| Research Article | ||
Open Vet. J.. 2025; 15(9): 4540-4547 Open Veterinary Journal, (2025), Vol. 15(9): 4540-4547 Research Article Electrochemotherapy for canine prostatic carcinoma treatmentFilomena Assunta Amato1, Enrico Pierluigi Spugnini2, Valeria Attorri3, Chiara Catalucci1, Francesco Menicagli4, Angela Vittoria De Magistris5 and Paola Valenti1*1Clinica Veterinaria Malpensa AniCura, Samarate, Italy 2Biopulse Srl, Roma, Italy 3Clinica RivieraVet-POVA, Grottammare, Italy 4Ecovet, Roma, Italy 5Ospedale Veterinario i Portoni Rossi AniCura, Zola Predosa, Italy *Corresponding Author: Paola Valenti. Clinica Veterinaria Malpensa AniCura, Via G. Marconi 27, 21017, Samarate, Italy. Tel.: + 39 0331228155; Fax: + 39 033120255. Email: paola.valenti [at] anicura.it Submitted: 11/04/2025 Revised: 21/07/2025 Accepted: 16/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
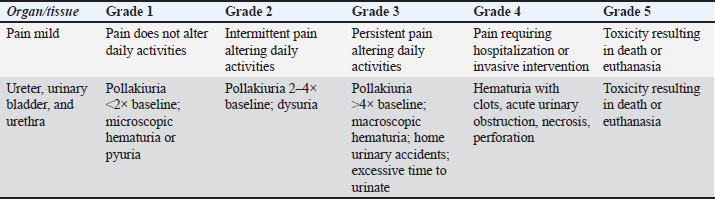
AbstractBackground: Canine prostatic carcinoma (PC) is an uncommon neoplasia characterized by aggressive biological behavior. Treatment options include surgery (partial or total prostatectomy), radiation therapy (RT), and medical treatment [non-steroidal anti-inflammatory drugs (NSAIDs), chemotherapy, or tyrosine kinase inhibitors]. Electrochemotherapy (ECT) is a local treatment modality increasingly used in human and veterinary oncology. Aim: This retrospective multi-institutional study aimed to assess the safety, feasibility, and preliminary efficacy of ECT as a treatment for canine PC. Methods: Dogs with cytologically or histologically confirmed PC treated with ECT as first-line therapy were included in this study. Dogs with distant metastases or those previously treated with surgery, RT, or chemotherapy were excluded. The clinical response was evaluated based on the improvement of clinical signs and reduction in tumor volume. Adverse effects, time to progression, and median survival time (MST) were recorded. Results: Nine dogs were enrolled in this study. All but two dogs were neutered. Regional lymph node metastases were present in two patients (22%). Metastatic lymph nodes were treated concurrently with ECT in one patient. Three patients (33%) had a partial response, three (33%) had stable disease, 2 (22%) had progressive disease, and one had a complete response. The overall response rate, including complete and partial responses, was 44%. The median TTP was 74 days, and MST was 70 days. The treatment was well tolerated, with no observed local or systemic adverse effects. Conclusion: This preliminary study showed that ECT is a safe and well-tolerated local therapy, although survival outcomes were modest compared with those of surgery, RT, or medical treatment. Further controlled, prospective studies are needed to better define its role as a therapeutic option. Keywords: Dog, Electrochemotherapy, Prostatic carcinoma, Safety, Treatment. IntroductionCanine prostatic carcinoma (PC) is a relatively uncommon tumor with a reported prevalence of 0.2%–0.6% (Bell et al., 1991). It is most often diagnosed in older dogs with a mean age of 10 years; an increased risk of developing this disease has been demonstrated in castrated males (Bryan et al., 2007). PC carries a poor prognosis because of its typically late diagnosis: the metastatic rate at diagnosis is >40%, rising to 80% at the time of death with spread primarily to locoregional lymph nodes, lungs, liver, spleen, and bone (Bell et al., 1991; Cornell et al., 2000; Hazzah et al., 2013). The most common clinical signs at diagnosis are hematuria, pollakiuria, stranguria, dyschezia, tenesmus, and constipation, which are a direct consequence of external compression of the urethra and descending colon by enlarged prostate and/or lymph nodes. Lameness, neurological signs, lethargy, inappetence, and weight loss are also associated with distant metastatic lesions (Krawiec and Helfin, 1992). Several techniques, including traumatic catheterization, prostatic massage, prostatic wash, ejaculation, ultrasound-guided fine needle aspiration (FNA), and percutaneous and surgical biopsy, have been described to achieve a diagnosis (Griffin et al., 2018). The median survival time (MST) in dogs with PC varies widely, ranging from 17 to 654 days, depending on the stage at diagnosis and treatment pursued (Griffin et al., 2018). Several therapeutic options for PC have been proposed, including surgery (Vlasin et al., 2006; Bennet et al., 2018; Stans, 2020), radiation therapy (RT) (Nolan et al., 2012; Griffin et al., 2018), and medical treatment (Sorenmo et al., 2004; Ravicini et al., 2018). Currently, a standard-of-care consensus for this neoplasia has not been established. As most dogs are euthanized due to local obstructive disease, research has focused on local treatment, such as complete or partial prostatectomy, photodynamic therapy, and RT. However, RT treatment is not always feasible, both due to financial issues and the availability of RT centers worldwide. Regarding surgery, owners might decline this option because of the reported high incidence of complications (Leroy and Northrup, 2009); also, the recurrence rate in some reports exceeds 80% (Vlasin et al., 2006; Bennet et al., 2018). Electrochemotherapy (ECT) is a locoregional anticancer therapeutic modality that combines the use of chemotherapeutic drugs (most frequently bleomycin) with electric pulses, leading to cell membrane permeabilization and drug uptake. Improved chemotherapy uptake by cancer cells induces apoptotic death (Silve and Mir, 2011). The available publications evaluating ECT have shown favorable outcomes, particularly in skin tumors, such as soft tissue sarcomas, mast cell tumors, and squamous cell carcinoma (Ramos et al., 2024). ECT is being increasingly explored for the treatment of deep-seated tumors in human medicine, although traditionally employed for cutaneous and subcutaneous tumors. A study demonstrated the feasibility and efficacy of ECT in patients with unresectable colorectal liver metastases, reporting a 55% complete response rate and favorable 6 months survival outcomes without significant complications (Edhemovic et al., 2014). Another study supported the safety and therapeutic potential of ECT in pancreatic and hepatic tumors (Tafuto et al., 2015). These findings suggest that ECT is a potential therapeutic option for deep-seated solid tumors, particularly in patients who are not eligible for standard treatments. Some data on the use of ECT in deep-seated tumors are also available in veterinary medicine (Spugnini et al., 2017; Valenti et al., 2021). In canine PC, the neoplastic prostate typically increases in volume and extends cranially into the abdominal cavity. This anatomical change might facilitate the access of the electrodes into the organ, allowing their insertion using a transcutaneous, ultrasound-guided approach. A detailed literature search of the terms “prostate,” “ECT,” and “dog” did not show any previously published information regarding the use of ECT as a therapy in canine PC. This study aimed to retrospectively assess the feasibility, safety, and preliminary efficacy of ECT as a treatment for canine PC. The response to therapy, time to progression (TTP), and MST were described. Materials and MethodsStudy designThis multi-institution, retrospective, preliminary study was conducted from January 2016 to December 2023, with cases recruited from four referral institutions. Dogs with a cytological or histological diagnosis of PC and treated with ECT as first-line therapy were included in the study. We excluded dogs with severe comorbidities precluding general anesthesia, dogs with evidence of distant metastases, and dogs that had previously received conventional treatments, such as surgery, RT, or chemotherapy were excluded. In all cases, standard therapy (surgery, RT, medical treatment) was discussed as the primary treatment option with the owners and, if declined, ECT was offered. ESOPE and veterinary guidelines were followed for patient selection and procedures (Mir et al., 2006; Tellado et al., 2022). Tumor staging included a complete blood cell count, serum biochemical profile, urine analysis, three-view thoracic radiographs combined with abdominal ultrasound or whole-body contrast-enhanced computed tomography (CT) scan, based on the clinician’s preference. The number of ECTs and the interval between sessions were individualized for each case, depending on the treatment response. Abdominal ultrasound was performed every 2 weeks after the first ECT session for at least 1 month to evaluate tumor response based on the response evaluation criteria in solid tumors criterion reassessment. Additional evaluations, such as thoracic radiographs or blood tests, were performed at different intervals based on the patient’s clinical reassessment and at the discretion of the case’s clinician. Data collectionPatient data retrieved included: neuter status, breed, age, body weight, presenting clinical signs, and concurrent comorbidities. Information about the methods of diagnosis (cytological/histological diagnosis), type of diagnostic imaging used, results of staging, urethral involvement, concomitant presence of urinary tract infection (UTI), any type of treatment before ECT, and any adjuvant therapy [non-steroidal anti-inflammatory drugs (NSAIDs), chemotherapy] were recorded. Regarding ECT treatment, we evaluated the date of initiation, treatment of any other metastatic site beyond the prostate, number of sessions, interval between sessions, and adverse events. Treatment tolerability was assessed based on the development of any local or systemic side effect, classified according to the Veterinary Radiation Therapy Oncology Group criteria (Poirier et al., 2023). Tables 1 and 2 illustrate the adverse events grading and adverse effect categories considered for the patients. Response to treatment was defined by the primary clinician’s reassessment of the patient combined with ultrasound re-evaluation of the primary tumor and any treated lesion, and was based on the Veterinary Cooperative Oncology Group RECST criteria (Nguyen et al., 2015). Table 1. VRTOG grading system for adverse events.
Table 2. The VRTOG acute radiation morbidity scoring scheme evaluated for the dogs included in the study.
Responses were classified as complete response, partial response, stable disease, or progressive disease. Treatment protocolBased on the staging results, the primary tumor and locoregional metastasis were treated with percutaneous ultrasound-guided ECT (Fig. 1), after the administration of intravenous bleomycin and with dogs under general anesthesia and with adequate analgesia. Ultrasonographic examinations were performed using 3 different machines (Esaote MyLab Seven, Esaote, Genoa, Italy; Logiq S8, GE Healthcare, Milwaukee, WI; Versana Premier, GE Healthcare, Milwaukee, WI) depending on the institution. Each ultrasound examination and procedure was performed by a single operator in each institution. The recorded ultrasonographic features included prostatic size (length and height), shape (rounded or irregular), parenchymal echogenicity and echotexture, and involvement of lymph nodes and other abdominal organs. Prostatic length and height were measured on longitudinal views and correlated with the dog’s age and body weight whenever feasible. A urinary bladder catheter was placed for better visualization of the prostatic urethra. Bleomycin was intravenously injected as a bolus at a dose of 15,000 IU/m2, as indicated by ESOPE and veterinary guidelines. Eight to ten minutes after the chemotherapy injection, with the patient in dorsal or lateral recumbency, two-needle 80-mm length 45-S shielded electrodes were inserted in the neoplastic prostatic parenchyma. The enlarged prostate dislocated in the abdominal cavity was visualized using an ultrasound guide, and the electrodes were positioned in the center of the tumor. Sequences of eight biphasic pulses lasting 100 μs each were delivered to the tumor at a voltage of 1,000–1,300 V/cm, depending on the electrical conduction of the tumor. Since the electric field is highest around the electrode and between the electrodes, tumors were treated by moving and placing electrodes nearby for each sequential electric pulse application in case of tumors larger than the distance between the electrodes. Pulses were generated using an electroporator certified for veterinary use (Onkodisruptor®). Statistical analysisA descriptive statistic was performed. Continuous data were expressed as mean ± SD, and categorical data were expressed as percentages and frequencies. TTP was defined as the interval between the first ECT session and progressive disease. The MST was defined as the time between the first ECT session and death. The date and cause of death (related or not to PC) were evaluated. Dogs lost to follow-up, deceased from unrelated causes, or still alive at the conclusion of the study were censored. The MST was calculated using the Kaplan–Meier product-limit method. Statistical analysis was performed using the statistical software (Jamovi Version 2.4). Ethical approvalThis retrospective study included client-owned dogs, and informed consent was obtained at admission. Patient care was provided in accordance with good clinical practice standards and institutional guidelines. Ethical approval was obtained from the lead authors’ institution.
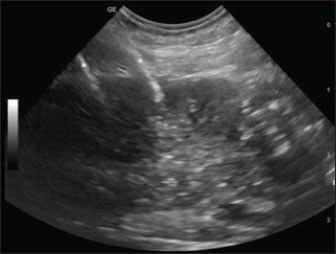
Fig. 1. Sagittal ultrasonographic image of a patient with neoplastic prostate. Right paramedian longitudinal scan, with a cranial orientation of the ultrasound marker (left side of the image). The right and left prostatic lobes are visualized along the near (superficial) and far (deep) aspects. A portion of the large intestine is visible caudally to the prostate. Despite relatively well-defined margins, the gland shows diffusely mild hypoechogenicity and coarse heterogeneous echotexture. Two hyperechoic lines (asterisks) located at the cranial and mid-levels of the right lobe correspond to the 45-S shielded electrodes inserted percutaneously. ResultsThe clinical data of the dogs included in the study are summarized in Table 3. Study populationNine dogs were included in this study. Breed distribution included five mixed breeds and one each of the following: American Cocker, Boxer, Jack Russell, and German Shepherd. All but two dogs were neutered (78.8%). The median age at diagnosis was 10.7 years (range: 5–14 years), and the median weight was 20.4 kg (range: 10–35 kg). The most common clinical sign at presentation was stranguria, present in six dogs (66.6%), followed by hematuria in three dogs (33.3%) and urinary tenesmus in two dogs (22.2%). Other clinical signs included dysuria, fecal tenesmus, and weight loss, each one in 11% of the patients. One dog presented with an abdominal wall mass, without any other clinical signs. This patient underwent omentalisation of a prostatic cyst months before presentation to the referral center; biopsy of the abdominal wall mass was compatible with adenocarcinoma. Based on the concurrent diagnosis of PC, a seeding metastasis was diagnosed. Two dogs presented with cardiologic comorbidities: myxomatous mitral valve disease and mitral and tricuspid valve disease. Diagnosis and stagingBlood tests were performed in all the dogs included in the study. Mild, non-regenerative anemia was present in two patients (11%). Biochemical abnormalities included an increase in blood urea nitrogen in four dogs (44.5%), an increase in serum creatinine in two dogs (22%), and an increase in liver enzymes in two dogs (22%). Concomitant UTI was present in one patient. Table 3. Staging, treatment details, and population outcome.
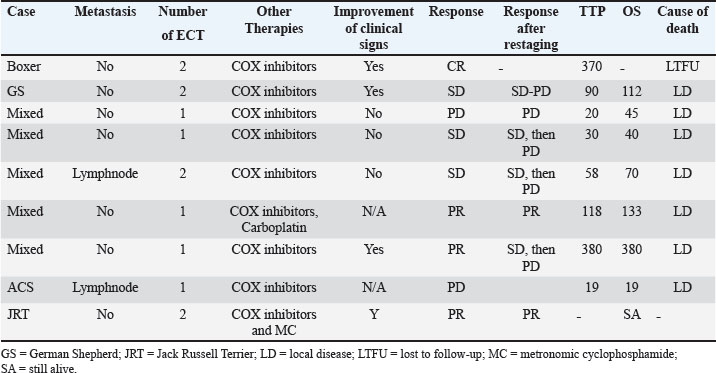
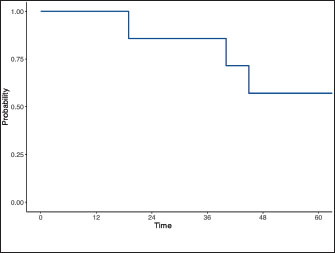
Histological diagnosis was available in five dogs, including adenocarcinoma in four (44.5%) and transitional cell carcinoma in one (11%). The diagnosis was based on cytology and was consistent with malignant epithelial tumor in the remaining four dogs (44%). A total of six dogs (66.6%) were staged using abdominal ultrasound and thoracic radiographs, while a contrast-enhanced total body CT scan was performed in three dogs (33%). All dogs had locoregional disease according to the staging at the time of diagnosis. Precisely, six dogs presented with prostate involvement only; two dogs presented with regional lymph node metastasis confirmed by cytology; and one dog presented with a lesion on the abdominal wall, most likely consistent with seeding. None of the dogs presented with urethral involvement based on diagnostic imaging evaluation. Medical therapyAll the dogs included in the study received NSAIDs, including piroxicam, firocoxib, and meloxicam. One dog received one carboplatin dose after the first ECT treatment. One dog received metronomic cyclophosphamide after two ECT sessions. Adjuvant chemotherapy was started only after the ECT treatment was discontinued. ECT treatment and associated adverse effectsFour dogs (44.5%) received two ECT sessions and five dogs (55%) received one session. In two patients, ECT was used to treat intra-abdominal metastatic lymph nodes, and in one dog, it was used to treat the abdominal wall mass concurrently with the primary lesion. No local or systemic side effects were documented in the patients. Tumor seeding along the electrode tract was not observed in any patient. Outcome and survivalThe overall response rate was 44%: three patients (33%) had a partial response, three (33%) had a stable disease, 2 (22%) had a progressive disease, and one patient (12%) had a complete response. In terms of clinical improvement, this information was available in 6 of 9 cases. Of these six dogs, four (66%) showed improvement in clinical signs. At the time of data collection, one dog (case 9) was alive 10 months after the first ECT. One dog (case 1) was lost to follow-up after PD was documented. Seven dogs (87%) died of tumor-related causes (progression of the local disease). One dog (case 6) was euthanized due to the progression of the abdominal wall lesion, despite being in partial remission in the prostate. The median TTP of the population was 74 days (95% CI, –0.577 to 229) and the overall MST was 70 days (95% CI, 8.76–262) (Fig. 2). DiscussionThis is the first preliminary study describing ECT treatment for PC. In our study, old, castrated male dogs were overrepresented, which is consistent with the previous literature and with Bryan et al.’s study (Bryan et al., 2007), which demonstrated a significant association between castration and PC development in companion dogs. Metastases are common in canine PC. One study of 76 dogs with PC documented metastatic disease in 80% of the dogs at necropsy (Cornell et al., 2000). The most common sites of metastasis were the lungs and lymph nodes. Bone and skeletal metastasis have also been reported in 22%–42% of dogs with PC. The most common sites of skeletal metastasis were the lumbar vertebrae and pelvis (Vail et al., 2019). In our study, the metastatic rate was lower than that in historical data. All dogs presented with locoregional disease, with only two patients presenting with regional lymph node involvement. None of the patients had distant metastasis. The incidence of metastasis in our study could have been underestimated due to the low number of cases (3) receiving a full staging using a total body CT scan, therefore, increasing the risk of underestimating skeletal or lung metastasis of small dimensions. Another reason to explain the low incidence of metastatic disease in our study is patient selection, as ECT was proposed in dogs without advanced disease, and dogs with distant metastasis were excluded from the study. A standard of care consensus therapy for dogs with PC does not currently exist, and therapy has historically been largely considered palliative.
Fig. 2. Kaplan–Meier survival curve depicting the population’s overall MST. The use of NSAIDs has resulted in an improved outcome in dogs with PC when compared to untreated dogs (7 vs. 0.7 months) (Sorenmo et al., 2004). The combination of NSAIDs and chemotherapy may improve quality of life and prolong survival. A recent study reported that dogs receiving NSAIDs combined with chemotherapy experienced signifsicantly longer MST (106 vs. 51 days) and TTP (76 vs. 44 days) (Ravicini et al., 2018). The reported MST following prostatectomy ranges from 19 to 231 days, with better survival time in dogs receiving adjuvant therapies such as NSAIDs and chemotherapy and with tumors confined to the prostate or prostatic urethra, without evidence of metastasis at the time of surgery (Goldsmid and Bellenger, 1991; Vlasin et al., 2006; Bennet et al., 2018). RT has also been described: a study on intensity-modulated and image-guided RT (IMRT) showed an MST of 317 days (Nolan et al., 2012). A more recent study reported a median survival of 220 days following definitive-intent IMRT and a median overall survival of 563 days (Walz et al., 2020). In our study, the median TTP and MST were 74 and 70 days, respectively, shorter than previously reported data in patients receiving surgery, RT, or medical treatment. The MST of our patients was only mildly improved compared to the MST of historical patients not receiving any form of treatment. Therefore, these findings do not provide sufficient evidence to support ECT as an effective therapeutic option for PC in dogs. Nonetheless, our results offer a preliminary basis for future investigations involving larger patient cohorts to further explore the potential role of ECT as a palliative treatment. Based on our study results, ECT was safe and well tolerated. We hypothesized that ECT might cause side effects similar to those reported with RT, such as uroabdomen, urethral stricture/obstruction, dysuria, and UTI, but none of our patients had local or systemic adverse effects. This finding could be related to the short follow-up period and MST because some of these adverse effects are more often observed after weeks or months. One dog was euthanized for severe prostate calcification causing dysuria and pain, which was not present at the time of presentation and detected during the follow-up period, despite the reduction in prostate volume after ECT treatment. Mineralization is a common pattern in epithelial tumors, and the presence of parenchymal mineralization in the prostate, as identified by either abdominal radiographs or ultrasound, is considered highly suspicious for prostatic neoplasia (Bradbury et al., 2009). In this case, we could not discriminate between an adverse local effect of the treatment or a sign of PD, which was considered less likely because the tumor volume remained stable. No late side effects were reported in the few dogs for whom long-term follow-up was available, but further studies are needed to confirm the low incidence of side effects associated with ECT. None of our patients developed tumor seeding along the electrode tract. Many clinicians avoid performing percutaneous aspirates and biopsies of suspected prostatic neoplastic lesions because of the risk of tumor seeding along the needle tract. Despite the clinical evidence of seeding, based on the current literature, seeding seems to be rare. Tumor-tract implantation is a rare complication of FNA in humans, occurring at an estimated frequency of only 0.009%. According to experimental studies, tumor cells may leak into the tissues and be deposited along the needle tract (Struve-Christensen, 1978). Only a few cells survive and are mainly destroyed by the host’s immune system (Smith, 1991). In our study, needle tract seeding was not reported, but it might be related to the short follow-up period of our patients and, in general, the short survival time in dogs with PC. We could also speculate that only a few cells survive the damage caused by ECT or that the local effect of ECT on the host’s immune system might play a beneficial role. Therefore, the risk of neoplastic dissemination because of needle puncture should be weighed against the treatment’s possible beneficial effect. In this study, ECT was used as the first-line therapy in all dogs. However, all patients received concurrent medical treatment with nonsteroidal anti-inflammatory drugs. Cyclooxygenase-2 (Cox-2) expression has been documented in several malignancies, both in humans and in dogs. Sorenmo’s study (Sorenmo et al., 2004) showed that 88.2% of canine prostate tumors expressed Cox-2, which is not expressed in normal prostate glands, suggesting that Cox-2 dysregulation may be involved in the pathogenesis of PC in dogs. The same study found a statistically significant improvement in survival times in dogs treated with piroxicam or carprofen compared with dogs not receiving NSAIDs (6.9 vs. 0.7 months). Therefore, we cannot exclude the influence of the use of NSAIDs on the response rate of our study. Only two patients received adjuvant chemotherapy: metronomic cyclophosphamide was started after two ECT sessions in one case. This dog was still alive at the end of the follow-up period of our study, with a disease-free interval of 10 months. In the second case, carboplatin was introduced in the treatment plan only after PD at the abdominal wall level was documented. The effect of chemotherapy on patient outcomes cannot be determined, even if it was introduced only after the completion of the ECT protocol. Therefore, further studies are needed to evaluate the effectiveness of ECT in the absence of possible interactions with other drugs on the response rate. Although the neoplastic prostate usually undergoes volumetric enlargement and dislocation in the abdominal cavity, making the organ accessible under ultrasound guidance, the procedure remains complex. Several steps need to be considered, such as careful pre-treatment planning for electrode positioning and the delivery of electric pulses, and a perfect positioning of the electrodes under ultrasound guidance, requiring a skilled radiologist. Compared with other ablative techniques, ECT has several advantages, including shorter treatment duration, the possibility of achieving larger tumor volumes, and its safety when treating tumors close to major vessels (Djokic et al., 2018; Zmuc et al., 2019). This study has several limitations related to its retrospective and multi-institutional design. The number of ECT sessions, the operators, the staging methods, the time to follow-up, and the imaging modality used to assess response were not standardized even though the treatment was performed with the same electroporator in the different centers. The absence of CT imaging in all patients may have underestimated the disease stage, potentially contributing to the observed short survival time. The small sample size limits the statistical power of our findings and does not allow us to assess the treatment effectiveness. The lack of a control group precludes direct comparison and makes isolating the specific impact of ECT from other potential influencing factors, such as concomitant and adjuvant therapies, challenging. This last topic complicates the interpretation of results and may obscure the effects of treatment. ConclusionThis preliminary study showed that ECT is safe and well tolerated for the treatment of canine PC. However, the available data do not provide sufficient evidence to support the effectiveness of ECT as a therapeutic modality for the treatment of PC. Nonetheless, the results offer a preliminary basis for future prospective studies involving larger cohorts to evaluate ECT as an alternative local palliative option, especially when RT or surgery is not feasible. AcknowledgmentsNone. Conflict of interestThe authors declare no conflicts of interest regarding any financial, personal, or other relationships with individuals or organizations related to the material discussed in this manuscript. FundingThis study received no specific grant. Authors’ contributionsFAA, EPS, ADFM, and PV conceived and supervised the study; ADM and MF performed the ultrasonographic evaluation of prostatic neoplasms and assisted with the ultrasonographic guidance for the ECT sessions; CC, VA, and EPS were directly responsible for the clinical cases and assisted during clinical outcome follow-up and data collection. All authors have read and approved the final version of the manuscript. Data availabilityThe data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. ReferencesBell, F.W., Klausner, J.S., Hayden, D.W., Feeney, D.A. and Johnston, S.D. 1991. Clinical and pathologic features of prostatic adenocarcinoma in sexually intact and castrated dogs: 31 cases (1970–1987). J. Am. Vet. Med. Assoc. 199, 1623–1630. Bennett, T.C., Matz, B.M., Henderson, R.A., Straw, R.C., Liptak, J.M., Selmic, L.E., Collivignarelli, F. and Buracco, P. 2018. Total prostatectomy as a treatment for prostatic carcinoma in 25 dogs. Vet. Surg. 47(3), 367–377. Bradbury, C.A., Westropp, J.L. and Pollard, R.E. 2009. Relationship between prostatomegaly, prostatic mineralization, and cytologic diagnosis. Vet. Radiol. &.Ultrasound 50(2), 167–171. Bryan, J.N., Keeler, M.R., Henry, C.J., Bryan, M.E., Hahn, A.W. and Caldwell, C.W. 2007. A population study of neutering status as a risk factor for canine prostate cancer. Prostate 67, 1174–1181. Cornell, K.K., Bostwick, D.G., Cooley, D.M., Hall, G., Harvey, H.J., Hendrick, M.J., Pauli, B.U., Render, J.A., Stoica, G., Sweet, D.C. and Waters, D.J. 2000. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: a retrospective analysis of 76 cases. Prostate 45, 173–183. Djokic, M., Cemazar, M., Popovic, P., Kos, B., Dezman, R., Bosnjak, M., Zakelj, M.N., Miklavcic, D., Potrc, S., Stabuc, B., Tomazic, A., Sersa, G. and Trotovsek, B. 2018. Electrochemotherapy as treatment option for hepatocellular carcinoma, a prospective pilot study. Eur. J. Surgical Oncol. 44, 651–657. Edhemovic, I., Brecelj, E., Gasljevic, G., Marolt Music, M., Gorjup, V., Mali, B., Jarm, T., Kos, B., Pavliha, D., Grcar Kuzmanov, B., Cemazar, M., Snoj, M., Miklavcic, D., Gadzijev, E.M. and Sersa, G. 2014. Intraoperative electrochemotherapy of colorectal liver metastases. J. Surgical Oncol. 110(3), 320–327. Goldsmid, S.E. and Bellenger, C.R. 1991. Urinary incontinence after prostatectomy in dogs. Vet. Surg. 20(4), 253–256. Griffin, M.A., Culp, W.T.N. and Rebhun, R.B. 2018. Lower urinary tract neoplasia. Vet. Sci. 5, 96. Hazzah, T.N., Kass, P.H., Brodsky, E.M., Elpiner, A.K., Silver, M.L., Boute, N.J. and Post, G.S. 2013. Evaluation of mitoxantrone with piroxicam as first line therapy for carcinomas of the prostate in dogs. J. Appl. Res. Vet. Med. 11, 16–24. Krawiec, D., R. and Heflin, D. 1992. Study of prostatic disease in dogs: 177 cases (1981-1986). J. Am. Vet. Med. Assoc. 200, 1119–1122. Leroy, B., E. and Northrup, N. 2009. Prostate cancer in dogs: comparative and clinical aspects. Vet. J. 180, 149–162. Mir, L.M., Gehld, J., Sersae, G., Collins, C.G., Garbay, J.R., Billard, V., Geertsend, P.F., Rudolfe, Z., O’Sullivan, G.C. and Marty, M. 2006. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the Cliniporator by means of invasive or non-invasive electrodes. EJC Supplements b, 14–25. Nguyen, S.M., Thamm, D.H., Vail, D.M. and London, C.A. 2015. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comparative. Oncol. 13(3), 176–183. Nolan, M.W., Kogan, L., Griffin, L.R., Custis, J.T., Harmon, J.F., Biller, B.J. and Larue, S.M. 2012. Intensity-modulated and image-guided radiation therapy for treatment of genitourinary carcinomas in dogs. J. Vet. Intern. Med. 26, 987–995. Poirier, V.J., Keyerleber, M., Gordon, I.K., Turek, M.M., Kent, M.S., Bentley, E. and Lawrence, J. 2023. ACVR and ECVDI consensus statement: reporting elements for toxicity criteria of the veterinary radiation therapy oncology group v2.0. Vet. Radiol. &.Ultrasound 64(5), 789–797. Ramos, S.C., Dias-Pereira, P., Luís, A.L., Macfarlane, M. and Santos, A.A. 2024. Electrochemotherapy in dogs and cats–A review. Vet. Comparative. Oncol. 22(3), 311–321. Ravicini, S., Baines, S.J., Taylor, A., Amores-Fuster, I., Mason, S.L. and Treggiari, E. 2018. Outcome and prognostic factors in medically treated canine prostatic carcinomas: a multi-institutional study. Vet. Comp. Oncol. 16(4), 450–458. Silve, A. and Mir, L.M. 2018. Cell electropermeabilization and cellular uptake of small molecules: the electrochemotherapy concept. Clinical aspects of electroporation. 1st. New York, NY, USA: pp. 69–82. Smith, E. and H. 1991. Complications of percutaneous abdominal fine-needle biopsy. Radiology 178, 253–258. Sorenmo, K.U., Goldschmidt, M.H., Shofer, F.S., Goldkamp, C. and Ferracone, J. 2004. Evaluation of cyclooxygenase-1 and cyclooxygenase-2 expression and the effect of cyclooxygenase inhibitors in canine prostatic carcinoma. Vet. Comp. Oncol. 2(1), 13–23. Spugnini, E., P. and Baldi, A. 2019. Electrochemotherapy in veterinary oncology state-of-the-art and perspectives. Vet. Clin. Small Anim. 49(5), 967–979. Spugnini, E.P., Menicagli, F., Pettorali, M. and Baldi, A. 2017. Ultrasound guided electrochemotherapy for the treatment of a clear cell thymoma in a cat. Open Vet. J. 7(1), 57–60. Stans, J. 2020. Prostatectomy as a treatment for canine prostate cancer: a literature review. Open Vet. J. 10(3), 317–322. Struve-Christensen, E. 1978. Iatrogenic dissemination of tumor cells. Dissemination of tumor cells along the needle track after percutaneous, transthoracic lung biopsy. Dan. Med. Bull. 25(2), 82–87. Tafuto, S., Von Arx, C., De Divitiis, C., Maura, C.T., Palaia, R., Albino, V., Fusco, R., Membrini, M., Petrillo, A., Granata, V. and Izzo, F. 2015. ENETS Center of Excellence Multidisciplinary Group for Neuroendocrine Tumors in Naples (Italy). Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int. J. Surg. 21(Suppl 1), S78–S82. Tellado, M., Mir, L.M. and Maglietti, F. 2022. Veterinary guidelines for electrochemotherapy of superficial tumors. Front. Vet. Sci. 9, 868989. Vail D.M., Thamm D.H., and Liptak J.M. 2019. editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 6th ed. Philadelphia: Saunders (Elsevier);.. Valenti, P., Menicagli, F., Baldi, A., Barella, G., Catalucci, C., Attorri, V. and Spugnini, E.P. 2021. Evaluation of electrochemotherapy in the management of apocrine gland anal sac adenocarcinomas in dogs: a retrospective study. Open Vet. J. 11, 100–106. Vlasin, M., Rauser, P., Fichtel, T. and Necas, A. 2006. Subtotal intracapsular prostatectomy as a useful treatment for advanced-stage prostatic malignancies. J. Small Anim. Pract. 47(9), 512–516. Walz, J.Z., Desai, N., Van Asselt, N., Poirier, V.J., Hansen, K. and Selmic, L. 2020. Definitive-intent intensity-modulated radiation therapy for treatment of canine prostatic carcinoma: a multi-institutional retrospective study. Vet. Comparative Oncol. 18, 381–388. Zmuc, J., Gasljevic, G., Sersa, G., Edhemovic, I., Boc, N., Seliskar, A. and et al. 2019. Large liver blood vessels and bile ducts are not damaged by electrochemotherapy with beomycin in pigs. Sci. Rep. 9, 3649. | ||
| How to Cite this Article |
| Pubmed Style Amato FA, Spugnini EP, Attorri V, Catalucci C, Menicagli F, Magistris AVD, Valenti P. Electrochemotherapy for canine prostatic carcinoma treatment. Open Vet. J.. 2025; 15(9): 4540-4547. doi:10.5455/OVJ.2025.v15.i9.60 Web Style Amato FA, Spugnini EP, Attorri V, Catalucci C, Menicagli F, Magistris AVD, Valenti P. Electrochemotherapy for canine prostatic carcinoma treatment. https://www.openveterinaryjournal.com/?mno=252217 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.60 AMA (American Medical Association) Style Amato FA, Spugnini EP, Attorri V, Catalucci C, Menicagli F, Magistris AVD, Valenti P. Electrochemotherapy for canine prostatic carcinoma treatment. Open Vet. J.. 2025; 15(9): 4540-4547. doi:10.5455/OVJ.2025.v15.i9.60 Vancouver/ICMJE Style Amato FA, Spugnini EP, Attorri V, Catalucci C, Menicagli F, Magistris AVD, Valenti P. Electrochemotherapy for canine prostatic carcinoma treatment. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4540-4547. doi:10.5455/OVJ.2025.v15.i9.60 Harvard Style Amato, F. A., Spugnini, . E. P., Attorri, . V., Catalucci, . C., Menicagli, . F., Magistris, . A. V. D. & Valenti, . P. (2025) Electrochemotherapy for canine prostatic carcinoma treatment. Open Vet. J., 15 (9), 4540-4547. doi:10.5455/OVJ.2025.v15.i9.60 Turabian Style Amato, Filomena Assunta, Enrico Pierluigi Spugnini, Valeria Attorri, Chiara Catalucci, Francesco Menicagli, Angela Vittoria De Magistris, and Paola Valenti. 2025. Electrochemotherapy for canine prostatic carcinoma treatment. Open Veterinary Journal, 15 (9), 4540-4547. doi:10.5455/OVJ.2025.v15.i9.60 Chicago Style Amato, Filomena Assunta, Enrico Pierluigi Spugnini, Valeria Attorri, Chiara Catalucci, Francesco Menicagli, Angela Vittoria De Magistris, and Paola Valenti. "Electrochemotherapy for canine prostatic carcinoma treatment." Open Veterinary Journal 15 (2025), 4540-4547. doi:10.5455/OVJ.2025.v15.i9.60 MLA (The Modern Language Association) Style Amato, Filomena Assunta, Enrico Pierluigi Spugnini, Valeria Attorri, Chiara Catalucci, Francesco Menicagli, Angela Vittoria De Magistris, and Paola Valenti. "Electrochemotherapy for canine prostatic carcinoma treatment." Open Veterinary Journal 15.9 (2025), 4540-4547. Print. doi:10.5455/OVJ.2025.v15.i9.60 APA (American Psychological Association) Style Amato, F. A., Spugnini, . E. P., Attorri, . V., Catalucci, . C., Menicagli, . F., Magistris, . A. V. D. & Valenti, . P. (2025) Electrochemotherapy for canine prostatic carcinoma treatment. Open Veterinary Journal, 15 (9), 4540-4547. doi:10.5455/OVJ.2025.v15.i9.60 |