| Research Article | ||
Open Vet. J.. 2025; 15(8): 3831-3837 Open Veterinary Journal, (2025), Vol. 15(8): 3831-3837 Research Article Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus)Viski Fitri Hendrawan1*, Alsya Pritama2, Aulia Firmawati1, Intan Firdha Olien Noor Al Ichsan2 and Ertika Fitri Lisnanti31Department of Veterinary Reproduction, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia 2Students of Undergraduate, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia 3Program of Animal Husbandry, Faculty of Agriculture, Universitas Islam Kadiri, Kediri, Indonesia *Corresponding Author: Viski Fitri Hendrawan. Department of Veterinary Reproduction, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia. Email: viski [at] ub.ac.id Submitted: 11/04/2025 Revised: 20/07/2025 Accepted: 30/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
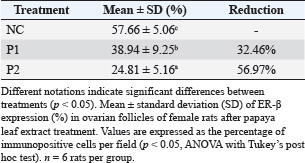
ABSTRACTBackground: Natural contraception is an affordable and accessible alternative to synthetic hormonal methods. One strategy for controlling overpopulation involves the use of plant-based agents that possess antifertility properties. Carica papaya (papaya) leaves have garnered scientific interest due to their rich content of bioactive compounds. Flavonoids, alkaloids, and saponins present in papaya leaves are known to influence reproductive physiology by modulating hormonal activity, disrupting follicular development, and altering uterine conditions. Aim: This study aimed to investigate the antifertility effects of papaya (Carica papaya L.) leaf extract by evaluating its influence on the length of the estrous cycle and the expression of estrogen receptor beta (ER-β) in the ovaries of female white rats (Rattus norvegicus). Methods: This study included six female albino rats (Rattus norvegicus) in the treatment group: negative control, P1 (3.15 mg/kgBW), and P2 (4.72 mg/kgBW) groups. Vaginal swab and Giemsa-stained epithelial histopathology were used to determine the estrous cycle duration. Immunohistochemistry was utilized to observe ovarian ER-β receptors. Results: The P2 dosage group demonstrated a 3-day increase in estrus cycle length and a 56.97% decrease in ovarian ER-β expression. This shows that the group can prolong the estrous cycle with a diestrus phase predominance and diminish ovarian ER-β expression, making it an anti-infertility drug. Conclusion: The P2 group best prolongs the estrous cycle of female white rats. The expression of ovarian ER-β decreases. Keywords: Antifertility, Extract of papaya leaf, ER-β, Vaginal Swab. IntroductionNatural contraception plays a significant role in reducing overpopulation due to its affordability and ease of use (Aritonang, 2019). Overpopulation of animal species, often due to the lack of natural predators or human intervention, can lead to habitat destruction, food source depletion, and negative impacts on biodiversity and ecosystem balance. In the context of animal population control, particularly among dogs and cats, veterinarians frequently employ both contraception and sterilization techniques. While sterilization such, as spaying and neutering, is effective, it is generally more expensive and invasive than natural contraceptive methods. According to Wang et al. (2021), natural contraception is a cost-effective alternative that can be more widely implemented, especially in communities with limited access to veterinary services or funding for surgical procedures. Tropical Indonesia is home to a rich diversity of flora and fauna, including a wide variety of medicinal plants with significant exhibiting antifertility effects, indicating a potential impact on reproductive function that warrants further investigation. Many indigenous herbs have been identified for their potential use in natural contraception. One such plant is papaya (Carica papaya), which is widely available and traditionally used for both nutritional and medicinal purposes. Various parts of the papaya plant, including its leaves, seeds, and fruit, are known to possess bioactive compounds with antifertility properties. According to Aritonang (2019), papaya leaves, in particular, contain substances that can be developed into natural contraceptive agents, offering a plant-based alternative to synthetic methods. These agents work by disrupting key physiological mechanisms in both the male and female reproductive systems, thereby inhibiting fertility. Post-copulation antifertility methods, in particular, act after mating to prevent fertilization from occurring. By lowering the chances of successful fertilization, these agents offer an effective, non-invasive approach to population control, especially in areas with limited access to surgical sterilization methods (Santana et al., 2019). Flavonoids, alkaloids, and saponins found in papaya leaves act as phytoestrogens by binding to estrogen receptors, which can mimic or modulate endogenous estrogen activity. This interaction may contribute to elevated circulating estrogen levels. Elevated estrogen exerts negative feedback on the hypothalamus, inhibiting the secretion of gonadotropin-releasing hormone (GnRH). As GnRH secretion decreases, the anterior pituitary reduces its release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), both of which are essential for follicular development. Suppressed FSH impairs follicle maturation, which may delay the onset of estrus. In addition, papaya steroidal alkaloids may influence gonadotropin balance, potentially altering ovarian structures such as follicle size, corpus luteum formation, and theca cell layer thickness and decreasing with steroid alkaloid compounds. Alkaloid compounds prevent ovulation and embryonic resorption (Dave and Trivedi, 2019). Saponin, a steroid derivative known for its antifertility properties, is one of the promising bioactive compounds found in various medicinal plants. Saponins can disrupt the activity of nerve cells in the hypothalamus, the part of the brain that produces the hormone GnRH. GnRH is a key hormone that regulates reproduction. Disruption of hypothalamic and pituitary activity disrupts the negative feedback loop on GnRH secretion. This hormonal imbalance affects the downstream release of LH and FSH, both of which are essential for normal reproductive function In addition to hormonal interference, saponins exert cytotoxic effects on reproductive cells. These compounds can compromise cell membrane integrity, leading to leakage and eventual cell lysis. As a result, ovarian follicle development may be significantly impaired, contributing to reduced fertility. Therefore, the presence of saponins in certain plants, such as papaya leaves, supports their potential application as natural contraceptive agents (Aritonang, 2019). Considering these factors, the potential of papaya leaf extract as a natural antifertility agent has become increasingly important. This research focuses on assessing its effects on both physiological aspects, such as the length of the estrous cycle, and molecular indicators, specifically the expression of estrogen receptor beta (ER-β) in the ovaries of female white rats (Rattus norvegicus). The findings are expected to offer scientific support for the use of papaya leaf extract in natural contraceptive applications. Materials and MethodsAnimalsThis study used Wistar-strain female white rats (Rattus norvegicus) from Brawijaya University’s Reproduction Laboratory. Giemsa-stained vaginal swabs were used to assess estrous cycle length by examining vaginal epithelial cell histology. Vaginal swabs are taken daily for 10 days. Ovarian organ samples were immunohistochemically analyzed for ER-β (Dissa et al., 2023). Preparation of the papaya leaf extractThe papaya leaf powder was extracted using distilled water by heating the mixture in a 60°C water bath for 30 minutes. The extract was then filtered using a standard Whatman filter paper to remove solid residues. The resulting filtrate was used for oral administration. The experimental animals were divided into two treatment groups based on the dosage. The first group (P1) received a dose of 3,15 mg/kg body weight (BW) of papaya leaf extract, whereas the second group (P2) received 4,72 mg/kg BW in 10 days. Each dose was diluted in distilled water to a final volume of 3 ml per rat and administered orally using a gastric sonde. The selected doses were adapted from Gong et al. (2012), the doses chosen (3.15 mg/kg and 4.72 mg/kg) are lower than the range used in that study (10–20 mg/kg). You could argue that your doses are an adjustment of the higher doses used for mice and that safety is improved. Euthanasia and organ collectionOn the 11th day of therapy, the rats were euthanized. Following cervical dislocation, rats were placed on a tray in dorsal recumbency for abdominal surgery and euthanized. The rat ovarium was surgically removed for estrogen receptor immunohistochemistry (IHC). Reproductive organs, including the ovaries, are removed when mice are euthanized. The organs are immersed in polyvinyl alcohol to avoid cell and shape deterioration (Ferreira et al., 2021). Vaginal swab and estrus cycle length observationVaginal swabs were collected daily from each rat using a moistened cotton swab gently inserted into the vaginal canal. The collected samples were then smeared onto glass slides, air-dried, and examined under a light microscope at 400× magnification to identify the phases of the estrous cycle based on the predominant cell types. The proestrus, estrus, metestrus, and diestrus phases were determined according to standard cytological criteria. The length of the estrous cycle was calculated by adding up the days in one complete cycle, including all four phases (Ferreira et al., 2021). Observation of ER-β expressionThe IHC method was used to observe ER-β expression in the ovaries. The immunohistochemical staining procedure can be done by soaking the ovarian preparation in xylol solution twice, then the preparation dehydration process is carried out using sequentially graded alcohol (96%, 90%, 80%, and 70%). Next, using phosphate buffered saline (PBS) solution with pH 7.4, the preparation is washed 3 times for 5 minutes each. The sample is soaked in 3% hydrogen peroxide (in distilled water) for 20 minutes, washed again using PBS solution with pH 7.4 3 times for 5 minutes each, then the preparation is soaked in bovine serum albumin (BSA) solution for approximately 10–30 minutes at room temperature, and washed again using PBS solution with pH 7.4 3 times for 5 minutes each. ER-β antibodies are added for 1 hour at room temperature and incubated for 1 night. After overnight, the slides were washed three times with PBS pH 7.4 for 5 minutes each. They were stained with hematoxylin and eosin for 5 minutes, then washed three times with distilled water for 5 minutes each. Entellan was used for mounting, and then observed using a microscope at 100 and 400 magnifications (Bintari and Yuliani, 2020). IHC preparations were observed using an optical microscope linked to a computer. The percentage of positive (brown) cells was calculated from 5 fields of view at 400× magnification. The ImmunoRatio plugin in ImageJ was used to quantify cell expression, yielding percentage values of positively stained nuclei (Hariono et al., 2021). Data analysisThe length of the estrous cycle and the expression of ER-β in the ovaries of female white rats (Rattus norvegicus) were examined in this study. Ethical approvalThis is an experimental laboratory study involving 78 experimental animal samples. The design used was a post-test only with control group design, meaning that measurements were only taken after treatment in the treatment and control groups. All research procedures were approved by the Animal Care and Use Ethics Committee, Brawijaya University, Malang, Indonesia, under approval number: 047-KEP-UB-2022. ResultsEffect of papaya leaf extract on estrus cycle length in white rats (Rattus norvegicus)The estrous cycle is a reproductive cycle associated with follicle development. The estrous cycle was observed using a vaginal swab to observe epithelial changes in each phase. The estrous cycle is linked to the hormone estrogen, which causes changes in vaginal epithelial cells. The estrus cycle begins with proestrus in the follicular phase, followed by estrus and ovulation. The estrous cycle is divided into four phases: proestrus, estrus, metestrus, and diestrus. The luteal phase refers to the part of the estrous cycle characterized by the presence and activity of the corpus luteum (Julianti et al., 2014) (Fig. 1). Based on the research results, the negative control group (A) had an estrus cycle of 4 days on average, while the P1 (3.15 mg/kgBW) research group (B) had an estrus cycle of 6 days, and the P2 group (4.72 mg/kgBW) (C) had an estrus cycle of 7 days. The estrus cycle length in female white rats (Rattus norvegicus) was measured by observing the estrus cycle using vaginal swabs for 10 days, and the results are presented in Table 1. Effect of papaya leaf extract on the expression of ER-β in the ovariesAccording to the research findings, the negative control group (A) had higher ER-β expression than groups P1 (3.15 mg/kgBB) and P2 (4.72 mg/kgBB) (C). There was a decrease in ER-β in the ovary cells of the group treated with papaya leaf extract (Fig. 2). Table 2 shows the average results of ER-β expression in the ovaries in each group. DiscussionPapaya leaf extract is rich in various bioactive compounds, including alkaloids, tannins, saponins, flavonoids, triterpenoids, and several volatile components (Rahmawati et al., 2023). Among these, flavonoids and alkaloids are classified as phytoestrogens, plant-derived compounds that mimic estrogen and exhibit steroid-like properties. These phytoestrogens, particularly alkaloids, triterpenoids, and terpenoids, influence the HPG axis. Their presence in the extract may facilitate its penetration into the central neuroendocrine system, disrupting normal hormonal signaling pathways. These compounds can interfere with the secretion of FSH and LH, both of which are crucial for initiating and regulating the reproductive cycle. FSH and LH inhibition during the early stages of the cycle leads to an imbalance characterized by a surge in estrogen dominance. This hormonal shift can result in alterations to the estrous cycle, such as delayed onset or an overall prolongation of the cycle duration. In certain conditions, such as axis disorders or complex hormonal therapy, the body’s feedback response can be nonlinear. Temporary hormonal blockade can cause the hypothalamus to release GnRH in a stronger pattern when the blockade is discontinued, leading to a secondary estrogen surge. The presence of phytoestrogens, such as flavonoids in papaya leaf extract, may bind to estrogen receptors and mimic endogenous estrogen activity. This receptor-level mimicry can create a state of plain estrogen dominance, altering the normal hormonal feedback mechanisms and potentially resulting in delayed estrus onset or prolonged estrous cycles (Widiana and Sumarmin, 2016). These findings highlight the potential of papaya leaf extract to modulate reproductive physiology through its phytochemical constituents. Phytoestrogens are naturally occurring plant compounds that exhibit structural and functional similarities to endogenous estrogen. These compounds can bind to estrogen receptors and modulate estrogenic activity within the body (Maheshwari et al., 2016). Depending on their concentration, affinity for estrogen receptor subtypes (ER-α and ER-β), and the hormonal status of the organism, phytoestrogens may act as estrogen agonists or antagonists. This dual action can result in a broad spectrum of physiological effects, including altered reproductive function, infertility, and even behavioral changes in animals (Aini et al., 2020).
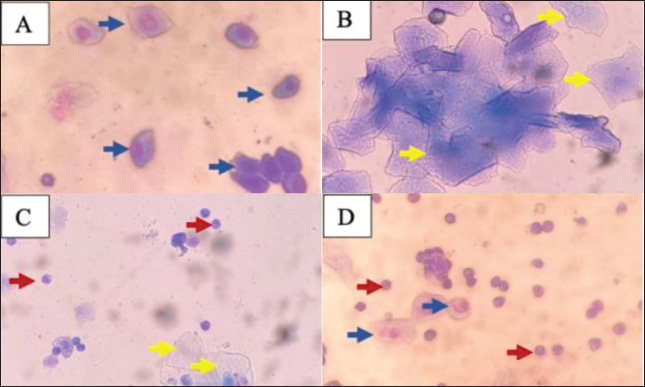
Fig. 1. Microscopic view of the estrous cycle in female rats (400×). A: Proestrus phase shows white blood cells, B: Estrus phase shows petiole cells with nuclei, C: Metestrus phase shows epithelial cells with nuclei and cornified cells, D: Diestrus phase shows white blood cells and cornified cells, ↑: epithelial cells with nucleus, ↑: cornified cells, ↑: leukocyte cells. Table 1. Average estrous cycle of female white rats (Rattus norvegicus).
One of the key reproductive impacts of phytoestrogens is their influence on the estrous cycle. Phytoestrogens can disrupt the normal hormonal regulation of the cycle by competing with endogenous estrogen for receptor binding sites. This receptor competition may inhibit normal estrogen signaling, resulting in a shortened overall estrous cycle and a prolonged estrus phase (Mostrom and Evans, 2018). Plants rich in phytoestrogens, such as papaya (Carica papaya), are of growing interest in the development of natural antifertility agents, especially in contexts where low-cost and accessible reproductive control is needed. Phytoestrogen flavonoids in papaya leaf bind to estrogen receptors and can exert either agonistic or antagonistic effects, depending on the concentration, receptor subtype (e.g., ER-α or ER-β), and physiological context. This interaction can influence estrogen-dependent processes, such as follicular development and reproductive hormone feedback. Negative feedback decreases hypothalamic GnRH. Lowering GnRH, FSH, and LH levels prolongs estrus. If the release of the FSH hormone decreases during diestrus, then follicular development and proestrus cannot occur. Follicular maturation occurs during the proestrus. Saponins kill fast-growing granulosa ovarian cells and remain in diestrus due to insufficient FSH, impairing ovarian follicle development. Saponins present in papaya leaf extract exhibit cytotoxic effects, particularly targeting granulosa cells, which actively proliferate during follicular development. Damage to these cells may impair folliculogenesis and contribute to follicular atresia. Follicle growth may slow down (Zou et al., 2022). Delayed follicle growth delays proestrus and estrus. Animal diestrus lasts 60–70 hours (Liu et al., 2018). Estrus: no pregnancy, no sexual activity, and the animal is calmer. Female animals reject male copulation except during estrus. Prolonging diestrus reduces female-male copulation and the risk of pregnancy (Man Le et al., 2022).
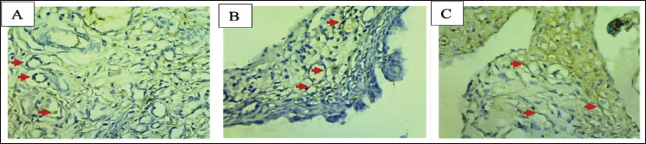
Fig. 2. Microscopic view of ER-β expression in ovarian cells using IHC (400×). A: Negative control, B: P1 dose (3.15 mg/kgBW of papaya leaf extract), C: P2 (4.72 mg/kgBW of papaya leaf extract), ↑: Expression of ER-β. Table 2. Average expression of ER-β in the ovaries.
The reduction in ER-β expression observed in treatment groups P1 (3.15 mg/kgBW) and P2 (4.72 mg/kgBW), which received papaya leaf extract, is likely attributed to antiestrogenic of the bioactive compounds present in the extract. The antiestrogenic components in papaya leaves may competitively bind to estrogen receptors, thereby inhibiting the binding of endogenous estrogen. This receptor competition impairs normal estrogen signaling, which is essential for follicular development and reproductive cycle regulation (Nafiu and Rahman, 2015). Antiestrogenic compounds can mimic estrogen structurally but lack the full biological efficacy required to activate receptor-mediated transcription. As a result, they occupy receptor sites without eliciting the same gene expression responses, effectively blocking the action of natural estrogen. This disruption leads to a decreased hormonal supply to the ovaries, which subsequently reduces the activation of estrogen receptor pathways critical for normal ovarian function, impairs the secretion of gonadotropins, particularly FSH and LH, which are essential for follicular development and estrogen production by the ovaries. As a result, reduced estrogen levels may lead to decreased activation of estrogen receptor pathways, particularly ER-β, which plays a critical role in regulating ovarian follicle maturation and function; then, diminished interaction between estrogen and its receptors results in weakened gene transcription and downregulation of ER-β expression (Oraebosi and Good, 2021). Consequently, downregulation or reduced activation of estrogen receptors, particularly in ovarian tissues, reflects impaired estrogen signaling. This compromised hormonal signaling environment can disrupt follicular development, reduce ovulation rates, and ultimately decrease fertility. Phytoestrogens have properties similar to the hormone estrogen. Phytoestrogens are plant-derived compounds that structurally resemble endogenous estrogens, particularly 17β-estradiol, allowing them to bind to estrogen receptors and exert estrogen-like or anti-estrogenic effects (Mastuti and Ciptono, 2017). Although estrogen is not found in plants, certain secondary metabolites—such as flavonoids, lignans, and coumestans—can act as phytoestrogens. Papaya leaf extract contains flavonoids that may influence circulating estrogen levels by mimicking estrogenic activity. This mimicry can trigger negative feedback mechanisms at the HPG axis, leading to reduced secretion of GnRH from the hypothalamus. Decreased GnRH levels can subsequently lower the secretion of FSH and LH by the anterior pituitary. Although flavonoids do not directly inhibit FSH production, their estrogenic activity may disrupt the hormonal balance necessary for normal follicular development. As a result, impaired FSH signaling can lead to follicular atresia where follicles fail to mature or undergo degeneration, ultimately inhibiting ovulation and potentially reducing fertility (Setiawan, 2022). ConclusionIn conclusion, the papaya leaf extract promotes the overall cycle length in female white rats. Estrous lasts 3–4 days longer than normal. The P2 (papaya leaf extract 4.72 mg/kg BB) group best prolongs the estrous cycle in female white rats. Additionally, papaya leaf extract reduced the expression of ER-β in the nuclei of ovarian follicles, as demonstrated by diminished brown immunohistochemical staining intensity. Although the P2 group showed the greatest reduction in ER-β expression, further statistical analysis is required to confirm whether this dose represents the optimal concentration for modulating receptor expression. AcknowlegmentsThe authors are grateful to the Faculty of Veterinary Medicine, Brawijaya University, for providing essential facilities to support this study. Conflict of interestThe author declares no conflicts of interest. FundingThe Faculty of Veterinary Medicine, Brawijaya University, provided funding. Data availabilityAll data supporting the findings of this study are available within the manuscript. Author’s contributionsVFH: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Original draft writing, Visualization, Supervision, Project administration, Funding acquisition. AF: Methodology, Validation, Investigation, Resources. AP: Writing an original draft, Visualization, Review, and Editing. ReferencesAini, H.A.N., Laksmi, D.N.D.I., Setiasih, N.L.E.S. and Purbantoro, S.D. 2020. Administration of black and red onco extracts extends the estrus cycle and thickens the endometrium of white mice. J. Vet. 21(4), e015050. Aritonang, E.A. 2019. Prospects for the use of papaya seeds as a biomaterial to control wild rat populations through antifertility mechanisms. Oc Biomed. J. 2, 15. Bintari, I. and Yuliani, M. 2020. Deteksi Aeromonas Hydrophila pada Ginjal Mencit Mus Musculus dengan Teknik Imunohistokimia. Agriekstensia J. Penelitian Terapan Bidang Pertanian 19(2), 114–120. Dave, H. and Trivedi, S. 2019. Carica papaya: potential implications in human health. Curr. Tradit. Med. 5, 321–336; doi:10.2174/2215083805666190705170022 Dissa, M., Yesuf, Y.K. and Belete, E. 2023. Effect of papaya (Carica papaya) seed as phytogenic feed additives on egg performance, egg quality and blood serum biochemical constituents of layer hens. Vet. Med. Sci. 9(6), 2747–2754. Ferreira, J.A.B., Ledo, C.A.S., Souza, F.V.D., Conceição, J.Q., Rossi, M.L. and Souza, E.H. 2021. Stigma structure and receptivity in papaya (Carica papaya L.). An. Acad. Bras. Ciênc. 93(1), e20190605. Gong, J.H., Shin, D., Han, S.Y., Kim, J.L. and Kang, Y.H. 2012. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J. Nutr. 142, 47–56. Hariono, M., Julianus, J., Djunarko, I., Hidayat, I., Adelya, L., Indayani, F., Auw, Z., Namba, G. and Hariyono, P. 2021. The future of Carica papaya leaf extract as an herbal medicine product. Molecules 26(22), 6922. Julianti, T., Oufir, M. and Hamburger, M. 2014. Quantification of the antiplasmodial alkaloid carpaine in papaya (Carica papaya) leaves. Planta Med. 80(13), 1138–1142. Liu, J., Sharma, A., Niewiara, M.J., Singh, R., Ming, R. and Yu, Q. 2018. Papain-like cysteine proteases in Carica papaya: lineage-specific gene duplication and expansion. BMC Genomics 19(1), 26. Maheshwari, H., Satyaningtijas, A.S., Harlina, E., Cahyaningsih, U., Effendi, M., Mustofa, M.A. and Bekalani, Y.K. 2016. The role of fennel infusion in estrous cycle and follicles development of white rats. J. Ilmu. Kefarm. Indones. 14(1), 19–25. Man Le, T., Huyen Tran, T.T., Quyen Vu, X., Duc Chu, H., Chau Pham, T., Thi Le, H., Thi Ngoc L, Q., Hong La, V. and Bang Cao, P. 2022. Genome-wide identification and analysis of genes encoding putative heat shock protein 70 in papaya (Carica papaya). Pak. J. Biol. Sci. 25(6), 468–475. Mastuti, R.Q.D. and Ciptono. 2017. Pengaruh pemberian ekstrak kacang merah (Phaseolus vulgaris, L.) terhadap perkembangan folikel ovarium tikus putih (Rattus norvegicus, L.). J. Prodi. Biol. 6(3), 13. Mostrom, M. and Evans, T.J. 2018. Phytoestrogens. 3rd ed. In Veterinary toxicology: basic and clinical principles. Hopkinsville, KY: Elsevier, pp: 817–833. Nafiu, A.B. and Rahman, M.T. 2015. Anti-inflammatory and antioxidant properties of unripe papaya extract in an excision wound model. Pharm. Biol. 53(5), 662–671. Oraebosi, M.I. and Good, G.M. 2021. Carica papaya augments anti-malarial efficacy of artesunate in Plasmodium berghei parasitized mice. Ann. Parasitol. 67(2), 295–303. Rahmawati, A.M., Anam, K. and Sasikirana, W. 2023. Potensi daun pepaya (Carica papaya L.) sebagai antikanker. J. Res. Pharm. 3(1), 27–35; doi:10.1016/j.jrp.2013.01.003 Santana, L.F., Inada, A.C., Espirito Santo, B.L.S.D., Filiú, W.F.O., Pott, A., Alves, F.M., Guimarães, R.D.C.A., Freitas, K.D.C. and Hiane, P.A. 2019. Nutraceutical potential of Carica papaya in metabolic syndrome. Nutrients 11(7), 1608. Setiawan, H. 2022. Pengaruh Ekstrak Etanol Daun Pepaya Calina terhadap Indeks Gonadosomatik dan Perkembangan Folikel Ovarium Tikus Wistar. Acta Vet. Indones. 10(3), 245–252. Wang, Z., Liu, P., Hu, M., Lu, S., Lyu, Z., Kou, Y., Sun, Y., Zhao, X., Liu, F. and Tian, J. 2021. Naoxintong restores ischemia injury and inhibits thrombosis via COX2-VEGF/NFκB signaling. J. Ethnopharmacol. 270, 113809. Widiana, R. and Sumarmin, R. 2016. Efek toksit dan teratogenik ekstrak Brotowali (Tinospora crispa L.) terhadap sistem reproduksi dan embrio mencit (Mus musculus L. Swiss Webster). BioCONCETTA 2(1), 1–11. Zou, Z., Guo, J., Zheng, Y., Xiao, Y. and Guo, A. 2022. Genomic analysis of LEA genes in Carica papaya and insight into lineage-specific family evolution in Brassicales. Life (Basel) 12(9), 1453. | ||
| How to Cite this Article |
| Pubmed Style Hendrawan VF, Pritama A, Firmawati A, , Lisnanti EF. Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus). Open Vet. J.. 2025; 15(8): 3831-3837. doi:10.5455/OVJ.2025.v15.i8.48 Web Style Hendrawan VF, Pritama A, Firmawati A, , Lisnanti EF. Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus). https://www.openveterinaryjournal.com/?mno=252103 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.48 AMA (American Medical Association) Style Hendrawan VF, Pritama A, Firmawati A, , Lisnanti EF. Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus). Open Vet. J.. 2025; 15(8): 3831-3837. doi:10.5455/OVJ.2025.v15.i8.48 Vancouver/ICMJE Style Hendrawan VF, Pritama A, Firmawati A, , Lisnanti EF. Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus). Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3831-3837. doi:10.5455/OVJ.2025.v15.i8.48 Harvard Style Hendrawan, V. F., Pritama, . A., Firmawati, . A., & Lisnanti, . E. F. (2025) Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus). Open Vet. J., 15 (8), 3831-3837. doi:10.5455/OVJ.2025.v15.i8.48 Turabian Style Hendrawan, Viski Fitri, Alsya Pritama, Aulia Firmawati, Intan Firdha Olien Noor Al Ichsan, and Ertika Fitri Lisnanti. 2025. Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus). Open Veterinary Journal, 15 (8), 3831-3837. doi:10.5455/OVJ.2025.v15.i8.48 Chicago Style Hendrawan, Viski Fitri, Alsya Pritama, Aulia Firmawati, Intan Firdha Olien Noor Al Ichsan, and Ertika Fitri Lisnanti. "Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus)." Open Veterinary Journal 15 (2025), 3831-3837. doi:10.5455/OVJ.2025.v15.i8.48 MLA (The Modern Language Association) Style Hendrawan, Viski Fitri, Alsya Pritama, Aulia Firmawati, Intan Firdha Olien Noor Al Ichsan, and Ertika Fitri Lisnanti. "Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus)." Open Veterinary Journal 15.8 (2025), 3831-3837. Print. doi:10.5455/OVJ.2025.v15.i8.48 APA (American Psychological Association) Style Hendrawan, V. F., Pritama, . A., Firmawati, . A., & Lisnanti, . E. F. (2025) Effectiveness of papaya leaf extract (Carica papaya L.) as an infertility agent on estrus cycle length and expression of estrogen receptor beta on ovaries of female white rats (Rattus norvegicus). Open Veterinary Journal, 15 (8), 3831-3837. doi:10.5455/OVJ.2025.v15.i8.48 |