| Review Article | ||
Open Vet. J.. 2025; 15(8): 3419-3427 Open Veterinary Journal, (2025), Vol. 15(8): 3419-3427 Review Article The incidence rates of human and animal echinococcosis: A systematic reviewVladimir Nicolaevich Domatskiy and Elena Ivanovna Sivkova*All-Russian Scientific Research Institute of Veterinary Entomology and Arachnology- Branch of Federal State Institution Federal Research Centre Tyumen Scientific Centre of Siberian Branch of the Russian Academy of Sciences, Tyumen, Russia *Corresponding Author: Elena Ivanovna Sivkova. All-Russian Scientific Research Institute of Veterinary Entomology and Arachnology- Branch of Federal State Institution Federal Research Centre, Tyumen, Russia. Email: sivkovaei73 [at] gmail.com Submitted: 08/04/2025 Revised: 01/07/2025 Accepted: 09/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
ABSTRACTThis review provides information about the prevalence of human and animal echinococcosis in various regions of the world. Many countries are endemic to this disease, especially in Eastern Europe, Asia, the North Caucasus, Kazakhstan, Kyrgyzstan, Uzbekistan, southern Russia, and other countries. The disease poses a biological threat to the world’s population, as the number of infected people around the globe increases every year, and the invasion progresses. Echinococcosis is a natural focal geohelminthiasis that is distributed among wild animals regardless of human presence; however, there are foci associated with human activity in cases in which the pathogen circulates in domestic animals and synanthropic mouse-like rodents. Two types of Echinococcus sp., namely E. multilocularis and E. granulosus, prevail in Central Europe and are causative agents of the two most significant forms: alveolar echinococcosis (AE) and cystic echinococcosis (CE) in humans. The liver and lungs are the most affected organs by the morbidity structure of the disease. The average annual incidence of human AE in Central Europe is 0.02–1.4 cases per 100,000 inhabitants. In the Russian Federation, the incidence of echinococcosis in 2023 was 494 cases (0.34 per 100,000 people). High incidence rates of animal echinococcosis have also been reported. In South America and Africa, the prevalence of echinococcosis in goats was 6.16%–10.85% and 13.27%, respectively. A high incidence of sheep echinococcosis (31%) was recorded in the northern Tajikistan region. In the Karachay–Cherkess Republic of the Russian Federation, in the period from 2012 to 2020, the incidence rate of echinococcosis of wolves increased from 38.4% to 68.5%, dogs increased from 73.6% to 100%; jackals increased from 46.9% to 90.8%. The prevalence of East multilocularis in foxes in Central Europe exceeds 40% in some areas. Cystic and AE continues to be prevalent in many regions of the world, requiring new or renewed efforts to treat and prevent the invasion. Keywords: Echinococcosis, Spread, Human, Animals. IntroductionHuman echinococcosis is a globally distributed disease that tends to relapse and develop complications and is often diagnosed at late stages (Araj and Mourad, 2014; Mjonnink et al., 2021). Echinococcus granulosus s.s., E. canadensis, and E. ortleppi are considered pathogens of human invasion (Harlaftis et al., 2009). The disease course depends on the localization of the process and the number and integrity of cysts. Echinococcus most commonly occurs in the liver (50%–70%) and less often in the lungs and other organs and tissues (20%–30%) (Yan et al., 2022; Gu et al., 2024; Mahmoudi et al., 2024). Many countries are endemic to this disease, especially in Eastern Europe, Asia, the North Caucasus, Kazakhstan, Kyrgyzstan, and Uzbekistan. A large number of regions in Russia are also affected, and the incidence is steadily increasing each year (Sadykova and Petrova, 2020; Chuelov and Rossina, 2023; Rustamova, 2023; Tishchenko, 2024). The limiting factors are abiotic (temperature, humidity, chemical and physical composition of soils, water mineralization, and so on) and biotic (the presence of certain intermediate hosts in the fauna, their number, life expectancy, and so on) in nature. It was established that echinococcosis is a natural focal geohelminthiasis that is distributed among wild animals regardless of human presence; however, there are foci associated with human activity in cases when the pathogen circulates in domestic animals and synanthropic mouse-like rodents (Fig. 1) (Tverdokhlebova et al., 2017). Currently, the invasion expands to new territories and affects more and more animals (Marshall, 2021; Bağcı and Bağcı, 2024). It can be confidently stated that no country or region is spared from this invasion (Ito and Budke, 2015; Alvi et al., 2023). The disease poses a biological threat to the world’s population, as the number of infected people around the globe increases every year, and the invasion progresses (Laipanov, 2019; Tikhaya and Ponamarev, 2022; Adamanyuk and Stepanova, 2023; Kokolova and Efremova, 2024; Agaeva et al., 2025).
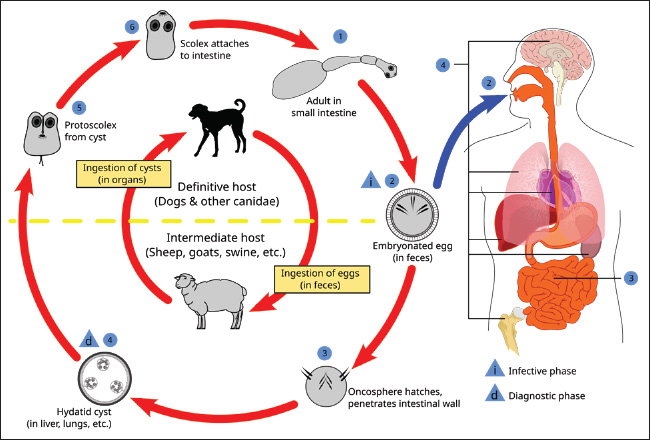
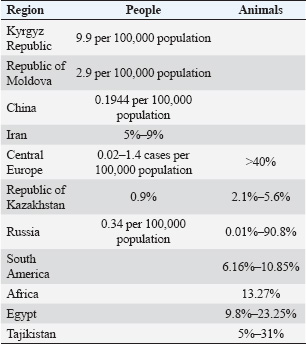
Fig. 1. Scheme of the development of Echinococcus granulosus. (https://nikulya.ru/stadii/jiznennogo/tsikla/exinokokka/?image=34) The purpose of this study was to analyze the scientific literature on the spread of human and animal echinococcosis in various regions of the world. MethodologyThe analysis and scientific approach were based on available information published at various times on the spread of human and animal echinococcosis and included in electronic databases (RSCI, Cyberleninka, PubMed, WoS, Scopus). Analytical, comparative, and systematic methods of scientific research were used. Keywords such as echinococcosis, spread, human, and animals were used. The selection of scientific papers is based on their scientific value in relation to the research topic. Abstracts of articles published at congresses and conferences were not considered because they did not contain a detailed presentation of the material. Dissertations, particular clinical cases, and diagnostic methods were not included in the review. We analyzed more than 150 publications, of which 61 contained content on the spread of human and animal echinococcosis in various regions of the world. The spread of echinococcosis in the worldCystic echinococcosis (CE) remains a significant public health problem in many regions of the northern and southern hemispheres. Alveolar echinococcosis (AE) is limited to the regions of the northern hemisphere of North America and Eurasia (Eckert and Deplazes, 2004). Studies have established that E. multilocularis, the causative agent of AE, is more widespread than previously thought. There are also some trends that show the growing importance of polycystic forms of the disease, which are limited to Central and South America (Grigoryan et al.,2024). Two types of Echinococcus, namely E. multilocularis and E. granulosus, are found in Central Europe and are causative agents of the two most important forms of AE and CE in humans. Studies have confirmed that in Central Europe, East multilocularis is found farther to the north, south, and east than previously thought. This parasite is endemic to Belgium, Luxembourg, France, Switzerland, Liechtenstein, Austria, Germany, Poland, and the Czech Republic (Kolářová et al., 2017). Prevalence rates of E. multilocularis in foxes are alarmingly high in some areas, with averages of >40%. The rates of invasion by dogs and cats are much lower. In recent years, accidental infections with E. multilocularis have been reported in various animal species (dogs, domestic pigs, wild boars, nutria, and monkeys) and humans. The average annual human incidence rates for AE are low, ranging from 0.02 to 1.4 cases per 100,000 people in several European countries and regions (Davidson et al., 2012). The prevalence of echinococcosis in Iran was 5%. At the same time, the incidence of the population was significantly higher in the north (9%) and the west of the country (6%) in patients below 40 years old (7%), and in rural residents and nomads (6%). Housewives were the most susceptible to the disease (31%), followed by illiterate people (14%) and farmers (12%). The liver (55%) and lungs (28%) were the most often invaded (Mahmoudi et al., 2019). Albania is considered endemic for CE, but there is little confirming evidence. A total of eight relevant papers were found on 540 patients infected with echinococcosis. A total of 347 additional hospitalizations were confirmed from 2011 to 2020, as well as 36 laboratory cases and 10 Albanian cases reported in Germany (Luga et al., 2023). CE is highly endemic among the nomadic pastoral tribes of East Africa, but it is rare in agricultural communities. E. granulosus infestations are common in dogs in all sub-Saharan African countries where examined. The transmission of E. granulosus to humans depends on several factors, such as the prevalence of the parasite in domestic dogs, human behavior toward dogs, heterogeneity of the parasite, and human susceptibility to invasion. Sheep and goats appear to be the most common domestic intermediate hosts, but research shows that camels are equally important intermediate hosts, especially in Sudan and Turkana. At least 5 out of the 10 E. granulosus genotypes are dangerous for humans in sub-Saharan Africa. The East granulosus strain of sheep and the camel strain cause the majority of cases of human invasion. Other strains found in the study area include lion, horse, and cattle strains (Magambo et al., 2006). The incidence of echinococcosis in the Kyrgyz Republic increased 2.6 times from 2001 to 2021, with an annual growth rate of 14.6%, reaching 19.2 per 100,000 people by 2015, followed by a decrease in 2021 to 9.9 per 100,000 people (Kasyev et al., 2023; Arstanbekova and Raimkulov, 2024; Raimkulov et al., 2024). The reduction in the number of new echinococcosis cases in 2019–2021 is due to an insufficient level of diagnostic measures, population migration, and deterioration of the socioeconomic and sanitary-epidemiological state of the country (Kundashev et al., 2024). Echinococcosis was more common in people of working age (35 ± 16 years average), rural residents mainly (67.3%), and those with close contact with carnivorous animals (58.4%). There was a predominance of complicated forms of echinococcosis among the surgical patients (64.7%), which increased the duration of hospitalization by 5.3 ± 2.4 days (Tursunov et al., 2022). In the Republic of Moldova, 788 surgically confirmed cases of echinococcosis were registered in the period 2011–2020. All cases were of local origin. Of these, 366 (46.4%) were men and 422 (53.6%) were women. The maximum number of disease cases (157) was registered in 2012, and the minimum (30) in 2020. The average incidence rates in the country were 2.9 per 100,000 people. 2.8 and 3.0 per 100,000 people in men and women, respectively. The breakdown by age shows that the majority of cases were registered in young people of working age: 40.2% in the 18–40 age group and 28.8% in the 41–60 age group. People over the age of 60 account for 20.2% of cases. The fact that a large proportion of the total number of diseases is registered in children under 17 is of concern. Rural areas account for the majority of cases (91.0%). The liver (78.5%) and lungs (17.1%) were the organs most affected by the morbidity structure of the disease. Other localizations were registered in 4.4% of cases, including kidney, spleen, and multiple organ localizations (Lungu and Lungu, 2023). Echinococcosis is widespread in many regions of the Republic of Kazakhstan. Thus, in the Kostonai region, the maximum number of sick people (26 cases) was registered in 2014, and the minimum, five cases, were registered in 2000. The maximum number of positive serological reactions to echinococcosis was recorded in humans in 2003, 2005, 2006, 2013–2016, when the incidence rates were 0.9%, 0.8%, 0.26%, 1.8%, 3.0%, 1.5%, and 0.9%, respectively (Domatsky and Aubakirov, 2022). The child echinococcosis incidence (aged 0–14 years) in 2006–2015 was 41 cases (1.3 per 100,000 people). Dog invasiveness with E. granulosus: for Rudny and Kostanay has not been established, and in Urpek village of Amangeldy district and in Arkalyk district, invasion was detected in two and three dogs, respectively. The study of farm animal infection established the maximum number of cases of echinococcosis in 2011–2017 the sheep. The extent of E. granulosus invasion was 3.1%, 3.5%, 4.2%, 3.1%, 5.6%, 3.0%, and 2.8%, respectively. In cattle, the largest amount of positive echinococcoses cases was noted in 2011–2014. The extent of E. granulosus invasion was 3.3%, 3.2%, 3.5%, and 3.6%, respectively. In pigs, the invasion peaked in 2012–2015 and amounted to 2.2%, 2.2%, 2.5%, and 2.1%, respectively. There were no cases of horse echinococcosis (Aubakirov et al., 2019; Aubakirov et al., 2020). In South America and Africa, the prevalence of echinococcosis in goats was 6.16%–10.85% and 13.27%, respectively. The prevalence of echinococcosis in goats before 2010 was significantly higher (9.76%) than from 2010 to 2014 (1.44%) or after 2014 (2.95%). The prevalence of echinococcosis in goats aged <12 months (4.48%) was higher than in goats aged ≥12 months (2.88%). The influence of geographical factors and climate on the prevalence of echinococcosis in goats was studied. The results showed that the prevalence of echinococcosis was higher in areas with higher altitudes and colder climates (Yan et al., 2022). Severe invasion of echinococcosis has been found in the regions of Northern Tajikistan, where the incidence of sheep reaches 31%. The Khatlon region has a significant incidence of echinococcosis in domestic ruminants. The regions of southwestern and central Tajikistan show an average degree of invasion, where the intensity of sheep invasion is 21%–22%. The districts of the Gorno-Badakhshan Autonomous Region have a low degree of invasion (less than 5%) (Mahmadshoeva and Anoyatbekov, 2023). An analysis of the research results obtained during the veterinary and sanitary post-slaughter examination of carcasses and organs of farm animals in the period 2012–2016 in the Khatlon region showed that the maximum number of cysts was found in the lungs and liver in cattle was 7,338 out of the examined 167,858 carcasses, in sheep—3,628 out of 60,385 carcasses and internal organs. According to medical reports in Tajikistan in recent years, human echinococcosis levels from 128 to 280 cases/year (Bobozhonov, 2023). Echinococcosis poses a significant threat to public health. The Chinese Government has taken preventive and control measures to mitigate the effects of the disease. The Chinese Center for Disease Control and Prevention and the State Council of the People’s Republic of China data analysis confirmed that the measures reduced the infection rate by almost 50% between 2004 and 2022 (from 0.3975 to 0.1944 per 100,000 person/years). However, some regions still carry a significant burden of the disease, and the lack of detailed information limits further assessment of the effectiveness of treatment for both alveolar and CE (Gu, 2024). Thus far, the actual incidence of CE in the Egyptian population remains unknown. Infection by E. granulosus (s.l.) is common among stray dogs in rural and suburban areas because of the spread of the parasite eggs. CE occurs in most parts of Egypt; however, available data are mostly from northern Egypt, particularly Cairo and Giza. In southern Egypt, the disease is likely to be underdiagnosed or underreported. A few risk factors were also studied. In the Egyptian population, residency in rural areas, farming, and age were significant factors for the development of CE. In livestock, age, sex, and season are associated with a high prevalence of CE. Several genotypes have been identified in livestock (G1, G4, G5, G6, and G7) and humans (G1, G6, and G7) (Abdelbaset et al.,2021). The results revealed that 39 sheep (9.8%), 74 cattle (18.4%), 95 buffaloes (21.8%), and 79 camels (23.25%) were infected. The prevalence of hydatidosis was higher in adult sheep, cattle, and camel; 32 (13.8%), 49 (26.3%), and 56 (24.5%) than the younger ones 7 (4.2%), 25 (11.6%), and 23 (20.5%), respectively. Hydatidosis infection in sheep and cattle was higher in winter 26 (9.01%) and 47 (27.5%) than in summer 13 (11.9%) and 27 (11.7%), respectively (Abo-Aziza et al.,2019). Hydatid cysts were found in 6 camels (6%) out of 100 inspected camels, while five hydatid cysts (0.87%) were detected in a total number of 574 cattle examined. The parasite was detected exclusively in the lungs of camels, whereas the lungs were the main organs infected by the parasite in cattle, and one hydatid cyst was found in the liver (0.17%). In camels, 66.7%, 16.65%, and 16.65% of detected cysts were fertile, sterile, and calcified, respectively, whereas in cattle, these percentages were 60%, 20%, and 20%, respectively (Gareh et al.,2021). The spread of echinococcosis in the Russian FederationIn the Russian Federation, the incidence of echinococcosis in 2023 did not exceed the long-term average (0.33). In total, 494 cases were registered in 2023, and the incidence was 0.34 per 100,000 people. In three cases, echinococcosis was fatal. A total of 63 cases were detected in children under 17 years of age (0.21 per 100 thousand children of this age), including 12 cases of echinococcosis (0.17 per 100 thousand children of this age) registered in 3–6-year-old children (State Report, 2024). The most disadvantaged regions are the southern regions—the Volgograd Region, the Republics of Karachay-Cherkessia and Dagestan—93.0%, 57.1%, and 34.3%, respectively. In other regions, the degree of infection varies from 0.6% to 33.3%. This phenomenon can be explained by the fact that the sheep industry prevails in the above-mentioned regions, where small cattle are grazed with the help of dogs, which infect ruminants. Dogs are reinfected by eating internal organs with blastocysts during uncontrolled sheep slaughter. Cattle echinococcosis was diagnosed in the Central region, and 6 out of 295 carcasses were infected, corresponding to 2.03%. The Central Black Earth economy and the Volga regions are safe, and the incidence in Russia was 1.2%. In sheep, the highest percentage of echinococcosis infections were diagnosed in the Volga region (41.9%) and the North Caucasus regions (31.5%). A single case of blastocysts in sheep was recorded in the Central Region (0.4%), with an incidence of 23.6% in Russia. Diagnostic for echinococcosis in deer and pigs showed infection in the period from 2020 to 2022; in the first quarter of 2023, larvocysts were identified in deer and sheep, and the animal echinococcosis leveled 0.09%, 3.2%, 2.5%, and 0.3% in 2020, 2021, 2022, and in the first quarter of 2023, respectively. The highest percentage of animal infection was detected in 2021, which is obviously due to the lower amount of livestock surveyed than in other reporting periods. It is also worth noting that of all the farm animals studied, deer were the most affected: the incidence was 6.6% in 2020, 7.4% in 2021, 6.4% in 2022, and 0.4% in 2023, which is due to close contact between deer and gazing and driving dogs as well as wild dogs. The pig’s echinococcosis level from 0.003% to 0.3% from 2020 to 2022. This proportion of infected animals falls on private farms, where pigs of various sexes and age groups freely come into contact with dogs and thus become infected. Echinococcal cysts were found in the parenchymal organs of sheep (liver and lungs) in 212 cases (23.6%). It is worth noting that blisters were identified in the liver in 11.7% of cases, in the lungs in 9.8% and in 2.1% of cases, and in both organs. In cattle, echinococcosis was diagnosed only in 6 cases in the lungs (1.2%). Echinococcosis was registered only in the Tula region, and the degree of infection is low—0.9% and 0.6% in cattle and 0.6% in sheep (Tsepilova et al., 2023). The Karachay-Cherkess Republic belongs to the unfavorable regions of the Russian Federation for carnivorous and ruminant echinococcosis. The incidence index of echinococcosis of wolves increased from 38.4% to 68.5% from 2012 to 2020, dogs increased from 73.6% to 100%; jackals increased from 46.9% to 90.8%. The incidence index of goat CE increased from 8.6% to 23.4% in the lowland zone; in the foothill zone, from 11.7% to 25.2%; and in the mountainous zone, from 6.9% to 20.6%, which indicates a 2–3-fold increase in the spread of the disease and indicates a risk to the biosafety of livestock due to the poor implementation of dog deworming measures. However, the incidence of CE in lowland, foothill, and mountain communities has decreased several-fold. The number of people with echinococcosis decreased by 6 times (from 6 to 1 people), which suggests an improvement in the epidemic situation thanks to the prevention and improvement of the sanitary and hygienic culture. At the same time, the soils of urban and rural sites are 100% disseminated with taeniidae eggs. The specific weight of soil samples containing viable taeniidae eggs is high and ranges from 58.7 ± 4.26% to 83 ± 6.90% in urban soils and from 82.4 ± 7.13% to 88.3 ± 7.59% in rural soils, which can lead to the spread of echinococcosis among intermediate hosts, including humans (Kabardiev et al., 2023). In the climatic subzones of the steppe zone of the Karachay-Cherkess Republic, dog echinococcosis poses a growing epizootiological threat to buffalo breeding. The average dog echinococcosis incidence was 70.00%. This is a high level of environmental echinococcal egg contamination in the region, echinococcosis. The incidence of buffaloes broken down by climatic subzones is 10.0%–30.0% (on average, 20.0%), indicating a high level of invasion. The adult buffalo populations showed an increase in the abundance index of E. granulosus acephalocyst from 11.6 ± 0.51 to 32.0 ± 0.95 per individual in the dynamics of research. However, sarcocyst fertility was 0.0% in all years with the complete absence of protoscolex in the echinococcal fluid (Gogushev et al., 2022). An analysis of epidemiological survey maps in the Karachay-Cherkess Republic established that the adult population accounted for 74.5% of cases of echinococcosis (Tverdokhlebova et al., 2022). Furthermore, 76.4% of patients in private households had farm animals, and 73.9% of patients had dogs and neglected regular deworming (Bolatchiev et al, 2019). A retrospective analysis of the long-term dynamics of infection in farm animals in the Novosibirsk region of the Russian Federation confirmed an uneven development of the invasive process with a significant downward trend. The average annual rate of decline was 13.9%. The epidemic situation is characterized by significant fluctuations in morbidity from 0.04 to 0.75 per 100,000 people and has an unreliable downward trend. The average long-term morbidity rate of the population for the period 1999–2020 was 0.265 per 100,000, which is two times lower than the same indicator in the Russian Federation (0.365) (Talovskaya and Efremova, 2020). For the period from 2009 to 2020, the average infection of farm animals with the echinococcosis pathogen was 0.58%, without any significant difference in cattle and sheep (1.16% and 1.15%, in pigs it leveled 0.36%, which is three times lower than in ruminants (Talovskaya et al., 2022). From 2017 to 2021, 72 cases of human echinococcosis were registered in the Astrakhan region of the Russian Federation. The majority of the cases were recorded in 2017–31.1% (n=28) and in 2015–23.0% (n=15). In 2013, 18.0% (n=10) became ill, and in subsequent 2016 and 2017, 19.7% (n=13) and 8.2% (n=6), respectively. Women accounted for 64.1% (n=39) of all echinococcosis cases. In the reviewed period, both adults and children were at risk of echinococcus infection. The proportion of children was 10.1% (n=5), including nursery-age children [2.9% (n=3)] and schoolchildren [6.6% (n=4)]. 90.9% (n=54) of infection cases were registered in the adult population, including production facilities workers [45.1% (n=28)], the temporarily unemployed [15.9% (n=9)] and retired [28.8% (n=18)] (Suleymanova and Rakisheva, 2024). Human infection with echinococcosis was registered in both urban and rural areas. Human echinococcosis most commonly develops in the liver and lungs, accounting for 96.6% of all cases (n=58). The liver was affected in 78.6% of cases (n=49), the lungs in 11.6% of cases (n=6), the combined liver and lung were damaged in 4.8% of cases (n=2), and damage to both lungs in 1.7% of cases (n=2). Rare localization of echinococcus was observed only in 3.4% of cases (n=3), including abdominal lesions and a combination of liver and spleen lesions [1.7% each (n=1 each)]. The information collected for medical histories showed that all patients diagnosed with echinococcosis had contact with dogs (Masyaninova et al., 2024). The dynamics of the incidence of echinococcosis in the Tyumen region of the Russian Federation over a 10-year period were characterized by a wave-like course with a moderate upward trend (growth rate—4.4%). The long-term annual average incidence rate in 2012–2021 was 0.68 per 100,000 people and was above the federal average (0.29 per 100,000 people) by 2.3 times. The incidence of echinococcosis ranged from 0.22 (2012) to 1.52 (2019) per 100,000 people. In total, 100 cases of echinococcosis were registered in the Tyumen region between 2012 and 2021. The number of infected people peaked in 2019 at 23 cases (1.52 per 100,000 people). The majority of epidemic processes occur in adult populations (94% of cases). During the study period, six cases of echinococcosis were registered in the child population. The majority of infected (85% of cases) kept dogs whose deworming was neglected or performed irregularly. The percentage of the larval stage of E. granulosus infection cases in farm animals (cattle, pigs, sheep, horses) over the 10-year period ranged from 0.01% to 0.9%. In terms of the structure of echinococcosis infection in farm animals, sheep accounted for 9.9% to 78%, cattle from 16.9% to 64.6%, and pigs from 0% to 46.6%. In 2013–2014, there were cases of echinococcosis in horses, with a proportion of 0.3% and 5.2%, respectively (Adamanyuk and Belyaeva, 2022). The problem of echinococcosis remains relevant in the Far Eastern Federal District of the Russian Federation. From 2010 to 2019, cases of the disease were registered in the territory of 10 subjects of the district (227 cases), in most of which the incidence is sporadic (Dragomeretskaya et al., 2020). In recent decades, tremendous progress has been made in the diagnosis, treatment, and control of diseases caused by taeniidae. The greatest interest in this group of parasites is due to the substantial human disease burden (Arkelova et al., 2021; Marshall et al., 2021; Harutyunyan et al., 2024). The most important achievements that have changed the treatment of human diseases caused by these parasites are the reduction of the transmission of taeniidae species to humans and the incidence of disease cases; understanding the biology of parasites with the prospect of better diagnosis or treatment in the foreseeable future (Goryacheva et al., 2024; Telicheva et al., 2024; Zubritskiy et al., 2024). The availability of new anti-cystic drugs for dogs and a detailed understanding of the dynamics of E. granulosus transmission formed the basis for successful programs that eliminated CE in some regions of the world and significantly reduced the incidence in others (Arkelova et al., 2024; Telicheva et al., 2024). Despite the achievements, cystic and AE continue to be prevalent in many regions of the world, requiring new or renewed efforts to treat and prevent the invasion (Kabardiev et al., 2024; Tishchenko et al., 2024) (Table 1). Table 1. Incidence rates of echinococcosis in humans and animals.
ConclusionHuman echinococcosis is a globally distributed disease that tends to relapse and develop complications and is often diagnosed at the late stages. Echinococcosis is a natural focal geohelminthiasis that is distributed among wild animals regardless of human presence; however, there are foci associated with human activity in cases in which the pathogen circulates among domestic animals and synanthropic mouse-like rodents. The transmission of E. granulosus to humans depends on several factors, such as the prevalence of the parasite in domestic dogs, human behavior toward dogs, heterogeneity of the parasite, and human susceptibility to invasion. Sheep and goats are the most common domestic intermediate hosts. E. granulosus s.s., E. canadensis, and East ortleppi are considered pathogens of invasion in humans. The disease course depends on the localization of the process and the number and integrity of cysts. Echinococcus most often affects the liver (50%–70%) and less often affects the lungs, other organs, and tissues (20%–30%). In most cases, echinococcosis occurs in people of working age, mainly rural residents who have close contact with carnivorous animals. AcknowledgmentsNone. Conflict of interestThe authors declare no conflict of interest. FundingThe research was carried out by the All-Russian Scientific Research Institute of Veterinary Entomology and Arachnology of the Tyumen Scientific Center of Siberian Branch of the Russian Academy of Sciences within the framework of the state assignment of the Ministry of Science and Higher Education of the Russian Federation: “Study and analysis of the epizootic state of diseases of invasive etiology of agricultural and non-productive animals, bees and birds, changes in species composition and bioecological patterns of the development cycle of parasites in conditions of shifting boundaries of their ranges” (FWRZ-2021-0018). Author’s contributionsDVN and SEI prepared the manuscript, revised it, and conducted a critical review. All authors have read and approved the final version of the manuscript. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesAbo-Aziza, F.A.M., Oda, S.S., Aboelsoued, D., Farag, T.K. and Almuzaini, A.M. 2019. Variabilities of Hydatidosis in domestic animals slaughtered at Cairo and Giza abattoirs, Egypt. Vet. World 12(7), 998–1007. Abdelbaset, A.E., Yagi, K., Nonaka, N. and Nakao, R. 2021. Cystic echinococcosis in humans and animals in Egypt: an epidemiological overview. Curr. Res. Parasitol. Vector Borne Dis. 1, 100061. Adamanyuk, S.V. and Belyaeva, M.I. 2022. Echinococcosis in the Tyumen region. Current issues in preventive medicine and sanitary and epidemiological welfare of the population: factors, technologies, management, and risk assessment: Collection of scientific papers. In Special Issue: Based on the Materials of the Interregional Scientific and Practical Conference, Nizhny Novgorod, 2022 Jun 07–08, Nizhny Novgorod: Medial. pp. 235–238. Adamanyuk, S.V. and Stepanova, K.B. 2024. Epidemiological features of cystic echinococcosis in the Omsk region. Nat. Priorit. Russ. 4(55), 178–182. Agaeva, A.N., Isaeva, K.K. and Ugur, I.O. 2025. Cestode in the internal organs of sheep. Curr. Res. 6–1(241), 11–14. Alvi, M.A., Athar Ali, R.M., Khan, S., Saqib, M., Qamar, W., Li, L., Fu, B.Q., Yan, H.B., Jia, W.Z. 2023. Past and present of diagnosis of echinococcosis: a review (1999–2021). Acta Trop. 243, 106925. Araj, G.F. and Mourad, Y. 2014. Hydatid disease: the Lebanese contribution. J. Med. Liban. 62(4), 217–226. Arkelova, M.R., Bolatchiev, K.K., Bolatchieva, E.K., Tverdokhlebova, T.I., Gogushev, Z.T. and Shemyakova, S.A. 2024. Statistical model of epidemiological monitoring of human echinococcosis in the subjects of the North Caucasus Federal District. Med. Parasitol. Parasit. Dis. 1, 14–18. Arstanbekova, B.A. and Raimkulov, K.M. 2024. Study of the prevalence of echinococcosis among the population of Zheti-Oguz district of the Issyk-Kul region of Kyrgyzstan. Endl. Light Sci. S4, 261–264. Aubakirov, M.Z., Mustafin, M.K., Selunskaya, L.S., Khassanova, M.A., Erenko, E.N., Khairov, G.K., Domatsky, V.N. and Murzakayeva, G. 2019. Technology for preventing ecological and economic damage caused by echinococcosis. J. Eng. Adv. Tech. 8(6), 2933–2936. Aubakirov, M.Z., Abdybekova, A.M., Mustafin, M.K., Yergazina, A.M., Murzakayeva, K.G., Domatsky, V.N., Mendybayeva, A.B. and Nalobina, L.V. 2020. Incidence of alveolar echinococcosis in humans and animals in the Kostanay region of the republic of Kazakhstan. Ukr. J. Ecol. 10(6), 203. Bağcı, U., Bağcı, Ö.U. 2024. A comprehensive bibliometric analysis of the literature between 1945 and 2024 about urinary tract echinococcosis. Parasitol. Int. 103, 102945. Bobozhonov, M.N. 2023. Epidemiological features of brain echinococcosis in the Republic of Tajikistan. Rep. Tajik Acad. Agric. Sci. 1(75), 58–61. Bolatchiev, K.H. 2019. Results of the epizootological and epidemiological monitoring of echinococcosis in the south of Russia. Vet. Med. 11, 34–37. Chuelov, S.B. and Rossina, A.L. 2023. Cystic echinococcosis. Child. Infect. 22.1(82), 50–55. Davidson, R.K., Romig, T., Jenkins, E., Tryland, M., Robertson, L.J. 2012. The impact of globalization on the distribution of Echinococcus multilocularis. Trends Parasitol. 28(6), 239–247. Domatsky, V.N. and Aubakirov, M.J. 2022. Parasitological situation of echinococcosis in Kostanay region of the Republic of Kazakhstan. In Integration of Science and Education in Agricultural Universities to Ensure Food Security in Russia: Proceedings of the National Scientific and Practical Conference, Tyumen, 2022 Nov 1–3, Tyumen: State Agrarian University of the Northern Urals, pp. 37–45. Dragomeretskaya, A.G., Bebenina, L.A. and Trotsenko, O.E. 2020. Cystic echinococcosis in the Far Eastern Federal District: the current state of the problem. Far East J. Infect. Pathol. 39(39), 148–149. Eckert, J. and Deplazes, P. 2004. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 17(1), 107–135. Federal Service for Supervision of Consumer Rights Protection and Human Welfare. 2024. The state of sanitary and epidemiological welfare of the population in the Russian Federation in 2023: State Report. Moscow: Federal Service for Supervision of Consumer Rights Protection and Human Welfare. p. 364. Gareh, A., Saleh, A.A., Moustafa, S.M., Tahoun, A., Baty, R.S., Khalifa, R.M.A., Dyab, A.K., Yones, D.A., Arafa, M.I., Abdelaziz, A.R., El-Gohary, F.A., Elmahallawy, E.K. 2021. Epidemiological, morphometric, and molecular investigation of cystic echinococcosis in camels and cattle in Upper Egypt: current status and zoonotic implications. Front. Vet. Sci. 8, 750640. Gogushev, Z.T., Arkelova, M.R., Bittirov, I.A., Bolatchiev, K.Kh., Bittirov, A.M. and Kaloshkin, I.V. 2022. Dog echinococcosis as a parasitic threat to buffalo breeding in the southern regions of the Russian Federation. Vet. Med. Kuban. 2, 36–38. Goryacheva M.V., Raimkulov K.M., Bobrovsky E.A., Sharsheeva B.K., Adambekova A.D., Mergenov A.Z., Usubalieva Zh. M., Trebukhov A.V. 2024. Echinococcosis: ultrasonic diagnostics as a screening method in regions with an increased risk of disease. J. Infectol. 16(2), 35. Grigoryan, A.S., Chopikyan, A.S., Yeranosyan, S.G., Sargsyan, L.G., Harutyunyan, A.A., Kulikova, K.A. and Tadevosyan, A.E. 2024. Main concerns regarding igg positivity for echinococcosis in Armenia during the period 2015-2023 based on the data of Blood Cells Laboratories. Euras. Health J. 2, 15–20. Gu, H., Hu, Y., Guo, S., Jin, Y., Chen, W., Huang, C., Hu, Z., Li, F. and Liu, J. 2024. China’s prevention and control experience of echinococcosis: a 19-year retrospective. J. Helminthol. 98, e16. Harutyunyan, H., Harutyunyan, A.A., Voskanyan, A., Barseghyan, A., Chopikyan, A. and Tadevosyan, A. E. 2024. Our experience in the treatment of residual cavity of the liver cystic echinococcosis (case report). Euras. Health J. 2, 84–89. Harlaftis, N.N., Aletras, H.A. and Symbas, P.N. 2009. Hydatid disease of the lung. Gen. Thorac. Surg. 7, 1187–1195. Ito, A. and Budke, C.M. 2015. The present situation of echinococcosis in Mongolia. J. Helminthol. 89(6), 680–688. Kabardiev, S.Sh., Bittirov, A.M., Aliev, A.Y. and Gogushev, Z.T. 2023. Echinococcosis of animals and humans as a socially dangerous problem in a densely populated region of the North Caucasus. Hyg. Sanit. 102(1), 34–39. Kabardiev, S.Sh., Bittirov, A.M., Karpushchenko, K.A. and Shapiev, B.I. 2024. Experimental trials of the drug Prazirex in cystic echinococcosis of sheep. Casp. Bull. Vet. Med. 2(7), 32–40. Kasyev, N., Bashirov, R., Aitnazarov M.S., Niyazov, B.S. and Emilbekov, U.E. 2023. Epidemiological aspects of echinococcosis in the Kyrgyz Republic. Bull. Kyrgyz State Med. Acad. Named after I.K. Akhunbaev 1, 73–80. Kokolova, L.M. and Efremova, M.D. 2023. Echinococcosis in Yakutia. Bull. Kyrgyz State Med. Acad. Named after I.K. Akhunbaev 5, 33–40. Kundashev, K.U., Ryskulbekova, A.B., Orokchieva, G.T. and Erkinbayeva, K.E. 2024. The epidemiological situation of echinococcosis in the population of Bishkek in 2012-2022. Euras. J. Health 1, 131–136. Kolářová, L., Matějů, J., Hozáková, L., Stejskal, F., Hrdý, J., Kolářová, H., Leissová, M., Skála, V. and Dundr, P. 2017. Human alveolar echinococcosis and an overview of the occurrence of Echinococcus multilocularis in animals in the Czech Republic. Epid. Microbiol. Imunol. 66(4), 163–172. Laipanov, B.K. 2019. Echinococcosis as a global problem and methods of combating it. In Actual Problems of Veterinary Medicine, Animal Science and Biotechnology: A Collection of Scientific Papers of the International Educational, Methodological and Scientific-Practical Conference Dedicated to the 100th Anniversary of the Founding of the K.I. Scriabin Moscow State Pedagogical University, 2019 Nov 20–22. Federal State Budgetary Educational Institution of Higher Education “Moscow State Academy of Veterinary Medicine and Biotechnology, pp. 140–142. Lightowlers, M.W., Gasser, R.B., Hemphill, A., Romig, T., Tamarozzi, F., Deplazes, P., Torgerson, P.R., Garcia, H.H. and Kern, P. 2021. Advances in the treatment, diagnosis, control, and scientific understanding of taeniid cestode parasite infections over the past 50 years. Int. J. Parasitol. 51(13–14), 1167–1192. Luga, P., Gjata, A., Akshija, I., Mino, L., Gjoni, V., Pilaca, A., Zobi, M., Martinez, G.E. and Richter, J. 2023. What do we know about the epidemiology and the management of human echinococcosis in Albania? Parasitol. Res. 122(8), 1811–1818. Lungu, V. and Lungu, L. 2023. Epidemiology of cystic echinococcosis in the Republic of Moldova. Bull. Kyrgyz State Med. Acad. Named after I.K. Akhunbaev 4, 196–201. Magambo, J., Njoroge, E. and Zeyhle, E. 2006. Epidemiology and control of echinococcosis in sub-Saharan Africa. Parasitol. Int. 55 Suppl, S193-S195. Mahmadshoeva, Z.A. and Anoyatbekov, Z.A. 2023. Echinococcosis in the Republic of Tajikistan. Probl. Vet. Sanit. Hyg. Ecol. 120, 142–148. Mahmoudi, S., Mamishi, S., Banar, M., Pourakbari, B. and Keshavarz, H. 2019. Epidemiology of echinococcosis in Iran: a systematic review and meta-analysis. BMC Infect. Dis. 19(1), 929; doi: 10.1186/s12879-019-4458-5 Manukyan, A.H. 2024. Assessment of Echinococcosis spp. surveillance systems using SWOT analysis in Armenia. Med. Sci. Armenia. 64, 142–148; doi: 10.54503/0514-7484-2024-64.1-142 Masyaninova, A.E., Mogilina, E.A., Lapina, A.S., Arakelian, R.S., Agadzhanova, M., Tashukhadzhieva, D.Sh., Guspanova, O.B., Serdarova, A., Shikhresedova, M.Z. and Sagaliev, F.R. 2024. Topical aspects of the epidemiology of pulmonary echinococcosis on the example of the Astrakhan region. Intern. Sci. Res. J. 3(141), 22. Mjonnink, G.L.E., Stijni, C., van Delden, O.M., Spijker, R., Grobusch, M.P. 2021. Percutaneous versus surgical interventions for hepatic cystic echinococcosis: a systematic review and meta-analysis. Cardio. Intervent Radiolog. 44, 1689–1696. Raimkulov, K.M., Kulzhabayeva, K.S., Toigombayeva, V.S., Kuttubaev, O.T. 2024. Epidemiological assessment of the prevalence of echinococcosis in Central Asia. Med. Parasitol. Parasit. Dis. 1, 19–24. Raimkulov, K.M, Toigombayeva, V.S., Kuttubaev, O.O. and Mergenov, A.E. 2024. Assessment of the invasion of echinococcosis and behavioral risk factors among the population of the Osh region of the Kyrgyz Republic. Euroas. J. Health 1, 137–145. Rustamova, S.I. 2023. Epidemiological situation of animal echinococcosis in Azerbaijan. Bull. Sci. Pract. 9(11), 195–197. Sadykova, V.P. and Petrova, O.G. 2020. EpizootiologicaL analysis, diagnosis, and prevention of echinococcosis in animals. In Actual Problems of the Development of Natural Sciences: A Collection of Articles by Participants of the XXIII Regional Scientific Research Competition “Scientific Olympus” in the Field of Natural Sciences. Ministry of Education and Youth Policy of the Sverdlovsk Region; State Educational Institution of Higher Education “House of Youth”; Ural Federal University named after the First President of Russia, B.N. Yeltsin Yekaterinburg: Ural Federal University named after the First president of Russia, B.N. Yeltsin, pp. 49–53. Suleymanova, D.R. and Rakisheva, K.B. 2024. Retrospective analysis of human echinococcosis in the Astrakhan region for 2017–2021. In Scientific Space: Current Issues, Achievements and Development Prospects: A Collection of Scientific Papers Based on the Materials of the XXIII International Scientific and Practical Conference, Anapa, 2024 Mar 18, Anapa: Limited Liability Company “Scientific Research Center of Economic and Social Processes” in the Southern Federal District, pp. 20–29. Talovskaya, O.B., Efremova, E.A. 2020. Features of the current epidemic situation of echinococcosis in the Novosibirsk region. In Agrarian Problems of Gorny Altai and Adjacent Regions: Proceedings of the All-Russian Scientific and Practical Conference Dedicated to the 90th Anniversary of the Gorno-Altaisk Research Institute of Agriculture and the 100th Anniversary of the Ministry of Agriculture of the Altai Republic, Gorno-Altaisk, 2020 Jun 30–02, Barnaul: ABC, pp. 339–345. Talovskaya, O.B., Efremova, E.A., Udaltsov, E.A. and Zubareva, 2022. Chronological and chorological features of the epizootic I. M. process of echinococcosis in the conditions of the Novosibirsk region. Sci. Notes Kazan St. Acad. Vet. Med. Named after N.E. Bauman. 252(4), 237–244. Tverdokhlebova, T.I., Kovalev, E.V., Ermakova, L.A., Dumbadze, O.S., Kondratenko, T.A., Bolatichev, K.Kh. and Chernigov, L.F. 2017. Epidemiological surveillance of helminthiasis in the southern part of Russia and the direction of its optimization. In Current issues in epidemiology, microbiology, and diagnostics of infectious and parasitic diseases in the Rostov region: Proceedings of the Scientific and Practical Conference, Rostov-on-Don, pp. 42–45. Tverdokhlebova, T.I., Kovalev, E.V., Karpushchenko, G.V., Boltachiev, Ph D., Dumbadze, O.S., Khutoryanina, I.V., Chernikova, M.P., Demidova, L.L., Shemyakova S.A. and Chernigov, L.F. 2022. Echinococcosis in southern Russia: epidemiological and epizootological aspects. Infect. Dise. 20(2), 68–74; doi 10.20953/1729-9225-2022-2-68-74 Telicheva V.O., Nagorny S.A., Ermakova L.A., Kornienko I.V., Kirtanasova E.Ya. and Khoronko E.Yu. 2024. Optimization of molecular genetic methods for diagnosis of echinococcosis. Klin. Lab. Diag. 69(5), 240–246; doi: 10.51620/0869-2084-2024-69-5-240-246 Telicheva, V.O., Nagorny, S.A., Ermakova, L.A., Golovchenko N.V., Kornienko I.V. and Kirtanasova E.I. 2024. Application of the PCR method to detect the DNA of the causative agent of cystic echinococcosis in the blood. Theory Pract. Comb. Parasit. Dis. 25, 405–411. Tishchenko, I.A. 2024. Human echinococcosis: current state of the problem and main trends. Moscow Surg. J. 3, 156–165. Tikhaya, N.V. and Ponamarev, N.M. 2022. Ecological features of animal echinococcosis spread in the Altai Territory. Bull. KrasGAU 4(181), 127–132. Tursunov, T.T., Isaev M.A., Ibragimova, Zh.A. and Nurpeishova, E.N. 2022. Problems of echinococcosis among animals and ways to solve them. Sci. New Technol. Innov. Kyrgyzstan 3, 163–166. Tsepilova, I.I., Shemyakova, S.A. and Velikaya A.V. 2023. Diagnosis of larval echinococcosis of farm animals in the territory of the Russian Federation. Bull. Kyrgyz State Med. Acad. Named after I.K. Akhunbaev 4, 223–229. Yan, W.L., Meng, J.X., Li, X.M., Zhao, J.P., Zhang M., Wang, X.Y., Sun, Y.Z., Ni, H.B., Ma, H.2022. Global prevalence of Echinococcosis in goats: a systematic review and meta-analysis. Foodborne Pathog. Dis. 19(10), 675–685. Zubritskiy, V.F., Pakhomova, R.A., Kochetova, L.V. and Tishchenko I.A. 2024. Modern approaches for the surgical treatment of echinococcosis. Bull. Med. Inst. Contin. Educ. 4(2), 56–62. | ||
| How to Cite this Article |
| Pubmed Style Domatskiy VN, Sivkova EI. The incidence rates of human and animal echinococcosis: A systematic review. Open Vet. J.. 2025; 15(8): 3419-3427. doi:10.5455/OVJ.2025.v15.i8.5 Web Style Domatskiy VN, Sivkova EI. The incidence rates of human and animal echinococcosis: A systematic review. https://www.openveterinaryjournal.com/?mno=251331 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.5 AMA (American Medical Association) Style Domatskiy VN, Sivkova EI. The incidence rates of human and animal echinococcosis: A systematic review. Open Vet. J.. 2025; 15(8): 3419-3427. doi:10.5455/OVJ.2025.v15.i8.5 Vancouver/ICMJE Style Domatskiy VN, Sivkova EI. The incidence rates of human and animal echinococcosis: A systematic review. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3419-3427. doi:10.5455/OVJ.2025.v15.i8.5 Harvard Style Domatskiy, V. N. & Sivkova, . E. I. (2025) The incidence rates of human and animal echinococcosis: A systematic review. Open Vet. J., 15 (8), 3419-3427. doi:10.5455/OVJ.2025.v15.i8.5 Turabian Style Domatskiy, Vladimir Nicolaevich, and Elena Ivanovna Sivkova. 2025. The incidence rates of human and animal echinococcosis: A systematic review. Open Veterinary Journal, 15 (8), 3419-3427. doi:10.5455/OVJ.2025.v15.i8.5 Chicago Style Domatskiy, Vladimir Nicolaevich, and Elena Ivanovna Sivkova. "The incidence rates of human and animal echinococcosis: A systematic review." Open Veterinary Journal 15 (2025), 3419-3427. doi:10.5455/OVJ.2025.v15.i8.5 MLA (The Modern Language Association) Style Domatskiy, Vladimir Nicolaevich, and Elena Ivanovna Sivkova. "The incidence rates of human and animal echinococcosis: A systematic review." Open Veterinary Journal 15.8 (2025), 3419-3427. Print. doi:10.5455/OVJ.2025.v15.i8.5 APA (American Psychological Association) Style Domatskiy, V. N. & Sivkova, . E. I. (2025) The incidence rates of human and animal echinococcosis: A systematic review. Open Veterinary Journal, 15 (8), 3419-3427. doi:10.5455/OVJ.2025.v15.i8.5 |