fouz et al., 2020). Anthropogenic activities and human wastewater discharge play a crucial role in the transmission of bacteria within the study area, contributing to the spread of waterborne pathogens. Untreated or inadequately treated sewage introduces high concentrations of fecal bacteria, including different strains of potential pathogens, such as Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa, into natural water bodies (Bukola et al., 2015; Xie et al., 2022). Manure from animals, birds, or even live fish could be another factor contributing to such biodiversity, particularly during the process of transferring live fish to different ponds (Elmberg et al., 2017; Delahoy et al., 2018). Manure from birds and livestock, or live fish, can carry a diverse range of bacteria that can contaminate water sources and fish-rearing environments (Bagumire et al., 2009; Ogello et al., 2019; Hasimuna et al., 2020). These bacteria may undergo genetic recombination, further enhancing their adaptability (Didelot and Maiden, 2010). This process underscores the importance of biosecurity measures and proper waste management practices in aquaculture to minimize the risk of bacterial transmission and maintain aquatic ecosystem health (Opiyo et al., 2020). Furthermore, floods may allow other P. aeruginosa strains from the surrounding environment to enter such farmed ponds (Talbot et al., 2018). Floodwaters often carry bacteria from the surrounding soil, sewage, and agricultural runoff into fish ponds. This introduces new genetically diverse pathogens into these ponds (Afia and Iwatt, 2023). Therefore, it is important to mitigate the impact of floods on fish farmers to minimize direct damage. These include constructing ponds above expected flood levels, building embankments, dikes, or concrete walls around fish farms, and constructing drainage systems to redirect excess water away from farms (Olutumise, 2023).
Fig. 2. The RAPD-PCR dendrogram displays the banding patterns of 35 P. aeruginosa strains isolated from cases of hemorrhagic septicemia in rainbow trout (Oncorhynchus mykiss) farms across the Sheladize and Amedi districts, Duhok, Iraq. Microbial genetic diversity primarily arises through point mutations and the insertion or deletion of specific DNA sequences. These genetic alterations can accumulate over time and contribute to the emergence of distinct strains within microbial populations. Such diversity often results from the adaptation of microorganisms to varying environmental conditions, including differences in temperature, salinity, nutrient availability, or host immune responses. Microbes are exposed to selective pressures that may favor certain mutations as they pass through different hosts or ecosystems, ultimately driving genomic variation (Jerome et al., 2011). Horizontal gene transfer plays a significant role in bacterial genetic diversity, as bacteria can exchange genetic material through conjugation, transformation, and transduction. This exchange allows the rapid acquisition of new traits (Arber, 2014). Biofilm formation also contributes to this diversity. Bacteria exhibit elevated rates of genetic exchange and mutations within biofilms due to their close proximity and protective microenvironment (Dopheide et al., 2015). This phenomenon provides a strong explanation for the high level of genetic diversity observed among P. aeruginosa isolates in this study, where diverse environmental factors and management practices across farms create dynamic conditions that promote genetic differentiation. Nevertheless, the two P. aeruginosa strains isolated across distinct farms and geographical areas exhibited the identical genotype, suggesting potential bacterial transmission between these farms. This could be attributed to several factors, including shared farming equipment (Olutumise, 2023; Clols-Fuentes et al., 2024). Such equipment can facilitate the spread of bacteria by transferring slimy, bacteria-laden biofilms that form on wet surfaces and are often resistant to cleaning between tanks or farms (Pandey et al., 2014; De Silva and Heo, 2023). In addition, human-mediated transfer without proper biosecurity measures (Muthu et al., 2020) or even sourcing fingerlings from the same supplier (Bondad-Reantaso et al., 2005) can also contribute to the spread of bacterial pathogens. ConclusionThe findings highlight the significant role of bacterial clonal differentiation in understanding strain variation and P. aeruginosa infection spread in fish farms. The presence of multiple clones within a single genotype is an indication of ongoing bacterial transmission from various sources, including anthropogenic activities, human wastewater discharge, and biological carriers such as birds, livestock, and live fish transfers. These factors contribute to the genetic diversity of P. aeruginosa strains, potentially enhancing their adaptability through genetic recombination. Key biosecurity practices include regular disinfection of equipment, tanks, and water systems and avoiding the sharing of tools between farms without proper sanitization to mitigate the spread of P. aeruginosa in aquaculture environments. In addition, strict quarantine procedures for new stock and fingerling sourcing from certified, disease-free suppliers are critical. Other essential measures include controlled access to farm facilities, constructing fish ponds away from human waste sources, and designing ponds to resist flooding, all of which help reduce the risk of bacterial contamination and disease transmission. Addressing these challenges will help safeguard fish health and maintain the sustainability of aquaculture systems. LimitationsThe study limitations include the small sample size, with only 35 isolates from seven farms in two districts, which may not capture the full genetic diversity of P. aeruginosa in aquaculture. In addition, the study did not correlate genetic findings with environmental or management factors that could influence bacterial spread. AcknowledgmentsThe “Duhok Research Center (DRC), College of Veterinary Medicine, University of Duhok, Duhok, Iraq,” supported this study. We extend our gratitude to Dr. Shaaban Tayar Sadiq for providing the necessary bacterial isolates for this study. Conflicts of interestThe author declares that there is no conflict of interest regarding the publication of this research. FundingSelf-funding. Authors contributionsZanan Mohamed-Ameen Taha designed the experiment, analyzed the results, and wrote the manuscript. Data availabilityThe author confirms that the data supporting the findings of this study are available in the manuscript. ReferencesAfia, O.E. and Iwatt, I.J. 2023. Impacts of flooding on the aquaculture sector. In Contemporary Discourse on Nigeria’s Economic Profile, A Festschrift in honour of Prof. Nyaudoh, U. Ndaeyo. A. Eds., Ayandele, I.A., Udom, G.N., Effiong, E.O., Etuk, U.R., Ekpo, I.E., Inyang, U.G., Edet, G.E. and Moffat, I. Uyo, Akwa Ibom State: Publication of University of Uyo, pp: 333–340. Ambreetha, S. and Singh, V. 2023. Genetic and environmental determinants of surface adaptation in Pseudomonas aeruginosa. Microbiology 169, 1–12; doi:10.1099/mic.0.001335 Arber, W. 2014. Horizontal gene transfer among bacteria and its role in biological evolution. Life 4(2), 217–224; doi:10.3390/life4020217 Bagumire, A., Todd, E.C.D., Nasinyama, G.W., Muyanja, C., Rumbeiha, W.K., Harris, C. and Bourquin, L.D. 2009. Potential sources of food hazards in emerging commercial aquaculture industry in sub‐Saharan Africa: a case study for Uganda. Int. J. Food Sci. Technol. 44(9), 1677–1687; doi:10.1111/j.1365-2621.2008.01904.x Baron, S., Granier, S.A., Larvor, E., Jouy, E., Cineux, M., Wilhelm, A., Gassilloud, B., Le Bouquin, S., Kempf, I. and Chauvin, C. 2017. Aeromonas diversity and antimicrobial susceptibility in freshwater—an attempt to set generic epidemiological cut-off values. Front. Microbiol. 8, 1–9; doi:10.3389/fmicb.2017.00503 Bondad-Reantaso, M.G., Subasinghe, R.P., Arthur, J.R., Ogawa, K., Chinabut, S., Adlard, R., Tan, Z. and Shariff, M. 2005. Disease and health management in Asian aquaculture. Vet. Parasitol. 132(3–4), 249–272; doi:10.1016/j.vetpar.2005.07.005 Bose, P., Sobur, K.A., Lijon, B., Rahman, Z. and Ahamed, T. 2025. Characterization of enterotoxins, antibiotic resistance genes, and antimicrobial susceptibility profiling of Staphylococcus aureus isolated from table eggs: implications for food safety and public health. Open Vet. J. 15(3), 1187–1205; doi:10.5455/OVJ.2025.v15.i3.11 Bukola, D., Zaid, A., Olalekan, E.I. and Falilu, A. 2015. Consequences of anthropogenic activities on fish and the aquatic environment. Fish Wildl. Sci. 3(2), 1–12; doi:10.4172/2375-446x.1000138 Clols‐Fuentes, J., Nguinkal, J.A., Unger, P., Kreikemeyer, B. and Palm, H.W. 2024. Bacterial communities from two freshwater aquaculture systems in northern Germany. Environ. Microbiol. Rep. 16(6), 1–17; doi:10.1111/1758-2229.70062 De Silva, L.A.D.S. and Heo, G. 2023. Biofilm formation by pathogenic bacteria isolated from aquatic animals. Arch. Microbiol. 205(1), 1–10; doi:10.1007/s00203-022-03332-8 Delahoy, M.J., Wodnik, B., McAliley, L., Penakalapati, G., Swarthout, J., Freeman, M.C. and Levy, K. 2018. Pathogens transmitted in animal feces in low- and middle-income countries. Int. J. Hyg. Environ. Health 221(4), 661–676; doi:10.1016/j.ijheh.2018.03.005 Didelot, X. and Maiden, M. 2010. The impact of recombination on bacterial evolution. Trends Microbiol. 18(7), 1–59; doi:10.1016/j.tim.2010.04.002 Dopheide, A., Lear, G., He, Z., Zhou, J. and Lewis, G.D. 2015. Functional gene composition, diversity and redundancy in microbial stream biofilm communities. PLoS One 10(4), e0123179–e0123121; doi:10.1371/journal.pone.0123179 Duman, M., Altun, S., Saticioglu, I.B. and Romalde, J.L. 2023. Bacterial disease outbreaks in rainbow trout (Oncorhynchus mykiss) reported from 2010 to 2022. J. Fish Dis. 46(9), 1–17; doi: 10.1111/jfd.13886 Elham, M.I., Mona, M.I., Maather, M.E. and Heba, I. 2017. Studies on Pseudomonas septicemia in some Ismailian tilapia. Suez Canal Vet. Med. J. 22(1), 107–117; doi:10.21608/scvmj.2017.62397 Elmberg, J., Berg, C., Lerner, H., Waldenström, J. and Hessel, R. 2017. Potential transmission of disease from wild geese and swans to livestock, poultry, and humans: a review of the scientific literature from a One Health perspective. Infect. Ecol. Epidemiol. 7(1), 1–21; doi:10.1080/20008686.2017.1300450 Fouz, N., Pangesti, K.N.A., Yasir, M., Al-Malki, A.L., Azhar, E.I., Hill-Cawthorne, G.A. and Abd El Ghany, M. 2020. The contribution of wastewater to the transmission of antimicrobial resistance in the environment: implications of mass gathering settings. Trop. Med. Infect. Dis. 5(1), 33–31; doi:10.3390/tropicalmed5010033 Fu, S., Wang, Q., Wang, R., Zhang, Y., Lan, R., He, F. and Yang, Q. 2024. Corrigendum to “Horizontal transfer of antibiotic resistance genes within the bacterial communities in aquacultural environment”. Sci. Total Environ. 922, 171434; doi:10.1016/j.scitotenv.2024.171434 Hasimuna, O.J., Maulu, S. and Mphande, J. 2020. Aquaculture health management practices in Zambia: status, challenges and proposed biosecurity measures. J. Aquac. Res. Dev. 11, 584; doi:10.35248/2155-9546.19.10.584 Hematzadeh, A. and Haghkhah, M. 2021. Biotyping of Pseudomonas aeruginosa isolates from human infections using RAPD and ERIC-PCR. Heliyon 7(9), 7967; doi:10.1016/j.heliyon.2021.e07967 Heras, J., Domínguez, C., Mata, E., Pascual, V., Lozano, C., Torres, C. and Zarazaga, M. 2015. GelJ – a tool for analyzing DNA fingerprint gel images. BMC. Bioinf. 16(1), s1–s8; doi:10.1186/s12859-015-0703-0 Jerome, J.P., Bell, J.A., Plovanich-Jones, A.E., Barrick, J.E., Brown, C.T. and Mansfield, L.S. 2011. Standing genetic variation in contingency loci drives the rapid adaptation of Campylobacter jejuni to a novel host. PLoS. One 6, e16399; doi:10.1371/journal.pone.0016399 Kadhim, H.M., Miah, A., Munn, C.B. and Gilpin, M.L. 2012. Development of a polymerase chain reaction (PCR) test for the detection of virulent forms of Vibrio parahaemolyticus. Eur. J. Clin. Microbiol. Infect. Dis. 31(4), 431–439; doi:10.1007/s10096-011-1324-9 Khor, W.C., Puah, S.M., Tan, J.A., Puthucheary, S.D. and Chua, K.H. 2015. Phenotypic and genetic diversity of Aeromonas species isolated from fresh water lakes in Malaysia. PLoS. One 10(12), 1–13; doi:10.1371/journal.pone.0145933 Muthu, M.P., George, M.R. and John, R. 2020. Biosecurity strategies in aquaculture for fish health management. J. Aquac. Trop. 35(9–26), 9–26. Ogello, E.O., Wullur, S. and Hagiwara, A. 2009. Blending fishpaste and chicken manure extract as a low-cost and stable diet for the mass culture of freshwater zooplankton, optimized for aquaculture. IOP Conf. Ser. Mater. Sci. Eng. 567(1), 1–11. doi:10.1088/1757-899X/567/1/012022 Olutumise, A.I. 2023. The impact of relaxing flood policy interventions on fish production: lessons from farmers in Southwest Nigeria. Aquac. Int. 31(4), 1855–1878; doi:10.1007/s10499-023-01062-2 Opiyo, M., Mziri, V., Kyule, D., Hinzano, S., Wainaina, M., Magondu, E., Werimo, K. and Ombwa, V. 2020. Fish disease management and biosecurity systems. In Status of aquaculture in Kenya. Mombasa, Kenya: Kenya Marine and Fisheries Research Institute (KMFRI). Available via https://www.researchgate.net/publication/351050775 Pandey, P.K., Vivekanand, B. and Kundan, K. 2014. Biofilm in aquaculture production. Afr. J. Microbiol. Res. 8(13), 1434–1443; doi:10.5897/ajmr2013.6445 Sadiq, S.T. 2024. A pathological and diagnostic studies of some bacterial gill diseases in rainbow trout (Oncorhynchus mykiss) cultured in Duhok Province. Sadiq, S.T., Al-Hamdani, A.H.A. and Taha, Z.M.A. 2024. Clonal dispersion and pathogenic potential of multidrug-resistant Aeromonas spp. isolated from Oncorhynchus mykiss with hemorrhagic septicemia. Vet. Res. 58(10), 529–536; doi:10.30466/vrf.2024.2010315.3998 Sadok, K., Mejdi, S., Nourhen, S. and Amina, B. 2013. Phenotypic characterization and RAPD fingerprinting of Vibrio parahaemolyticus and Vibrio alginolyticus isolated during fish farm outbreaks in Tunisia. Folia Microbiologica 58(1), 17–26; doi:10.1007/s12223-012-0174-x Stefańska, I., Kwiecień, E., Górzyńska, M., Sałamaszyńska-Guz, A. and Rzewuska, M. 2022. RAPD-PCR-based fingerprinting method as a tool for epidemiological analysis of Trueperella pyogenes infections. J. Microbiol. Biotechnol. Biotechnol. Pathogens. 11(5), 1–10; doi:10.3390/pathogens11050562 Taha, Z.M., Ahmed, M.S., Jwher, D.M.T., Abdulrahman, R.F. and Rahma, H.Y. 2024. Diversity analysis of livestock-associated Staphylococcus aureus nasal strains between animals and humans. Open Vet. J. 14(9), 2256–2260; doi:10.5455/OVJ.2024.v14.i9.13 Talbot, C.J., Bennett, E.M., Cassell, K., Hanes, D.M., Minor, E.C., Paerl, H., Raymond, P.A., Vargas, R., Vidon, P.G., Wollheim, W. and Xenopoulos, M.A. 2018. Impact of flooding on aquatic ecosystem services. Biogeochemistry 141(3), 439–461; doi:10.1007/s10533-018-0449-7 Xie, Y., Liu, X., Wei, H., Chen, X., Gong, N., Ahmad, S., Lee, T., Ismail, S. and Ni, S.Q. 2022. Insight into impact of sewage discharge on microbial dynamics and pathogenicity in river ecosystem. Scientific Rep. 12(1), 1–12; doi:10.1038/s41598-022-09579-x | ||
| How to Cite this Article |
| Pubmed Style Zanan Mohamed-Ameen Taha. Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemia. Open Vet. J.. 2025; 15(8): 3780-3786. doi:10.5455/OVJ.2025.v15.i8.42 Web Style Zanan Mohamed-Ameen Taha. Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemia. https://www.openveterinaryjournal.com/?mno=253012 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.42 AMA (American Medical Association) Style Zanan Mohamed-Ameen Taha. Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemia. Open Vet. J.. 2025; 15(8): 3780-3786. doi:10.5455/OVJ.2025.v15.i8.42 Vancouver/ICMJE Style Zanan Mohamed-Ameen Taha. Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemia. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3780-3786. doi:10.5455/OVJ.2025.v15.i8.42 Harvard Style Zanan Mohamed-Ameen Taha (2025) Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemia. Open Vet. J., 15 (8), 3780-3786. doi:10.5455/OVJ.2025.v15.i8.42 Turabian Style Zanan Mohamed-Ameen Taha. 2025. Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemia. Open Veterinary Journal, 15 (8), 3780-3786. doi:10.5455/OVJ.2025.v15.i8.42 Chicago Style Zanan Mohamed-Ameen Taha. "Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemia." Open Veterinary Journal 15 (2025), 3780-3786. doi:10.5455/OVJ.2025.v15.i8.42 MLA (The Modern Language Association) Style Zanan Mohamed-Ameen Taha. "Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemia." Open Veterinary Journal 15.8 (2025), 3780-3786. Print. doi:10.5455/OVJ.2025.v15.i8.42 APA (American Psychological Association) Style Zanan Mohamed-Ameen Taha (2025) Random amplified polymorphic DNA polymerase chain reaction was used to analyze the genotype and diversity of Pseudomonas aeruginosa isolated from salmon trout farms with hemorrhagic septicemia. Open Veterinary Journal, 15 (8), 3780-3786. doi:10.5455/OVJ.2025.v15.i8.42 |