| Research Article | ||
Open Vet. J.. 2025; 15(9): 4505-4519
Open Veterinary Journal, (2025), Vol. 15(9): 4505-4519 Research Article The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytesRimayanti Rimayanti1*, Sri Pantja Madyawati1, Fedik Abdul Rantam2, Aswin Rafif Khairullah3, Widjiati Widjiati4, Pudji Srianto1, Adeyinka Oye Akintunde5, Imam Mustofa1, Muhammad Fajar Amrullah6, Laily ‘Ulya Nurul ‘Ilmi7, Riza Zainuddin Ahmad3 and Siti Rani Ayuti81Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia 2Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 4Division of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria 6Faculty of Animal Science, Universitas Hasanuddin, Makassar, Indonesia 7Department of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram, Indonesia 8Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia *Corresponding Author: Rimayanti Rimayanti. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: rimayanti [at] fkh.unair.ac.id Submitted: 12/04/2025 Revised: 22/07/2025 Accepted: 10/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
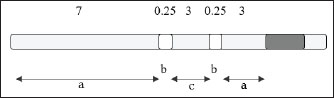
AbstractBackground: The application of biotechnology in the field of animal husbandry in Indonesia is continuously being developed to increase livestock productivity and reproductivity. Embryo transfer technology is closely related to in vitro fertilization (IVF) because IVF is used to produce high-efficiency embryos. Aim: This study aims to demonstrate that heat shock protein 70 (HSP70) plays a role in providing cell protection against temperature stress during oocyte vitrification. Methods: This experimental laboratory research was conducted divided into three research stages: The first stage is a descriptive study aimed at describing the presence of HSP70 expression in bovine oocytes. The second stage is an examination of cytochrome c expression and caspase 3 expression in fresh oocytes, post-in vitro maturation (IVM) oocytes, and post-thawing oocytes. The third stage is an examination of apoptosis using the Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling method to determine the occurrence of apoptosis in fresh oocytes, post-IVM oocytes, and post-thawing oocytes. Results: Post-IVM oocytes showed a decrease in the percentage of oocytes with normal morphology, followed by an increase in apoptosis, HSP70, Cyt-c, and caspase-3 expression. The addition of HSP70 to the vitrification medium resulted in the highest percentage of apoptotic oocytes. The percentage of oocytes with normal morphology and undergoing apoptosis was not linear with the expression of Cyt-c and caspase 9. Conclusion: HSP70 plays a role in providing cell protection against temperature stress during oocyte vitrification. HSP70 expression is observed in fresh oocytes, post-IVM oocytes, and post-vitrification oocytes without HSP70 supplementation. Cytochrome c expression in various oocyte preparations Keywords: Apoptosis, Bovine oocyte, Caspase 3, Cytochrome-c. IntroductionBiotechnology applications in the livestock sector in Indonesia are being developed to increase livestock productivity and reproductivity (Said, 2020). The use of biotechnology to improve crops and livestock is one of the agribusiness development programs (Khan et al., 2017). The development of reproductive biotechnology in the field of animal husbandry through artificial insemination and embryo transfer will provide the advantage of obtaining superior offspring and shortening the generation interval so that the genetic quality of livestock can be improved more quickly (Crowe et al., 2021). Embryo transfer depends on in vitro fertilization (IVF) to efficiently produce embryos, thereby reducing costs (D’Angelo et al., 2022). In human IVF programs, embryo cryopreservation has been widely performed with satisfactory success rates (Lee et al., 2019). However, frozen embryos create new problems because freezing embryos is considered unethical and is prohibited in several countries due to the presence of remaining embryos (Kamerlin, 2024). The advantages of IVF embryos have become a topic of thought for scientists to develop toward stem cell production (Kushnir et al., 2022). The cryopreservation of oocytes for human IVF programs is related to cost savings and the availability of oocytes in case of IVF program failure. In addition, the provision of oocytes for mothers who postpone pregnancy, as well as storage of oocytes before a woman undergoes therapy or chemotherapy, can result in oocytes losing their function or even damage to the oocytes (Resetkova et al., 2013). In the development of reproductive biotechnology, especially for the benefit of livestock, various programs and research have been conducted on embryo freezing (Hernandez Gifford and Gifford, 2013; Fernández et al., 2021). However, embryo freezing has been developed using the vitrification method, which is more efficient, simpler, and cheaper because it does not require a long time in the freezing procedure, besides not using expensive equipment like the freezing methods that have been carried out previously (Rezazadeh Valojerdi et al., 2009). Several research results on cow oocyte cryopreservation using vitrification techniques show a fairly high viability rate of around 80%–90%, but it turns out that its developmental competence is very disturbed (Hwang and Hochi, 2014; Tharasanit and Thuwanut, 2021; Ledwaba et al., 2025). The average fertilization and division rates are only 30% and 7.4%, respectively. Mavrides and Morroll (2002) showed that the survival rate of the vitrification method was 90.5% and that of slow freezing was 54.4%, but the success rate after IVF was still relatively low. Cleavage rates after IVF on fresh oocytes were 49.5%, with slow freezing 15.4%, and with vitrification 25.8%. Meanwhile, Gómez-Guzmán et al. (2024) conducted vitrification of bovine oocytes with three different rewarming stages, and the results showed that the viability of vitrified oocytes was 83%–91% but the penetration percentage was only around 7%–16%, and cleavage rates were 3%–13%. Cryopreservation procedures can cause damage due to DNA fragmentation and transcription of apoptosis-related genes, thereby reducing the ability of oocytes or embryos to develop after freezing and thawing (Tamburrino et al., 2023). The presence of DNA fragmentation and apoptosis-related gene transcription are biochemical marker of apoptosis. In addition to these, visible cytoplasmic condensation, cytoplasmic fragmentation, and the formation of apoptotic bodies are also characteristic features (Elmore, 2007). Damage that occurs due to temperature changes from hot – cold – reheating in the vitrification process series, namely in vitro maturation, vitrification, and especially rewarming or thawing, requires attention to the possibility of damage to cell organelles and cell protein structures (Jia et al., 2020). The concept of damage to DNA and cell protein structures that will greatly affect oocyte viability, both after vitrification, during IVF, and during the embryonic growth and development phase, provides an opportunity to explain the mechanism of cell protection against temperature stress in relation to the varying quality of vitrified oocytes (Chang et al., 2019). Extreme temperature changes cause oocytes to experience stress (Gómez-Guzmán et al., 2024). Stressed cells will increase the need for heat shock protein 70 (HSP70) (Stamperna et al., 2021a). The induced response of HSP70 is considered a protective mechanism. The role of HSP70 in cell protection in oocytes that experience extreme temperature changes during the vitrification process must be studied by providing HSP70 supplementation into vitrification cryoprotectants. The viability of vitrified oocytes determined based on their normal morphology is quite high, but these oocytes do not necessarily have good quality to be able to carry out the fertilization process for IVF programs, grow, and develop. This indicates a variation in the quality of vitrified oocytes that is not yet satisfactory. This study aims to prove that HSP70 plays a role in providing cell protection against temperature stress during oocyte vitrification. Materials and MethodsResearch designThis research was conducted at the In Vitro Fertilization Laboratory and the Veterinary Pathology Laboratory, Faculty of Veterinary Medicine, Airlangga University, Indonesia. The research implementation period lasted for 12 months from February 2023 to January 2024. This research is an experimental laboratory research carried out divided into three research stages. The first stage is a descriptive study that aims to describe the presence of HSP70 expression in bovine oocytes by examining HSP70 expression in fresh oocytes, post-in vitro maturation (IVM) oocytes, and post-thawing oocytes without HSP70 supplementation using the immunofluorescence method to determine the occurrence of HSP70 induction in oocytes. The second stage is the examination of cytochrome c expression and caspase 3 expression in fresh oocytes, post-IVM oocytes, and post-thawing oocytes with various doses of HSP70 supplementation using the immunofluorescence method to determine the expression of cytochrome c and caspase 3 expression in oocytes. The research design used was the post-test-only control group design. The third stage is the examination of apoptosis using the Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling (TUNEL) method to see the occurrence of apoptosis in fresh oocytes, post-IVM oocytes, and post-thawing oocytes with various doses of HSP70 supplementation. The research design used was the post-test-only control group design. Ovary collection from the slaughterhouseFresh ovaries were taken from the Pegirian Slaughterhouse Surabaya, with a total of 20–25 ovaries each time. The obtained ovaries were washed with 0.9% NaCl solution or physiological saline that had been added with 50 µg/ml of gentamycin sulfate antibiotic. Furthermore, the ovaries were taken to the laboratory in a thermos containing physiological saline solution at a temperature of 36°C–37°C for a maximum of 5 hours after collection. Oocyte aspiration and oocyte observationBefore performing oocyte aspiration, the floor and work table were first de-pestified using 70% alcohol or organic antiseptic solution. The ovaries are washed 2–3 times with physiological saline solution at 38°C, then placed in a baker’s glass and stored in a water bath at 38°C. 1 The ovary was taken with sterile tweezers, and the surface was dried with sterile tissue. Next, the syringe was filled with 1–1.5 ml of phosphate-buffered saline (PBS) + media using an 18 Gauge syringe needle inserted into the ovarian parenchyma near the follicle. Follicles with a diameter of 3–6 mm are performed near the needle puncture point (without removing the needle first). After all the follicle fluid has been sucked or when the syringe contents have reached 3–4 ml, the fluid is transferred into a sterile test tube and stored in a water bath. When sucking fluid or transferring it to a cup, it must be done carefully so that no damage occurs to the cumulus. The aspiration fluid was poured from the test tube into a 90-mm diameter Petri dish and then examined under a microscope. If the oocyte can be found, it is taken with a Pasteur pipette with a tip diameter equal to the oocyte diameter. The oocyte is placed in a small 35 mm diameter Petri dish containing PBS+ media to be washed three times, then washed again in tissue culture medium (TCM) 199 three times. Furthermore, it is examined under a microscope, and immature oocytes are taken, which are in phase 3, namely complex oocytes with cumulus oophorus and compact corona radiata; the oocyte appears faint (Turathum et al., 2021). Then, the in vitro maturation process will be carried out on this oocyte. IVMAfter being washed in TCM 199 medium three times in a row, the oocytes were transferred into TCM 199 maturation medium supplemented with 3% bovine serum albumin (BSA), 0.01 mg/ml follicle-stimulating hormone, 5 µg/ml luteinizing hormone, and 50 µg/ml gentamicin sulfate. Each 50 µl drop of medium was filled with 30–35 oocytes, then matured for 22 hours in an incubator containing 5% CO2 at 38.5°C. After maturation, the oocytes were examined under a microscope to determine the stage of maturation. Oocyte vitrificationAfter maturation for 22 hours, the mature oocytes were transferred to a petri dish containing vitrification medium with various doses of HSP70 supplementation in cryoprotectants, namely P0: Ethylene Glycol + Sucrose (EG+S) cryoprotectant, T1: EG+S+HSP70 cryoprotectant 0.25 µg/ml, T2: EG+S+HSP70 cryoprotectant 0.5 µg/ml, T3: EG+S+HSP70 cryoprotectant 1.0 µg/ml, and T4: EG+S+HSP70 cryoprotectant 2.5 µg/ml. Furthermore, the oocytes in this cryoprotectant were exposed to room temperature, with an exposure time of 20 minutes. After exposure, the oocytes are ready to be packed in a 0.25 cc transparent ministra (French straw) that has undergone open-pulled straw or straw-pulling technique so that it is elongated and narrowed, and its walls become thinner, for the vitrification process. The packaging method is as follows: first, 1 M sucrose diluent is aspirated into a 3-cm straw, then a 0.25-cm air cavity is created. A total of 6–16 oocytes in the cryoprotectant were taken from the Petri dish and then aspirated 3 cm, after which they were separated again with a 0.25 cm air cavity. The remaining cavity was filled with 1 M sucrose diluent in PBS (Fig. 1). The ministraw is immediately dipped into liquid nitrogen after packaging. During immersion/plunging, the color of the sucrose diluent solution changes to milk, while the vitrification medium remains transparent.
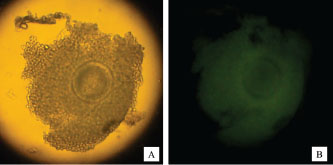
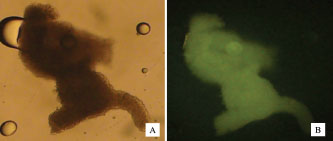
Fig. 1. Scheme of oocyte packaging in straw for vitrification (Supriatna and Pasaribu, 1992). Description (units in cm). a : diluent (1 M sucrose in PBS). b : air cavity. c : oocytes in vitrification medium. Thawing of the cryoprotectantsAfter being frozen and stored for 2–4 weeks, it is thawed again by placing the ministra in a 30°C water bath until the ice crystals in the diluent disappear, then the entire contents of the ministra are removed into a petri dish. Cryoprotectant flushOocytes that had been removed from the ministra were rinsed 2× with 0.5 M sucrose to remove cryoprotectants, as per the procedure described by Fabbri et al. (2001). Examination of oocyte morphologyA total of 20–25 oocytes were collected and morphological quality was evaluated under a microscope. After undergoing cryoprotectant flushing, oocytes were examined under an inverted microscope to observe their morphology with 200× and 400× magnification. The quality of oocytes after re-thawing and cryoprotectant flushing was determined based on the following normal morphological assessments: intact plasma membrane, homogeneous granulated ooplasm, and clearly demarcated zona pellucida and ooplasm. Abnormal oocyte morphology showed irregular shapes and degeneration with dark and fragmented ooplasm. Examination of HSP70, cytochrome c, and caspase 3 expression by immunofluorescenceFor the immunofluorescence analysis, the following primary antibodies were used: anti-HSP70 (rabbit polyclonal, Abcam, Cat# ab79852) at a dilution of 1:200, anti-cytochrome c (mouse monoclonal, Santa Cruz Biotechnology, Cat# sc-13156) at 1:100, and anti-cleaved caspase-3 (rabbit monoclonal, Cell Signaling Technology, Cat# 9661) at 1:250. Alexa Fluor® 488-conjugated goat anti-rabbit IgG (Thermo Fisher Scientific, Cat# A-11008) and Alexa Fluor® 594-conjugated goat anti-mouse IgG (Thermo Fisher Scientific, Cat# A-11005) were used as secondary antibodies at a dilution of 1:500. All antibodies were diluted in blocking buffer (PBS containing 1% BSA), and incubations were performed according to standard immunofluorescence protocols. Examination of HSP70 expression in oocyte organelles was examined using the immunofluorescent method (Stamperna et al., 2021a). Oocytes were placed on a glass object coated with poly-L-lysine and covered with vaseline on both sides. Oocytes were fixed with 10% formalin to stick to the glass object (20°C for 15 minutes), then washed with PBS containing 1% fetal calf serum and left for 15 minutes. After that, rinse with PBS for 5 minutes. The next step is coating with primary antibodies (HSP70, cytochrome c, and caspase 3 antibodies). Conjugate 1: 100 µl was dropped as much as 20 µl was dropped on the glass object after that it was placed in a box and wet paper. Incubated at 37°C for 45 minutes. Then, it was washed with PBS for 5 minutes. Fluorescein Isothiocyanate (FITC)-labeled secondary antibody was added and incubated at 37°C for 45 minutes in a box and wet paper, then washed with PBS for 5 minutes. Immunofluorescence results were examined using a fluorescence microscope at 400× magnification. DNA fragmentation examination using the TUNNEL methodThe Biovison Apo-BrdU-IHCTM In Situ DNA Fragmentation Assay Kit was used in this study, with the procedure as described below. Oocytes on the glass object were washed with PBS (pH 7.4) and incubated with permeability cell buffer and Proteinase K for 20 minutes at room temperature. The mixture was washed once with PBS (pH 7.4), incubated in 3% H2O2 at room temperature for 5 minutes, and then washed once with PBS (pH 7.4). The mixture was then incubated with Reaction Buffer at room temperature for 15 min. After incubation with Tunel fragmented DNA labeling for 60 min at 37°C, the samples were washed once with PBS (pH 7.4). Blocking Buffer was incubated for 10 minutes at room temperature, then dripped with antibody solution and incubated in a dark container for 1 hour at room temperature. After that, it was washed with PBS once, dripped with blocking buffer, given conjugate, and incubated for 30 minutes at room temperature. Washed with PBS once, then dripped with substrate for Peroxidase (Diamino Benzidine) for 15 minutes at room temperature. Continued by washing with H2O. Counterstaining was done with Methyl Green for 3 minutes and then washed with dH2O. Furthermore, the staining was observed under an inverted microscope with magnifications of 200× and 400×. The presence of brown staining in the oocyte nucleus, indicating DNA fragmentation detected by the TUNEL assay, identified apoptotic oocytes. TUNEL-positive oocytes were quantified manually under a microscope at 400× magnification. A total of 20–25 oocytes were examined, and the percentage of apoptotic oocytes was calculated by dividing the number of TUNEL-positive oocytes by the total number of oocytes observed. Data analysisThe data obtained in the first and second phases of the study were statistically analyzed using Fisher’s exact test. Meanwhile, the data obtained from the third phase of the study were statistically analyzed using the multivariate analysis of ANOVA (Manova) test followed by Hotelling’s Trace. The results of the hypothesis test are significant if the p value is ≤ 0.05. Ethical approvalNot needed for this study. The use of tissue samples obtained from a local slaughterhouse does not require ethical approval, as no live animals were involved in the experimental procedures in accordance with institutional and national guidelines. ResultsDescriptively, post-IVM oocytes showed a decrease in the percentage of oocytes with normal morphology from 100.00% to 84.39%, followed by an increase in the percentage of apoptosis from 7.22% to 9.22% (Table 1), HSP70, Cyt-c, and caspase-3 expression (Table 2). Figure 2 shows normal oocyte morphology, and Figure 3 shows a degenerated oocyte.
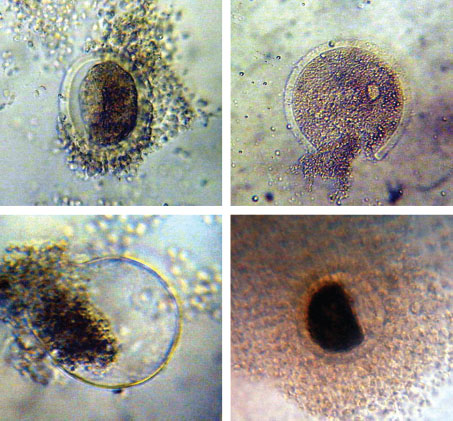
Fig. 2. Normal morphology of oocytes after vitrification.
Fig. 3. Some forms of oocytes that undergo degeneration after vitrification. Table 1. Percentage of normal morphology and apoptosis in fresh oocytes (before) and after in vitro maturation.
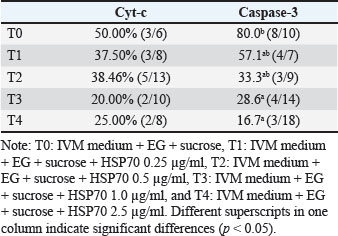
Table 2. Expression of HSP70+, Cyt-c, and Caspase 3 in fresh oocytes (before) and after in vitro maturation.
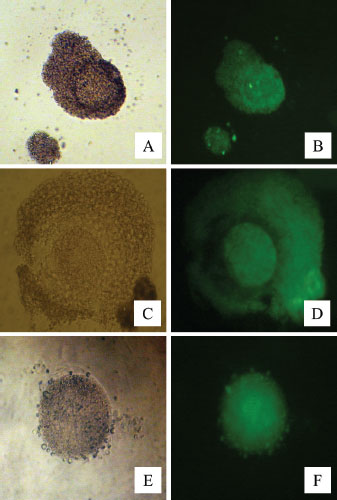
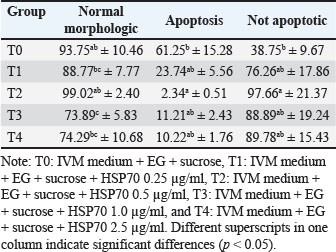
Figure 4 shows HSP70 expression in fresh oocytes, post-IVM oocytes, and post-vitrification oocytes. Without the addition of HSP70 in the vitrification medium, the percentage of oocytes undergoing apoptosis was the highest (Table 3). The addition of 0.5 µg/ml HSP70 (T2 group) resulted in the highest percentage of oocytes with normal morphology (99.02% ± 2.40%) and the lowest percentage of apoptotic oocytes (2.34% ± 0.51%), with both differences being statistically significant (p < 0.05) compared to the other treatment groups. Figure 5 shows the expression of cytochrome c in oocytes using the immunofluorescence method with the FITC marker. Figure 6 shows the expression of caspase-3 in oocytes by immunofluorescence using the FITC marker.
Fig. 4. HSP70 expression in fresh oocytes (A,B), post-IVM oocytes (C,D), and post-vitrification oocytes (E,F) by immunofluorescence using FITC marker. Normal (A,C, and E) and fluorescent (B, D, and F) light.
Fig. 5. Cytochrome c expression in oocytes by immunofluorescence with FITC marker Normal (A) and fluorescence (B) light.
Fig. 6. Caspase-3 expression in oocytes by immunofluorescence using FITC marker Normal (A) and fluorescent (B) light. Table 3. Percentage of normal morphology and apoptosis of post-thawed oocytes vitrified in medium with the addition of several doses of HSP70+.
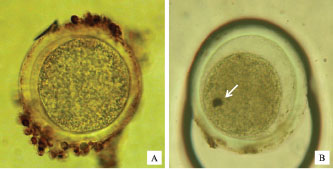
Figure 7 shows oocytes that do not undergo apoptosis, oocytes that undergo apoptosis, and DNA fragmentation in the nucleus. The percentage of oocytes undergoing apoptosis and those with normal morphology does not follow a linear trend with the expression levels of Cyt-c and Caspase-9. Interestingly, the lowest Cyt-c expression was observed in the group supplemented with 1.0 µg/ml HSP70, despite not having the lowest apoptosis percentage. This nonlinearity may be due to the complex regulation of the intrinsic apoptotic pathway, in which Cyt-c release is tightly controlled by mitochondrial membrane stability, reactive oxygen species (ROS) levels, and the balance between pro- and anti-apoptotic proteins. At certain HSP70 concentrations, protective mechanisms such as enhanced mitochondrial integrity or stress protein interaction thresholds may be activated, leading to suppression of Cyt-c release without a proportional reduction in downstream apoptosis. In addition, post-translational modifications or feedback inhibition within the apoptotic cascade may contribute to this disconnect between early signaling (Cyt-c) and morphological outcomes. The percentage of cells with caspase-3 expression was found in IVM+ethylene glycol+BSA+HSP70 2.5 µg/ml medium (Table 4).
Fig. 7. Non-apoptotic oocytes (A), apoptotic oocytes (B), DNA fragmentation in the nucleus (arrow) using the TUNEL method. Table 4. Cyt-c and Caspase 3 expression in post-thawed oocytes vitrified in medium with the addition of several doses of HSP70+.
DiscussionFreezing oocytes is a challenge for cryobiologists due to their complex structure (Brambillasca et al., 2013). The large size, presence of spindles in metaphase, and specific cytoplasm in each species pose unique problems in freezing and thawing oocytes (Blengini and Schindler, 2022). Optimal oocyte vitrification conditions vary across species due to differences in cell characteristics, such as size, shape, membrane properties, and sensitivity to cryoprotectants (Caliskan et al., 2024). Vitrification is still rarely applied due to the fact that the most appropriate packaging or protocol for placing cells during the vitrification process has not been obtained, in addition to the fact that the most appropriate cryoprotectant formulation has not been obtained because until now research is still being conducted and various types of cryoprotectants are being formulated for vitrification (Kattera and Chen, 2006). HSP70 expressionHSP70 expression was detected in both fresh, post-IVM, and post-vitrification oocytes. In oocytes, HSP70 is synthesized during growth in the germinal vesicle (GV) stage and maturation until the metaphase II stage; therefore, HSP70 expression can be observed in all three oocyte conditions after immunofluorescence examination (Le Masson and Christians, 2011). As stated by Lánská et al. (2006), during the growth period, oocytes with a size of 80–99 μm synthesize HSP70 even though it is not triggered by temperature stress conditions but has the ability to increase HSP70 synthesis when experiencing heat stress for 1 hour. Likewise, during the oocyte growth period with a size of 100–115 μm, the oocyte continues to synthesize HSP70 but no longer synthesizes HSP70 when experiencing heat stress. Meanwhile, as added by Stamperna et al. (2021b), large amounts of HSP70 can be found in mature oocytes at the end of growth, but the HSP70 content does not increase when temperature stress occurs. The ability to synthesize HSP70 decreases at the end of oocyte growth, and oocytes no longer induce HSP70 synthesis under temperature stress. HSP70 is synthesized and stored during oocyte growth. Lánská et al. (2006) concluded that HSP70 plays an important role in oocyte growth and maturation. The results of this study indicate no difference in HSP70 expression between post-IVM and post-vitrification oocytes. Although both groups of oocytes were exposed to procedures that potentially induce temperature-related stress, such treatments did not sufficiently stimulate endogenous HSP70 synthesis. The description of HSP70 expression in the vitrification process series is in accordance with the results of Turathum et al.’s (2010) research on dog oocytes. HSP70 expression was not significantly different between fresh and post-vitrification oocytes. Kabakov and Gabai (2021) stated that heat shock protein (HSP) can be classified into constitutive and inducible forms. The constitutive form of heat shock cognate (HSC) is HSP that is constantly synthesized without any stress stimulation, while the inducible form of HSC can only be synthesized when there is heat stimulation or other stress conditions (Stetler et al., 2010). One of the major limitations of this study is the lack of evaluation of embryo development after fertilization. Although the results showed improved oocyte morphology and decreased apoptosis after HSP70 supplementation, these parameters are insufficient to guarantee optimal developmental competence. Therefore, further research is needed to evaluate the developmental potential of embryos, including blastocyst formation and viability after embryo transfer. In terms of field applications, vitrification media formulations containing HSP70 have the potential to be applied in assisted reproductive technology (ART) in animals, especially in field conditions with limited laboratory facilities and frequent exposure to environmental stress. HSP70 supplementation can serve as a protective strategy to improve oocyte quality during cryopreservation and can be utilized in animal conservation programs, superior breeding, and gene banks to maintain long-term fertility (Stamperna et al., 2021a). Cytochrome C expressionBiomarkers are important for determining changes that occur at the cellular and molecular levels based on biochemical, histological, morphological, and physiological changes in organisms (Ahmad et al., 2023). The release of cytochrome c from mitochondria into the cytoplasm is studied as a biomarker of cell death or apoptosis in cells undergoing apoptosis (Abu-Qare and Abou-Donia, 2001). Mitochondria are organelles that provide energy for cells and are essential for cell development and life (Casanova et al., 2023). Mitochondria in oocytes can provide ATP for fertilization and embryo development. In addition, mitochondria store intracellular calcium and proapoptotic factors (Wang et al., 2009). Mitochondria are a part of the cell that plays an important role in initiating apoptosis. Several proteins located in the mitochondrial intermembrane space are released during the early stages of apoptosis (Wang and Youle, 2009). Some of these proteins include AIF, AK-2, Smac/DIABLO, and cytochrome c (Gupta et al., 2009). The release of cytochrome c is preceded by changes in the permeability of the cell membrane (Martinou et al., 1999). The Bcl2 family acts on the mitochondrial membrane, and although the mechanism of action is unknown, it is likely that Bcl-2 forms channels in the membrane (Shamas-Din et al., 2013). The mitochondrial membrane surface in normal cells expresses Bcl2. This Bcl-2 protein binds to the Apoptosis Protease Activating Factor (APAF-1) protein. When an apoptosis signal occurs, the bond between the Bcl-2 protein and APAF-1 and cytochrome c is released, and both proteins are transported to the cytosol (Qian et al., 2022). Cytochrome c and the APAF-1 protein bind to caspase 9 to form a complex with ATP called the apoptosome (Bratton and Salvesen, 2010). Caspase 9 then breaks down procaspase 3 to activate caspase 3, which is the effector stage of this apoptotic pathway. Caspase 3 can cause DNA fragmentation by breaking down the DNA Fragmentation Factor subunit, which then activates nuclease to degrade DNA (Elmore, 2007). Zhang and Wu (2023) showed that mitochondrial changes can indicate changes in oocyte quality, while mitochondrial dysfunction, such as structural and genetic abnormalities, will affect embryonic development. Galeati et al. (2011) observed changes in the ultrastructure of pig oocytes after vitrification and proved that mitochondrial and microtubule damage are factors in cryopreservation failure. The results showed that approximately 52.5% of oocytes experienced disruption in the location of microtubules and mitochondrial disorganization that affected the fertilization process and embryonic development. Disturbances that occur in mitochondria increase cell permeability to calcium, causing an increase in intracellular calcium, which activates hydrolytic enzymes, damages energy production, and ultimately causes cell death or apoptosis (Walkon et al., 2022). Cytochrome c expression did not differ significantly among all groups, including fresh oocytes, post-IVM oocytes, and post-vitrification oocytes supplemented with various doses of HSP70, although a trend toward variation was observed. This lack of significant change in Cyt-c expression, despite a reduction in apoptotic oocytes, suggests the involvement of compensatory mechanisms at other points in the intrinsic or extrinsic apoptotic pathways. For example, HSP70 inhibits apoptosis downstream of Cyt-c release by directly interfering with apoptosome formation or caspase-9 activation. Alternatively, HSP70 could exert its anti-apoptotic effects by stabilizing mitochondrial membranes, thereby reducing the actual release of Cyt-c into the cytosol, even if total Cyt-c expression remains unchanged. These possibilities indicate that apoptosis regulation in oocytes is multifactorial, and changes in cell fate may not always directly correlate with single-marker expression levels. The condition of post-vitrification oocytes at T0 showed a tendency for higher percentage cytochrome c expression compared to fresh oocytes and post-IVM oocytes, then decreased at T1, T2, T3, and T4. Caspase 3 expressionIn cells undergoing apoptosis, in addition to the release of cytochrome c from the mitochondria into the cytoplasm, the ongoing activation of caspase-3 is also a biomarker of cell death or apoptosis (Abu-Qare and Abou-Donia, 2001). As stated by Szyller and Bil-Lula (2021), if stress occurs in the physiological conditions of cells, such as hyperthermia, hypothermia, hypoxia, hyperoxia, viral infections, acidosis, ROS, energy loss, and ischemia-reperfusion, it will activate the inductive form of HSP70 and increase its expression in cells. However, Lánská et al. (2006) revealed that HSP70 can be found in large amounts in mature oocytes, but the HSP70 content does not increase when temperature stress occurs. The ability to synthesize HSP70 decreases at the end of oocyte growth, and oocytes no longer induce HSP70 synthesis when temperature stress occurs. According to Beere (2004), increased HSP synthesis inhibits apoptosis that occurs due to temperature changes as a thermotolerant activity. The inhibition of caspase 3 polymerase cleavage by HSP70 indicates that HSP blocks the apoptosis process in cells. In the results of this study, extreme temperature changes that occur in the vitrification process are thought to cause HSP70 to be stored and prepared by oocytes to protect cells from environmental influences to be insufficient to provide protection against temperature stress in the form of chilling and rewarming injury (hot - cold - hot), so that stored HSP70 in oocytes was insufficient to inhibit caspase 3 activation observed in the T0 group. This condition is thought to require HSP70 supplementation to inhibit the activation of caspase 3. HSP70 supplementation of as much as 0.25 µg/ml for T1 and 0.5 µg/ml for T2 can inhibit caspase 3 activation, although not significantly different from T0. The expression of caspase 3 in fresh oocytes, post-IVM oocytes, and post-vitrification oocytes with HSP70 supplementation of as much as 1.0 µg/ml for T3 and 2.5 µg/ml for T4 significantly differed from that in post-vitrification oocytes without HSP70 supplementation in cryoprotectants, so it is suspected to inhibit apoptosis. Oocyte morphologyThe morphological condition of oocytes after thawing or rewarming can be used as a basis for determining oocyte viability. Normal oocytes or those in good condition are seen from the normal shape, diameter, and size of cells, intact zona pellucida and oolemma, clear perivitelline space, and no visible ooplasmic degeneration (Nottola et al., 2011). According to Chi et al. (2002), EG is effectively used as a cryoprotectant for embryo cryopreservation and is also applied to oocyte cryopreservation. The low molecular weight of EG (62.07) provides a beneficial effect in the form of higher permeability. Some researchers also say that the advantage of EG as a cryoprotectant is due to its low toxicity. The use of EG as an intracellular cryoprotectant in vitrification media is believed to be a major factor in increasing the success of fertilization, as also expressed by Chian et al. (2014). In the EG+S+HSP70 0.25 µg/ml cryoprotectant formulation, the cryoprotectant concentration did not change much, but HSP70 was not sufficient to maintain the morphology of the oocytes so that they did not experience abnormalities. HSP70 supplementation given with the cryoprotectant formulation EG+S+HSP70 0.5 µg/ml did not change the permeability of the cell membrane because the hyperosmolar state was maintained for the viability of the oocytes after vitrification (Alvarez-Rodríguez et al., 2013). Meanwhile, HSP70 supplementation with the cryoprotectant formulation EG+S+HSP70 1.0 µg/ml and cryoprotectant EG+S+HSP70 2.5 µg/ml affects the condition of the oocytes due to changes in the concentration of cryoprotectants, thus facilitating the formation of intracellular ice crystals, which can damage the oocytes due to the cells not being in a hyperosmolar medium, which is one of the requirements for successful vitrification (Reddy et al., 2018). Concentration and type of cryoprotectant have an important influence on the development competence of vitrified oocytes. As proven by García-Martínez et al. (2021) demonstrated the use of EG cryoprotectants with various formulas and concentrations for oocyte vitrification. Yassin et al. (2022) showed that the use of 40% and 50% EG cryoprotectants had a better effect on the viability of mature bovine oocytes after the vitrification process than 20% and 30% EG. According to Chen et al. (2022), the speed of freezing and re-thawing, as well as the concentration of the cryprotectant, influence the success of vitrification. This radical vitrification technique is expected to eliminate intracellular ice crystal formation. Increasing the freezing rate reduces freezing damage, such as damage to intracellular lipid droplets, membrane lipids, and the cytoskeleton (Bouchnak et al., 2023). Mature oocytes are at phase 5 as classified by Gregory et al. (1994) showed complex oocytes with cumulus oophorus and loose corona radiata, quite slimy. The oocytes were clearly visible and already had polar bodies I, a sign that they had entered metaphase II. Dai et al. (2015) vitrified porcine oocytes in the Metaphase II (MII) phase and observed changes in microtubules, chromosomes, and mitochondria. The ultrastructural evaluation of oocytes showed an increase in spindle abnormalities, no microtubules, and irregular chromosome arrangement and decondensation, which can later affect embryonic development. Tamura et al. (2013) also stated that the main damage in MII oocyte vitrification is spindle disorganization followed by microtubule depolymerization. Madan et al. (2022) also provided an overview of mitochondrial distribution disorders in the cytoplasm and cytoskeleton of cells that can lead to cell shape changes. Cortical granule exocytosis and zona pellucida hardening can inhibit fertilization. Problems related to the zona pellucida are overcome by micromanipulation techniques such as intracytoplasmic sperm injection. In some embryos and oocytes, osmotic damage renders the embryos of some species unable to be cryopreserved efficiently at some developmental stages. This is especially true for oocytes, which are more sensitive to cryopreservation than embryos (Konc et al., 2014). Osmotic damage is thought to make vitrification of embryos or oocytes difficult to succeed because normal cells immediately lose their normal condition and degenerate after being returned to their original condition in isotonic solution. Cryopreserved cells are more sensitive to osmotic damage than fresh cells (Mullen et al., 2007). Apoptosis in oocytesThe results of this study indicate that the lowest average percentage of oocytes without apoptosis was obtained in vitrification with EG+S cryoprotectant without HSP70 supplementation. Despite a high percentage of normal morphology (93.75%), the apoptosis examination did not yield good results. This normal morphological condition, but apoptosis, is also shown in experiments conducted by Men et al. (2003) using vitrification techniques on cow oocytes, and Caspase 3 activity was observed to detect apoptosis events. The results of the research showed a degeneration mechanism in oocytes that underwent cryopreservation. This study evaluated the viability of most oocytes after freezing and thawing by observing their morphological condition. Degeneration occurred during subsequent culture, as indicated by the morphology of oocytes undergoing apoptosis, such as cytoplasmic condensation, cytoplasmic fragmentation, and apoptotic body formation. Biomarkers of apoptosis, such as DNA fragmentation and caspase activation, could be detected not only in oocytes with apoptotic conditions but also in oocytes with normal morphology. This experiment concluded that degeneration via apoptosis occurred in vitrified oocytes. HSP70 supplementation with EG+S+HSP70 0.5 µg/ml cryoprotectant formulation showed the highest average percentage of oocytes without apoptosis. HSP70 supplementation of 0.5 µg/ml is thought to be effective in providing protection to oocytes undergoing vitrification. The amount of HSP70 0.5 µg/ml does not change the concentration of the cryoprotectant much so that the hyperosmolarity condition is maintained for the viability of oocytes after vitrification to prevent the formation of intracellular ice crystals that can damage oocytes. In addition, the supplemented HSP70 is capable of providing protection against chilling and rewarming injury with extreme temperature changes that occur in the vitrification process. According to Liu et al. (2004), HSP accumulated or stored in the early stages of oocyte development, namely at the GV stage, is needed to protect the oocyte from environmental influences that occur in the ovary, and HSP is thought to be provided for zygote gene activation and protective effects against apoptosis. In the results of this study, extreme temperature changes that occur in the vitrification process are thought to cause HSP70 stored and prepared by oocytes to protect cells from environmental influences to be insufficient to provide protection against temperature stress in the form of chilling and rewarming injury (hot - cold - hot), so that HSP70 supplementation of 0.5 µg/ml in cryoprotectants can inhibit the occurrence of apoptosis. Hendrey and Kola (1991) conducted a study on mouse oocytes injected with HSP70 mRNA and observed the viability of these oocytes after heating. The number of viable oocytes was significantly higher in the group injected with HSP70 mRNA and then heated compared with oocytes injected with HSP70 antisense mRNA. The research conducted by these two researchers also showed that unfertilized oocytes are sensitive to excessive temperatures, but the oocytes did not induce HSP70 synthesis. Supplementation of HSP70 into vitrification cryoprotectants is thought to inhibit the occurrence of apoptosis in post-vitrification oocytes, but the precise mechanism by which this apoptosis inhibition process occurs has not been explained. Spinaci et al. (2005) demonstrated that HSPs play an important role in the fertilization and early development of mammalian embryos by demonstrating that the presence of anti-HSP70 antibodies significantly reduced the strong binding of spermatozoa to the zona pellucida of bovine oocytes and interrupted the achievement of meiosis II and pronuclear formation. The presence of anti-HSP70 in the culture medium on days 3–9 will increase the incidence of apoptosis and significantly reduce the number of embryos reaching the blastocyst stage. Thus, the reduction in embryo development is a response to HSP70 inhibition related to increased apoptosis due to temperature stress (Olexikova et al., 2010). The inhibitory effect of anti-HSP70 on sperm-oocyte interaction can occur at all stages of the fertilization process, from the beginning of initiation to the fusion of the spermatozoa plasma membrane with the oocyte (Rosyada et al., 2022). The mechanism of antibody inhibition against HSP70 has not been precisely explained, but it is suspected that anti-HSP70 receptors are present in the zona pellucida and plasma membrane of the embryo. Immunolabeling for the presence of anti-HSP70 showed that the antibody can penetrate the zona pellucida and membranes of living embryonic cells and cause intracellular effects (Leng et al., 2013). A cell can provide protection through repair and apoptosis mechanisms. For vitrified oocytes that cannot undergo repair, they will experience apoptosis or experience failure of fertilization, growth, and development, while those that are able to survive will continue to grow and develop (Elmore, 2007). According to Lanneau et al. (2008), HSPs can also control the apoptosis mechanism that occurs in these cells. Based on the statement from Vasaikar et al. (2015), the mechanism of the apoptosis inhibition process is suspected to be that HSP70 added to the vitrification medium in this study can interact with oocytes through the presence of HSP70 receptors in the zona pellucida and cause intracellular reactions and internalization. The existence of the HSP70 receptor (HSP70-R) is also explained by Calderwood et al. (2007), who reported that the effects of extracellular protein stress on cell signaling and immunity are mediated through receptors on the cell surface. In the circulatory system, HSP60 and HSP70 can enter the bloodstream through interactions with TLR receptors 2 and 4, CD40, CCR5, LOX-1, and SREC-1 (Calderwood et al., 2012). Similarly, Ferat-Osorio et al. (2014) explained that the HSP70 release mechanism can occur both actively and passively. The passive release of HSP70 occurs when there is a viral infection, trauma, or necrosis. While active release of HSP70 occurs when there is psychological stress, stimulants such as heat, ultraviolet light, radiation, and heavy metals cause stress, as well as the presence of inflammatory mediators (IFN, TNF, and IL) and mediation through receptors, in this case ligation of HSP with its receptor (Lubkowska et al., 2021). The description presented by Murshid et al. (2011) regarding the presence of HSP receptors or HSP-R in the HSP release mechanism is the basis for supplementing HSP70 into vitrification cryoprotectants so that they can carry out their function as oocyte protection by internalizing after the supplemented external HSP70 binds to HSP-R. The intracellular reactions described by Roh et al. (2008) indicate that extreme temperature changes during vitrification activate the inductive form of HSP70 and increase its expression in cells. The cytosolic heat shock factor (HSF-1) is bound to group with HSP in an inactive state. HSF-1 is a protein responsible for inducing the expression of group with HSP both in physiological conditions and in cells experiencing stress (Alagar Boopathy et al., 2022). The presence of stressors activates HSF-1 and causes HSF-1 to separate from HSP70. Protein kinase phosphorylates HSF-1, which then undergoes trimerization in the cytosol (Hentze et al., 2016). This HSF-1 trimer complex enters the cell nucleus and binds to the heat shock element (HSE) in the promoter region of the HSP70 gene. HSP70 mRNA undergoes transcription and enters the cytoplasm to be synthesized and translated into new HSP70 (Milarski and Morimoto, 1986). This cell protection mechanism includes the function of HSPs as chaperone molecules that help the translocation process of proteins synthesized in cells and help the repair and refolding process of damaged proteins, as well as inhibit apoptosis (Hu et al., 2022). HSP70 shows a cell protection mechanism by preventing the activation of proapoptotic caspases (Stankiewicz et al., 2005). It begins with oocytes experiencing stress due to a series of vitrification processes, which will cause mitochondria to release cytochrome c, resulting in APAF-1 activation and proapoptotic caspase 9 (Cao et al., 2022). Activation of APAF-1 and caspase-9 will break down procaspase-3 to activate caspase-3. Apoptosis will occur if the process continues (Zou et al., 2003). However, because oocytes experiencing stress can produce HSP70, HSP70 suppresses the activation of APAF-1, caspase 9, and caspase 3 to inhibit apoptosis in oocytes (Parrish et al., 2013). Although the phenomenon of apoptosis in bovine embryos has been reported, very little information is available regarding apoptosis and HSP, and the exact mechanisms of signaling pathways during temperature stress in oocytes and embryos are still largely unknown (Vandaele et al., 2006). This study shows that HSP70 supplementation with EG+S+HSP70 0.5 µg/ml cryoprotectant formulation is an effective dose for oocyte protection during vitrification. The HSP70 dose of 0.5 µg/ml does not significantly change the concentration of cryoprotectant so that post-vitrification oocyte viability is maintained. In addition, HSP70 supplementation has been shown to maintain normal morphology and suppress caspase-3 activation, thereby reducing the incidence of apoptosis. This small dose is also more economical compared to the relatively expensive price of HSP70. The new findings obtained from this study are that HSP70 supplementation into bovine oocyte vitrification cryoprotectants provides a protective effect on oocytes against temperature stress during the vitrification process by suppressing the occurrence of apoptosis in post-vitrification oocytes. The cryoprotectant formulation of Ethylene Glycol 40% + 0.3 M Sucrose + HSP70 0.5 μg / ml has a significant effect on post-vitrification oocyte viability, as indicated by the normal morphology that can still be maintained and suppresses caspase-3 activation, thereby reducing the occurrence of apoptosis. The combination of 40% EG as an intracellular cryoprotectant and 0.3 M sucrose as an extracellular cryoprotectant, the use of which is recommended by many previous researchers, as well as HSP70 supplementation at a dose of 0.5 μg/ml as a stress protein to provide a protective effect on post-vitrification oocytes that experience very extreme temperature changes, provides new hope for the development of oocyte cryopreservation techniques (Bautista and Kanagawa, 1998). This newly discovered cryoprotectant formulation provides the potential to increase the success of reproductive biology techniques, especially in the development of ART. The potential protective mechanism of HSP70 during vitrification is a novel finding; however, this conclusion is based on indirect evidence, as no direct assessment of receptor binding or cellular internalization was conducted. HSP70 supplemented into cryoprotectants can interact with oocytes through the presence of HSP70-R in the zona pellucida and cause an intracellular reaction in the form of cell signaling (Spinaci et al., 2005). Extreme temperature changes as stressors during the vitrification process will cause HSF-1 in the cytosol to bind to HSPs that were originally inactive to become active (Anckar and Sistonen, 2007). HSF-1 is separated from HSP70. Protein kinase phosphorylates HSF-1, which then undergoes trimerization in the cytosol (Lu et al., 2022). This HSF-1 trimer complex enters the cell nucleus and binds to the HSE in the promoter region of the HSP70 gene. The HSP70 mRNA undergoes transcription and enters the cytoplasm to be synthesized and translated into new HSP70 (Xing et al., 2004). This cell protection mechanism includes the function of HSPs as chaperone molecules which together will help the translocation process of proteins synthesized in cells and help the repair and refolding process of damaged proteins, as well as inhibit apoptosis (Hu et al., 2022). HSP70 shows a cell protection mechanism by preventing the activation of caspases that work proapoptotically (Stankiewicz et al., 2005). Oocytes that experience stress due to a series of vitrification processes will cause mitochondria to release cytochrome c, resulting in the activation of APAF-1 and proapoptotic caspase 9 (Wen et al., 2025). Activation of APAF-1 and caspase 9 will break down procaspase 3 to activate caspase 3. Apoptosis will occur if the process continues (Zou et al., 2003). The presence of HSP70 in vitrification cryoprotectants suppresses the activation of APAF-1, caspase 9, and caspase 3 to inhibit apoptosis in oocytes (Beere et al., 2000). The present findings indicate an association between HSP70 supplementation and reduced apoptosis, as evidenced by lower caspase 3 expression and improved oocyte morphology. However, these results should be interpreted with caution. Although HSP70 has been proposed to interfere with caspase activation pathways, our study did not directly assess the mechanism of action, such as protein-protein interactions or transcriptional regulation. Thus, causal relationships—particularly HSP70-mediated inhibition of the intrinsic apoptosis pathway—remain speculative. Furthermore, a critical limitation of this study is the lack of data on subsequent embryo development following fertilization. Although oocyte morphology and apoptotic status are important quality indicators, they do not accurately predict developmental competence. Future studies incorporating IVF and blastocyst formation and viability assessment are essential to confirm the functional benefits of HSP70 supplementation in vitrification medium. ConclusionHSP70 plays a role in providing cell protection against temperature stress during oocyte vitrification. HSP70 expression is observed in fresh oocytes, post-IVM oocytes, and post-vitrification oocytes without HSP70 supplementation. Cytochrome c expression in various oocyte preparations Cytochrome c expression in fresh oocytes, post-maturation oocytes, and post-vitrification oocytes with various doses of HSP70 supplementation into cryoprotectants was not significantly different. HSP70 supplementation into vitrification medium will suppress caspase activation. Apoptosis occurs during the series of oocyte vitrification processes after in vitro maturation and post-thawing. HSP70 supplementation with an optimum dose of 0.5 μg/ml in vitrification cryoprotectants has a significant effect on the viability of bovine oocytes after vitrification with a decrease in the incidence of apoptosis and normal morphology that can be maintained. AcknowledgmentsThe authors would like to thank the Faculty of Veterinary Medicine, Universitas Airlangga. The author also thanks Win Darmanto, Aucky Hinting, and Aulanni’am Aulanni’am for their expertise. Conflict of interestThe authors declare no conflict of interest. FundingThe authors funded this study. Author’s contributionsRR, SPM, ARK, and WW: conceived the idea and drafted the manuscript. PS, SRA, IM, and AOA: acquisition, analysis, and interpretation of data. FAR, MFA, L’UN’I, and RZA: critically read and revised the manuscript for intellectual content. All authors have read and approved the final version of the manuscript. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityAll data are available in the revised manuscript. ReferencesAbu-Qare, A., W., Abou-Donia, M. and B. 2001. Biomarkers of apoptosis: release of cytochrome c, activation of caspase-3, induction of 8-hydroxy-2’-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene. J. Toxicol. Environ. Health B. Crit. Rev. 4(3), 313–332. Ahmad, A., Imran, M. and Ahsan, H. 2023. Biomarkers as biomedical bioindicators: approaches and techniques for the detection, analysis, and validation of novel biomarkers of diseases. Pharmaceutics 15(6), 1630. Alagar Boopathy, L.R., Jacob-Tomas, S., Alecki, C. and Vera, M. 2022. Mechanisms tailoring the expression of heat shock proteins to proteostasis challenges. J. Biol. Chem. 298(5), 101796. Alvarez-Rodríguez, M., Alvarez, M., Borragan, S., Martinez-Pastor, F., Holt, W.V., Fazeli, A., De Paz, P. and Anel, L. 2013. The addition of heat shock protein HSPA8 to cryoprotective media improves the survival of brown bear (Ursus arctos) spermatozoa during chilling and after cryopreservation. Theriogenology 79(3), 541–550. Anckar, J. and Sistonen, L. 2007. Heat shock factor 1 as a coordinator of stress and developmental pathways. Adv. Exp. Med. Biol. 594(1), 78–88. Bautista, J.A. and Kanagawa, H. 1998. Current status of vitrification of embryos and oocytes in domestic animals: ethylene glycol as an emerging cryoprotectant of choice. Jpn. J. Vet. Res. 45(4), 183–191. Beere, H.M. 2004. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J. Cell. Sci. 117(Pt 13), 2641–2651. Beere, H.M., Wolf, B.B., Cain, K., Mosser, D.D., Mahboubi, A., Kuwana, T., Tailor, P., Morimoto, R.I., Cohen, G.M. and Green, D.R. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biol. 2(8), 469–475. Blengini, C. and Schindler, K. 2022. Acentriolar spindle assembly in mammalian female meiosis and the consequences of its perturbations on human reproduction. Biol. Reprod. 106(2), 253–263. Bouchnak, I., Coulon, D., Salis, V., D’Andréa, S. and Bréhélin, C. 2023. Lipid droplets are versatile organelles involved in plant development and plant response to environmental changes. Front. Plant Sci. 14(1), 1193905. Brambillasca, F., Guglielmo, M.C., Coticchio, G., Mignini Renzini, M., Dal Canto, M. and Fadini, R. 2013. The current challenges to efficient immature oocyte cryopreservation. J. Assist. Reprod. Genet. 30(12), 1531–1539. Bratton, S.B. and Salvesen, G.S. 2010. Regulation of the Apaf-1-caspase-9 apoptosome. J. Cell Sci. 123(Pt 19), 3209–3214. Calderwood, S.K., Mambula, S.S., Gray, P.J. and Theriault, J.R. 2007. Extracellular heat shock proteins in cell signaling. FEBS Lett. 581(19), 3689–3694. Calderwood, S.K., Murshid, A. and Gong, J. 2012. Heat Shock Proteins: conditional Mediators of Inflammation in Tumor Immunity. Front. Immunol. 3(1), 75. Caliskan, S., Liu, D., Oldenhof, H., Sieme, H. and Wolkers, W.F. 2024. Use of membrane transport models to design cryopreservation procedures for oocytes. Anim. Reprod. Sci. 267(1), 107536. Cao, B., Qin, J., Pan, B., Qazi, I.H., Ye, J., Fang, Y. and Zhou, G. 2022. Oxidative stress and oocyte cryopreservation: recent advances in mitigation strategies involving antioxidants. Cells 11(22), 3573. Casanova, A., Wevers, A., Navarro-Ledesma, S. and Pruimboom, L. 2023. Mitochondria: it is all about energy. Front. Physiol. 14(1), 1114231. Chang, H., Chen, H., Zhang, L., Wang, Y., Xie, X., Zhang, Y. and Quan, F. 2019. Effect of oocyte vitrification on DNA damage in metaphase II oocytes and the resulting preimplantation embryos. Mol. Reprod. Develop. 86(11), 1603–1614. Chen, H., Zhang, L., Meng, L., Liang, L. and Zhang, C. 2022. Advantages of vitrification preservation in assisted reproduction and potential influences on imprinted genes. Clin. Epigenetics 14(1), 141. Chi, H.J. 2002. Cryopreservation of human embryos using ethylene glycol in controlled slow freezing. Hum. Reprod. 17(8), 2146–2151. Chian, R.C., Wang, Y. and Li, Y.R. 2014. Oocyte vitrification: advances, progress and future goals. J. Assist. Reprod. Genet. 31(4), 411–420. Crowe, A.D., Lonergan, P. and Butler, S.T. 2021. Invited review: use of assisted reproduction techniques to accelerate genetic gain and increase value of beef production in dairy herds. J. Dairy Sci. 104(12), 12189–12206. Dai, J., Wu, C., Muneri, C.W., Niu, Y., Zhang, S., Rui, R. and Zhang, D. 2015. Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 71(2), 291–298. D’Angelo, A., Panayotidis, C., Alteri, A., Mcheik, S. and Veleva, Z. 2022. Evidence and consensus on technical aspects of embryo transfer. Hum. Reprod. Open 2022(4), hoac038. Elmore, S. 2007. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35(4), 495–516. Fabbri, R., Porcu, E., Marsella, T., Rocchetta, G., Venturoli, S. and Flamigni, C. 2001. Human oocyte cryopreservation: new perspectives regarding oocyte survival. Hum. Reprod. 16(3), 411–416. Ferat-Osorio, E., Sánchez-Anaya, A., Gutiérrez-Mendoza, M., Boscó-Gárate, I., Wong-Baeza, I., Pastelin-Palacios, R., Pedraza-Alva, G., Bonifaz, L.C., Cortés-Reynosa, P., Pérez-Salazar, E., Arriaga-Pizano, L., López-Macías, C., Rosenstein, Y. and Isibasi, A. 2014. Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor-1/constitutive heat shock element-binding factor-dependent mechanism. J. Inflamm. (Lond). 11(1), 19. Fernández, J.V.R., Gallardo, H.A., Duarte, D.U., Islas, A.F., Pelayo, M.A.A., Utrera, A.R., Reynozo, S.P. and Sánchez, J.F.D.L.T. 2021. Reproductive biotechnologies in beef cattle: five decades of research in Mexico. Rev. Mex. Cienc. Pecu. 12(Supl 3), 39–78. Galeati, G., Spinaci, M., Vallorani, C., Bucci, D., Porcu, E. and Tamanini, C. 2011. Pig oocyte vitrification by cryotop method: effects on viability, spindle and chromosome configuration and in vitro fertilization. Anim. Reprod. Sci. 127(1–2), 43–49. García-Martínez, T., Mogas, T., Mullen, S.F., Martínez-Rodero, I., Gulieva, R.E. and Higgins, A.Z. 2021. Effect of cryoprotectant concentration on bovine oocyte permeability and comparison of two membrane permeability modelling approaches. Sci. Rep. 11(1), 15387. Gómez-Guzmán, J.A., Parra-Bracamonte, G.M. and Velazquez, M.A. 2024. Impact of heat stress on oocyte developmental competence and pre-implantation embryo viability in cattle. Animals 14(15), 2280. Gregory, L., Booth, A.D., Wells, C. and Walker, S.M. 1994. A study of the cumulus-corona cell complex in in-vitro fertilization and embryo transfer; a prognostic indicator of the failure of implantation. Hum. Reprod. 9(7), 1308–1317. Gupta, S., Kass, G.E., Szegezdi, E. and Joseph, B. 2009. The mitochondrial death pathway: a promising therapeutic target in diseases. J. Cell. Mol. Med. 13(6), 1004–1033. Hendrey, J. and Kola, I. 1991. Thermolability of mouse oocytes is due to the lack of expression and/or inducibility of Hsp70. Mol. Reprod. Develop. 28(1), 1–8. Hentze, N., Le Breton, L., Wiesner, J., Kempf, G. and Mayer, M.P. 2016. Molecular mechanism of thermosensory function of human heat shock transcription factor Hsf1. eLife 5(1), 11576. Hernandez Gifford, J.A. and Gifford, C.A. 2013. Role of reproductive biotechnologies in enhancing food security and sustainability. Anim. Front. 3(3), 14–19. Hu, C., Yang, J., Qi, Z., Wu, H., Wang, B., Zou, F., Mei, H., Liu, J., Wang, W. and Liu, Q. 2022. Heat shock proteins: biological functions, pathological roles, and therapeutic opportunities. MedComm. (2020). 3(3), e161. Hwang, I.S. and Hochi, S. 2014. Recent progress in cryopreservation of bovine oocytes. Biomed. Res. Int. 2014(1), 570647. Jia, B., Xiang, D., Fu, X., Shao, Q., Hong, Q., Quan, G. and Wu, G. 2020. Proteomic changes of porcine oocytes after vitrification and subsequent in vitro maturation: a tandem mass tag-based quantitative analysis. Front. Cell Dev. Biol. 8(1), 614577. Kabakov, A.E. and Gabai, V.L. 2021. HSP70s in breast cancer: promoters of tumorigenesis and potential targets/tools for therapy. Cells 10(12), 3446. Kamerlin, S.C.L. 2024. In vitro fertilization and the ethics of frozen embryos. EMBO Rep. 25(7), 2817–2818. Kattera, S. and Chen, C. 2006. Cryopreservation of embryos by vitrification: current development. Int. Surg. 91(5 Suppl), S55–S62. Khan, F.F., Ahmad, K., Ahmed, A. and Haider, S. 2017. Application of medical images for diagnosis of diseases-review article. World J. Biol. Biotechnol. 2(1), 135–138. Konc, J., Kanyó, K., Kriston, R., Somoskői, B. and Cseh, S. 2014. Cryopreservation of embryos and oocytes in human assisted reproduction. BioMed. Res. Int. 2014(1), 1–9. Kushnir, V.A., Smith, G.D. and Adashi, E.Y. 2022. The future of IVF: the new normal in human reproduction. Reprod. Sci. 29(3), 849–856. Lanneau, D., Brunet, M., Frisan, E., Solary, E., Fontenay, M. and Garrido, C. 2008. Heat shock proteins: essential proteins for apoptosis regulation. J. Cell. Mol. Med. 12(3), 743–761. Lánská, V., Chmelíková, E., Sedmíková, M., Petr, J., Rajmon, R., Jeseta, M. and Rozinek, J. 2006. Expression of heat shock protein70 in pig oocytes: heat shock response during oocyte growth. Anim. Reprod. Sci. 96(1–2), 154–164. Le Masson, F. and Christians, E. 2011. HSFs and regulation of Hsp70.1 (Hspa1b) in oocytes and preimplantation embryos: new insights brought by transgenic and knockout mouse models. Cell Stress Chaperones 16(3), 275–285. Ledwaba, M.R., O’Neill, H.A., Thema, M.A., Maqhashu, A. and Mphaphathi, M.L. 2025. Techniques for in vitro fertilisation of vitrified cattle oocytes: challenges and new developments. Agriculture 15(4), 363. Lee, S., Ryu, K.J., Kim, B., Kang, D., Kim, Y.Y. and Kim, T. 2019. Comparison between Slow freezing and vitrification for human ovarian tissue cryopreservation and xenotransplantation. Int. J. Mol. Sci. 20(13), 3346. Leng, X., Wang, X., Pang, W., Zhan, R., Zhang, Z., Wang, L., Gao, X. and Qian, L. 2013. Evidence of a role for both anti-Hsp70 antibody and endothelial surface membrane Hsp70 in atherosclerosis. Cell Stress Chaperones 18(4), 483–493. Liu, C.H., Yang, C.C., Lin, D.C., Wu, M.H. and Tsai, K.J. 2004. Stored of Hsp72/Hsp73 in germinal vesicle‐stage mouse oocytes. Reprod. Domestic Animals 39(1), 19–24. Lu, W.C., Omari, R., Ray, H., Wang, J., Williams, I., Jacobs, C., Hockaden, N., Bochman, M.L. and Carpenter, R.L. 2022. AKT1 mediates multiple phosphorylation events that functionally promote HSF1 activation. FEBS J. 289(13), 3876–3893. Lubkowska, A., Pluta, W., Strońska, A. and Lalko, A. 2021. Role of heat shock proteins (HSP70 and HSP90) in viral infection. Int. J. Mol. Sci. 22(17), 9366. Madan, S., Uttekar, B., Chowdhary, S. and Rikhy, R. 2022. Mitochondria lead the way: mitochondrial dynamics and function in cellular movements in development and disease. Front. Cell Dev. Biol. 9(1), 781933. Martinou, I., Desagher, S., Eskes, R., Antonsson, B., André, E., Fakan, S. and Martinou, J.C. 1999. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J. Cell Biol. 144(5), 883–889. Mavrides, A. and Morroll, D. 2002. Cryopreservation of bovine oocytes: is cryoloop vitrification the future to preserving the female gamete?. Reprod. Nutr. Dev. 42(1), 73–80. Men, H., Monson, R.L., Parrish, J.J. and Rutledge, J.J. 2003. Degeneration of cryopreserved bovine oocytes via apoptosis during subsequent culture. Cryobiology 47(1), 73–81. Milarski, K.L. and Morimoto, R.I. 1986. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc. Natl. Acad. Sci. U. S. A. 83(24), 9517–9521. Mullen, S.F., Rosenbaum, M. and Critser, J.K. 2007. The effect of osmotic stress on the cell volume, metaphase II spindle and developmental potential of in vitro matured porcine oocytes. Cryobiology 54(3), 281–289. Murshid, A., Theriault, J., Gong, J. and Calderwood, S.K. 2011. Investigating receptors for extracellular heat shock proteins. Methods Mol. Biol. Mol. Chaperones. 787(1), 289–302. Nottola, S.A., Cecconi, S., Bianchi, S., Motta, C., Rossi, G., Continenza, M.A. and Macchiarelli, G. 2011. Ultrastructure of isolated mouse ovarian follicles cultured in vitro. Reprod. Biol. Endocrinology 9(1), 3. Olexikova, L., Makarevich, A.V., Pivko, J. and Chrenek, P. 2010. Antibody to Hsp70 alters response of rabbit preimplantation embryos to hyperthermia in vitro. Anim. Reprod. Sci. 119(1–2), 130–136. Parrish, A.B., Freel, C.D. and Kornbluth, S. 2013. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 5(6), a008672. Qian, S., Wei, Z., Yang, W., Huang, J., Yang, Y. and Wang, J. 2022. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 12(1), 985363. Reddy, V.S., Yadav, B., Yadav, C.L., Anand, M., Swain, D.K., Kumar, D., Kritania, D., Madan, A.K., Kumar, J. and Yadav, S. 2018. Effect of sericin supplementation on heat shock protein 70 (HSP70) expression, redox status and post thaw semen quality in goat. Cryobiology 84(1), 33–39. Resetkova, N., Hayashi, M., Kolp, L.A. and Christianson, M.S. 2013. Fertility preservation for prepubertal girls: update and current challenges. Curr. Obstet. Gynecol. Rep. 2(4), 218–225. Rezazadeh Valojerdi, M., Eftekhari-Yazdi, P., Karimian, L., Hassani, F. and Movaghar, B. 2009. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J. Assist. Reprod. Genet. 26(6), 347–354. Roh, B.H., Kim, D.H., Cho, M.K., Park, Y.L. and Whang, K.U. 2008. Expression of heat shock protein 70 in human skin cells as a photoprotective function after UV exposure. Ann. Dermatol. 20(4), 184–189. Rosyada, Z.N.A., Ulum, M.F., Tumbelaka, L.I.T.A., Solihin, D.D., Purwantara, B. and Memili, E. 2022. Implications of sperm heat shock protein 70-2 in bull fertility. Vet. World 15(6), 1456–1466. Said, S. Integrated livestock business and industry in Indonesia. IOP Conf. Ser.: Earth Environ. Sci. 2020 465(1), 12003. Shamas-Din, A., Kale, J., Leber, B. and Andrews, D.W. 2013. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 5(4), a008714. Spinaci, M., Volpe, S., Bernardini, C., De Ambrogi, M., Tamanini, C., Seren, E. and Galeati, G. 2005. Immunolocalization of heat shock protein 70 (Hsp 70) in boar spermatozoa and its role during fertilization. Mol. Reprod. Dev. 72(4), 534–541. Stamperna, K., Giannoulis, T., Dovolou, E., Kalemkeridou, M., Nanas, I., Dadouli, K., Moutou, K., Mamuris, Z. and Amiridis, G.S. 2021a. Heat shock protein 70 improves in vitro embryo yield and quality from heat stressed bovine oocytes. Animals (Basel). 11(6), 1794. Stamperna, K., Giannoulis, T., Dovolou, E., Kalemkeridou, M., Nanas, I., Dadouli, K., Moutou, K., Mamuris, Z. and Amiridis, G.S. 2021b. The effects of heat shock protein 70 addition in the culture medium on the development and quality of in vitro produced heat shocked bovine embryos. Animals (Basel). 11(12), 3347. Stankiewicz, A.R., Lachapelle, G., Foo, C.P., Radicioni, S.M. and Mosser, D.D. 2005. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing bax translocation. J. Biol. Chem. 280(46), 38729–38739. Stetler, R.A., Gan, Y., Zhang, W., Liou, A.K., Gao, Y., Cao, G. and Chen, J. 2010. Heat shock proteins: cellular and molecular mechanisms in the central nervous system. Prog. Neurobiol. 92(2), 184–211. Szyller, J. and Bil-Lula, I. 2021. Heat shock proteins in oxidative stress and ischemia/reperfusion injury and benefits from physical exercises: a review to the current knowledge. Oxid. Med. Cell. Longev. 2021(1), 6678457. Tamburrino, L., Traini, G., Marcellini, A., Vignozzi, L., Baldi, E. and Marchiani, S. 2023. Cryopreservation of human spermatozoa: functional, molecular and clinical aspects. Int. J. Mol. Sci. 24(5), 4656. Tamura, A.N., Huang, T.T. and Marikawa, Y. 2013. Impact of vitrification on the meiotic spindle and components of the microtubule-organizing center in mouse mature oocytes. Biol. Reprod. 89(5), 112. Tharasanit, T. and Thuwanut, P. 2021. Oocyte cryopreservation in domestic animals and humans: principles, techniques and updated outcomes. Animals (Basel). 11(10), 2949. Turathum, B., Gao, E.M. and Chian, R.C. 2021. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 10(9), 2292. Turathum, B., Saikhun, K., Sangsuwan, P. and Kitiyanant, Y. 2010. Effects of vitrification on nuclear maturation, ultrastructural changes and gene expression of canine oocytes. Reprod. Biol. Endocrinol. 8(1), 70. Vandaele, L., Mateusen, B., Maes, D., De Kruif, A. and Van Soom, A. 2006. Is apoptosis in bovine in vitro produced embryos related to early developmental kinetics and in vivo bull fertility?. Theriogenology 65(9), 1691–1703. Vasaikar, S.V., Ghosh, S., Narain, P., Basu, A. and Gomes, J. 2015. HSP70 mediates survival in apoptotic cells-Boolean network prediction and experimental validation. Front. Cell. Neurosci. 9(1), 319. Walkon, L.L., Strubbe-Rivera, J.O. and Bazil, J.N. 2022. Calcium overload and mitochondrial metabolism. Biomolecules 12(12), 1891. Wang, C. and Youle, R.J. 2009. The role of mitochondria in apoptosis*. Annu. Rev. Genet. 43(1), 95–118. Wang, L.Y., Wang, D.H., Zou, X.Y. and Xu, C.M. 2009. Mitochondrial functions on oocytes and preimplantation embryos. J. Zhejiang Univ. Sci. B. 10(7), 483–492. Wen, H., Deng, H., Li, B., Chen, J., Zhu, J., Zhang, X., Yoshida, S. and Zhou, Y. 2025. Mitochondrial diseases: from molecular mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 10(1), 9. Xing, H., Mayhew, C.N., Cullen, K.E., Park-Sarge, O.K. and Sarge, K.D. 2004. HSF1 modulation of Hsp70 mRNA polyadenylation via interaction with symplekin. J. Biol. Chem. 279(11), 10551–10555. Yassin, H.B., Abu-Elnaga, N.A., Farrag, B. and El-Bahrawy, K.A. 2022. Effect of cryoprotectants on camel oocytes vitrification. Al-Azhar Bull. Sci. Sec. C 33(1), 91–104. Zhang, W. and Wu, F. 2023. Effects of adverse fertility-related factors on mitochondrial DNA in the oocyte: a comprehensive review. Reprod. Biol. Endocrinol. 21(1), 27. Zou, H., Yang, R., Hao, J., Wang, J., Sun, C., Fesik, S.W., Wu, J.C., Tomaselli, K.J. and Armstrong, R.C. 2003. Regulation of the Apaf-1/Caspase-9 apoptosome by Caspase-3 and XIAP. J. Biol. Chem. 278(10), 8091–8098. | ||
| How to Cite this Article |
| Pubmed Style Rimayanti R, Madyawati SP, Rantam FA, Khairullah AR, Widjiati W, Srianto P, Akintunde AO, Mustofa I, Amrullah MF, ‘ilmi L�N, Ahmad RZ, Ayuti SR. The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytes. Open Vet. J.. 2025; 15(9): 4505-4519. doi:10.5455/OVJ.2025.v15.i9.56 Web Style Rimayanti R, Madyawati SP, Rantam FA, Khairullah AR, Widjiati W, Srianto P, Akintunde AO, Mustofa I, Amrullah MF, ‘ilmi L�N, Ahmad RZ, Ayuti SR. The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytes. https://www.openveterinaryjournal.com/?mno=252315 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.56 AMA (American Medical Association) Style Rimayanti R, Madyawati SP, Rantam FA, Khairullah AR, Widjiati W, Srianto P, Akintunde AO, Mustofa I, Amrullah MF, ‘ilmi L�N, Ahmad RZ, Ayuti SR. The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytes. Open Vet. J.. 2025; 15(9): 4505-4519. doi:10.5455/OVJ.2025.v15.i9.56 Vancouver/ICMJE Style Rimayanti R, Madyawati SP, Rantam FA, Khairullah AR, Widjiati W, Srianto P, Akintunde AO, Mustofa I, Amrullah MF, ‘ilmi L�N, Ahmad RZ, Ayuti SR. The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytes. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4505-4519. doi:10.5455/OVJ.2025.v15.i9.56 Harvard Style Rimayanti, R., Madyawati, . S. P., Rantam, . F. A., Khairullah, . A. R., Widjiati, . W., Srianto, . P., Akintunde, . A. O., Mustofa, . I., Amrullah, . M. F., ‘ilmi, . L. �. N., Ahmad, . R. Z. & Ayuti, . S. R. (2025) The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytes. Open Vet. J., 15 (9), 4505-4519. doi:10.5455/OVJ.2025.v15.i9.56 Turabian Style Rimayanti, Rimayanti, Sri Pantja Madyawati, Fedik Abdul Rantam, Aswin Rafif Khairullah, Widjiati Widjiati, Pudji Srianto, Adeyinka Oye Akintunde, Imam Mustofa, Muhammad Fajar Amrullah, Laily ‘ulya Nurul ‘ilmi, Riza Zainuddin Ahmad, and Siti Rani Ayuti. 2025. The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytes. Open Veterinary Journal, 15 (9), 4505-4519. doi:10.5455/OVJ.2025.v15.i9.56 Chicago Style Rimayanti, Rimayanti, Sri Pantja Madyawati, Fedik Abdul Rantam, Aswin Rafif Khairullah, Widjiati Widjiati, Pudji Srianto, Adeyinka Oye Akintunde, Imam Mustofa, Muhammad Fajar Amrullah, Laily ‘ulya Nurul ‘ilmi, Riza Zainuddin Ahmad, and Siti Rani Ayuti. "The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytes." Open Veterinary Journal 15 (2025), 4505-4519. doi:10.5455/OVJ.2025.v15.i9.56 MLA (The Modern Language Association) Style Rimayanti, Rimayanti, Sri Pantja Madyawati, Fedik Abdul Rantam, Aswin Rafif Khairullah, Widjiati Widjiati, Pudji Srianto, Adeyinka Oye Akintunde, Imam Mustofa, Muhammad Fajar Amrullah, Laily ‘ulya Nurul ‘ilmi, Riza Zainuddin Ahmad, and Siti Rani Ayuti. "The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytes." Open Veterinary Journal 15.9 (2025), 4505-4519. Print. doi:10.5455/OVJ.2025.v15.i9.56 APA (American Psychological Association) Style Rimayanti, R., Madyawati, . S. P., Rantam, . F. A., Khairullah, . A. R., Widjiati, . W., Srianto, . P., Akintunde, . A. O., Mustofa, . I., Amrullah, . M. F., ‘ilmi, . L. �. N., Ahmad, . R. Z. & Ayuti, . S. R. (2025) The addition of heat shock protein 70 to in vitro maturation medium improves the post-thaw quality of vitrified oocytes. Open Veterinary Journal, 15 (9), 4505-4519. doi:10.5455/OVJ.2025.v15.i9.56 |