| Research Article | ||
Open Vet. J.. 2025; 15(7): 3054-3062 Open Veterinary Journal, (2025), Vol. 15(7): 3054-3062 Research Article Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applicationsNungki Hapsari Suryaningtyas1,2*, Raden Wisnu Nurcahyo3, Beni Ernawan4, Hadian Iman Sasmita5, Sunaryo Sunaryo4, Tri Ramadhani4, Supriyono Supriyono6 and Triwibowo Ambar Garjito41Postgraduate Veterinary Science, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia 2Center for Biomedical Research, Research Organization for Health, National Research and Innovation Agency (BRIN), Cibinong Science Center, Cibinong, Bogor, Indonesia 3Department of Parasitology, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia 4Research Center for Public Health and Nutrition, Research Organization for Health, National Research and Innovation Agency (BRIN), Cibinong Science Center, Bogor, Indonesia 5Research Center for Radiation Process Technology, Research Organization for Nuclear Energy, National Research and Innovation Agency (BRIN), Jakarta, Indonesia 6Division of Parasitology and Medical Entomology, School of Veterinary Medicine and Biomedicine, IPB University, Bogor, Indonesia *Corresponding Author: Nungki Hapsari Suryaningtyas. Center for Biomedical Research, Research Organization for Health, National Research and Innovation Agency (BRIN), Cibinong Science Center, Bogor, Indonesia. Email: nungkihapsari36 [at] gmail.com Submitted: 27/03/2025 Revised: 28/05/2025 Accepted: 03/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Culex quinquefasciatus is a major vector of filariasis and other mosquito-borne diseases. The sterile insect technique (SIT) has been widely used to suppress mosquito populations, but its effectiveness depends on the dispersal, survival, and competitiveness of sterile males. Aim: This study evaluated the dispersal range, survival rate, and recapture success of gamma-irradiated Cx. quinquefasciatus under field conditions. Methods: A mark-release-recapture (MRR) experiment was conducted using sterile male and female Cx. quinquefasciatus. Two release events were conducted, and recapture data were collected over seven days using BG-Sentinel-2 traps baited with octanol placed within a 250-m radius. Results: The irradiated males traveled an average of 143.18 m (FR50: 92.78 m; FR90: 220.02 m), and the females dispersed 146.26 m (FR50: 95.26 m; FR90: 227.25 m). Dispersal distance was significantly influenced by release site in males (p=0.0089) and females (p=0.0042) but not by recapture day (p > 0.89). Recapture location significantly affected dispersal in both sexes (p < 0.0001). The daily survival probabilities of males and females were 0.88 and 0.69, respectively, with corresponding life expectancies of 7.57 and 2.71 days. Conclusion: The dispersal and survival of sterile Cx. quinquefasciatus are affected by release strategies. To optimize SIT, further studies should refine the marking techniques, explore a combination of trapping methods, and evaluate the dispersal patterns across varied landscapes. These findings offer valuable insights into improving the implementation of SIT for Cx. quinquefasciatus population control. Keywords: Culex quinquefasciatus, Sterile insect technique, Mark-release-recapture, Dispersal, Survival, Vector control. IntroductionCulex quinquefasciatus (Diptera: Culicidae) is a primary vector of several zoonotic diseases and parasitic infections affecting human and animal health. This species plays a critical role in the transmission of lymphatic filariasis, West Nile virus, and Japanese encephalitis, impacting millions of people globally each year (Bartholomay et al., 2010; Farajollahi et al., 2011; Surasa et al., 2024). The widespread distribution of Cx. quinquefasciatus in both urban and rural environments, combined with its ability to breed in polluted habitats, presents a significant challenge to effective control (Simonsen and Mwakitalu, 2013). Conventional vector control strategies, including chemical insecticides and habitat modification, have been widely implemented but are increasingly constrained by insecticide resistance, ecological concerns, and limited long-term efficacy (Ranson and Lissenden, 2016; Benelli and Beier, 2017). In response to these challenges, the Sterile Insect Technique (SIT) has emerged as a promising genetic-based vector control method, particularly for mosquito species such as Aedes aegypti and Anopheles arabiensis (Dyck et al., 2005; Bouyer et al., 2020). The SIT involves the release of males sterilized via irradiation into wild populations to disrupt reproduction and progressively reduce population density. However, the application of SIT to Cx. quinquefasciatus remains understudied, particularly with respect to the dispersal patterns and survival of sterile males under field conditions. The mark-release-recapture (MRR) method has been widely used to evaluate mosquito movement, survival, and mating competitiveness in the field (Reisen et al., 1992; Guerra et al., 2014). Nonetheless, most prior research has focused on Aedes and Anopheles, with limited data available for Cx. quinquefasciatus. This study aimed to assess the dispersal patterns, survival rates, and release efficiencies of irradiated male Cx. quinquefasciatus in the context of the SIT. Specifically, the flight range of sterile mosquitoes was quantified using Flight Range 50 (FR50) and Flight Range 90 (FR90) metrics to determine their spatial spread from the release point. Additionally, the probability of daily survival (PDS) and average life expectancy (ALE) weree estimated to evaluate postrelease survival under natural conditions. The findings of this study are expected to provide critical insights into the postrelease dynamics of Cx. quinquefasciatus, offering valuable guidance for optimizing SIT-based mosquito control programs. Materials and MethodsStudy siteThis study focused on the urban area of Pasirkratonkramat subdistrict, Pekalongan City, Central Java Province, Indonesia (109° 38’ 50” East Longitude to 109° 40’ 13” East Longitude and 6° 52’39” South Latitude to 6° 53’ 41” South Latitude). The study site covers an area of 1.8 km² and has a population of 15,996. MRR trials were conducted on two plots within the affected area (Fig. 1). Mosquito colonyCulex quinquefasciatus colonies used for all releases were obtained from the Entomology Laboratory, Faculty of Veterinary Medicine, Bogor Agricultural Institute, Bogor, West Java Province, Indonesia. The colonies were maintained in a culture room at 28–30°C with a relative humidity of 60%–70% and a photoperiod of 16:8 (L:D) hours. Approximately 28,000 L1 larvae were reared in clear plastic trays (31 × 24 × 4 cm) containing 1 L of water and were fed chicken liver until pupation. Adult mosquitoes were reared in an adult cage with a continuous supply of 10% glucose solution. Postmating female mosquitoes were fed guinea pig bait for blood feeding. Culex quinquefasciatus was subjected to pathogen screening before release. For irradiation and release, male and female pupae were sexed using a larval–pupal separator (John West Hoc Company, USA). Sex separation of Cx. quinquefasciatus was conducted at the pupal stage, aged 24–30 hours. Harvested pupae were placed in a plastic container (500 ml) containing 200 ml of water and transported to the Irradiator Unit of the National Research and Innovation Agency of Indonesia (BRIN), Jakarta, for irradiation. Gamma irradiation procedureIrradiation treatments were performed using a Gammacell 220 irradiator (initially manufactured by Atomic Energy of Canada Ltd., Ottawa, Canada, in 1968 and upgraded by the Institute of Isotopes, Co., Ltd., Budapest, Hungary, in 2015) with the Co-60 isotope as the irradiation source. The radioisotope activity during the study ranged from 3,743 to 3,738 curie, with a dose rate of 2,734.375 Gy/h Gamma irradiation was conducted following the guidelines for the irradiation of mosquitoes by the Food and Agriculture Organization/International Atomic Energy Agency (FAO/IAEA, 2020) and a previous study of Ernawan et al. (2022). Briefly, the pupae were placed in a cup with a diameter of 12 cm filled with a small amount of water (to keep the pupae damp) and put into an irradiator tube. Pupae were irradiated with a dose of 70 Gy. Mosquito marking procedureApproximately 1,000 gamma-irradiated male and female Cx. quinquefasciatus pupae were transferred to a 30x30x30 cm cage (Bugdorm-1 cage, Megaview Science Co., Ltd., Taichung, Taiwan) for emergence. A 10% sucrose solution was subsequently administered. Forty-eight to ninety-six hours postemergence, the irradiated males and females were marked using fluorescent dust (RADGLO(R) JST, Radiant NV, Houthalen, Belgium) with blue and red colors for the first and second releases, respectively, following the guidelines of the FAO/IAEA (2023). Containers measuring 5.5 cm in diameter and 7 cm high were used for the marking process. The inner surface of the container was rubbed with sandpaper to create a rough surface. As much as 15 mg of fluorescent dust was added to the container. The container was subsequently subjected to intensive agitation to achieve a uniform coating of dust on its internal surface. The coated containers were kept at 10 °C until use. Approximately 3000 adult mosquitoes were freeze-immobilized in Memmert climate chambers (HPP 260, Memmert GmbH + Co. KG, Schwabach, Germany) for 15 minutes. The immobilized mosquitoes were quickly transferred to a fluorescent-coated container and rotated for 10 seconds, equivalent to approximately 20 rotations. After all the marking processes, the immobilized mosquitoes were gently placed back in their original containers. To confirm successful marking, a sample of 50 mosquitoes was collected under a stereomicroscope (Model SMZ 745, Nikon Corp., Minato-ku, Tokyo, Japan). Release and recapture proceduresThe irradiated-marked mosquitoes were placed in cages measuring 30x30x30 cm (Bugdorm-1 cage, Megaview Science Co., Ltd., Taichung, Taiwan) for 6 hours of land transportation from the irradiation facility to the release study. The MRR experiments were performed using two releases differentiated by color. The interval between the first day of the two releases was three days. The release at dusk from the single release point was determined based on the nature of the breeding habitat for Cx. quinquefasciatus. Irradiated-marked mosquitoes were released by simply opening the lid of the cages. Mosquitoes remaining in the cage after 30 minutes were considered poor flyers and were counted as nonreleaser. The recording events lasted seven days for both releases. Nineteen BG-Sentinel-2 traps (Biogents Regensburg, Germany) with lures (octanol – Merck, Germany) were positioned 50, 100, 150, 200, and 250 m from the release point. The data were collected daily by changing the collection bags at intervals of 12 hours. The collected adult mosquitoes were analyzed microscopically for species determination and coloration.
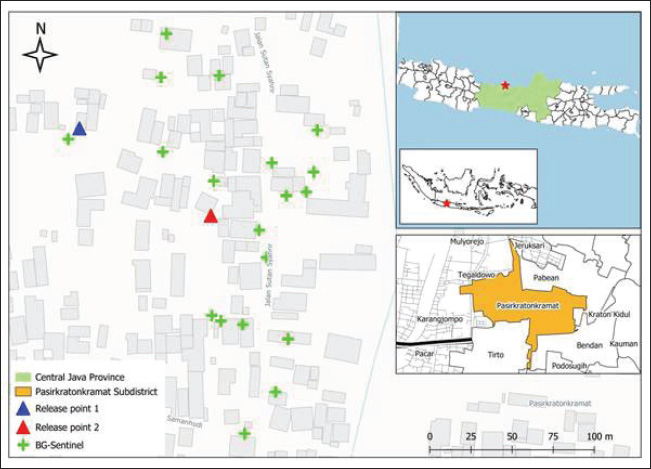
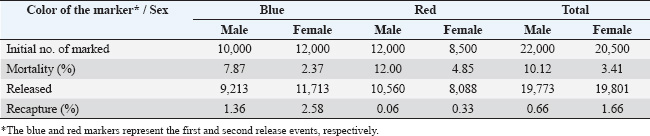
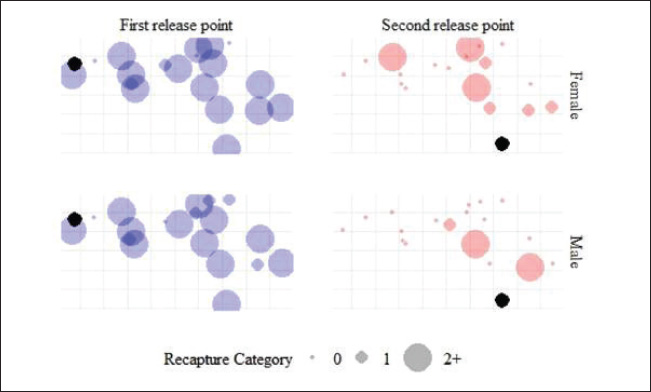
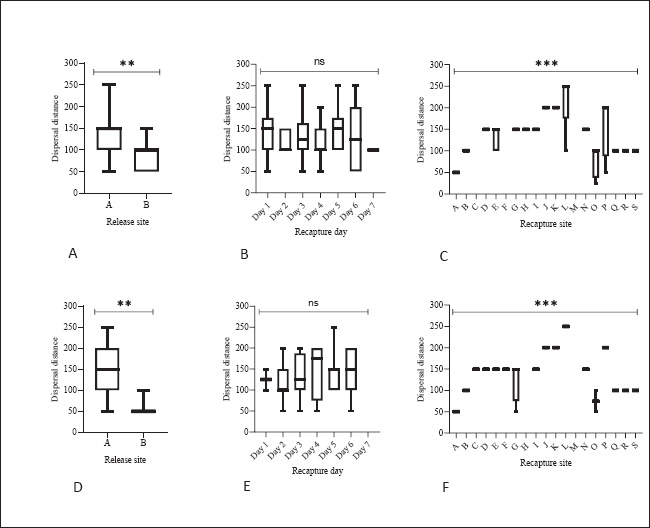
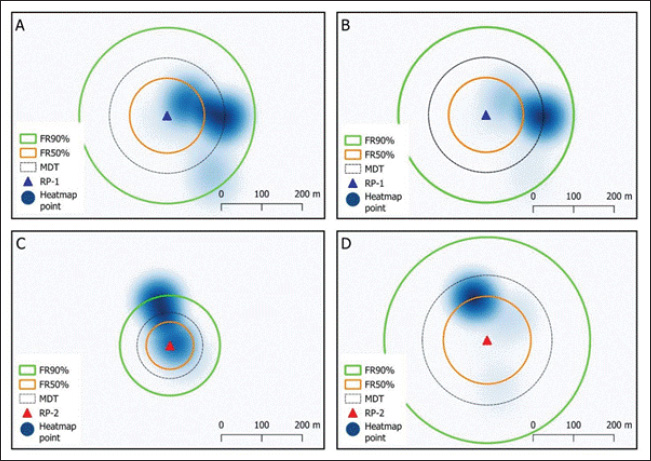
Fig. 1. Location of the study site was located in Pasirkratonkramat subdistrict, Pekalongan City, Central Java Province, Indonesia. Statistical data analysisMosquito dispersal patterns can be quantitatively assessed by calculating the mean distance traveled (MDT) and flight range (FR). The mean distance traveled was measured by drawing circles around the release points to estimate dispersal distances (Le Goff et al., 2019; Trewin et al., 2021). The flight range, estimated using a linear regression model, represents the maximum flight distance that mosquitoes are likely to achieve. The specific values of FR 50 and FR 90 indicate the distances that 50% and 90% of the released mosquitoes can reach, respectively. These metrics offer valuable insights into the population dynamics of mosquitoes and their potential role in disease transmission (Trewin et al., 2021). A regression analysis was conducted to estimate the PDS of individuals in the field. The number of recaptures was transformed using a logarithmic function and then analyzed in relation to the time elapsed since the recapture. The slope of the regression line was used to calculate the PDS, which was then used to determine the ALE in the field. To estimate mosquito abundance in the study area, a modified Lincoln index was used. This method considers both the number of marked individuals and their daily survival rates, providing a more accurate estimate of population size (Le Goff et al., 2019). Statistical analyses were performed using GraphPad Prism version 8.0.2 (GraphPad Software Inc., San Diego, CA, USA). The kernel density estimates (heat maps) were generated with QGIS version 3.34.13 Prizren, using OpenStreetMap (CC-BY-SA license) as the background. Analysis of variance (ANOVA) was used to evaluate dispersal distance parameters based on release site, capture day, and recapture site. Ethical approvalThe MRR study designs and protocols, including animal use, were reviewed and approved by the ethics committee of the BRIN: 029/KE.03/SK/04/2023, April 4, 2023. ResultsApproximately 20,500 and 22,000 irradiated female and male Cx. quinquefasciatus specimens were marked and released into the field at two different times. Pre-release mortality was higher in males (10.12%) than in females (3.41%). After excluding weak flyers, the final number of released males and females was 39,576 individuals, with 19,773 males (89.9%) and 19,803 females (96.6%). The first release (blue marker) exhibited higher survival rates than the second (red marker) (Table 1). During the recapture period, 329 females (1.66%) and 131 males (0.66%) were successfully recaptured, with females showing a higher recapture rate. The first release was more effective, particularly among females (2.58%). Among males, there was a significant difference between release batches, with a notably lower recapture rate in the second release (0.06%) compared with the first (1.36%). Recapture data for irradiated male and female specimens, along with those of wild Cx. quinquefasciatus, are presented (Fig. 2). Overall, recapture rates were lower for marked mosquitoes than for wild individuals. Temporal differences were observed in peak recapture days: both irradiated and wild females peaked on day 4 post-release, whereas wild males peaked on day 3 and irradiated males peaked on day 5. By day 7, a sharp decline in recapture was observed for both sexes. Recapture rates were higher near the first release site, likely due to closer trap placement. In contrast, traps at the second site captured fewer mosquitoes, particularly males (Fig. 3). Mosquitoes from the first release exhibited wider dispersal, whereas those from the second release remained more localized. Dispersal patterns differed between sexes and release sites (Fig. 4). At Site A, both sexes had a greater flight range, reaching up to 250 m. At Site B, dispersal was more limited among males (50–100 m range). The release site significantly influenced dispersal distance in both males {F(2.769, 35)=2.684, p=0.0089} and females {F(2.983, 57)=2.764, p=0.0042}. Female Cx. quinquefasciatus were capable of flying up to 250 m within 1–6 days, although most remain within 50–170 m. A notable decrease in mosquito concentration was observed within 100 m at the end of the observation period. Males showed a relatively homogeneous distribution within 100–150 m on day 1, with increasing dispersal up to 250 m by days 2–6, followed by a reduction post day 7. There was no significant variation in mean dispersal distance across recapture days for either sex {males: F(5, 31)=0.3248, p=0.8941; females: F(6, 51)=0.3462, p=0.9089}. The maximum recorded dispersal distance (250 m) occurred at Location L, whereas shorter distances were observed at Location A. Some sites were not recaptured. Recapture location was significantly associated with dispersal distance in both females {F(15, 41)=7.146, p < 0.0001} and males {F(16, 20)=11.90, p < 0.0001}. The spatial dispersal patterns based on the trap data showed differences in heatmap intensity and dispersal radius between Release Points 1 and 2 (RP-1 and RP-2) (Fig. 5). Darker blue areas indicate higher concentrations of mosquitoes near the release point, whereas lighter areas indicate lower recapture. The MDT by marked sterile males was 143.18 m (min: 82.81 m, max: 144.85 m) and 146.26 m for females (min: 144.10 m, max: 163.10 m). The 90% flight range (FR90) was 221.33 m for males and 258.83 m for females, indicating that most individuals remained within these distances. The 50% flight range (FR50) was concentrated near the release points: 92.78 m for males and 95.26 m for females. The estimated daily survival probabilities (PDSs) for males and females were 0.88 and 0.69, respectively, with life expectancies of 7.57 and 2.71 days. The estimated total populations were 18,803 males and 13,108 females. With a study area of 19.635 ha, this corresponded to population densities of 958 males and 668 females per hectare. Table 1. A total of gamma-irradiated Culex quinquefasciatus mosquitoes were released and recaptured.
Fig. 2. Comparison of capture rates between marked and unmarked males (A) and females (B) with Cx. quinquefasciatus.
Fig. 3. Comparison of recapture between male and female mosquitoes at two release sites. DiscussionThe MRR method employed in this study provided valuable insights into the dispersal, survival, and population density of irradiated Cx. quinquefasciatus mosquitoes. The overall recapture rate was relatively low (0.32%–1.8%), consistent with findings from previous MRR studies in California and Hawaii (Reisen et al., 1991; Lapointe, 2008). This low recapture rate may reflect high mobility, high mortality, or a combination of both, as similarly reported in Aedes albopictus dispersal studies (Medeiros et al., 2017; Verdonschot and Besse-Lototskaya, 2014). The maximum observed dispersal distance (FR90) of up to 250-m aligns with previous reports on other Culex species (Tsuda et al., 2008). However, significant differences between the two release sites suggest that environmental factors—such as dense vegetation and urban barriers—likely restricted mosquito movement, corroborating findings by Marini et al. (2010), who reported that dense vegetation and urban barriers significantly reduced mosquito flight distances. The dispersal patterns observed in this study have direct implications for the effectiveness of SIT, particularly in ensuring that sterile males reach wild-type females in sufficient numbers. Limited dispersal can reduce the probability of mating between sterile males and wild females, ultimately diminishing the long-term effectiveness of SIT (Bouyer et al., 2020; Carvalho et al., 2022). The differences in dispersal patterns between RP-1 and RP-2 further emphasize that strategic site selection is crucial to maximizing sterile male distribution, as landscape features such as vegetation and urban structures can significantly influence mosquito movement (Marini et al., 2010).
Fig. 4. Dispersal distance of irradiated Cx. quinquefasciatus females (A–C) and males (D–F). Dispersal is a function of the release site (A, D), the recapture day (B, E), and the recapture location (C, F). Although the high daily survival probability (PDS=0.88) and average life expectancy (ALE=7.57 days) indicate post-release viability, the mating competitiveness of sterile males remains a major challenge for SIT success. Previous studies have demonstrated that irradiation can negatively impact male mosquito mating behavior, reducing their ability to locate and successfully mate with wild females (Helinski et al., 2009; Hossain et al., 2022). For example, in A. albopictus, sterilized males exhibited reduced flight performance and lower mating success compared to wild-type males (Bellini et al., 2013). These findings suggest that adjustments to irradiation protocols or prerelease conditioning may be necessary to enhance the effectiveness of sterile Cx. quinquefasciatus males. Furthermore, environmental conditions such as temperature, humidity, and wind direction can significantly impact mosquito dispersal and survival. The differences in recapture rates between RP-1 and RP-2 may be attributed to microclimatic variations, particularly related to vegetation density and thermal exposure. Similar patterns have been observed in Culex populations, where higher temperatures and increased wind speeds are associated with reduced dispersal and survival (Marini et al., 2010; Simonsen and Mwakitalu, 2013). Future studies should integrate real-time environmental monitoring to better understand the relationship between climatic variables and sterile male longevity.
Fig. 5. Spatial distribution and dispersal patterns of marked sterile male (A, C) and female (B, D) Cx. quinquefasciatus mosquitoes relative to their release site. FR90% and FR50% represent the flight ranges of 50% and 90%, respectively. MDT represents the mean distance traveled. RP-1 and RP-2 represent release points 1 and 2, respectively. One limitation of this study is the use of a single trap type (BG-Sentinel-2) baited with octanol for recapture, which may have limited the numbers of recaptured mosquitoes. The use of only one type of attractant could result in biased recapture data, as not all sterile males will respond equally to octanol. This may lead to an underestimation of dispersal or survival rates. Additionally, although fluorescent powder marking is widely used, it has been reported to slightly alter mosquito flight behavior or survival (Culbert et al., 2020). Future studies should consider evaluating a combination of trapping methods and more refined marking techniques to improve the accuracy and reliability of postrelease monitoring. The findings of this study have important implications for SIT program design and implementation. More frequent releases in smaller numbers may be a more effective strategy than large, infrequent releases, as this approach has been shown to increase sterile male presence in the target area and improve population suppression effectiveness (FAO/IAEA, 2020; Hapugoda et al., 2024). Additionally, drone-based release systems could serve as a viable solution to optimizing sterile male distribution across complex landscapes (Carvalho et al., 2022). Future research should consider integrating genetic monitoring techniques, such as molecular xenomonitoring, to assess the long-term impact of SIT on Cx. quinquefasciatus populations (Ramesh et al., 2018). ConclusionThis study successfully applied the MRR method to assess the dispersal and survival of gamma-irradiated Cx. quinquefasciatus. The results show that sterile males and females dispersed up to 250 meters, with most remaining within 92–95 meters of the release site. The survival analysis indicated that males lived longer than females, with life expectancies of 7.57 and 2.71 days, respectively. However, due to the limited trap placement range (250 m), the full dispersal potential may not have been captured, and the results may vary under different environmental conditions. The differences between release sites highlight the importance of selecting optimal locations to improve SIT effectiveness. Vegetation and environmental barriers can restrict dispersal, whereas irradiation can reduce male mating competitiveness. Further research is needed to optimize radiation doses or prerelease treatments to enhance sterile male performance. More frequent, smaller scale releases are likely to be more effective than large, infrequent releases. Drone-based release systems and improved monitoring methods, such as molecular xenomonitoring and enhanced trapping techniques, could further refine SIT applications for Cx. quinquefasciatus control and reduce mosquito-borne disease transmission risks. AcknowledgmentsThe authors would like to acknowledge the people of Pasirkratonkramat district for their willingness to release mosquitoes during the study period. FundingThis study was supported by the National Research and Innovation Agency of Indonesia (BRIN). Authors' contributionsNHS: conceptualization, data curation, formal analysis, and writing of the original draft. BE: methodology, software, validation, and writing – review & editing. HIS: methodology, software, validation, and writing – review and editing. SS: supervision and project administration. TR: investigation and validation. SS: resources and writing–review & editing. TAG: writing–review & editing and methodology. RWN: writing–review & editing and conceptualization. All authors have read and approved the final manuscript. Conflict of interestThe authors declare no conflicts of interest regarding the publication and/or funding of this manuscript. Data availabilityAll data required to support the findings of this study are available in the manuscript. ReferencesBartholomay, L.C., Waterhouse, R.M., Mayhew, G.F., Corey, L.C., Michel, K., Zou, Z., Ramirez, J.L., Das, S., Alvarez, K., Arensburger, P., Bryant, B., Chapman, S.B, Dong, Y., Erickson, S.M., Karunaratne, S.H.P.P., Kokoza, V., Kodira, C.D., Pignatelli, P., Shin, S.W., Vanlandingham, D.L., Atkinson, P.W., Birren, B., Christophides, G.K., Clem, R.J., Hemingway, J., Higgs, S., Megy, K., Ranson, H., Zdobnov, E.M., Raikhel, A.S., Christensen, B.M., Dimopoulos, G. and Muskavitch, M.A.T. 2010. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science 330(6000), 88–90. Bellini, R., Balestrino, F., Medici, A., Gentile, G., Veronesi, R. and Carrieri, M. 2013. Mating competitiveness of Aedes albopictus radio-sterilized males in large enclosures exposed to natural conditions. J. Med. Entomol. 50(1), 94–102. Benelli, G and Beier, J.C. 2017. Current vector control challenges in the fight against malaria. Acta Trop. 174, 91–96. Bouyer, J., Yamada, H., Pereira, R., Bourtzis, K., and Vreysen, M. J. B. 2020. Phased conditional approach for mosquito management using sterile insect technique. Trends Parasitol. 36(4), 325–336. Carvalho, D.O., Morreale, R., Stenhouse, S., Hahn, D.A., Gomez, M., Lloyd, A. and Hoel, D. 2022. A sterile insect technique pilot trial on Captiva Island: Defining mosquito population parameters for sterile male releases using mark–release–recapture. Parasit. Vectors 15(1), 1–14. Culbert, N.J., Kaiser, M., Venter, N., Vreysen, M.J.B., Gilles, J.R.L. and Bouyer, J. 2020. A standardised method of marking male mosquitoes with fluorescent dust. Parasit. Vectors 13(1), 1–11. Ernawan, B., Anggraeni, T., Yusmalinar, S., Sasmita, H.I., Fitrianto, N. and Ahmad, I. 2022. Assessment of compaction, temperature, and duration factors for packaging and transporting of sterile male Aedes aegypti (Diptera: Culicidae) under laboratory conditions. Insects 13(9), 1–13. Farajollahi, A., Fonseca, D.M., Kramer, L.D. and Kilpatrick, A. 2011. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 11(7), 1577–1585. Food and Agriculture Organization/International Atomic Energy Agency. 2020. Guidelines for irradiation of mosquito pupae in sterile insect technique programmes, version 1 Eds., Yamada, H., Parker, A., Maiga, H., and Bouyer, J. Food and Agriculture Organization of the United Nations and International Atomic Energy Agency. Available via https://www.iaea.org/sites/default/files/2020-guidelines-for-irradiation.pdf (Accessed 15 May 2025). Food and Agriculture Organization/International Atomic Energy Agency. 2023. Guidelines for mark-release-recapture procedures of Aedes mosquitoes version 2, Eds., Bouyer, J. and Mamai, W. Food and Agriculture Organization of the United Nations and International Atomic Energy Agency. Available via https://www.iaea.org/sites/default/files/guidelinesmrr_v2.pdf (Accessed 15 May 2025). Guerra, C.A., Reiner Jr, R.C., Perkins, T.A., Lindsay, S.W., Midega, J.T., Brady, O.J., Barker, C.M., Reisen, W.K., Harrington, L.C., Takken, W., Kitron, U., Lloyd, A.L., Hay, S.I., Scott, T.W. and Smith, D.L. 2014. A global assembly of adult female mosquito mark-release-recapture data to inform the control of mosquito-borne pathogens. Parasit. Vectors 7(1), 1–15. Hapugoda, M., Gunawardena, N.S., Ranathunge, T., Bouyer, J., Maiga, H., Karunathilake, K., Withanage, G.P., Weerasinghe, I., Sow, B.B.D and Harishchandra, J. 2024. Mark–release–recapture (MRR) of sterile male Aedes albopictus (Skuse) in Sri Lanka: field performance of sterile males and estimation of the wild mosquito population density. Insects 15(7), 1–12. Helinski, M.E.H., Parker, A.G. and Knols, B.G.J. 2009. Radiation biology of mosquitoes. Malar. J. 8(SUPPL. 2), 1–13. Hossain, M.F., Ghosh, A., Sultana, N., Momen, M., Hossain, M.A., Khan, S.A. and Seheli, K. 2022. Optimization of irradiation sterility dose of the male Aedes aegypti (Linnaeus) mosquito: A laboratory study in Bangladesh. Int. J. Trop. Insects Sci. 42(2), 1421–1428. Klassen, W. 2005. Use of SIT in implementing pest management strategies. In Sterile insect technique: principles and practice in area-wide integrated pest management, Eds., Dyck, V.A, Hendrichs, J. and Robinson, A.S. Dordrecht, The Netherlands: Springer, pp: 55-60. Lapointe, D.A. 2008. Dispersal of Culex quinquefasciatus (Diptera: Culicidae) in a Hawaiian rain forest. J. Med. Entomol. 45(4), 600–609. Le Goff, G., Damiens, D., Ruttee, A.H., Payet, L., Lebon, C., Dehecq, J.S. and Gouagna, L.C. 2019. Field evaluation of seasonal trends in relative population sizes and dispersal pattern of Aedes albopictus males in support of the design of a sterile male release strategy. Parasit. Vectors 12(1),1–10. Marini, F., Caputo, B., Pombi, M., Tarsitani, G. and Torre, A. 2010. Study of Aedes albopictus dispersal in Rome, Italy, using sticky traps in mark-release-recapture experiments. Med. Vet. Entomol. 24(4), 361–368. Medeiros, M.C.I., Boothe, E.C., Roark, E.B. and Hamer, G.L. 2017. Dispersal of male and female Culex quinquefasciatus and Aedes albopictus mosquitoes using stable isotope enrichment. PLoS Negl. Trop. Dis. 11(1), 1–24. Ramesh, A., Cameron, M., Spence, K., Spaans, R.H., Melo-Santos, M.A.V., Paiva, M.H.S., Guedes, D.R.D., Barbosa, R.M.R., Oliveira, C.M.F., Sa, A., Jeffries, C.L. Castanha, P.m.S., Oliveira, P.A.S., Walker, T., Alexander, N. and Braga, C. 2018. Development of an urban molecular xenomonitoring system for lymphatic filariasis in the Recife Metropolitan Region, Brazil. PLoS negl. Trop. Dis. 12(10), 1–24. Ranson, H. and Lissenden, N. 2016. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32(3), 187–196. Reisen, W.K., Milby, M.M. and Meyer, R.P. 1992. Population dynamics of adult Culex mosquitoes (Diptera: Culicidae) along the Kern River, Kern County, California, in 1990. J. Med. Entomol. 29(3), 531–543. Reisen, W.K., Milby, M.M., Meyer, R.P., Pfuntner, A.R., Spoehel, J., Hazelrigg, J.E. and Webb Jr, J.P. 1991. Mark-release-recapture studies with Culex mosquitoes (Diptera: Culicidae) in Southern California. J. Med Entomol. 28(3), 357–371. Simonsen, P.E. and Mwakitalu, M.E. 2013. Urban lymphatic filariasis. Parasitol. Res. 112(1), 35–44. Surasa, W., Pientong, C., Ekalaksananan, T., Overgaard, H.J., Aromseree, S. and Phanthanawiboon, S. 2024. Interepidemic xenosurveillance of Japanese encephalitis virus and Zika virus in Culex mosquitoes from Ubon Ratchathani Province, Thailand. Vet. World 17(7), 1555–1561 Trewin, B.J., Pagendam, D.E., Johnson, B.J., Paton, C., Snoad, N., Ritchie, S.A., Staunton, K.M., White, B.J., Mitchell, S., Beebe, N.W. 2021. Mark-release-recapture of male Aedes aegypti (Diptera: Culicidae): Use of rhodamine B to estimate movement, mating and population parameters in preparation for an incompatible male program. PLoS Negl. Trop. Dis. 15(6), 1–21. Tsuda, Y., Komagata, O., Kasai, S., Hayashi, T., Nihei, N., Saito, K., Mizutani, M., Kunida, M., Yoshida, M. and Kobayashi, M. 2008. A mark-release-recapture study on dispersal and flight distance of Culex pipiens pallens in an urban area of Japan. J. Am. Mosq. Control Assoc. 24(3), 339–343. Verdonschot, P.F.M. and Besse-Lototskaya, A.A. 2014. Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica 45, 69–79. | ||
| How to Cite this Article |
| Pubmed Style Suryaningtyas NH, Nurcahyo RW, Ernawan B, Sasmita HI, Sunaryo S, Ramadhani T, Supriyono S, Garjito TA. Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applications. Open Vet. J.. 2025; 15(7): 3054-3062. doi:10.5455/OVJ.2025.v15.i7.16 Web Style Suryaningtyas NH, Nurcahyo RW, Ernawan B, Sasmita HI, Sunaryo S, Ramadhani T, Supriyono S, Garjito TA. Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applications. https://www.openveterinaryjournal.com/?mno=249589 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i7.16 AMA (American Medical Association) Style Suryaningtyas NH, Nurcahyo RW, Ernawan B, Sasmita HI, Sunaryo S, Ramadhani T, Supriyono S, Garjito TA. Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applications. Open Vet. J.. 2025; 15(7): 3054-3062. doi:10.5455/OVJ.2025.v15.i7.16 Vancouver/ICMJE Style Suryaningtyas NH, Nurcahyo RW, Ernawan B, Sasmita HI, Sunaryo S, Ramadhani T, Supriyono S, Garjito TA. Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applications. Open Vet. J.. (2025), [cited January 25, 2026]; 15(7): 3054-3062. doi:10.5455/OVJ.2025.v15.i7.16 Harvard Style Suryaningtyas, N. H., Nurcahyo, . R. W., Ernawan, . B., Sasmita, . H. I., Sunaryo, . S., Ramadhani, . T., Supriyono, . S. & Garjito, . T. A. (2025) Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applications. Open Vet. J., 15 (7), 3054-3062. doi:10.5455/OVJ.2025.v15.i7.16 Turabian Style Suryaningtyas, Nungki Hapsari, Raden Wisnu Nurcahyo, Beni Ernawan, Hadian Iman Sasmita, Sunaryo Sunaryo, Tri Ramadhani, Supriyono Supriyono, and Triwibowo Ambar Garjito. 2025. Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applications. Open Veterinary Journal, 15 (7), 3054-3062. doi:10.5455/OVJ.2025.v15.i7.16 Chicago Style Suryaningtyas, Nungki Hapsari, Raden Wisnu Nurcahyo, Beni Ernawan, Hadian Iman Sasmita, Sunaryo Sunaryo, Tri Ramadhani, Supriyono Supriyono, and Triwibowo Ambar Garjito. "Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applications." Open Veterinary Journal 15 (2025), 3054-3062. doi:10.5455/OVJ.2025.v15.i7.16 MLA (The Modern Language Association) Style Suryaningtyas, Nungki Hapsari, Raden Wisnu Nurcahyo, Beni Ernawan, Hadian Iman Sasmita, Sunaryo Sunaryo, Tri Ramadhani, Supriyono Supriyono, and Triwibowo Ambar Garjito. "Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applications." Open Veterinary Journal 15.7 (2025), 3054-3062. Print. doi:10.5455/OVJ.2025.v15.i7.16 APA (American Psychological Association) Style Suryaningtyas, N. H., Nurcahyo, . R. W., Ernawan, . B., Sasmita, . H. I., Sunaryo, . S., Ramadhani, . T., Supriyono, . S. & Garjito, . T. A. (2025) Dispersal and survival of gamma-irradiated Culex quinquefasciatus: Implications for sterile insect technique applications. Open Veterinary Journal, 15 (7), 3054-3062. doi:10.5455/OVJ.2025.v15.i7.16 |