| Short Communication | ||
Open Vet. J.. 2025; 15(8): 3888-3898 Open Veterinary Journal, (2025), Vol. 15(8): 3888-3898 Short Communication Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025Anh Duc Truong1†, Ha Thi Thanh Tran1, Duy Le Khac1, Uyen Trung Nguyen3, Hiep Van Dang1,2, Nhu Thi Chu1, Hieu Minh Nguyen1, Linh Thi Nguyen1, Kien Van Le2 and Hoang Vu Dang1*1Department of Biochemistry and Immunology, National Institute of Veterinary Research, Hanoi, Vietnam 2Sub-Department of Livestock and Animal Health, Thanh Hoa Department of Agriculture and Rural Development, Thanh Hoa, Vietnam 3Faculty of Agriculture and Environment, Ha Tinh University, Ha Tinh, Vietnam †Both authors contributed equally to this work *Corresponding Author: Hoang Vu Dang. Department of Biochemistry and Immunology, National Institute of Veterinary Research, Hanoi, Vietnam. Email: dangvuhoang [at] nivr.gov.vn Submitted: 16/04/2025 Revised: 26/06/2025 Accepted: 04/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
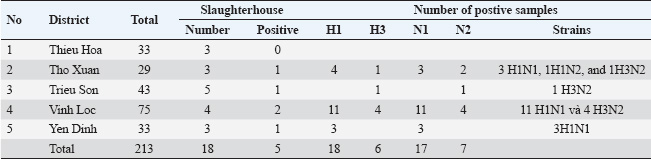
ABSTRACTBackground: Swine influenza viruses (SIVs) is a highly infectious viral disease in pig population. This disease is caused by the influenza A virus. According to the World Organization for Animal Health (WOAH), three subtypes of SIVs have been officially reported, including H1N1, H1N2, and H3N2. However, information about this pathogen circulated in Vietnam is limited, especially during the last 5 years. Aim: This research aimed to examine the molecular characteristics of SIVs circulating in Vietnam. A total of 213 swab samples from 18 slaughterhouses in Thanh Hoa province, North Central Vietnam, were collected between 2024 and early 2025, and SIVs were detected by realtime RT-PCR. The characteristics of these viruses were further determined based on the phylogenetic analysis of the Hemagglutination (HA) and Neuraminidase (NA) genes. Methods: SIV subtypes were determined by realtime RT-PCR. The full-length HA and NA genes were amplified by PCR. Phylogenetic and characterization analysis of HA and NA nucleotides and proteins were performed using bioinformatics. Virus isolation and titration were performed in the Madin–Darby canine kidney (MDCK) cell line. Results: Of 213 samples, 24 were positive for SIVs, representing an 11.27% prevalence at the slaughterhouses (SIVs were confirmed in 5 out of 18 slaughterhouses). These samples included 17 H1N1, 1 H1N2, and 6 H3N2 subtype samples. Phylogenetic analysis of the HA genes showed that four H1N1 subtypes belonged to the H1N1pdm09 lineage, three to the Eurasian “avian-like” (EA) H1N1 lineage, and one to the TR-H1N2 lineage. Additionally, four H3N2 subtypes were classified into clade IV of the recent human-line (RH) H1N1. Based on the NA gene sequences, seven viruses belonged to the EA H1N1 lineage, three to the RH-H3N2 lineage, and one to the TR-H1N2 lineage. Receptor-binding and antigenic site analysis revealed that all isolates could bind to both human- and avian-like receptors. Furthermore, in vitro analysis of the growth kinetics of SIVs showed that 12 SIV isolates could efficiently replicate in MDCK cell lines. Conclusion: Our study demonstrated that all three WOAH subtypes have been circulating in Vietnam during 2024 and early 2025. H1N1 and H3N2 subtypes are predominant, suggesting a potential threat to human health. This highlights the importance of active surveillance as a critical step for swine influenza prevention and control in Vietnam. Keywords: Swine influenza viruses H1N1, H3N2, Phylogenetic analysis, Subtypes, Lineages. BackgroundInfluenza A viruses (IAVs) pose a substantial One Health threat due to their capacity to circulate across various host species, often spilling over into new populations, creating risks for both human and animal health (Coker et al., 2011; WOAH, 2022). Pigs are especially critical in this process, acting as “mixing vessels” where both avian and mammalian influenza viruses can infect and reassort, leading to the creation of novel viral strains with the potential for pandemics (Mak et al., 2011; Janke, 2013). The 2009 H1N1 pandemic is a prime example, as it was caused by a reassortant H1N1 virus that incorporated gene segments from classical swine flu, Eurasian avian-like swine flu (EA), and human seasonal flu lineages. This event underscores the pivotal role of pigs in the generation of new influenza strains capable of crossing species barriers and triggering global health threats (Cisneros et al., 1996; Zhu et al., 2013; Ozawa et al., 2015). Swine influenza viruses (SIVs) of three subtypes—H1N1, H3N2, and H1N2—are found in swine populations worldwide, exhibiting regional variation in their antigenic characteristics (Vijaykrishna et al., 2011). The emergence of contemporary SIV lineages, including H1N1, H1N2, and H3N2 triple reassortant viruses (TR), occurred in the 1990s as a result of reassortment between classical swine, avian, and human influenza viruses (Coker et al., 2011; Zhu et al., 2013). Additionally, EA-H1N1 SIVs were identified and characterized in pigs in the 1970s, and various human-derived strains, such as H1N1pdm09, pre-2009 H1N1, and H3N2-variant viruses, have been identified in swine populations (Trevennec et al., 2011b; Nguyen et al., 2015). The expanding genetic diversity of SIVs in pigs is concerning as it could lead to the emergence of new viral strains with pandemic potential (Mak et al., 2011). Human infections with emerging SIVs are most commonly reported in the USA, where all non-human influenza viruses are nationally notifiable, with outbreaks occurring at agricultural fairs (Baudon et al., 2015; Ozawa et al., 2015). However, sporadic cases of SIVs infections have also been reported in Asia, Europe, and Australia (Coker et al., 2011; Trevennec et al., 2011a; Vijaykrishna et al., 2011; Zhu et al., 2013; Baudon et al., 2015; Nelson et al., 2015). Between 2013 and 2019, surveillance at a collective slaughterhouse in Hanoi, Vietnam, revealed the co-circulation of several SIV strains, including H1N1pdm09, H1N2 with pre-2009 seasonal influenza-derived H1 hemagglutinin (HA), H3N2 from human seasonal influenza, and “triple-reassortant” (TR) H1N2 and H3N2 viruses (Takemae et al., 2013; Baudon et al., 2017). Additionally, the first recorded human infection with the A(H1N1) virus occurred in the Northern province of Son La, Vietnam (Bansal et al., 2024). In North Central Vietnam, more than 50% of the Vietnamese population lives in rural areas and relies on small-scale animal husbandry for their livelihood. Close and frequent contact with animals increases the risk of SIV transmission. SIVs transmitted between pigs and humans can spread through direct contact with infected animals, exposure to their waste, contaminated water, soil, or other environmental sources. Furthermore, North Central Vietnam, which has a high concentration of human, pig, and poultry populations, lacks information on the surveillance and subtype of SIVs circulating in the region. To address this, we conducted epidemiological surveillance in slaughterhouses in Thanh Hoa province, North Central Vietnam, to identify the 2009 pandemic H1N1 influenza A virus in pigs and mitigate the risk of pig-to-human transmission. Materials and MethodsSamplingA total of 213 nasal swab samples from 18 different slaughterhouses of six districts in Thanh Hoa province of North Central Vietnam were collected from pigs by veterinary officials during 2024 and early 2025 (Table 1). Nasal swab samples were collected immediately after the pigs were stunned, before the slaughter process. Individual swab samples were stored in a viral transport medium with an ice box to ensure the stability and preservation of the samples. The swab samples were kept at 4°C and sent to the National Institute of Veterinary Research for virology diagnosis by realtime RT-PCR and virus isolation. RNA extraction, PCR, and sequencingViral RNA from swab samples was isolated using the QIAamp Viral RNA Kits (QIAgen, Hilden, Germany) according to the manufacturer’s instructions. RNA isolation from Nasal swab samples was initially screened for the detection of SIV genomes using a realtime RT-PCR assay targeting the matrix (M) gene, following previously established methods with the qScript XLT 1-Step RT-qPCR ToughMix Kit (Quantabio, Beverly, MA, USA) (WOAH, 2018). The positive RNA isolation from nasal swab samples was subsequently subtypes using realtime RT-PCR assays designed for the specific identification of the HA and NA genetic lineages (H1, H3, N1, and N2) of SIVs (Richt et al., 2004). Realtime RT-PCR was carried out on a QuantStudio 5 system (Applied Biosystems, USA) according to the World Organization for Animal Health (WOAH)-recommended procedure described earlier or previously method (Richt et al., 2004; WOAH, 2018). Furthermore, cDNA was made from each RNA-positive extract using ThermoScript RT-PCR System for First-Strand cDNA Synthesis (Invitrogen) with Uni12 primer, then 5 µl of the cDNA was amplified in a 50 µl volume using the Platinum Taq DNA High Fidelity Polymerase kit (Invitrogen, CA, USA) using specific primers as described by Hoffmann et al. (2001). The PCR reaction and thermo cycles were processed as previously described by (Hoffmann et al., 2001). PCR products of correction size were purified using the QIAquick Gel Extraction Kit (QIAgen) and then sequenced with the Apical Scientific Sdn. Bhd (Seri Kembangan, Selangor, Malaysia). Table 1. Results of realtime RT-PCR detection of IAVs in nasal samples in Thanh Hoa, Vietnam.
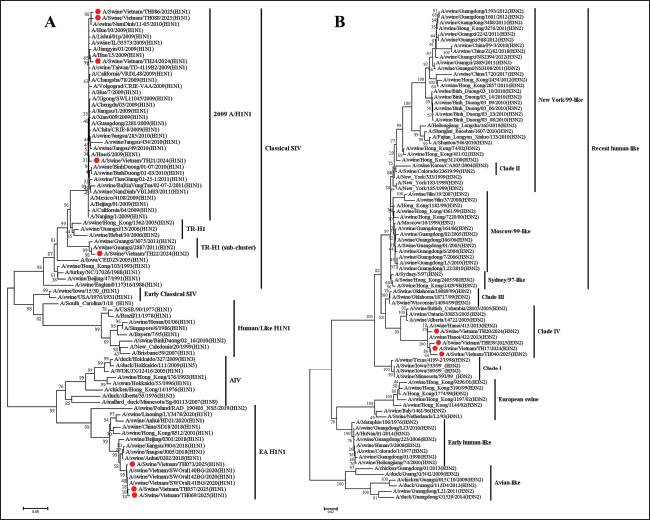
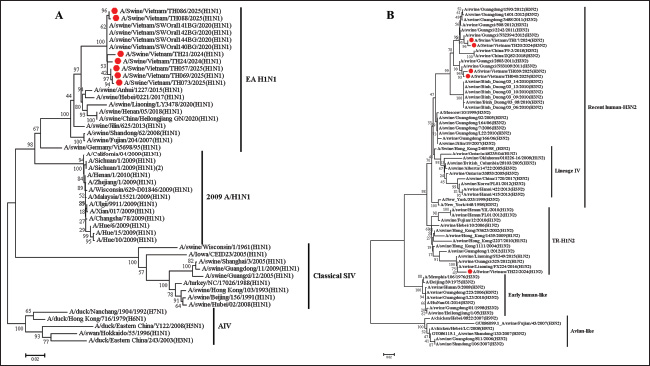
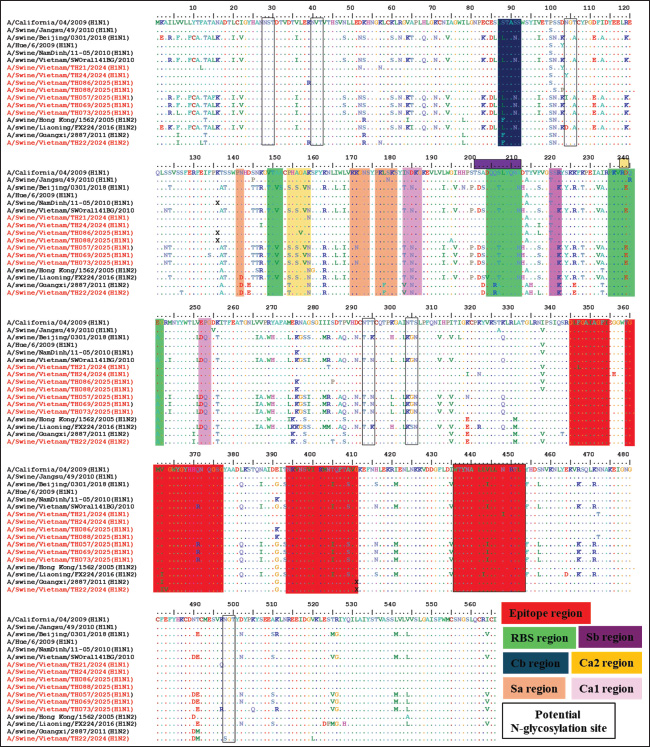
Isolation and titration of virusesThe positive nasal swab samples were further processed for virus isolation by inoculating them with Madin–Darby canine kidney (MDCK) cell lines. The MDCK cell line was grown in minimum essential medium with trypsin (2 µg/ml) and 10% Fetal bovine serum (Invitrogen, CA, USA) with 1% antibiotic (Sigma-Aldrich, St. Louis, Missouri, USA) according to the WOAH instructions (WOAH, 2018). Following inoculation, the cells were monitored for developing cytopathic effect (CPE) at 3 days post inoculation. The suspected cytopathogenic in the MDCK were collected and tested by the realtime RT-PCR or HA. Cultures demonstrating a positive CPE or a positive result in the haemagglutination test underwent a second and third passage on fresh MDCK cells. When CPE was identified on the third passage, the cells were collected, aliquoted, and tested by the realtime RT-PCR or HA to confirm positive CPE. The cells were stored at −80°C. The virus titration assay was performed using the MDCK cell line as described previously (WOAH, 2018; Tran et al., 2020). The MDCK cell line was seeded in 96-well plates. The positive samples on the third passage were subsequently added to the plates and titrated in triplicate using 10 ×dilutions. The quantity of SIVs was observed by CPE development. Virus titration was observed for 3–4 days, and 50% of TCID50 were measured using Reed and Muench’s method (Reed and Muench, 1938). Bioinformatic analysisThe obtained complete HA and NA gene sequences were compared with available SIV sequences in NCBI databased using the Basic Local Alignment Search Tool. Phylogenetic and evolutionary analyses of the HA and NA genes from Vietnam SIVs and reference SIVs sequences were conducted using MEGA 7.0 software (Kumar et al., 2016). Phylogenetic trees were constructed using the Maximum Likelihood method with 1,000 bootstrap replicates, and the evolutionary distances were computed using the Kimura 2-parameter method with transition-transversions. The rate among sites was gamma distributed, and the partial deletion was used to fill gaps/missing data. N-glycosylation of HA protein sequences was predicted using NetOGlyc 4.0 Server (http://www.cbs.dtu.dk/services/NetOGlyc). The similarity and identity of the HA protein sequence was analysis by using the SIAS online tool (http://imed.med.ucm.es/Tools/sias.html). Ethical approvalThe research is not related to the use of animals. Ethical approval was not obtained because this study did not involve laboratory animals and involved only non-invasive procedures. Results and discussionInfluenza was first recognized as a disease affecting swine during the 1918 pandemic, when it was noted that families infected with the virus often had sick pigs. Although the 1918 A(H1N1) pandemic virus emerged in both humans and swine around the same time, whether the virus was initially transmitted from swine to humans, from humans to swine, or if both species were simultaneously infected remains unclear. In 1930, the first A(H1N1) viruses isolated from swine in the United States in 1930 became known as classical swine influenza A(H1N1) viruses. Later, A(H3N2) viruses were first detected in swine during an influenza surveillance study conducted in Taiwan in 1970 (Trevennec et al., 2011a). Pigs play a crucial role in the ecology of IAVs because they are susceptible to avian, porcine, and human IAV infections. A variety of SIV lineages have been isolated from pigs globally, including avian/human, human/swine, and human/avian/swine triple-reassortant lineages. Additionally, Eurasian avian-like (EA) and classical swine lineage SIVs have been identified in pigs around the world (Mak et al., 2011; Zhu et al., 2013). Since the emergence of the 2009 H1N1 pandemic virus (pdm/09), there has been frequent reassortment with local SIVs, resulting in the development of new viral strains. Some of these newly formed strains have the ability to infect humans, which increases the potential for zoonotic transmission and increases the risk of future pandemic outbreaks (Vijaykrishna et al., 2011; Pomorska-Mol et al., 2017). Between 2010 and 2019, the prevalent SIVs in Vietnam included pre-2009 human-derived H1 viruses, which were further separated into H1-δ-like and H1-δ1a lineages. In addition, EA and TR viruses of both the H1 and H3 subtypes have also been identified in this region. These SIVs generated 15 genotypes, with genotype 1 being the most commonly detected in Vietnam (Baudon et al., 2017; Cheung et al., 2023). Furthermore, the first case of human infection with the A(H1N1) virus was reported in Son La, Vietnam, in 2024, as it could lead to the emergence of the A(H1N1) pandemic potential in humans in Vietnam (Bansal et al., 2024). Thus, SIV surveillance in slaughterhouses and farms plays an important role in observing genetic diversity, reassorting, and novel influenza A (H1N1, H3N2 or H1N2) and supporting the control and surveillance of SIVs in pigs and humans. In this study, a total of 213 nasal swab samples from 18 slaughterhouses screened, 5/18 (27.78%) slaughterhouses and 24 (11.28%) from 213 samples were detected the positive for influenza A viruses using realtime RT-PCR (Table 1). On subtyping of 24 IAV-positive samples using realtime RT-PCR, 17 (70.83%) samples were positive for A (H1N1) pdm09, 6 (25.0%) samples were positive for A(H3N2), and 1 (4.17%) was positive for the A(H1N2) subtype. Our results indicated that H1N1, H1N2, and H3N2 SIVs remain the predominant circulating subtype in Thanh Hoa, Vietnam. Our results indicated that in 5 slaughterhouses positive with SIVs were at 4 districts of Thanh Hoa province but concentrated on a few. Pigs in positive slaughterhouses mainly originated from households or small farms. This suggests that the emergence transmitted between pigs and humans can spread through direct contact with infected animals, exposure to their waste, or through contaminated water, soil, or other environmental sources. Furthermore, 13 slaughterhouses were negative for SIV, where pigs mainly came from big or industry farms. Our results suggest that further research on the active surveillance of SIV circulating in North Center of Vietnam with large farm scale and sample size is needed to control and prevent SIVs in the pig population. From the positive samples, 7 of A(H1N1) pdm09, 4 of A(H3N2), and 1 of A(H1N2) strains were randomly selected for virus isolation, and subsequent sequencing analyses were conducted. Phylogenetic analysis of the HA gene indicated that all 12 isolates from Thanh Hoa province, North Central Vietnam, were of the H1 and H3 subtypes and were grouped into four distinct lineages. The identity of nucleotide sequence among the 8 H1 genes ranged from 72.80% to 99.41% (Fig. 1A). In the H1 subtype, three isolates were grouped with the EA-H1N1 lineage, four with the pdm09/H1N1 lineage, and one with the TR-H1 lineage (Fig. 1A). Four isolates (TH21/2024, TH24/2024, TH086/2025, and TH088/2025) were grouped with the pdm09/H1N1 strain. The similarity between the HA genes of these four isolates and the human strains (A/Jiangsu/1/2009 and A/Beijing/01/2009) ranged from 98.88% to 99.23% at the nucleotide level and from 98.9% to 99.12% at the amino acid level. In comparison, the homology rates between the HA genes and the A(H1N1) pandemic strain (A/California/04/2009) were 98.43%–98.89% for nucleotides and 98.12%–98.78% for amino acids. These results suggest that the isolates in Thanh Hoa, Vietnam, including TH21/2024, TH24/2024, TH086/2025, and TH088/2025, have evolved from the same or a close relationship origin. Thus, our results indicated that the four viruses isolated in Thanh Hoa, Vietnam, showed a close relationship with the swH1N1 viruses isolated in China in 2009−2011 and the human isolation in Vietnam in 2009–2011 (Fig. 1A). On the other hand, based on the HA sequence analysis, one isolation (TH22/2024) was clustered with TR-H1 (TR-H1N2), which was closely related to the swine H1N2 viruses isolated in Guangxi province in 2011−2012 with the homologous rates between 97.76% and99.35% for nucleotide sequence (Fig. 1A). Finally, three isolates (TH073/2025, TH057/2025, and TH069/2025) were clustered to the EA-H1N1 lineage and closely related to swine EA-H1N1 isolated in China 2018–2020 and Vietnam 2020, with homologous nucleotide sequence rates between 93.94% and 98.11% and 98.33%–99.82% (Fig. 1A). In the H3 subtype, four isolates belonged to the clade IV of SIVs, and the nucleotide and amino acid homology of the HA genes from the seven H3N2 isolates in this study were 98.35%–99.35% and 98.18%–99.13%, respectively (Fig. 1B). In comparison between isolates in clade IV of SIVs, the amino acid identifies and similarities of four isolated in Vietnam (TH17/2024, Th20/2024, TH039/2025, and TH040/2025) were 93.82%–95.29% and 93.57%–95.18% with other strain of clade IV of H3N2 SIV such as A/swine/British-Columbia/28105/2005, A/swine/Ontario/33853/2005, or A/swine/Alberta/14722/2005. On the other hand, the homology of the HA genes in this study with previously research in Vietnam showed that the homologous of nucleotide sequence rates between 87.88% and 88.94% and 98.35%–99.58% with H3N2 isolated in Binh Duong/2010 (South of Vietnam) and Hanoi/2013 (North of Vietnam), respectively. On the NA genes, the nucleotide sequences of the seven N1 NA genes had a 98.67%–99.86%, clustered in the EA H1N1 lineage, and a nucleotide sequence homology with EA H1N1 group of 95.03%–96.09% (Fig. 2A). Thus, based on NA genes, our results indicated that four isolated in Thanh Hoa, Vietnam, showed a close relationship to EA H1N1 viruses isolated in China in 2018–2020 (Fig. 2A). Four N2 NA genes were clustered into the RH-H3N2 lineage, and one N2 NA gene was clustered in the TR-H1N2 lineage and closely related to pig H3N2 from China isolates in 2016-2018 (Fig. 2B). Our results indicate that the isolates from Thanh Hoa, Vietnam, were genetically similar to the 2009 H1N1 pandemic virus (Figs. 1 and 2). In particular, the HA genes were classified within the lineage of classical swine viruses, whereas the NA genes were identified as EA-H1N1 viruses, consistent with their viral precursors. Furthermore, H3N2 and H1N1 isolates from Thanh Hoa, Vietnam, showed a genetically close relationship with viral strain isolates in China from 2016 to 2018. The nucleotide sequence of the HA genes of the isolates is 1701 bp in length, encoding 566 amino acids, including a 17-amino-acid signal peptide. The HA protein consisted of HA1 (326 aa) and HA2 (223 aa), linked by a basic amino acid residue (R). The HA cleavage sites, where the HA0 protein is cleaved by specific protease(s) into HA1 and HA2 to activate the protein for facilitating IAV entry into host cells, were identical (PSIQSR↓GLFGAI) across all analyzed strains, which is characteristic of low-pathogenic avian IAVs (Sun et al., 2010; Burke and Smith, 2014). Furthermore, the residues Asp204, Asp239, Gln240, and Gly242 in the receptor-binding site (RBS), responsible for viral attachment to the host cell receptor—a crucial step for viral entry—were conserved across the five H1 isolates (Shi et al., 2022). A mutation at Asp239 was found in the TH57/2025, TH69/2025, and TH73/2025 isolates, similar to the mutation seen in isolates from China (Beijing/0310/2018) and Vietnam (SWOral141BG/2010) (Fig. 3). Notably, these four residues have previously been identified as crucial for the HA protein’s specific binding to the host cell receptor (Santos et al., 2019; Chang et al., 2021; Shi et al., 2022). In contrast, amino acid variations were observed at several positions within the HA RBS among different pdm09/H1N1 (A/California/04/2009) viruses, including positions 149, 151, 206, 207, and 212 (Fig. 3). Further biological investigation is needed to determine whether these variations affect the virus’s infectivity in specific hosts.
Fig. 1. The phylogenetic tree of the HA genes of swH1N1, H1N2 (A), and H3N2 (B) viruses isolated in the Thanh Hoa province of North Central Vietnam. Phylogenetic tree of HA genes based on 30–1730 nt. The sequences were aligned using the ClustalW and Maximum Likelihood method (Ml), and the tree was created with 1,000 bootstrap replicates using MEGA 7. The sequence of this study in the Thanh Hoa province of North Central Vietnam is indicated by red dots. The antigenic properties of the eight H1 isolates from Thanh Hoa, Vietnam, were compared with those of pdm09/H1N1 (A/California/04/2009) by analyzing previously identified antigenic epitope regions. Two groups of epitope regions were examined: highly conserved and highly variable regions (Munoz-Medina et al., 2015; Guo et al., 2019). As illustrated in Figure 3, four highly conserved epitope regions in HA2, include region 1 the amino acid residues from 345 to 354, region 2 from 359 to 376, region 3 from 394 to 411, and region 4 from 436 to 453, were observed in the 8H1 isolates of H1N1 and H1N2 viruses. However, the highly variable regions in HA1, including Cb (site residues from 86 to 91), Sa (site residues from 141 to 142, 170 to 174, and 176 to 181), Sb (site residues from 201 to 212), Ca1 (site residues from 183 to 187, 220 to 222, and 252 to 254), and Ca2 (site residues from 154 to 159 and 238–239) (Fig. 3) (Thomas et al., 1990; Wan et al., 2015; Guo et al., 2019; Decker et al., 2022), exhibited significant differences between the 8H1 isolates from Thanh Hoa and the pdm09/H1N1 (A/California/04/2009) and the similarity with pig H1N1 in Vietnam and China 2016–2018 (Figs. 1 and 3). Furthermore, the HA protein of the 8H1 isolates contained six potential N-glycosylation sites: NST (28-30), NVT (40-42), NCT (104-106), NTT (293-295), NTS (304-306), and NGT (498-500) (Thomas et al., 1990).
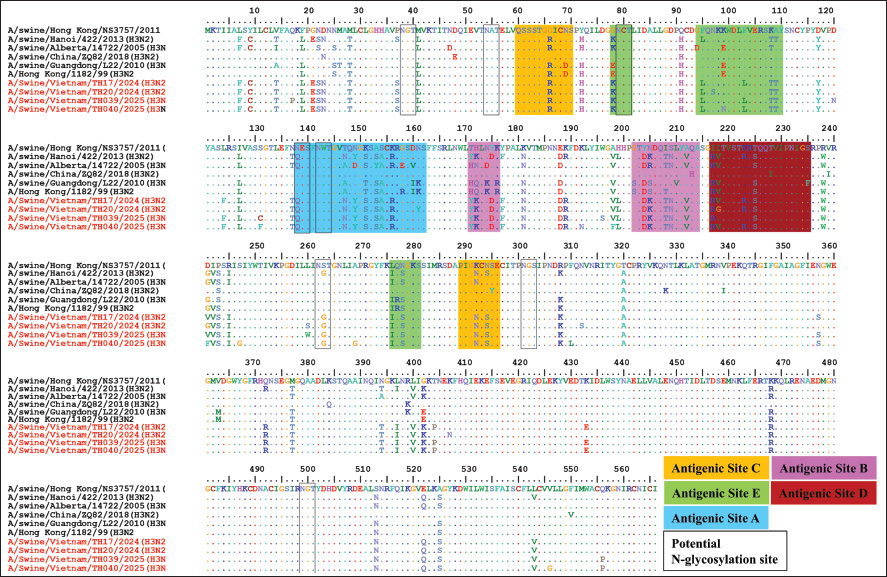
Fig. 2. Phylogenetic tree of the NA genes of swH1N1, H1N2 (A), and H3N2 (B) viruses isolated in the Thanh Hoa province of North Central Vietnam. Phylogenetic tree of NA genes based on 20–1429 nucleotides. The sequences were aligned using the ClustalW and Maximum Likelihood method (Ml), and the tree was created with 1,000 bootstrap replicates using MEGA 7. The sequence of this study in the Thanh Hoa province of North Central Vietnam is indicated by red dots. The cleavage site motif of the H3 HA is PSIQSR↓GLFGAI, suggesting that the four isolates in Thanh Hoa have low pathogenicity (Burke and Smith, 2014). The HA protein of 4 H3 isolates in Thanh Hoa province contains eight potential N-glycosylation sites, including NGT (site residues from 38 to 40), NAT (site residues from 54 to 56), NCT (site residues from 79 to 81), NES (site residues from 138 to 140), NWT (site residues from 142 to 144), NST (site residues from 262 to 264), NGS (site residues from 301 to 303), and NGT (site residues from 499 to 501) (Fig. 4). Compared with A/swine/Alberta/14722/2005 (H3N2) in antigenic site A indicated that 4 H3 isolates in Thanh Hoa province has 5 amino acids mutated (A147N, D149Y, Y153S, E158G, and V160D) but no mutation when compared with isolates in Hanoi, Vietnam (A/swine/Hanoi/422/2013 (H3N2)) (Fig. 4). There is 3 out of 5 amino acids were changed in antigenic site B at position 171–176 and conserved at antigenic site B position 202–214 when compared to A/swine/Alberta/14722/2005 (H3N2), but there is no mutation between isolated in Thanh Hoa province when compared to isolates in Hanoi, Vietnam (A/swine/Hanoi/422/2013 (H3N2)) (Fig. 4). Additionally, the amino acid mutation A212V, located in antigenic site B of HA, enhances the replication of human H3N2 influenza viruses in eggs (Yamashita et al., 2010; Iba et al., 2014). In antigenic site C, only one mutation (N294S) was found, while antigenic site D contained a mutation at K223R (Munoz-Medina et al., 2015; Zhang et al., 2022) (Fig. 4). Our analysis of the antigenic relationships between the eight H1 and four H3 isolates from Thanh Hoa province, as well as other H1N1, H1N2, and H3N2 influenza viruses (including those from swine and humans), revealed that viruses from the same host origin exhibit similar antigenicity. However, swine-origin H1N1, H1N2, and H3N2 viruses displayed significantly different antigenicity from human-origin SIVs. The NA genes of all 12 isolates (7 N1 and 5 N2) were 1410 bp in length and encoded 469 amino acids. No mutations were found at positions 119 (E), 152 (R), 275 (H), and 295 (N) of the NA protein, suggesting that these isolates in Thanh Hoa, Vietnam may be sensitive to neuraminidase inhibitors, such as Oseltamivir phosphate (Huang et al., 2013; Comas et al., 2015; Wan et al., 2015) (data not shown).
Fig. 3. Molecular analysis of the HA gene of the 8 H1N1 and H1N2 SIVs and reference strains. The black box is a potential glycosylation site; the epitope region in HA2 is presented in box red; the RBS in HA1 is presented in box green; the antigen site Cb is presented in box dark blue; the antigen site Sa is presented in box orange; the antigen site Sb is presented in box purple; the antigen site Ca1 is presented in box plum and the antigen site Ca2 is presented in box light yellow. Dots denote amino acids similar to the consensus and red color of virus strains are isolated from slaughterhouse in this study.
Fig. 4. Amino acid comparison of the HA domains from the four H3N2 influenza viruses. Dots represent amino acids similar to the consensus, whereas squares indicate conserved amino acid residues at the receptor-binding site. The N-glycosylation sites are highlighted in boxes. Amino acid residues at previously defined antigenic sites A, B, C, D, and E are shown in blue, purple, yellow, pink, and green, respectively. The red color is isolated from slaughterhouses in this study.
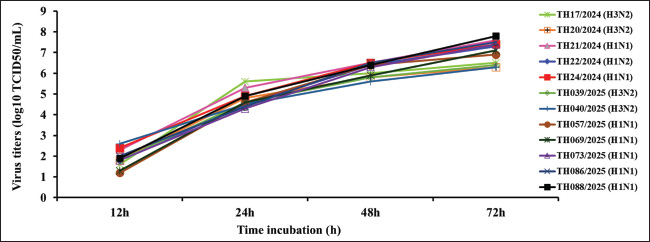
Fig. 5. Growth kinetics of isolated SIVs in mammalian cells. The MDCK cell line was infected with equal amounts of viruses at an MOI of 0.01. Cell supernatants were harvested at 12, 24, 48, and 72 hpi and titrated in MDCK cells. The data shown are the mean virus titers ± SD in log10 TCID50/ml. To assess the in vitro replication capability of the H1 and H3 subtype SIV isolates, the growth kinetics of various SIVs were analyzed in MDCK cells. The results demonstrated that the H1 and H3 subtype SIV isolates in this study are replicated efficiently in MDCK cells. Thus, virus replication peaked between 48 and 72 hours post-inoculation (hpi), with average peak tiers ranging from 6.3 to 7.6 log10 TCID50/ml. Several EA-H1N1 SIVs exhibited replication kinetics and showed medium viral titers, whereas one pandemic-like H1N2 virus, TH22/2024 (H1N2), and three H3 subtype SIVs showed lower viral titers compared with H1N SIVs (Fig. 5). ConclusionIn summary, our findings indicate that all three WOAH subtypes have been circulating in Vietnam between 2024 and early 2025. The H1N1 and H3N2 SIV subtypes are predominant, suggesting a potential threat to human health. It remains uncertain whether, through further adaptation and reassortment, these viruses could evolve into strains capable of efficient transmission among human populations in the future. Given the significant public health risk posed by these circulating SIVs, ongoing surveillance and genetic characterization are crucial for informing vaccine strain selection and updating control and prevention strategies. Limitations of this studyThis study only investigated samples from the upper respiratory tract (nasal swabs) of healthy pigs, without considering samples from unhealthy pigs or the lower respiratory tract. Furthermore, this study only focuses on the analysis of HA and NA genes of influenza viruses. In the future, our groups will explore the large-scale research of samples in other provinces of North Center of Vietnam and more analysis of whole-genome sequencing of SIVs circulating to contribute of a full understanding the genetically of SIVs circulating in this region. AcknowledgmentsThis work was supported by the Sustainable Food Systems Ireland Project No. VIPFAST-NIVR-18012024), Ministry for Agriculture, Food and the Marine, Republic of Ireland. Conflict of interestThere are no potential conflicts of interest to declare. Data availability statementAll sequences generated in this study were submitted to GenBank under accession nos. PV786625-36 for HA genes and PV786613-24 for NA genes. Authors’ contributionsADT, HTTT, and HVD conceived and designed the experiments. ADT, HTTT, DLK, HVD, CTN, HMN, UTN, LTN, KVL, and VNT performed the experiments. ADT, HTTT, and HVD analyzed the data. ADT, HTTT, and HVD contributed reagents, materials, and analytical tools. ADT, HTTT, and HVD wrote the manuscript. All authors have read and approved the final version of the manuscript. ReferencesBansal, N.K., Sah, S., Shabil, M., Verma, A. and Ndabashinze, R. 2024. First human infection with the influenza A(H1N1) variant virus in Vietnam. New Microbes New Infect. 62, 101519. Baudon, E., Peyre, M., Peiris, M. and Cowling, B.J. 2017. Epidemiological features of influenza circulation in swine populations: a systematic review and meta-analysis. PLoS One 12 (6), e0179044. Baudon, E., Poon, L.L., Dao, T.D., Pham, N.T., Cowling, B.J., Peyre, M., Nguyen, K.V. and Peiris, M. 2015. Detection of novel reassortant influenza A (H3N2) and H1N1 2009 pandemic viruses in swine in Hanoi, Vietnam. Zoonoses Public Health 62(6), 429–434. Burke, D.F. and Smith, D.J. 2014. A recommended numbering scheme for influenza A HA subtypes. PLoS One 9, e112302. Chang, Y.J., Yeh, C.Y., Cheng, J.C., Huang, Y.Q., Hsu, K.C., Lin, Y.F. and Lu, C.H. 2021. Potent sialic acid inhibitors that target influenza A virus hemagglutinin. Sci. Rep. 11, 8637. Cheung, J., Bui, A.N., Younas, S., Edwards, K.M., Nguyen, H.Q., Pham, N.T., Bui, V.N., Peiris, M. and Dhanasekaran, V. 2023. Long-term epidemiology and evolution of swine influenza viruses, Vietnam. Emerg. Infect. Dis. 29, 1397–1406. Cisneros, R.L., Gibson, F.C. 3rd and Tzianabos, A.O. 1996. Passive transfer of poly-(1-6)-beta-glucotriosyl-(1-3)-beta-glucopyranose glucan protection against lethal infection in an animal model of intra-abdominal sepsis. Infect. Immun. 64, 2201–2205. Coker, R.J., Hunter, B.M., Rudge, J.W., Liverani, M. and Hanvoravongchai, P. 2011. Emerging infectious diseases in southeast Asia: regional challenges to control. Lancet (London, England) 377, 599–609. Comas, V., Moratorio, G., Sonora, M., Goni, N., Pereyra, S., Ifran, S., Moreno, P. and Cristina, J. 2015. Phylogenetic analysis of the neuraminidase gene of pandemic H1N1 influenza A virus circulating in the South American region. Virus Res. 197, 1–7. Dea, S., Gagnon, C.A., Mardassi, H., Pirzadeh, B. and Rogan, D. 1999. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: comparison of the North American and European isolates. Arch. Virol. 145(4), 659–688. Decker, C.H., Rapier-Sharman, N. and Pickett, B.E. 2022. Mutation in Hemagglutinin Antigenic Sites in Influenza A pH1N1 Viruses from 2015 to 2019 in the United States Mountain West, Europe, and the Northern Hemisphere. Genes (Basel) 13(5), 909. Guo, C.Y., Zhang, H.X., Zhang, J.J., Sun, L.J., Li, H.J., Liang, D.Y., Feng, Q., Li, Y., Feng, Y.M., Xie, X. and Hu, J. 2019. Localization analysis of heterophilic antigen epitopes of H1N1 influenza virus hemagglutinin. Virol. Sin. 34, 306–314. Hoffmann, E., Stech, J., Guan, Y., Webster, R.G. and Perez, D.R. 2001. Universal primer set for full-length amplification of all influenza A viruses. Arch. Virol. 146, 2275–2289. Huang, P., Yu, S., Wu, C. and Liang, L. 2013. Highly conserved antigenic epitope regions of hemagglutinin and neuraminidase genes between 2009 H1N1 and seasonal H1N1 influenza: vaccine considerations. J. Transl. Med. 11, 47. Iba, Y., Fujii, Y., Ohshima, N., Sumida, T., Kubota-Koketsu, R., Ikeda, M., Wakiyama, M., Shirouzu, M., Okada, J., Okuno, Y., Kurosawa, Y. and Yokoyama, S. 2014. Conserved neutralizing epitope at the globular head of hemagglutinin in H3N2 influenza viruses. J. Virol. 88, 7130–7144. Janke, B.H. 2013. Clinicopathological features of swine influenza. Curr. Top. Microbiol. Immunol. 370, 69–83. Kumar, S., Stecher, G. and Tamura, K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. Mak, P.W.Y., Wong, C.K.S., Li, O.T.W., Chan, K.H., Cheung, C.L., Ma, E.S., Webby, R.J., Guan, Y., Peiris, J.S.M. and Poon, L.L.M. 2011. Rapid genotyping of swine influenza viruses. Emerg. Infect. Dis. 17, 691–694. Munoz-Medina, J.E., Sanchez-Vallejo, C.J., Mendez-Tenorio, A., Monroy-Munoz, I.E., Angeles-Martinez, J., Santos Coy-Arechavaleta, A., Santacruz-Tinoco, C.E., Gonzalez-Ibarra, J., Anguiano-Hernandez, Y.M., Gonzalez-Bonilla, C.R., Ramon-Gallegos, E. and Diaz-Quinonez, J.A., 2015. In silico identification of highly conserved epitopes of influenza A H1N1, H2N2, H3N2, and H5N1 with diagnostic and vaccination potential. BioMed Res. Int. 2015, 813047. Nelson, M.I., Stratton, J., Killian, M.L., Janas-Martindale, A. and Vincent, A.L. 2015. Continual reintroduction of human pandemic H1N1 influenza A viruses into swine in the United States, 2009 to 2014. J. Virol. 89, 6218–6226. Nguyen, H.K.L., Nguyen, P.T.K., Nguyen, T.C., Hoang, P.V.M., Le, T.T., Vuong, C.D., Nguyen, A.P., Tran, L.T.T., Nguyen, B.G. and Lê, M.Q. 2015. Virological characterization of influenza H1N1pdm09 in Vietnam, 2010-2013. Influenza Other Respir. Viruses 9, 216–224. Ozawa, M., Matsuu, A., Yonezawa, K., Igarashi, M., Okuya, K., Kawabata, T., Ito, K., Tsukiyama-Kohara, K., Taneno, A. and Deguchi, E. 2015. Efficient isolation of swine influenza viruses by age-targeted specimen collection. J. Clin. Microbiol. 53(4), 1331–1338. Pomorska-Mol, M., Dors, A., Kwit, K., Czyzewska-Dors, E. and Pejsak, Z. 2017. Co-infection modulates inflammatory responses, clinical outcome and pathogen load of H1N1 swine influenza virus and Haemophilus parasuis infections in pigs. BMC Vet. Res. 13, 376. Reed, L.J. and Muench, H. 1938. A simple method for estimaing fifty percent endpoints. Am. J. Epidemiol. 27, 493-497. Richt, J.A., Lager, K.M., Clouser, D.F., Spackman, E., Suarez, D.L. and Yoon, K.J. 2004. Real-time reverse transcription-polymerase chain reaction assays for the detection and differentiation of North American swine influenza viruses. J. Vet. Diagn. Invest. 16, 367–373. Santos, J.J.S., Abente, E.J., Obadan, A.O., Thompson, A.J., Ferreri, L., Geiger, G., Gonzalez-Reiche, A.S., Lewis, N.S., Burke, D.F., Rajao, D.S., Paulson, J.C., Vincent, A.L. and Perez, D.R. 2019. Plasticity of amino acid residue 145 near the receptor binding site of H3 swine influenza A viruses and its impact on receptor binding and antibody recognition. J. Virol. 93(2), e01413–e01418. Shi, R., Zeng, J., Xu, L., Wang, F., Duan, X., Wang, Y., Wu, Z., Yu, D., Huang, Q., Yao, Y.G. and Yan, J. 2022. A combination vaccine against SARS-CoV-2 and H1N1 influenza based on receptor binding domain trimerize by six-helix bundle fusion core. EBioMedicine 85, 104297. Sun, X., Tse, L.V., Ferguson, A.D. and Whittaker, G.R. 2010. Modifications to the cleavage site of hemagglutinin control the virulence of a neurotropic H1N1 influenza virus. J. Virol. 84, 8683–8690. Takemae, N., Nguyen, T., Ngo, L.T., Hiromoto, Y., Uchida, Y., Pham, V.P., Kageyama, T., Kasuo, S., Shimada, S., Yamashita, Y., Goto, K., Kubo, H., Le, V.T., Van Vo, H., Do, H.T., Nguyen, D.H., Hayashi, T., Matsuu, A. and Saito, T. 2013. Antigenic variation of H1N1, H1N2, and H3N2 influenza viruses in Japan and Vietnam. Arch. Virol. 158, 859–876. Thomas, D.B., Hodgson, J., Riska, P.F. and Graham, C.M. 1990. The role of the endoplasmic reticulum in antigen processing. N-glycosylation of influenza hemagglutinin abrogates CD4+ cytotoxic T cell recognition of endogenously processed antigen. J. Immunol. 144, 2789–2794. Tran, H.T.T., Truong, A.D., Ly, D.V., Vu, T.H., Hoang, V.T., Nguyen, T.C., Chu, T.N., Nguyen, T.H., Pham, N.T., Nguyen, T., Yersin, A.G. and Dang, H.V. 2020. Genetic characterization of African swine fever virus in outbreaks in Ha Nam province, Red River Delta Region of Vietnam, and activity of antimicrobial products against virus infection in contaminated feed. J. Vet. Res. 64, 207–213. Trevennec, K., Cowling, B.J., Peyre, M., Baudon, E., Martineau, G.-P. and Roger, F. 2011a. Swine influenza surveillance in East and Southeast Asia: a systematic review. Rev. Anim. Health Res. 12(2), 213–223. Trevennec, K., Leger, L., Lyazrhi, F., Baudon, E., Cheung, C.Y., Roger, F., Peiris, M. and Garcia, J.M. 2011b. Transmission of pandemic influenza H1N1 (2009) in Vietnamese swine in 2009–2010. Influenza Other Respir. Viruses 6, 348–357. Vijaykrishna, D., Smith, G.J.D., Pybus, O.G., Vijaykrishna, D., Bhatt, S., Poon, L.L.M., Riley, S., Bahl, J., Ma, S.K., Cheung, C.L., Perera, R.A.P.M., Chen, H., Shortridge, K.F., Webby, R.J., Webster, R.G., Guan, Y. and Peiris, J.S.M. 2011. Long-term evolution and transmission dynamics of swine influenza A virus. Nature 473(7348), 519–522. Wan, H., Yang, H., Shore, D.A., Garten, R.J., Couzens, L., Gao, J., Jiang, L., Carney, P.J., Villanueva, J., Stevens, J. and Eichelberger, M.C. 2015. Structural characterization of a protective epitope spanning A(H1N1)pdm09 influenza virus neuraminidase monomers. Nat. Commun. 6, 6114. WOAH. 2018. Chapter 3.8.7. Influenza A virus of swine. In OIE terrestrial manual 2018. Ed., Brown, I. Paris: World Organisation for Animal Health, pp. 1954–1607. WOAH. 2022. Chapter 3.9.7. Influenza A virus of swine. In: Manual of diagnostic tests and vaccines for terrestrial animals. Ed., I. Brown. Paris: World Organisation for Animal Health, pp: 1–18. Yamashita, A., Kawashita, N., Kubota-Koketsu, R., Inoue, Y., Watanabe, Y., Ibrahim, M.S., Ideno, S., Yunoki, M., Okuno, Y., Takagi, T., Yasunaga, T. and Ikuta, K. 2010. Highly conserved sequences for human neutralization epitope on hemagglutinin of influenza A viruses H3N2, H1N1, and H5N1: implications for human monoclonal antibody recognition. Biochem. Biophys. Res. Commun. 393:614–618. Zhang, Z., Li, S., Zhu, X., Hou, J., Zhang, H., Zhao, B. and Tian, Z. 2022. Increased genetic variation of A(H3N2) virus from influenza surveillance at the end of the 2016/2017 season for Shanghai port, China. Sci. Rep. 12, 17089. Zhu, H., Webby, R., Lam, T.T.Y., Smith, D.K., Peiris, J.S.M. and Guan, Y. 2013. History of swine influenza viruses in Asia. Curr. Top. Microbiol. Immunol. 370, 57–68. | ||
| How to Cite this Article |
| Pubmed Style Truong AD, Tran HTT, Khac DL, Nguyen UT, Dang HV, Chu NT, Nguyen HM, Nguyen LT, Le KV, Dang HV. Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025. Open Vet. J.. 2025; 15(8): 3888-3898. doi:10.5455/OVJ.2025.v15.i8.55 Web Style Truong AD, Tran HTT, Khac DL, Nguyen UT, Dang HV, Chu NT, Nguyen HM, Nguyen LT, Le KV, Dang HV. Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025. https://www.openveterinaryjournal.com/?mno=252893 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.55 AMA (American Medical Association) Style Truong AD, Tran HTT, Khac DL, Nguyen UT, Dang HV, Chu NT, Nguyen HM, Nguyen LT, Le KV, Dang HV. Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025. Open Vet. J.. 2025; 15(8): 3888-3898. doi:10.5455/OVJ.2025.v15.i8.55 Vancouver/ICMJE Style Truong AD, Tran HTT, Khac DL, Nguyen UT, Dang HV, Chu NT, Nguyen HM, Nguyen LT, Le KV, Dang HV. Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3888-3898. doi:10.5455/OVJ.2025.v15.i8.55 Harvard Style Truong, A. D., Tran, . H. T. T., Khac, . D. L., Nguyen, . U. T., Dang, . H. V., Chu, . N. T., Nguyen, . H. M., Nguyen, . L. T., Le, . K. V. & Dang, . H. V. (2025) Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025. Open Vet. J., 15 (8), 3888-3898. doi:10.5455/OVJ.2025.v15.i8.55 Turabian Style Truong, Anh Duc, Ha Thi Thanh Tran, Duy Le Khac, Uyen Trung Nguyen, Hiep Van Dang, Nhu Thi Chu, Hieu Minh Nguyen, Linh Thi Nguyen, Kien Van Le, and Hoang Vu Dang. 2025. Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025. Open Veterinary Journal, 15 (8), 3888-3898. doi:10.5455/OVJ.2025.v15.i8.55 Chicago Style Truong, Anh Duc, Ha Thi Thanh Tran, Duy Le Khac, Uyen Trung Nguyen, Hiep Van Dang, Nhu Thi Chu, Hieu Minh Nguyen, Linh Thi Nguyen, Kien Van Le, and Hoang Vu Dang. "Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025." Open Veterinary Journal 15 (2025), 3888-3898. doi:10.5455/OVJ.2025.v15.i8.55 MLA (The Modern Language Association) Style Truong, Anh Duc, Ha Thi Thanh Tran, Duy Le Khac, Uyen Trung Nguyen, Hiep Van Dang, Nhu Thi Chu, Hieu Minh Nguyen, Linh Thi Nguyen, Kien Van Le, and Hoang Vu Dang. "Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025." Open Veterinary Journal 15.8 (2025), 3888-3898. Print. doi:10.5455/OVJ.2025.v15.i8.55 APA (American Psychological Association) Style Truong, A. D., Tran, . H. T. T., Khac, . D. L., Nguyen, . U. T., Dang, . H. V., Chu, . N. T., Nguyen, . H. M., Nguyen, . L. T., Le, . K. V. & Dang, . H. V. (2025) Phylogenetic analysis of swine influenza viruses circulating in slaughterhouses in Thanh Hoa province, Vietnam, during 2024 and early 2025. Open Veterinary Journal, 15 (8), 3888-3898. doi:10.5455/OVJ.2025.v15.i8.55 |