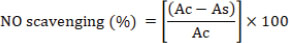| Research Article | ||
Open Vet. J.. 2025; 15(9): 4671-4680 Open Veterinary Journal, (2025), Vol. 15(9): 4671-4680 Research Article Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpaJuma Al-Mutaani1,2, Tahani Zorgui3, Abdalla A. Mohamed4,5, Iryna Smetanska6, Lazhar Zourgui3* and Nabiha Missaoui11Research Laboratory LR21ES03, Oncogenesis and Tumor Progression, Faculty of Medicine of Sousse, University of Sousse, Sousse, Tunisia 2Primary Health Care, Jaalan Bani Bu Hassan Hospital, Oman Ministry of Health, Muscat, Oman 3Research Laboratory BMA LR22ES02, Higher Institute of Applied Biology of Medenine, University of Gabes, Gabes, Tunisia 4Laboratory of Plant Biotechnology, Faculty of Science, University of Sfax, Sfax, Tunisia 5Department of Medical Nutrition, Faculty of Medical Technology, Biomedical Research Team, University of Zawia, Zawia, Libya 6Plant Production and Processing, University of Applied Sciences Weihenstephan-Triesdorf, Berlin, Germany *Corresponding Author: Lazhar Zourgui. Research Laboratory LR21ES03, Oncogenesis and Tumor Progression, Faculty of Medicine of Sousse, University of Sousse, Sousse, Tunisia. Email: lazhar.zourgui [at] gmail.com Submitted: 05/04/2025 Revised: 21/07/2025 Accepted: 10/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
AbstractBackground: Recent studies have linked various types of cancer to free radicals, driving research into plant-derived compounds that may mitigate oxidative damage. Aim: To evaluate the antioxidant potential of ethanolic (EE) and water extracts (WEs) from four Omani plant species: Ocimum basilicum, Teucrium polium, Cleome amblyocarpa, and Caralluma arabica. Methods: Antioxidant activity was assessed using the following five assays: 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical-scavenging, nitric oxide (NO) radical scavenging, ferric reducing antioxidant power (FRAP), Trolox equivalent antioxidant capacity (TEAC), and total antioxidant capacity (TAC). Ascorbic acid and Trolox were used as reference antioxidants. Results: Both extracts exhibited dose-dependent DPPH scavenging, with O. basilicum EE showing the highest activity (IC50=0.25 ± 0.022 mg/ml). Ocimum basilicum EE also displayed the strongest NO scavenging activity (IC50=0.02 ± 0.002 mg/ml), surpassing that of ascorbic acid. WE demonstrated superior reducing power (p=0.01) in the FRAP assay, with C. amblyocarpa WE exhibiting the highest activity (7.42 ± 0.015 mg/ml). TEAC and TAC assays revealed species- and solvent-dependent differences, with EE generally showing greater antioxidant activity, except for C. amblyocarpa WE, which exhibited a significantly higher TAC (p < 0.001). Two-way ANOVA identified plant species as the primary determinant of antioxidant potential (p < 0.0001). Conclusion: The strong antioxidant properties of selected Omani plants, particularly O. basilicum and C. arabica, highlight their potential for therapeutic and nutraceutical applications. Keywords: Antioxidant activity, DPPH free radical scavenging, Ethanolic extract, Oman, Water extract. IntroductionOxidative stress drives cancer progression by promoting tumor cell invasion, inflammation, and DNA damage (Jovankić et al., 2022; Bardelčíková et al., 2023). Although antioxidants help mitigate oxidative damage, cancer treatment remains challenging due to high recurrence rates and chemotherapy resistance (Sheng et al., 2017; Xiang et al., 2020). Plant-derived polyphenols and flavonoids have shown promising anticancer effects by inhibiting cancer progression (Affi et al., 2021). Effective therapies target cancer cell proliferation, apoptosis, and migration by modulating key signaling pathways such as NF-κB and epithelial-to-mesenchymal transition (Sheng et al., 2017; Khajeh et al., 2020). Ongoing research continues to explore the use of natural compounds as alternative cancer treatments (Jovankić et al., 2022). During molecular biodegradation, reactive oxygen species (ROS) are primarily produced by the mitochondrial electron transport chain and cytochrome oxidase (Petrova et al., 2023). Reactive nitrogen species (RNS) act as potent oxidants capable of damaging various biological components. Under normal physiological conditions, low ROS and RNS levels regulate signal transduction, cellular proliferation, and apoptosis. However, excessive ROS and RNS concentrations induce oxidative damage to DNA, proteins, and lipids, increasing the risk of mutagenesis and cancer development (Jovankić et al., 2022). Oxidative stress arises from an imbalance between ROS and RNS production, disrupting cellular homeostasis and contributing to the pathogenesis of complex diseases (Jovankić et al., 2022). Conversely, antioxidants neutralize ROS and free radicals, protecting cells from oxidative damage and slowing the progression of chronic diseases (Koksal et al., 2017). Tumor recurrence and metastasis remain major obstacles to successful cancer treatment and long-term patient survival (Sheng et al., 2017). Plant-derived natural antioxidants are used in traditional and modern medicine to treat complex diseases due to their safety, affordability, availability, and therapeutic potential (Koksal et al., 2017; Jovankić et al., 2022). Potent antioxidants typically contain high concentrations of polyphenols and flavonoids, which play a crucial role in neutralizing free radicals. Recently, Almutaani et al. (2025) showed that Ocimum basilicum, Teucrium polium, Cleome amblyocarpa, and Caralluma arabica contain significant amounts of polyphenolic and flavonoid compounds. Therefore, this study aims to evaluate the antioxidant properties of ethanolic and water-based extracts (EE/WE) from four Omani plant species, including O. basilicum, T. polium, C. amblyocarpa, and C. arabica, using five distinct methodologies to assess their potential antioxidant capacity. Materials and MethodsSolvents and chemicals usedThe study used various solvents and chemicals, including ethanol, 70% ethanol spray, distilled water (H2O), 0.2 M phosphate buffer (pH 6.6), 0.5 M phosphate buffer (pH 7.4), Griess reagent (1% sulfanilamide, 2% H3PO4, and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride), sodium nitroprusside (Na2[Fe(CN)5NO]·2H2O), potassium ferricyanide {1% (w/v) K3[Fe(CN)6]}, trichloroacetic acid [10% (w/v) TCA], ferric chloride [0.1% (w/v) FeCl3], 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ascorbic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and 2.456 mM potassium persulfate (K2S2O8), which were provided by the Faculty of Sciences, University of Gabes, Tunisia. In the current study, we used the following materials and equipment: a biosafety cabinet, a UV-Vis spectrophotometer, and an electrical oscillator (Whatman No. 2 filter paper, a vacuum rotary evaporator, personal protective equipment (sterile gloves, lab coat, safety visor), a water bath, a centrifuge, waste liquid and waste containers, pipettes, wrapped disposable pipettes, and a tube rack (Huh et al., 2018; Moloudi et al., 2018; Daham, 2021; Padauleng et al., 2023). Plant materialsThe plants were harvested from the South Al Sharqiyah Governorate, Sultanate of Oman, between July and September 2023. The collected species included C. amblyocarpa, T. polium, C. arabica, and O. basilicum (Fig. 1). The plant specimens were authenticated by Professor Mustapha Gorai, a botanical expert at the Higher Institute of Applied Biology of Medenine, University of Gabes (Tunisia), where a voucher specimen was also deposited. Upon collection, the plants were shade-dried at room temperature for several days, transported to the laboratory, chopped into small pieces, and rinsed with distilled water (Zourgui et al., 2020; Al-Mutaani et al., 2025). Subsequently, an electric grinder was used to grind only the dried leaves and stems (excluding roots) into a fine powder. Preparation of the plant extractThe plant extracts were prepared as previously described (Al-Mutaani et al., 2025). Briefly, 200 g of powdered plant material was mixed with 800 ml of either ethanol or distilled water in separate flasks. The mixtures were placed on a rotary shaker for 24 hours, followed by filtration and centrifugation to remove solid residues and enhance clarity. The supernatants were concentrated by evaporation at room temperature and subsequently lyophilized at 4°C. The dried extracts were stored at 4°C until further analysis. Antioxidant activitiesDPPH free radical scavenging assayThe free radical-scavenging activity of DPPH was assessed using the method described by Sanchez et al. (2014). In brief, 1.5 ml of a freshly prepared DPPH solution (2.5 mg in 100 ml of methanol) was mixed with 1 ml of each extract at varying concentrations. The reaction mixture was incubated in the dark at room temperature for 30 minutes, and the absorbance was measured at 517 nm. A blank was prepared for each concentration by omitting the DPPH solution. Ascorbic acid served as the positive control, whereas the control tube contained only DPPH solution. The DPPH radical scavenging activity (%) was calculated as follows:
where Ac: absorbance of the control, Ab: absorbance of the blank, and As: absorbance of the sample. A lower absorbance indicated higher DPPH radical-scavenging activity. The experiments were performed in triplicate (Zourgui et al., 2020; Ben Lataief et al., 2021). Nitric oxide (NO) radical-scavenging assayThe NO scavenging activity was evaluated using the method described by Jagetia and Baliga (2004). Under aerobic conditions, NO was generated from sodium nitroprusside (10 mM in 0.5 M phosphate buffer, pH 7.4), and the nitrate ion concentration was determined using Griess reagent. Briefly, 250 µl of each sample at varying concentrations was mixed with 250 µl of sodium nitroprusside and incubated at 25°C for 140 minutes. Subsequently, 150 µl of Griess reagent (1% sulfanilamide, 1% N-(1-naphthyl) ethylene diamine dihydrochloride, and 2.5% phosphoric acid) was added to 150 µl of the incubated mixture. After 30 minutes, the absorbance was measured at 546 nm, and the NO scavenging activity was calculated using the following formula:
Fig. 1. Representative specimens of plant species. A: Ocimum basilicum; B: Caralluma arabica; C: Cleome amblyocarpa; D: Teucrium polium.
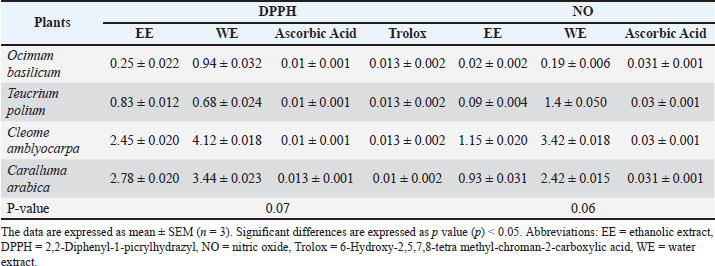
where Ac: absorbance of the control and As: absorbance of the sample. The experiments were performed in triplicate (Zourgui et al., 2020; Ben Lataief et al., 2021). Ferric reducing antioxidant power assay (FRAP)The FRAP of the extracts was evaluated using the method described by Yildirim et al. (2001). Briefly, 1 ml of each plant extract was mixed with 2.5 ml of 1% (w/v) potassium ferricyanide solution and 2.5 ml of 0.2 M phosphate buffer (pH 6.6). After incubation at 50 °C for 30 minutes, 2.5 ml of 10% (w/v) trichloroacetic acid was added to the solution. The mixture was then centrifuged at 5,000 rpm for 10 minutes. The supernatant (2.5 ml) was mixed with 0.5 ml of 0.1% (w/v) ferric chloride and 2.5 ml of distilled water. The absorbance was measured at 700 nm. A higher absorbance indicates greater reducing power. The experiment was repeated three times. ABTS radical scavenging activityThe ABTS radical scavenging activity was evaluated using the Trolox equivalent antioxidant capacity (TEAC) assay according to Chang et al. (2008). ABTS radicals were generated by mixing 5 ml of ABTS+ solution (7 mM) with 88 µl of potassium persulfate (2.456 mM). The mixture was incubated in the dark for 12–16 hours to allow complete radical formation. The ABTS radical solution was then diluted with ethanol to achieve an absorbance of 0.70 ± 0.02 at 734 nm. For the assay, 200 µl of each extract (1 mg/ml) was mixed with 2 ml of the ABTS radical solution. The absorbance was recorded after 6 minutes, and antioxidant activity was expressed as mM Trolox equivalents. Lower absorbance values indicate stronger antioxidant activity. All experiments were conducted in triplicate (Zourgui et al., 2020; Ben Lataief et al., 2021). Total antioxidant capacity (TAC) determined using the phosphomolybdenum methodThe TAC was measured using the phosphomolybdenum method described by Phatak and Hendre (2014), Izuegbuna et al. (2019), Kumari et al. (2023), and Kumari et al. (2024). Briefly, 300 µl of each extract (1 mg/ml) was mixed with 3 ml of a freshly prepared reagent solution containing 28 mM sodium phosphate, 0.6 M sulfuric acid, and 4 mM ammonium molybdate. The mixture was then incubated at 95°C for 90 minutes. Absorbance was measured at 695 nm against a blank reagent. Results were expressed as mg of AAE per gram of extract (mg AAE/g) (Zourgui et al., 2020; Ben Lataief et al., 2021). Statistical analysisThe experimental results were obtained from three independent replicates and are presented as mean ± standard error of the mean (n=3). A one-way analysis of variance was used to analyze differences among groups, followed by the least significant difference post-hoc test to assess significance at a 5% probability level (p ≤ 0.05). Ethical approvalNot needed for this study. ResultsTable 1 summarizes the antioxidant activity of ethanolic and water extracts from four Omani plants, assessed using five assays: DPPH, NO radical scavenging, FRAP, TEAC, and TAC. Ascorbic acid and Trolox were used as standard reference antioxidants. DPPH free radical scavenging activityAll plant extracts and standards exhibited dose-dependent DPPH scavenging activity, with lower absorbance indicating higher activity. Ascorbic acid (IC50=0.01 ± 0.001 mg/ml) and Trolox (IC50=0.013 ± 0.002 mg/ml) exhibited the highest activity. Among the extracts, O. basilicum EE displayed the strongest DPPH scavenging activity (IC50=0.25 ± 0.022 mg/ml). In general, EE exhibited greater DPPH scavenging activity than WE, except for T. polium, where WE (IC50=0.68 ± 0.024 mg/ml) outperformed EE (IC50=0.83 ± 0.012 mg/ml). However, the difference between EE and WE was not statistically significant (p=0.07). NO radical scavenging activityTable 1 presents NO radical scavenging activity, reported as IC50 values. Ocimum basilicum EE exhibited the strongest NO scavenging effect (IC50=0.02 ± 0.002 mg/ml), exceeding that of ascorbic acid (IC50=0.031 ± 0.001 mg/ml). The next most active extracts were T. polium EE (IC50=0.09 ± 0.004 mg/ml), C. arabica EE (IC50=0.93 ± 0.031 mg/ml), and C. amblyocarpa EE (IC50=1.15 ± 0.020 mg/ml). Aqueous extracts showed slightly lower NO scavenging activity, with IC50 values of 0.19 ± 0.006, 1.4 ± 0.050, 3.42 ± 0.018, and 2.42 ± 0.015 mg/ml for O. basilicum, T. polium, C. amblyocarpa, and C. arabica, respectively. However, the difference between EE and WE was not statistically significant (p=0.06). Table 1. DPPH and NO radical-scavenging activities of O. basilicum, T. polium, C. arabica, and C. amblyocarpa ethanolic and water extracts.
FRAP activityThe reducing power of the extracts was evaluated using the FRAP assay. At a concentration of 1 mg/ml, C. amblyocarpa WE exhibited the highest reducing activity (7.42 ± 0.015 mg/ml), followed by C. arabica WE (6.44 ± 0.021 mg/ml) and T. polium WE (3.4 ± 0.032 mg/ml), all of which surpassed the activity of ascorbic acid (2.43 ± 0.009 mg/ml). Among the ethanolic extracts, C. amblyocarpa showed the highest reducing activity (Table 2). Overall, aqueous extracts showed significantly greater reducing power than ethanolic extracts across all plant species (p=0.01). ABTS radical scavenging activityTable 2 presents the TEAC assay results of different plant extracts. A lower absorbance corresponds to stronger antioxidant activity. A two-way analysis of variance (ANOVA) confirmed significant differences among plant species (p < 0.0001), with ethanolic extracts generally exhibiting superior antioxidant activity. However, C. amblyocarpa EE exhibited the highest TEAC value (1.13 ± 0.012 mM), whereas O. basilicum WE exhibited the lowest (0.38 ± 0.009 mM). A significant plant-solvent interaction was observed (p=0.002), indicating solvent-dependent variations in antioxidant extraction efficiency. Total antioxidant capacityOur findings revealed species- and solvent-dependent variations for TAC (Table 2). Although ethanolic extracts generally demonstrated higher TAC values (p=0.010), C. amblyocarpa WE was an exception, showing 23% greater activity than its EE counterpart (98.12 ± 0.012 mg AAE/g dw vs. 76.16 ± 0.021 mg AAE/g dw; p < 0.001). A two-way analysis of variance identified plant species as the primary determinant of TAC variation (p < 0.0001), with a significant plant-solvent interaction (p < 0.0001), emphasizing species-specific extraction efficiency (Table 2). Notably, C. arabica EE displayed the highest TAC value (98.12 ± 0.015 mg AAE/g dw), highlighting its antioxidant potential. DiscussionRecent studies have linked diverse malignancies to free radicals, prompting research into compounds capable of mitigating oxidative damage (Affi et al., 2021; Brandão et al., 2022). In this study, we employed five complementary colorimetric assays, including DPPH and NO radical-scavenging activity, FRAP, TEAC, and TAC, alongside two reference antioxidants, ascorbic acid and Trolox, to assess the electron-donating and radical-scavenging potential of Omani plant extracts. Given the limitations of individual assays, this multi-method approach offers a more comprehensive assessment of O. basilicum, T. polium, C. amblyocarpa, and C. arabica’s antioxidant capacity, yielding deeper insights into their underlying mechanisms of action (Zourgui et al., 2020; Ben Lataief et al., 2021; Kızıltaş et al., 2021). The DPPH assay is commonly used to evaluate antioxidant activity by measuring the ability of compounds to inhibit lipid oxidation through free radical scavenging. This method is favored for its simplicity, sensitivity, and compatibility with UV-Vis spectrophotometry, ensuring reliable results (Hikmawanti et al., 2021; Kızıltaş et al., 2021; Baliyan et al., 2022). The antioxidant efficacy of plant extracts largely depends on the number and position of hydroxyl groups in polyphenols and flavonoids. Strong correlations have been reported between DPPH radical-scavenging activity and polyphenol content (Lohvina et al., 2021). Table 2. FRAP, TEAC, and TAC of ethanolic and water extracts of O. basilicum, T. polium, C. arabica, and C. amblyocarpa.
DPPH, a crystalline compound with a characteristic dark blue or purple hue, consists of stable free radicals that are detectable under ultraviolet (UV) light. Antioxidants donate hydrogen atoms or electrons to DPPH radicals, converting them into diphenylpicrylhydrazine (DPPH-H), which shifts the solution from violet to colorless or pale yellow, thereby reducing absorbance and indicating free radical suppression (Hatami et al., 2014; Hikmawanti et al., 2021; Baliyan et al., 2022; Fadhila et al., 2023). The IC50 value represents the concentration required to inhibit 50% of DPPH radicals and is determined through linear regression analysis. A lower IC50 value reflects greater antioxidant potency, with antioxidant efficiency generally increasing with concentration (Zourgui et al., 2020; Ben Lataief et al., 2021; Hikmawanti et al., 2021; Molole et al., 2022). Ascorbic acid and Trolox exhibited greater scavenging efficiency than the plant extracts, as indicated by lower IC50 values, consistent with previous findings (Zourgui et al., 2020). Several factors, including antioxidant structure (e.g., oxygen content, aromatic rings, or conjugated double bonds), solvent polarity, and extract concentration, influence radical-scavenging activity (Molole et al., 2022). In this study, ethanolic plant extracts showed higher DPPH inhibition than aqueous extracts, a trend also reported by Lohvina et al. (2021). However, some studies have suggested that certain ethanol dilutions exhibit greater antioxidant activity than pure ethanol, likely due to the improved solubility of diverse phytochemicals in mixed solvents (Yashin et al., 2017; Hikmawanti et al., 2021; Fadhila et al., 2023). Lohvina et al. (2021) attributed the strong antioxidant effects of aqueous and ethanolic extracts to their ability to neutralize both phenolic and non-phenolic antioxidants. However, they cautioned against using ethanolic extracts with less than 30% concentration, as higher water content reduces total phenolic and antioxidant levels, which are key contributors to free radical scavenging and iron chelation (Lohvina et al., 2021). Similarly, Himawan et al. (2021) and Molole et al. (2022) reported that combined extracts, such as 70% ethanolic extracts of basil and jinzhong leaves (IC50=0.0243 mg/ml), may exhibit superior antioxidant properties compared to single-plant extracts (Himawan et al., 2021; Molole et al., 2022). Kızıltaş et al. (2021) recommended using 100% ethanol for extraction, as it preserves antioxidant activity, a conclusion supported by Shekhar and Anju (2014) and Lohvina et al. (2021). These studies examined absolute ethanol extracts from Ageratum conyzoides Linn., Limnophila (aromatic), and O. basilicum, which exhibited strong DPPH radical scavenging with IC50 values of 0.07, 0.0243, and 0.25 mg/ml, respectively. Nonetheless, Shalaby and Shanab (2013) reported that 100% methanol yielded the highest antiradical activity per mg of extract, likely due to its direct interaction with phenolic and phycobiliprotein compounds. Fadhila et al. (2023) considered that the abundance of plant metabolites may interact synergistically or antagonistically, influencing antioxidant activity. The composition and concentration of phytochemicals, which vary based on plant species, solvent type, and extraction conditions, determine the antioxidant efficacy (Gawron-Gzella et al., 2012; Zourgui et al., 2020; Ouahhoud et al., 2022; Almutaani et al., 2025). In our study, ethanolic plant extracts exhibited lower IC50 values than aqueous extracts, indicating their greater free radical-scavenging capacity. Although T. polium WE showed slightly stronger DPPH activity than its EE counterpart (0.83 ± 0.012 mg/ml and 0.68 ± 0.024 mg/ml, respectively), the difference was not statistically significant. Our recent data showed that O. basilicum EE contained the highest polyphenol and flavonoid content among the studied Omani species, followed by T. polium (Al-Mutaani et al., 2025). The EE of O. basilicum also exhibited the strongest DPPH radical-scavenging activity (IC50=0.25 ± 0.022 mg/ml), followed by the WE of T. polium (IC50=0.68 ± 0.024 mg/ml), consistent with the findings of Himawan et al. (2021). NO scavenging activity was assessed using ascorbic acid as a positive control for all Omani plant extracts. A lower IC50 value indicates greater NO radical elimination and stronger antioxidant activity (Ben Lataief et al., 2021). The highest NO radical-scavenging activity was observed in the EE of O. basilicum (IC50=0.02 ± 0.002 mg/ml), followed by ascorbic acid (IC50=0.031 ± 0.001 mg/ml) and T. polium (IC50=0.09 ± 0.004 mg/ml), consistent with the findings of Izuegbuna et al. (2019). Although EEs generally exhibited stronger NO radical-scavenging activity than aqueous extracts, the difference was not statistically significant (p > 0.05), as reported by Ben Lataief et al. (2021). Overall, the NO radical-scavenging assay revealed the strongest antioxidant activity, followed by the DPPH assay, which agrees with previous studies (Izuegbuna et al., 2019; Ben Lataief et al., 2021). Compared with WEs, EEs contain higher concentrations of polyphenolic and flavonoid compounds, which enhance their radical-scavenging performance by donating hydrogen atoms and electrons, respectively (Zourgui et al., 2020; Al Mutaani et al., 2025). The results of the FRAP, ABTS, and TAC assays further supported the strong antioxidant activity of these plant extracts. A significant correlation between reducing power and antioxidant activity has been reported (Zourgui et al., 2020). However, the relationship between DPPH radical-scavenging activity, TAC, and iron-chelating ability appears to be weaker than that proposed by Lohvina et al. (2021). The FRAP assay is considered a reliable method for quantifying total antioxidants in edible plants because it does not react with low concentrations of thiols present in these sources. Unlike assays that assess free radical suppression, the FRAP method directly measures antioxidants (reductants) in a sample. However, impaired oxidative phosphorylation can increase ROS accumulation, leading to biomolecular degradation, disruption of the electron transport chain, and alteration of cellular homeostasis (Payne et al., 2013; Affi et al., 2021; Baliyan et al., 2022; Ouahhoud et al., 2022). The extraction conditions play a critical role in the antioxidant yield. Yashin et al. (2017) warned that a considerable fraction of polyphenols might undergo oxidation when exposed to elevated temperatures for extended periods, highlighting the importance of precise extraction protocols. Furthermore, oxidized polyphenols likely involve hydroxyl groups in phenolic compounds, which act as electron donors and contribute to antioxidant mechanisms such as the reduction or inactivation of oxidants (Ouahhoud et al., 2022). The quality and yield of the extract depend on its chemical stability in the solvent, incubation time, and temperature conditions (Hikmawanti et al., 2021). Several researchers have used the potassium ferricyanide method to evaluate the chelating efficacy of plant extracts, comparing the results with control samples (Sudan et al., 2014; Affi et al., 2021; Kızıltaş et al., 2021). As a ferric salt, potassium ferricyanide serves as an oxidizing agent. Iron reduction occurs when an antioxidant donates an electron, converting ferric iron (Fe³+) to ferrous iron (Fe²+), which is detected by absorbance at 700 nm. Impaired oxidative phosphorylation can lead to ROS accumulation, resulting in biomolecular degradation and affecting the electron transport chain (Payne et al., 2013; Affi et al., 2021; Baliyan et al., 2022; Ouahhoud et al., 2022). Yashin et al. (2017) reported that prolonged exposure to high temperatures (exceeding 3 hours) could oxidize a substantial fraction of polyphenols, emphasizing the importance of controlled extraction conditions. This oxidation likely affects the hydroxyl groups in phenolic compounds, which serve as electron donors and contribute to antioxidant mechanisms, including oxidant reduction and inactivation (Ouahhoud et al., 2022). The quality and yield of the extract depend on its chemical stability in the solvent, incubation time, and temperature conditions (Hikmawanti et al., 2021). Higher extract concentrations correlate with increased FRAP values, indicating greater antioxidant potential. Studies have shown that samples with higher absorbance exhibit stronger free radical-scavenging activity (Zourgui et al., 2020; Affi et al., 2021; Ben Lataief et al., 2021; Kızıltaş et al., 2021; Ouahhoud et al., 2022). Cleome amblyocarpa EE exhibited the highest reducing power (7.42 ± 0.015 mg/ml), followed by C. arabica WE. Previously, Khlifi et al. (2020) reported that the leaves and stems of C. amblyocarpa EE showed significant antioxidant activity against DPPH, ABTS, FRAP, and thiobarbituric acid reactive species (TBARS), likely due to their high polyphenol content. Similar findings were reported by Khasawneh et al. (2014), Affi et al. (2021), and Lohvina et al. (2021), demonstrating strong antioxidant activity associated with high ascorbic acid content (2.43 ± 0.009 mg/ml) and low Trolox levels (1.86 mg/ml), consistent with Koksal et al. (2017) and Affi et al. (2021). However, our results were significantly lower than those of the WEs. Lohvina et al. (2021) reported significant differences between aqueous and ethanolic extracts (p < 0.05). The FRAP assay conducted by Sudan et al. (2014) revealed that 0.1 mg/ml ethanolic crude extracts exhibited a chelating capacity of 49.7%. TEAC values were determined based on the reaction between the extract and ABTS radicals rather than on the inhibition of oxidation. ABTS radicals interact with antioxidants to form a colorless solution, enabling the quantification of ABTS radical scavenging activity relative to Trolox within a defined timeframe. The TEAC value was calculated by measuring the reduction in absorbance compared with that of Trolox at 734 nm (Baliyan et al., 2022). A greater decrease in absorbance indicates a higher TEAC value, reflecting enhanced antioxidant activity. In our study, the TEAC values for WEs ranged from 0.38 ± 0.009 to 0.95 ± 0.012 mM Trolox/g, whereas those for EEs ranged from 0.53 ± 0.0012 to 1.13 ± 0.012 mM Trolox/g. Notably, WEs exhibited lower TEAC values than EEs. For instance, O. basilicum WE had a TEAC value of 0.38 ± 0.009 mM Trolox/g dry extract, whereas O. basilicum EE recorded 0.53 ± 0.0012 mM Trolox/g. Furthermore, O. basilicum and T. polium extracts showed lower absorbance than the positive control (0.85 ± 0.02 mM Trolox/g), which is consistent with the results of Ben Lataief et al. (2021) but contradicts those of Zourgui et al. (2020). TEAC values from the ABTS assay exhibited a significant positive correlation. The strong ABTS-scavenging activity of the selected Omani plant extracts aligns with the findings of Kızıltaş et al. (2021). A recent study highlighted the correlation between TAC and antioxidant activity, demonstrating that higher TAC values reflect greater radical-scavenging potential and enhanced antioxidant efficacy (Zourgui et al., 2020; Ben Lataief et al., 2021). Our findings are expressed in AAE per gram of dry extract. Ethanolic plant extracts generally exhibited higher TAC values than WEs, except for C. amblyocarpa, which recorded 76.16 ± 0.021 AAE/g and 98.12 ± 0.012 AAE/g for its ethanolic and aqueous extracts, respectively. The positive control (ascorbic acid) had a TAC value of 81.24 ± 0.14 mg AAE/g, consistent with the results reported by Ben Lataief et al. (2021). However, our results differed from the control values and lower TAC values reported by Zourgui et al. (2020) and Affi et al. (2021). Furthermore, the difference in TAC values between EEs and WEs was not statistically significant. In conclusion, the antioxidant potential of ethanolic and aqueous extracts from four Omani plants, O. basilicum, T. polium, C. amblyocarpa, and C. arabica, was evaluated using five complementary assays: DPPH, NO scavenging, FRAP, TEAC, and TAC. Both extract types exhibited significant antioxidant activity, with notable variations based on plant species and extraction solvent. Ethanol extracts generally showed superior efficacy, particularly O. basilicum, which excelled in DPPH and NO scavenging capacity. Conversely, the aqueous extracts of C. amblyocarpa and C. arabica exhibited strong reducing power and TAC. These results underscore the therapeutic and nutraceutical potential of these plant species, attributed to their potent antiradical properties. AcknowledgmentsWe would like to express our sincere gratitude to the Research Laboratory of Plant Physiology and Biotechnology, Faculty of Sciences, University of Gabes (Tunisia), for their continuous support, laboratory assistance, and provision of essential resources and facilities throughout the research. Our heartfelt thanks go to Professor Mustapha Gorai, from the Higher Institute of Applied Biology of Medenine, University of Gabes (Tunisia), for authenticating the Omani plant specimens used in this study. FundingNot applicable. Authors’ contributionsJAM: conceptualization, data collection, and data curation. TZ, AAM, and IS: experiments and data analysis. LZ: conceptualization, experimental design, resources, and supervision. NM: conceptualization, visualization, supervision, and paper writing. All authors have reviewed and approved the final version of the manuscript. Conflict of interestThe authors declare that there is no conflict of interest. Data availabilityThe supplementary data will be made available by the corresponding author upon reasonable request. ReferencesAffi, W., Zourgui, M.N., Ben Lataief, S., Agil, A. and Zourgui, L. 2021. Comparative study of phenolic compound antioxidant and antimicrobial activities of fruits peel and cladodes from Tunisian Opuntia stricta. J. Altern. Complem. Integr. Med. 7(5), 1–7; doi:10.24966/ACIM-7562/100202 Al Mutaani, J., Al Hasani, B., Al Hattali, S., Al Oraimi, M., Al Alawi, N., Kumar, S., Al Sadi, K., Al Alawi, S., Al Harbi, M., Al Faraji, A., Al Rajhi, A., Al Jaffari, M., Al Jaffari, S., Al Alawi, M., Al Suri, M., Al Satmi, F., Al Saadi, M., Al Rottali, B., Al Wihabi, F., Hmissa, S., Ouahchi, I. and Missaoui, N. 2024. Colorectal cancer detection during a screening awareness campaign in a high-risk region in Oman. Asian Pac. J. Cancer Prev. 25(8), 2831–2840; doi:10.31557/APJCP.2024.25.8.2831 Al-Mutaani, J., Zourgui, L. and Missaoui, N. 2025. Phytochemicals, bioactive compounds, and antimicrobial activities of Ocimum basilicum, Teucrium polium, Cleome amblyocarpa, and Caralluma arabica extracts: a comparative Omani study. Cell. Mol. Biol. (Noisy-le-grand). 71(3), 134–145; doi:10.14715/cmb/2025.71.3.16 Baliyan, S., Mukherjee, R., Priyadarshini, A., Vibhuti, A., Gupta, A., Pandey, R.P. and Chang, C.M. 2022. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 27(4), 1326; doi:10.3390/molecules27041326 Bardelčíková, A., Šoltys, J. and Mojžiš, J. 2023. Oxidative stress, inflammation and colorectal cancer: an overview. Antioxidants 12(4), 901; doi:10.3390/antiox12040901 Ben Lataief, S., Zourgui, M.N., Rahmani, R., Najjaa, H., Gharsallah, N. and Zourgui, L. 2021. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of bioactive compounds extracted from Opuntia dillenii cladodes. J. Food Meas. Characterization 15(1), 782–794; doi:10.1007/s11694-020-00671-2 Brandão, L.B., Santos, L.L., Martins, R.L., Rodrigues, A.B.L., Pena Da Costa, A.L., Faustino, C.G. and Da Silva De Almeida, S.S.M. 2022. The potential effects of species Ocimum basilicum L. on health: a review of the chemical and biological studies. Pharmacogn. Rev. 16(31), 22–26; doi:10.5530/phrev.2022.16.4 Chang, S.F., Hsieh, C.L. and Yen, G.C. 2008. The protective effect of Opuntia dillenii Haw fruit against low-density lipoprotein peroxidation and its active compounds. Food Chem. 106(2), 569–575; doi:10.1016/j.foodchem.2007.06.017 Dahham, S.S., Tabana, Y., Asif, M., Ahmed, M., Babu, D., Hassan, L.E., Ahamed, M.B.K., Sandai, D., Barakat, K., Siraki, A. and Majid, A.M.S.A. 2021. β-Caryophyllene induces apoptosis and inhibits angiogenesis in colorectal cancer models. Int. J. Mol. Sci. 22(19), 10550; doi:10.3390/ijms221910550 Fadhila, S.I., Hayati, E.K., Rafi, M. and Sabarudin, A. 2023. Effect of ethanol-water concentration as extraction solvent on antioxidant activity of Acalypha indica. Al-Kimiya 10(2), 133–142; doi:10.15575/ak.v10i2.30081 Gawron-Gzella, A., Dudek-Makuch, M. and Matławska, I. 2012. DPPH radical scavenging activity and phenolic compound content in different leaf extracts from selected blackberry species. Acta Biol. Cracoviensia Ser. Botanica 54(2), 32–38; doi:10.2478/v10182-012-0017-8 Gülçin, I., Kızıltaş, H., Bingöl, Z., Gören, A.C., Pinar, S.M. and Alwasel, S.H. 2021.J. Chem. Metrology 2(2), 135–151; doi:10.25135/jcm.62.2107.2155 Hatami, T., Emami, S.A., Miraghaeea, S.S. and Mojarrab, M. 2014. Total phenolic contents and antioxidant activities of different extracts and fractions from the aerial parts of Artemisia biennis Willd. Iranian J. Pharm. Res. 13(2), 551–558. Hikmawanti, N.P., Fatmawati, S. and Asri, A.W. 2021. The eEffect of ethanol concentrations as the extraction solvent on antioxidant activity of Katuk (Sauropus androgynus (L.) Merr.) leaves extracts. In IOP Conference . Series: . Earth and Environmental . Science,2021 . 755(1), p 12060. ; doi:10.1088/1755-1315/755/1/012060 Himawan, H.C., Isa, A.F. and Wiharja, D.S. 2021. Antioxidant activity of 70% ethanol extract combination of kemangi leaf (Ocimum Americanum Linn) and binahong leaf (Anredera cordifolia (Ten.) Steenis) using DPPH. In J. Phys. Conf. Ser,. 20211764(1), p 12009. ; doi:10.1088/1742-6596/1764/1/012009 Huh, M.K., Park, S.J., Ahn, N.K., Yoo, S.J. and Kim, M.J. 2018. DPPH and no radical scavenging activity effect using extraction of four halophyte species in Korea. Eur. J. Basic Appl. Sci. 5(2), 15–23. Izuegbuna, O., Otunola, G. and Bradley, G. 2019. Chemical composition, antioxidant, anti-inflammatory, and cytotoxic activities of Opuntia stricta cladodes. PLoS. One 14(1), e0209682; doi:10.1371/journal.pone.0209682 Jagetia, G.C. and Baliga, M.S. 2004. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: a preliminary study. J. Med. Food 7(3), 343–348; doi:10.1089/jmf.2004.7.343 Jovankić, J., Nikodijević, D., Blagojević, S., Radenković, N., Jakovljević, D., Grbović, F. and Cvetković, D. 2022. The biological activity of Ocimum minimum L. flowers on redox status parameters in HCT-116 colorectal carcinoma cells. Kragujevac J. Sci. 44, 155–168; doi:10.5937/KgJSci2244155J Khajeh, E., Rasmi, Y., Kheradmand, F., Malekinejad, H., Aramwit, P., Saboory, E., Daeihassani, B. and Nasirzadeh, M. 2020. Crocetin suppresses the growth and migration in HCT-116 human colorectal cancer cells by activating the p-38 MAPK signaling pathway. Res. Pharm. Sci. 15(6), 592https://doi.org/; doi: 10.4103/1735-5362.301344 Khasawneh, M., Elwy, H.M., Fawzi, N.M., Hamza, A.A., Chevidenkandy, A.R. and Hassan, A.H. 2014. Antioxidant activity and lipoxygenase inhibitory effect of Caralluma Arabica and related polyphenolic constituents. Am. J. Plant Sci. 05(11), 1623–1631; doi:10.4236/ajps.2014.511176 Khlifi, A., Chrifa, A.B., Lamine, J.B., Thouri, A., Adouni, K., Flamini, G., Oleszek, W. and Achour, L. 2020. Gas chromatography-mass spectrometry (GC-MS) analysis and biological activities of the aerial part of Cleome amblyocarpa Barr. and Murb. Environ. Sci. Pollut. Res. 27(18), 22670–22679; doi:10.1007/s11356-020-08764-7 Kızıltaş, H., Bingöl, Z., Gören, A.C., Pinar, S.M., Alwasel, S.H. and Gülçin, S.H. 2021. LC-HRMS profiling of phytochemicals, antidiabetic, anticholinergic and antioxidant activities of evaporated ethanol extract of Astragalus brachycalyx Fischer. J. Chem. Metrol. 2(2), 135–151; doi:10.25135/jcm.62.2107.2155 Köksal, E., Tohma, H., Kılıç, O., Alan, Y., Aras, A., Gülçin, I. and Bursal, E. 2017. Assessment of antimicrobial and antioxidant activities of Nepeta trachonitica: analysis of its phenolic compounds using HPLC-MS/MS. Sci. Pharm. 85(2), 24; doi:10.3390/scipharm85020024 Kumari, T., Phogat, D. and Shukla, V. 2023. Exploring the multipotentiality of plant extracts for the green synthesis of iron nanoparticles: a study of adsorption capacity and dye degradation efficiency. Environ. Res. 229, 116025; doi:10.1016/j.envres.2023.116025 Kumari, T., Phogat, D., Jakhar, N. and Shukla, V. 2024. Effectiveness of copper oxychloride coated with iron nanoparticles against earthworms. Sci. Rep. 14(1), 23150; doi:10.1038/s41598-024-73794-x Lohvina, H., Sándor, M. and Wink, M. 2021. Effect of ethanol solvents on total phenolic content and antioxidant properties of seed extracts of fenugreek (Trigonella foenum-graecum L.) varieties and determination of phenolic composition by HPLC-ESI-MS. Diversity 14(1), 7; doi:10.3390/d14010007 Molole, G.J., Gure, A. and Abdissa, N. 2022. Determination of total phenolic content and antioxidant activity of Commiphora mollis (Oliv.) Engl. resin. BMC. Chem. 16(1), 48; doi:10.1186/s13065-022-00841-x Moloudi, R., Oh, S., Yang, C., Teo, K.L., Lam, A.T.L., Warkiani, M.E. and Naing, M.W. 2018. Inertial-based filtration method for removal of microcarriers from mesenchymal stem cell suspensions. Sci. Rep. 8(1), 12481; doi:10.1038/s41598-018-31019-y Ouahhoud, S., Khoulati, A., Kadda, S., Bencheikh, N., Mamri, S., Ziani, A., Baddaoui, S., Eddabbeh, F.E., Lahmass, I., Benabbes, R., Addi, M., Hano, C., Asehraou, A. and Saalaoui, E. 2022. Antioxidant activity, metal chelating ability and DNA protective effect of the hydroethanolic extracts of Crocus sativus stigmas, tepals and leaves. Antioxidants 11(5), 932; doi:10.3390/antiox11050932 Padauleng, N., Mustofa, M., Wahyuningsih, T.D. and Purnomosari, D. 2023. Chalcone-3 inhibits the proliferation of human breast cancer MDA-MB-231 cell line. Asian Pac. J. Cancer Prev. 24(2), 683–691; doi:10.31557/APJCP.2023.24.2.683 Payne, A.C., Mazzer, A., Clarkson, G.J.J. and Taylor, G. 2013. Antioxidant assays - consistent findings from FRAP and ORAC reveal a negative impact of organic cultivation on antioxidant potential in spinach but not watercress or rocket leaves. Food Sci. Nutr. 1(6), 439–444; doi:10.1002/fsn3.71 Petrova, M., Dimitrova, L., Dimitrova, M., Denev, P., Teneva, D., Georgieva, A., Petkova-Kirova, P., Lazarova, M. and Tasheva, K. 2023. Antitumor and antioxidant activities of in vitro cultivated and wild-growing Clinopodium vulgare L. Plants 12(8), 1591; doi:10.3390/plants12081591 Phatak, R., S., Hendre, A. and S. 2014. Total antioxidant capacity (TAC) of fresh leaves of Kalanchoe pinnata. J. Pharmacogn. Phytochem. 2(5), 32–35. Sánchez, E., Dávila-Aviña, J., Castillo, S.L., Heredia, N., Vázquez-Alvarado, R. and García, S. 2014. Antibacterial and antioxidant activities in extracts of fully grown cladodes of 8 cultivars of Cactus pearcactus pear. J. Food Sci. 79(4), M659–M664; doi:10.1111/1750-3841.12416 Shalaby, E., .A.,. and Shanab, S., and M..M. 2013. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Marine Sci. 42(5), 556–564. Shekhar, T., C. and Anju, G. 2014. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn. leaves. Am. J. Ethnomed. 1(4), 244–249. Sheng, X., Zhu, P.F. and Qin, J.M. 2017. Study of the biological effect of bufalin on anti proliferation, adhesion and invasion in liver cancer cells. Clin. Surg. 2(1), 1363. Sudan, R., Bhagat, M., Gupta, S., Singh, J. and Koul, A. 2014. Iron (FeII) chelation, ferric reducing antioxidant power, and immune modulating potential of Arisaema jacquemontii(Himalayan Cobra Lily). BioMed. Res. Int. 2014, 1–7; doi:10.1155/2014/179865 Xiang, L., He, B., Liu, Q., Hu, D., Liao, W., Li, R., Peng, X., Wang, Q. and Zhao, G. 2020. Antitumor effects of curcumin on the proliferation, migration and apoptosis of human colorectal carcinoma HCT-116 cells. Oncol. Rep. 44(5), 1997–2008; doi:10.3892/or.2020.7765 Yashin, A., Yashin, Y., Xia, X. and Nemzer, B. 2017. Antioxidant activity of spices and their impact on human health: a review. Antioxidants 6(3), 70; doi:10.3390/antiox6030070 Yildirim, A., Mavi, A. and Kara, A.A. 2001. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 49(8), 4083–4089; doi:10.1021/jf0103572 Zourgui, M.N., Hfaiedh, M., Brahmi, D., Affi, W., Gharsallah, N., Zourgui, L. and Amri, M. 2020. Phytochemical screening, antioxidant and antimicrobial activities of Opuntia streptacantha fruit skin. J. Food Meas. Charact. 14(5), 2721–2733; doi:10.1007/s11694-020-00518-w | ||
| How to Cite this Article |
| Pubmed Style Al-mutaani J, Zorgui T, Mohamed AA, Smetanska I, Zourgui L, Missaoui N. Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpa. Open Vet. J.. 2025; 15(9): 4671-4680. doi:10.5455/OVJ.2025.v15.i9.71 Web Style Al-mutaani J, Zorgui T, Mohamed AA, Smetanska I, Zourgui L, Missaoui N. Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpa. https://www.openveterinaryjournal.com/?mno=250875 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.71 AMA (American Medical Association) Style Al-mutaani J, Zorgui T, Mohamed AA, Smetanska I, Zourgui L, Missaoui N. Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpa. Open Vet. J.. 2025; 15(9): 4671-4680. doi:10.5455/OVJ.2025.v15.i9.71 Vancouver/ICMJE Style Al-mutaani J, Zorgui T, Mohamed AA, Smetanska I, Zourgui L, Missaoui N. Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpa. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4671-4680. doi:10.5455/OVJ.2025.v15.i9.71 Harvard Style Al-mutaani, J., Zorgui, . T., Mohamed, . A. A., Smetanska, . I., Zourgui, . L. & Missaoui, . N. (2025) Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpa. Open Vet. J., 15 (9), 4671-4680. doi:10.5455/OVJ.2025.v15.i9.71 Turabian Style Al-mutaani, Juma, Tahani Zorgui, Abdalla A. Mohamed, Iryna Smetanska, Lazhar Zourgui, and Nabiha Missaoui. 2025. Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpa. Open Veterinary Journal, 15 (9), 4671-4680. doi:10.5455/OVJ.2025.v15.i9.71 Chicago Style Al-mutaani, Juma, Tahani Zorgui, Abdalla A. Mohamed, Iryna Smetanska, Lazhar Zourgui, and Nabiha Missaoui. "Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpa." Open Veterinary Journal 15 (2025), 4671-4680. doi:10.5455/OVJ.2025.v15.i9.71 MLA (The Modern Language Association) Style Al-mutaani, Juma, Tahani Zorgui, Abdalla A. Mohamed, Iryna Smetanska, Lazhar Zourgui, and Nabiha Missaoui. "Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpa." Open Veterinary Journal 15.9 (2025), 4671-4680. Print. doi:10.5455/OVJ.2025.v15.i9.71 APA (American Psychological Association) Style Al-mutaani, J., Zorgui, . T., Mohamed, . A. A., Smetanska, . I., Zourgui, . L. & Missaoui, . N. (2025) Comparative antioxidant activity of four Omani medicinal plants: Ocimum basilicum, Teucrium polium, Caralluma arabica, and Cleome amblyocarpa. Open Veterinary Journal, 15 (9), 4671-4680. doi:10.5455/OVJ.2025.v15.i9.71 |