| Research Article | ||
Open Vet. J.. 2025; 15(8): 3823-3830 Open Veterinary Journal, (2025), Vol. 15(8): 3823-3830 Research Article Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy catsAndrea Núñez1, Victor Silva2, María Gabriela Pereira3 and Rodrigo Castro3*1Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias y Forestales, Universidad Católica del Maule, Curicó, Chile 2VSV-Consulting-LATAM, Pucón, Chile 3Escuela de Medicina Veterinaria, Facultad de Recursos Naturales y Medicina Veterinaria, Universidad Santo Tomás, Santiago, Chile *Corresponding Author: Rodrigo Castro. Escuela de Medicina Veterinaria, Facultad de Recursos Naturales y Medicina Veterinaria, Universidad Santo Tomás, Talca, Chile. Email: rodrigocastro [at] santotomas.cl Submitted: 12/04/2025 Revised: 30/06/2025 Accepted: 15/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
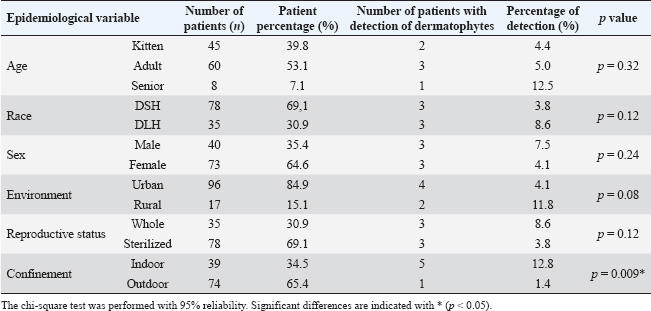
ABSTRACTBackground: Dermatophytes are fungi that invade keratinized tissues, causing superficial skin lesions in both animals and humans. Although Microsporum canis (M. canis) is the most frequent dermatophyte in companion animals, and cats are considered carriers and disseminators, few reports have determined its prevalence and antifungal susceptibility in healthy cats, which could represent a public health problem. Aim: This study aimed to determine the prevalence and antifungal susceptibility of dermatophyte strains obtained from dermatologically healthy cats in the city of Talca, Chile. Methods: From March 2024 to August 2024, skin or hair samples were collected from three anatomical zones (facial, abdomen, and limbs) of 113 dermatologically healthy cats using the mat technique. Samples were seeded in Sabouraud dextrose agar enriched with chloramphenicol plus cycloheximide and incubated at 25ºC for 21 days. Then, the antifungal sensitivity of the isolated strains was determined by disc diffusion testing, and amphotericin B (10 µg), fluconazole (25 µg), clotrimazole (10 µg), caspofungin (5 µg), voriconazole (1 µg), and nystatin (100 µg) were used as antifungals. The chi-square independence test (p < 0.05) was used to determine the association between the anatomical site of the sample and isolation of dermatophytes, as well as between dermatophyte carriage and the epidemiological variables of the study population. Results: Of the 113 healthy cats included in this study, six (5.3%) were found to be fungal carriers, with M. canis being the only isolated dermatophyte species. While 100% of the strains were susceptible to clotrimazole, voriconazole, caspofungin, amphotericin B, and nystatin, all were fluconazole-resistant. Confinement status was the only epidemiological variable associated with fungal carriage (p=0.009), with indoor cats having a 10.73-fold greater risk of carrying dermatophytes than outdoor cats. No association was found between the anatomical site and the isolation of dermatophytes. Conclusion: Microsporum canis was the only dermatophyte isolated from the skin and hair of dermatologically healthy cats in Talca, with a prevalence of 5.3%. All the isolates were fluconazole-resistant. Keywords: Cats, Dermatophytes, Microsporum canis, Antifungal resistance. IntroductionDermatophytosis is the infection of keratinized structures such as hair, nails, and the stratum corneum of the skin (Cafarchia et al., 2004; Baghza, 2016). Dermatophytes are the fungi that cause the disease, with more than 50 species described. These are divided into three ecological niches, anthropophilic, zoophilic, and geophilic, depending on the main habitat or host (Zhan et al., 2018). Microsporum canis is a zoophilic dermatophyte, and worldwide, it is the most frequently described agent of dermatophytosis in the scalp of humans and animals (Silva et al., 2003; Ginter-Hanselmayer et al., 2007; Moriello et al., 2017). In animals, M. canis infection has been associated with multifocal alopecia, desquamation, and circular-looking lesions (Degreef, 2008). In humans, M. canis infection presents as tinea capitis, tinea corporis, tinea pedis, and onychomycosis less frequently (Ginter-Hanselmayer et al., 2007). The distribution of this fungus can vary according to geography and other epidemiological factors, such as age and sex (Cafarchia et al., 2004; Wiegand et al., 2016). Transmission occurs through direct contact with sick animals (Sparkes et al., 1994), and companion animals, mainly cats, can act as carriers of Microsporum spores or arthroconidia, which do not invade healthy skin and can remain viable in the environment for up to 18 months (Sparkes et al., 1994; Weitzman and Summerbell, 1995). Cats represent the main source of infection for humans, and close contact with cats and dogs can result in 20% of human diseases (Thompson, 2000; Lund et al., 2014). The carrier stage can progress to infection depending on predisposing factors such as young age, immunosuppression, skin trauma, nutritional deficiency, increased humidity, and environmental temperature. After penetrating through damaged skin, spores or conidia germinate in the stratum corneum, and dermatophyte metabolites induce an inflammatory reaction at the site of infection (Weitzman and Summerbell, 1995). Asymptomatic animals are estimated to transmit the disease in approximately 50% of infected human cases, representing a public health concern (Mancianti et al., 2013). From a zoonotic point of view, studies have reported infection in 9% of the human population in the United States (Moriello, 2014). Direct microscopic examination of clinical samples is used together with in vitro isolation and identification through fungal culture for the diagnosis of dermatophytosis (Nardoni et al., 2010). Owing to the high risk of M. canis infection, it is important to have therapeutic protocols in place to prevent transmission to other hosts. Topical and oral antifungals used in dogs and cats include itraconazole, terbinafine, griseofulvin, and fluconazole (Gupta and Del Rosso, 2000; Adimi et al., 2013; Moriello et al., 2017, Bishnoi et al., 2018). Some authors describe therapeutic failures in 25%–40% of patients with dermatophytosis, probably due to failure to penetrate the drug into the skin, variation in drug bioavailability, and emergence of strains with antifungal resistance (Bueno et al., 2010; Alcazar-Fuoli and Mellado, 2014). Debnath et al. (2016) reported antifungal resistance to fluconazole and miconazole in M. canis strains, whereas Singh et al. (2021) reported resistance to fluconazole and griseofulvin. This study aimed to determine the prevalence and antifungal susceptibility of dermatophyte strains obtained from dermatologically healthy cats in the city of Talca, Chile. Materials and methodsAnimalsConvenience sampling was conducted on 113 dermatologically healthy cats from veterinary clinics in the city of Talca, without distinction of breed, sex, or age. Cats that presented any underlying disease and had lesions on the skin and hair coat or that received antifungal treatment at least 2 weeks before joining the study were excluded from the study. To this end, before taking a sample, a complete anamnesis was taken for each patient, recording antecedents such as age, sex, breed, and coexistence with other animals, general clinical examination, visual inspection, and palpation. The cats sampled were those that attended healthy and preventive control consultations, such as vaccinations, deworming, and surgical procedures, such as castrations or sterilizations, after the guardian signed the informed consent. SamplingSamples were collected from March 2024 to August 2024. The mat technique of Mariat and Tapia (1966) was used, which consists of rubbing a piece of sterile sponge of 4 × 4 cm for 30 seconds on the skin or hair of three anatomical sites: the facial area, abdominal area, and fore and hind limbs, and then being transported in sterile packaging to the mycology laboratory. Sample processingThe samples were inoculated in Sabouraud dextrose agar culture medium with chloramphenicol and cycloheximide. They were then incubated in an aerobiosis laboratory incubator at 25°C for 21 days. The dermatophytes were identified using the classical method in medical mycology, such as macroscopic and microscopic analysis of the mature dermatophyte colony, analyzing the fruiting structures as macroconidia and microconidia, and then using dichotomous identification keys (Silva and Zaror, 2015). Antifungal susceptibility was determined using the disc diffusion method on Müller–Hinton agar without supplements, following the Clinical and Laboratory Standards Institute (CLSI, 2010) M51A guidelines. A conidial suspension corresponding to the 0.5 McFarland standard was prepared from a pure colony inoculated on potato agar and incubated for 7–10 days at 25°C, supported by a spectrophotometer measuring the turbidity at λ 530 nm, with an optical density range of 0.09–0.11. This procedure will produce a suspension of 1−5 × 106 CFU/ml of conidia, according to CLSI M38 (CLSI, 2017) and CLSI M44 (CLSI, 2018). The antifungals used were as follows: amphotericin B (10 µg), fluconazole (25 µg), clotrimazole (10 µg), caspofungin (5 µg), voriconazole (1 µg), and nystatin (100 µg). Trichophyton mentagrophytes ATCC 9533 was used as a quality control strain, using the same materials and methods described earlier (Agarwal et al., 2015; Gupta et al., 2015). Ethical approvalNo ethical approval was required for this study. Statistical analysisThe association between dermatophyte carriage and the epidemiological variables of the 113 cats sampled was determined using the chi-square independence test, considering a 95% reliability through the use of the statistical software InfostatTM (Di Rienzo et al., 2008). The following epidemiological variables were evaluated: age (kittens: 0–1 year; adults: 1.1–8 years; seniors: from 8.1 years of age onwards); sex (male or female); reproductive status (whole, neutered, or no information); breed [Domestic Short Haired (DSH); Domestic Long Haired (DLH)]; environment (rural or urban); and confinement (indoor or outdoor). Table 1. Absolute (n) and relative (%) frequency distribution of the epidemiological variables and detection of dermatophytes in the patients included in the study.
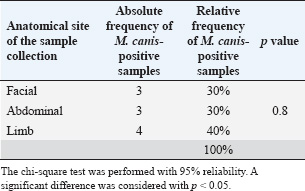
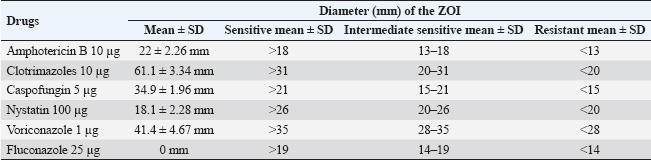
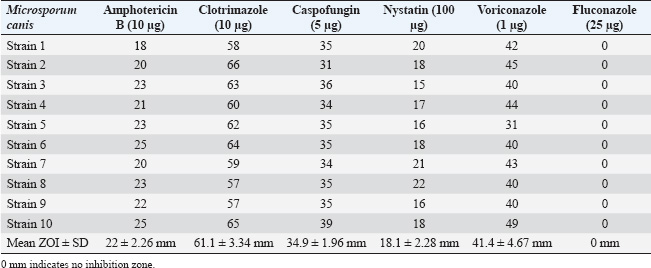
ResultsOf the 113 dermatologically healthy cats that were included in the study, dermatophyte isolation was obtained in six of them (5.3%), obtaining 10 strains, all of which were identified as M. canis. These strains presented yellowish-white colonies with a hairy or woolly texture with flat and radiated edges. A dark yellow pigment was observed on the reverse side. Microsporum canis presented a septate, hyaline, branched mycelium, presenting hyphae in the form of rackets with numerous long, tapered, thick-walled, equinulated macroconidia, with pointed ends and more than six septa, plus microconidia with a sharp end, in accordance with both the macro and microscopic characteristics of this species (Silva and Zaror, 2015; Rodríguez, 2016). Table 1 presents the epidemiological variables and their frequency in the total number of patients. Thus, adult cats (53.1%), DSH (69.1%), female (64.6%), urban (84.9%), sterilized reproductive condition (69.1%), and outdoor confinement (65.4%) were the most frequent distributions. The animals that tended to colonize M. canis were senior, DLH, male, rural, and whole reproductive cats, but without statistical difference. Cats in indoor confinement had a statistically higher frequency of dermatophyte colonization compared with outdoor animals (p=0.009), with an odds ratio of 10.73 (95% confidence interval=1.11–24.2). This indicates that these cats have a 10 times higher risk of carrying dermatophytes. Approximately 40% of the strains were isolated from the limbs (fore and hind limbs), 30% from the facial area, and 30% from the abdomen. There was no association between the anatomical site and the isolation of dermatophytes (p=0.8) (Table 2). The strains were classified into sensitive, intermediate sensitive, or resistant based on their mean diameter (mm) zone of growth inhibition (ZOI ± SD) (Table 3) (Agarwal et al., 2015; Gupta et al., 2015; Idris et al., 2025; Pakshir et al., 2009). The 10 M. canis strains were sensitive to the antifungals amphotericin B (22 ± 2.26 mm), clotrimazole (61.1 ± 3.34), caspofungin (34.9 ± 1.96 mm), nystatin (18.1 ± 2.28 mm), and voriconazole (41.4 ± 4.67 mm), while all of these were resistant to fluconazole (0 mm) (Table 4). Table 2. Absolute (n) and relative (%) frequencies of isolation of 10 Microsporum canis strains from the skin of six dermatologically healthy cats from Talca, Chile, according to the anatomical site of the sample.
Table 3. Breakpoint values for ZOI for each antifungal under study.
Table 4. Diameters (mm) of the ZOI of the dermatophyte strains by the antifungals under study.
DiscussionDermatophytosis is the most common fungal infection that affects domestic animals. It can be transmitted to other animal species and is an important zoonosis (Frymus et al., 2013). Cats are considered the main reservoir of dermatophytes, and the infected animal may present symptoms such as alopecia, blisters, papules, scabs, dander, scales, erythema, follicular obstruction, hyperpigmentation, and abnormal nail growth. Cats can cause trauma lesions during scratching or grooming, which can lead to purulent dermatitis or eosinophilic ulcerative lesions. In general, the lesions occur in the facial area, paws, and other anatomical sites (DeBoer and Moriello, 1994). In the present study, the extremities were the sites with the greatest isolation of M. canis, followed by the facial and abdominal areas, with no statistical differences between them. This information is relevant because no previous data have determined the isolation of dermatophytes by anatomical site. Microsporum canis is the main dermatophyte species in healthy cats (Betancourt et al., 2009; Moriello and DeBoer, 2012; Debnath et al., 2016). Regarding subclinical infections, a relatively low prevalence is described in long-haired animals over 2 years of age. Therefore, M. canis should not be considered as part of the normal skin microbiota of cats (Moriello et al., 2017), and if isolated from an animal without dermatological lesions, it should be interpreted as a subclinical infection or carrier status (Moriello and DeBoer, 2012). Microsporum canis transmission can occur through contact between clinically infected or dermatologically healthy animals, mainly cats, by spores or arthroconidia that remain viable in the environment for up to 18 months (Sparkes et al., 1994). Arthroconidia transmission occurs through contact with sick cats, as infected hairs become brittle and hair follicle fragments contain these infective fungal elements, which are very effective in spreading the infection. Uninfected cats, which can passively carry fungal cells in their fur, are another form of dissemination. Therefore, avoiding the introduction of new animals into hatchery environments or cat shows is important. Transmission can also occur by indirect contact, through collars, brushes, and toys, since arthroconidia are easily spread in dust particles (Frymus et al., 2013). Several authors have shown that the frequency of isolation varies between 0.5% and 47.3% in clinically healthy cats, with M. canis being the most isolated dermatophyte (Alpun and Osgun, 2009), which is ratified by our study that identified M. canis in all isolates of 5.3% of cats. The results of the present study are similar to those described by Ilhana et al. (2016), who isolated 7.1% of dermatophytes from Van cats without dermatological lesions. However, they differ from a previous study in Chile, where Betancourt et al. (2009) described a 60% isolation from healthy cats, highlighting their role in the dissemination of dermatophytes to humans and other animals. According to the epidemiological variables, only the indoor confinement condition presented a significant value, with a higher risk of carrying dermatophytes. However, within the limitations of this study, the lack of significant associations with other epidemiological variables could reflect the independence of the condition with respect to these factors or could be due to limitations in the study sample size, in particular the small group of six cats for comparison, which could reduce the study’s ability to detect small effects and limit the generalizability of the findings. This is concerning because dermatophytes can coexist with animals without causing injury, transforming these hosts into asymptomatic carriers (Silva et al., 2003). In recent years, an increase in cases of human dermatophytosis due to zoophilic agents has been reported. In this situation, the epidemiological importance of pets becomes relevant due to close contact with guardians. This frequent and close coexistence could explain the increase in human infections by dermatophytes (Levy et al., 2006). The active search for dermatophytes in healthy animals contributes to the knowledge of the frequency of isolation in our country (Zaror et al., 1988) and, more importantly, to reducing the risk of zoonoses. López et al. (2012) described the isolation of 10.8% (4 of 37 cats) in animals without dermatological lesions and 25% (2/8) in animals with dermatological lesions, with no statistical differences between the variables of age, sex, and breed. Microsporum canis was isolated in 83.3% of the samples (5/6) and T. mentagrophytes in 16.6% (1/6). The authors highlight the importance of these results being framed in the close and direct contact that cats had with older adults and children, which would be related to the increased risk of developing dermatophytosis. There are a variety of oral and topical antifungal protocols to treat M. canis infection, such as azoles, polyenes, allylamine, and griseofulvin (Mahajan et al., 2017; Aneke et al., 2018). In the triazole group, fluconazole, itraconazole, and voriconazole stand out as widely used agents due to their low side effects and high antifungal activity (Or et al., 2000; Nocua-Báez et al., 2020). However, the efficacy of these drugs is variable, and the number of patients with treatment-refractory dermatophytosis has increased (Aneke et al., 2018; Bishnoi et al., 2018). One reason may be the cutaneous pharmacokinetics of the main antifungals used in dermatophytosis, as they penetrate the stratum corneum for at least 3–4 weeks after treatment completion (Piérard, 2016). Relapses, drug interactions, lack of compliance or incorrect treatment administration by the guardian, difficulty in accessing the infection site, and lack of environmental control are among the main causes of therapeutic failure (ESCCAP, 2011; Vitiello et al., 2023). In vitro antifungal resistance is associated with a high probability of therapeutic failure (Aneke et al., 2018). This is further aggravated in the case of the detection of strains resistant to various classes of antifungals, a situation that greatly limits therapy and therefore hinders the patient’s management and recovery (Singh et al., 2021). In this study, all strains were sensitive to clotrimazole, voriconazole, caspofungin, amphotericin B, and nystatin and were resistant to the antifungal fluconazole. Target modification is one of the mechanisms of resistance to azoles, in which changes in the quantity or quality of 14α-demethylase or other enzymes involved in ergosterol synthesis cause some species to require a higher concentration of azoles to inhibit ergosterol synthesis (Orozco et al., 1998). The most frequently described enzymatic alterations are found in the CYP51/ERG11 gene product, in which the most characterized mutation is the substitution of arginine by lysine at amino acid 46766. There are strains of fungi that increase the synthesis of ergosterol in relation to a high microsomal content of cytochrome P450, as a mechanism of resistance. The second resistance mechanism is active efflux. In this case, the target fungi of azole action have proteins belonging to the superfamily of ATP binding cassette proteins, such as CDR1 and CDR2, and the main facilitating superfamily, such as MDR1, which are responsible for the expulsion of azole molecules (Vanden et al., 1992; Fuentes et al., 2014), overexposure of membrane transporters, and alteration of ergosterol synthesis (Bhanderi et al., 2009; Yegenoglu, 2012; Moriello, 2020). Mereles et al. (2020) reported lower antifungal activity of fluconazole in M. canis strains. These data show the need to consider mycological studies in pet cats to determine their status as dermatophyte carriers and thus facilitate the adoption of appropriate preventive behaviors by veterinarians. ConclusionIn Talca, Chile, healthy cats have a dermatophyte carrying of 5.3%, mainly associated with the indoor condition, with M. canis being the only isolated species, showing antifungal sensitivity to clotrimazole, voriconazole, caspofungin, amphotericin B, and nystatin, but resistant to fluconazole. AcknowledgmentsThe authors would like to thank the veterinary clinics in the city of Talca that agreed to participate in this study. Conflicts of interestThe authors declare no conflicts of interest. Authors’ contributionAHN and RAC contributed to the conception and design of the study; MGP collected the samples and performed all laboratory assays; AHN, VSV, and RAC performed statistical analyses and wrote the first draft of the manuscript; and AHN, VSV, and RAC critically revised the manuscript. All authors contributed to the revision of the manuscript and read and approved the submitted version. ReferencesAdimi, P., Hashemi, S.J., Mahmoudi, M., Mirhendi, H., Shidfar, M.R., Emmami, M., Rezaei-Matehkolaei, A., Gramishoar, M. and Kordbacheh, P. 2013. In-vitro activity of 10 antifungal agents against 320 dermatophyte strains using microdilution method in Tehran. Iran. J. Pharm. Res. 12, 537–545. Agarwal, R., Gupta, S., Mittal, G., Khan, F., Roy, S. and Agarwal, A. 2015. Antifungal susceptibility testing of dermatophytes by agar based disk diffusion method. Int. J. Curr. Microbiol. App. Sci. 4, 430–436. Alcazar-Fuoli, L. and Mellado, E. 2014. Current status of antifungal resistance and its impact on clinical practice. Br. J. Haematol. 166, 471–484. Alpun, G. and Ozgur N.Y. 2009. Mycological examination of Microsporum canis infection in suspected dermatophytosis of owned and ownerless cats its asymptomatic carriage. J. Anim. Vet. Adv. 8, 803–806. Aneke, C.I., Otranto, D. and Cafarchia, C. 2018. Therapy and antifungal susceptibility profile of Microsporum canis. J. Fungi. 4, 107. Baghza, M.N. 2016. Isolation and identification of potential zoonotic dermatophytes from domestic camels in Dhamar Area, Yemen. Am. J. Health Res. 4, 46. Betancourt, O., Salas, V., Otarola, A., Zaror, L., Salas, E. and Neumann, J. 2009. Microsporum canis in dermatologically healthy cats in Temuco, Chile. Rev. Iberoam. Micol. 26, 206–210. Bhanderi, B., Yadav, M. and Roy, A. 2009. Antifungal drug resistance - concerns for veterinarians. Vet. World 2, 204. Bishnoi, A., Vinay, K. and Dogra, S. 2018. Emergence of recalcitrant dermatophytosis in India Lancet Infect. Dys. 18, 250–251. Bueno, J.G., Martinez, C., Zapata, B., San Clemente, G., Gallego, M. and Mesa, A.C. 2010. In vitro activity of fluconazole, itraconazole, voriconazole and terbinafine against fungi causing onychomycosis. Clin. Exp. Dermato. 35, 658–663. Cafarchia, C., Romito, D., Sasanelli M., Lia, R., Capelli, G. and Otranto, D. 2004. The epidemiology of canine and feline dermatophytoses in southern Italy. Mycoses 47, 508–513. CLSI. 2010. Method for antifungal disk diffusion susceptibility testing of nondermatophyte filamentous fungi: approved guideline. CLSI document M51-A. Wayne, PA: Clinical and Laboratory Standards Institute. CLSI. 2017. Reference method for broth diffusion antifungal susceptibility testing of filamentous fungi. 3rd ed. CLSI standard M38. Wayne, PA: Clinical and Laboratory Standards Institute. CLSI. 2018. Method for antifungal disk diffusion susceptibility testing of yeasts. 3rd ed. CLSI guideline M44. Wayne, PA: Clinical and Laboratory Standards Institute. Debnath, C., Mithras, T., Kumar, A. and Samanta, I. 2016. Detection of dermatophytes in healthy companion dogs and cats in eastern India. IJVR. 17, 20–24. DeBoer, D.J. and Moriello, K.A. 1994. Development of an experimental model of Microsporum canis infection in cats. Vet. Microbiol. 42, 289–295. Degreef, H. 2008. Clinical forms of dermatophytosis (ringworm infection). Mycopathologia 166, 257–265. Di Rienzo, J., Cassanoves, F., Balzarini, M., González, L., Tablada, M. and Robledo, C. 2008. InfoStat. Córdoba, Spain: National University of Córdoba. European Scientific Counsel Companion Animal Parasites (ESCCAP) Guideline. 2011. Superficial mycoses in dogs and cats. Available via http://www.esccap.org/ Frymus, T., Gruffydd-Jones, T., Pennisi, M.G., Addie, D., Belák, S., Boucraut-Baralon, C., Egberink, H., Hartmann, K., Hosie, M., Lloret, A., Lutz, H., Marsilio, F., Möstl, K., Radford, A., Thiry, E., Truyen, U. and Horzinek, M.C. 2013. Dermatophytosis in cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 15, 598–604. Fuentes, M., Hermosilla, G., Alburquenque, C., Falconer, MA., Amaro, J. and Tapia, C. 2014. Characterization of azole resistance mechanisms in Chilean clinical isolates of Candida albicans. Rev. Chilean Infectol. 31, 511–517. Ginter-Hanselmayer, G., Weger, W., Ilkit, M. and Smolle, J. 2007. Epidemiology of tinea capitis in Europe: current state and changing patterns. Mycoses 50, 6–13. Gupta, S., Agarwal, R., Mittal, G., Roy, S., Khan, F. and Agarwal, A. 2015. Comparison of broth micro dilution and disk diffusion method for susceptibility testing of dermatophytes. Int. J. Curr. Microbiol. App. Sci. 4, 24–33. Gupta, A.K. and Del Rosso, J.Q. 2000. An evaluation of intermittent therapies used to treat onychomycosis and other dermatomycoses with the oral antifungal agents. Int. J. Derm. 39, 401–411. Idris, A., Getso, M., Hafiz, T. and Kabuga, A. 2025. Recommendations on selection of antifungal agents for the treatment of superficial mycoses in people living with diabetes mellitus. Niger. J. Med. 33, 223–227. Ilhana, Z., Karacab, M., Hakki Ekina, I., Solmazc, H., Akkanb, H.A. and Tutuncud, M. 2016. Detection of seasonal asymptomatic dermatophytes in Van cats. Braz. J. Microbiol. 47, 225–230. Levy, H., Luzes, J., Ramiro, S., Friciello, R. and Acqua, S. 2006. Isolation of Microsporum gypseum from the haircoat of health wild felids kept in captivity in Brazil. Brazi. J. Microbiol. 37, 148–152. López, M.F., Grilli, D., Degarbo, S., Arenas, G. and Teleche, A. 2012. Frequency of dermatophytes in a sample of felines from the urban area of Greater Mendoza, Argentina. Rev. Iberoam. Micol. 29, 238–240. Lund, A., Bratberg, A.M., Nass, B. and Gudding, R. 2014. Control of bovine ringworm by vaccination in Norway. Vet. Immunol. Immunopathol. 158, 37–45. Mahajan, S., Tilak, R., Kaushal, S., Mishra, R. and Pandey S. 2017. Clinico-mycological study of dermatophytic infections and their sensitivity to antifungal drugs in a tertiary care center. Indian J. Dermatol. Venereol. Leprol. 83, 436–440. Mancianti, F., Nardoni, S., Corazza, M., D’achille, P. and Ponticelli, C. 2013. Environmental detection of Microsporum canis arthrospores in the households of infected cats and dogs. J. Feline Med. Surg. 5, 323–328. Mariat, F. and Tapia, G. 1966. Dénombrement des champignons keratinophiles de une population de Cynocephales (Papio papio). Ann. Parasitol. 41, 627–634. Mereles, B., Fiedler, J., Bruquetas, A. and Chade, M. 2020. Evaluación de la sensibilidad de hongos dermatofitos aislados de muestras clínicas a los antifúngicos. Rev. Cienc. Technol. 34, 101–106. Moriello, K. 2014. Feline dermatophytosis: aspects pertinent to disease management in single and multiple cat situations. J. Feline Med. Surg. 16, 419–431. Moriello, K.A. 2020. Dermatophytosis. In Feline dermatology. Eds., Chiara Noli and Silvia Colombo. Cham, Switzerland: Springer Nature Switzerland AG. Moriello, K.A., Coyner, K., Paterson, S. and Mignon, B. 2017. Diagnosis and treatment of dermatophytosis in dogs and cats. Vet. Dermatol. 28, 266–e68. Moriello, K.A. and DeBoer, D.J. 2012. Dermatophytosis. In Infectious diseases of the dog and cat. 4th ed. Ed. Greene, C.E. St Louis, MO: Elsevier, pp: 588–602. Nardoni, S., Papini, R., Verin, R. and Mancianti, F. 2010. Survey on the role of brown hares (Lepus europaeus, Pallas 1778) as carriers of zoonotic dermatophytes. Italian J. Anim. Sci. 9, 126–128. Nocua-Báez, L.C., Uribe-Jerez, P., Tarazona-Guaranga, L., Robles, R. and Cortés, J.A. 2020. Azoles then and now: a review. Rev. Chilena Infectol. 37, 219–230. Or, M.E., Bakirel, U., Baran, A., Gurel, A., Ak, K., Dodurka, T., Ayyildiz, G. and Tan, H. 2000. The effects of oral antimycotics on serum testosterone levels, spermatogenesis, and other semen characteristics in dogs. Istanbul Univ. Vet. Fak. Derg. 26, 77–98. Orozco, A.S., Higginbotham, L.M., Hitchcock, C.A., Parkinson, T., Falconer, D. and Ibrahim, A.S. 1998. Mechanism of fluconazole resistance in Candida krusei. Antimicrob. Agents Chemother. 42, 2645–2649. Pakshir, K., Bahaedinie, L., Rezaei, Z., Sodaifi, M. and Zomorodian, K. 2009. In-vitro activity of six antifungal drugs against clinically important dermatophytes. Jundishapur J. Microbiol. 2, 158–163. Piérard, G.E. 2016. Dermatomycoses due to dermatophytes. Rev. Med. Liege. 71, 147. Rodríguez, B. 2016. Atlas of mycological identification. Virtual gallery. Available via https://atlasdemicologia.wordpress.com/2016/03/29/microsporum-spp/ Silva, V. and Zaror, L. 2015. Mycological diagnosis in the laboratory. 1st ed. Chile, Universidad Mayor. Silva, V., Thomson, P., Maier L. and Anticevic, S. 2003. Infection and colonization by dermatophytes in canids from the southern area of Santiago, Chile. Rev. Iberoam. Micol. 20, 145–148. Singh, A.D., Debnath, C. and Banerjee, A. 2021. Epidemiological investigation, characterization and antifungal susceptibility profile of Microsporum canis isolated from pet animals. Veterinarski Arhiv. 91, 339–347. Sparkes, A.H., Werrett, G., Stokes, C.R. and Gruffydd-Jones, T.J. 1994. Microsporum canis: inapparent carriage by cats and the viability of arthrospores. J. Small Anim. Pract. 35, 397–401. Thompson, R.C.A. 2000. Giardiasis as a reemerging infectious disease and its zoonotic potential. Inter. J. Parasitol. 30, 1257–1267. Vanden Bossche, H., Marichal, P., Odds, F.C., Le Jeune, L. and Coene, M.C. 1992. Characterization of an azole-resistant Candida glabrata isolate. Antimicrob. Agents Chemother. 36, 2602–2610. Vitiello, A., Ferrara, F., Boccellino, M., Ponzo. A., Cimmino. C., Comberiati. E., Zovi. A., Clemente, S. and Sabbatucci, M. 2023. Antifungal drug resistance: an emergent health threat. Biomedicines 11, 1063. Weitzman, I. and Summerbell, R.C. 1995. The dermatophytes. Clin. Microbiol. Rev. 8, 240–259. Wiegand, C., Mugisha, P., Mulyowa, GK., Elsner, P., Hipler, UC., Gräser, Y., Uhrlaß, S. and Nenoff, P. 2016. Identification of the causative dermatophyte of tinea capitis in children attending Mbarara Regional Referral Hospital in Uganda by PCR-ELISA and comparison with conventional mycological diagnostic methods. Med. Mycol. 55, 660–668. Yegenoglu, Y. 2012. Fungi with antifungal resistance. ANKEM J. 26, 254–260. Zaror, L., Casas, S., Martín, R., Thibot, J. and Fishman, O. 1998. Dermatophytes in healthy dogs and cats in Valdivia, Chile. Arch. Med. Vet. 20, 140–141. Zhan, P., Dukik, K., Li, D., Sun, J., Stielow, J.B., Van den Ende, B.G., Brankovics, B., Menken, S.B.J., Mei, H. and Bao, W. 2018. Phylogeny of dermatophytes with genomic character evaluation of clinically distinct Trichophyton rubrum and T. violaceum. Stud. Mycol. 89, 153–175. | ||
| How to Cite this Article |
| Pubmed Style Núñez A, Silva V, Pereira MG, Castro R. Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy cats. Open Vet. J.. 2025; 15(8): 3823-3830. doi:10.5455/OVJ.2025.v15.i8.47 Web Style Núñez A, Silva V, Pereira MG, Castro R. Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy cats. https://www.openveterinaryjournal.com/?mno=252252 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.47 AMA (American Medical Association) Style Núñez A, Silva V, Pereira MG, Castro R. Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy cats. Open Vet. J.. 2025; 15(8): 3823-3830. doi:10.5455/OVJ.2025.v15.i8.47 Vancouver/ICMJE Style Núñez A, Silva V, Pereira MG, Castro R. Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy cats. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3823-3830. doi:10.5455/OVJ.2025.v15.i8.47 Harvard Style Núñez, A., Silva, . V., Pereira, . M. G. & Castro, . R. (2025) Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy cats. Open Vet. J., 15 (8), 3823-3830. doi:10.5455/OVJ.2025.v15.i8.47 Turabian Style Núñez, Andrea, Victor Silva, María Gabriela Pereira, and Rodrigo Castro. 2025. Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy cats. Open Veterinary Journal, 15 (8), 3823-3830. doi:10.5455/OVJ.2025.v15.i8.47 Chicago Style Núñez, Andrea, Victor Silva, María Gabriela Pereira, and Rodrigo Castro. "Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy cats." Open Veterinary Journal 15 (2025), 3823-3830. doi:10.5455/OVJ.2025.v15.i8.47 MLA (The Modern Language Association) Style Núñez, Andrea, Victor Silva, María Gabriela Pereira, and Rodrigo Castro. "Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy cats." Open Veterinary Journal 15.8 (2025), 3823-3830. Print. doi:10.5455/OVJ.2025.v15.i8.47 APA (American Psychological Association) Style Núñez, A., Silva, . V., Pereira, . M. G. & Castro, . R. (2025) Antifungal susceptibility testing of Microsporum canis isolated from the skin of dermatologically healthy cats. Open Veterinary Journal, 15 (8), 3823-3830. doi:10.5455/OVJ.2025.v15.i8.47 |