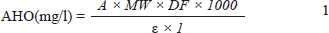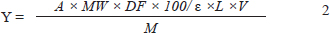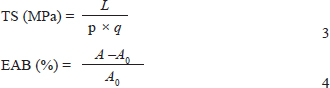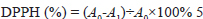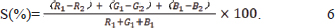| Research Article | ||
Open Vet. J.. 2025; 15(4): 1836-1847 Open Veterinary Journal, (2025), Vol. 15(4): 1836-1847 Research Article Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meatsJin-Kui Ma1, Qian-Ru Lin1, Zhi-Yao Liu1, Jia-Min Wang1, Xin Peng1, Yu-Tong Lin1, Li-Zhen Nie2 and Xiao-Chen Huang1*1School of Food and Pharmaceutical Engineering, Zhaoqing University, Zhaoqing, China 2Hengyang Market Supervision, Inspection and Testing Center, Hengyang City, China *Corresponding Author: Xiao-Chen Huang. School of Food and Pharmaceutical Engineering, Zhaoqing University, Zhaoqing, China. Email: xiaochenhuang423 [at] gmail.com Submitted: 24/03/2025 Accepted: 06/04/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
AbstractBackground: Food spoilage, particularly in meat products, presents a significant economic and health concern, necessitating the development of reliable and user-friendly monitoring methods. Aim: This study presents the development and evaluation of novel colorimetric films for real-time monitoring of meat freshness. Methods: Films were formulated using anthocyanin (AHO) and betacyanin (BTA) from purple cabbage and red dragon fruit, respectively, embedded within polyvinyl alcohol and carboxymethyl cellulose matrix. Two pigment ratios, AHO:BTA (1:3) and (3:1), were compared to evaluate mechanical properties, antioxidant activity, and ammonia sensitivity. Results: The AHO:BTA (1:3) film demonstrated higher tensile strength, whereas the (3:1) film exhibited greater antioxidant capacity and ammonia responsiveness. Colorimetric changes were assessed across the pH range, showing sensitivity to pH shifts. The films were then applied to beef, lamb, fish, and pork samples, and their color alterations during storage under ambient and refrigerated conditions were correlated with total volatile basic nitrogen and pH levels. Conclusion: The results show a strong correlation between film color changes and established meat spoilage indicators, demonstrating the potential of these natural pigment-based films for effective, nontoxic, and visual monitoring of meat freshness. Keywords: Colorimetric films, Meat freshness Monitoring, anthocyanins and Betacyanins, Food packaging, Real-time indicators. IntroductionGrowing consumer awareness of food safety and health concerns has increased the demand for effective and reliable methods of monitoring food quality and freshness, especially for perishable products such as meat (Majid et al., 2018). Meat, a staple in many diets, is highly susceptible to spoilage due to microbial activity and enzymatic reactions, leading to undesirable changes in color, odor, texture, and nutritional value (Jay et al., 2008). Traditional methods for assessing meat freshness, such as sensory evaluation and chemical analyses (e.g., total volatile basic nitrogen (TVB-N), pH, and thiobarbituric acid reactive substances, are often subjective, time-consuming, laborious, and require specialized equipment and skilled personnel (Singh et al., 2011). Therefore, rapid, convenient, and accurate technologies for real-time monitoring of meat freshness are urgently needed. In recent years, intelligent packaging technologies, particularly colorimetric indicator films, have offered a promising solution for real-time monitoring of freshness (Ghaani et al., 2016). These films respond to changes in their environment by exhibiting a visually discernible color shift, directly signaling the condition of the packaged product (Mohammadian et al., 2020, Alizadeh-Sani et al., 2020). Conventional colorimetric indicators often rely on synthetic dyes such as cresol red, bromocresol green, methyl red, and methyl orange (Azman et al., 2022). However, concerns about the potential toxicity of these synthetic dyes and their unsuitability for direct food contact applications have driven the search for natural, nontoxic alternatives (Yadav et al., 2023). Anthocyanins (AHO) and betacyanins (BTA), natural pigments found in plants, have garnered increasing interest in the food science domain due to their unique physicochemical properties, excellent antioxidant capacity, and pH-sensitive color variations (Xu et al., 2024a). AHO, water-soluble pigments widely distributed in plants such as blueberries and black goji berries, exhibit a diverse range of colors depending on pH (Wrolstad et al., 2005). BTA, predominantly sourced from beetroot and red dragon fruit, also possess vibrant colors and strong stability, making them suitable for use as food colorants (Lim et al., 2022). The combination of these two pigments is expected to be synergistic and provide a wider range of color variations based on pH. Water-soluble polyvinyl alcohol (PVA) is a popular choice for film production because of its flexibility, film-forming properties, and biodegradability (Marin et al., 2014). However, PVA’s inherent hydrophilicity and lack of antibacterial properties can limit its use in high-humidity environments (Mittal et al., 2021). Sodium carboxymethyl cellulose (CMC-Na), an anionic polymer, is also widely used as a film-forming agent, enhancing product stability and preventing precipitation (McMullen et al., 2022). The combination of PVA and CMC-Na can potentially overcome some limitations while creating a film with good mechanical properties. While studies have explored the use of AHO and BTA individually as indicators (Kanha et al., 2022, Kossyvaki et al., 2022), research on the combined use of these two natural pigments in colorimetric indicator films is limited. Previous studies have focused on similar mixtures for various food applications, mainly for single pigment types. For instance, Chen et al. (2020) developed films that combined anthocyanins and curcumin for fish-freshness monitoring, demonstrating enhanced performance in the composite film (Chen et al., 2020). Similarly, Zhou et al. (2021) fabricated a dual-layer indicator film for monitoring muscle freshness, indicating the potential to enhance the stability of anthocyanins using a combination with other pigments (Zhou et al., 2021). Based on this, this study intends to investigate the mixture of both to enhance stability. To address the need for effective meat-freshness monitoring, this research explores the development of a novel colorimetric film. The film is composed of AHO and BTA incorporated into a PVA and CMC-Na film-forming base and is designed to indicate the spoilage level of beef, lamb, fish, and pork. The synergistic effects of AHO and BTA are explored in this study, along with the effectiveness of the films in detecting meat spoilage. The goal is to develop a simple, visual, and reliable method for assessing meat freshness, addressing the limitations of existing methods and providing a more effective method to ensure food safety and quality. Materials and MethodsMaterialsPurple cabbage and red dragon fruits were purchased from a local supermarket in Zhaoqing, Guangdong, China, and stored at 4°C prior to use. Fresh beef, tilapia, pork, and lamb were purchased from the local Dongjiang market in Zhaoqing City and stored in ice bags before testing. Polyvinyl alcohol (PVA, average molecular weight 89,000–98,000, 99+% hydrolyzed), sodium carboxymethyl cellulose (CMC-Na, molecular weight 90,000, substitution degree 0.65–0.90), ethanol (95%), methanol, KCl, sodium acetate (CH3COONa), NaOH, HCl, MgO, citric acid, glycine), Na2HPO4, NH3 (25%–28%), boric acid, bromocresol green, methyl red, and DPPH were purchased from Aladdin Biochemical Technology (Shanghai, China), while macroporous resins AB-8 and HPD-100A were obtained from Solarbio Science & Technology (Beijing, China). All chemicals were of analytical grade and were used as received without further purification. EquipmentAbsorbance measurements were performed using a UV-Vis spectrophotometer (721N, Shanghai Instrument Co., Ltd., Shanghai, China). A temperature-controlled water bath (SB-1200, Ailang Instrument Co., Ltd., Shanghai, China) was used for extraction, and a sonicator (G-24A, Songneng Ultrasonic Equipment Co., Ltd., Shenzhen, China) was used to remove air bubbles. A household juice extractor (HH-200C, Midea Group, Foshan, China) was used to prepare dragon fruit pulp. An electronic analytical balance (YH-M6001, Yingheng Intelligent Equipment Co., Ltd., Dongyang, China) was used for all mass measurements. A texture analyzer (XLW (M), Jinan Languang Electromechanical Technology Co., Ltd., Jinan, China) was used for the mechanical testing of the films. Solvent evaporation was carried out using a rotary evaporator (RE-52AA, Yarong Biochemistry Instrument Factory, Shanghai, China). A digital heating magnetic stirrer (DF-101S, Yu Hua Instrument Co., Zhengzhou, China) was used for solution mixing, a drying oven (DHG-9030A, Shanghai Yiheng Scientific Instrument Co., Shanghai, China) for drying the films, and a high-speed centrifuge (5804R, Eppendorf, Germany) for phase separations. The pH was measured using a digital pH meter (PHS-3C, Leici Instrument Co., Shanghai, China), and the TVB-N values were measured using a low-temperature thermostatic bath (YRDC-0506, Yarong Biochemical Instrument Factory, Shanghai, China) and a refrigerator (SL-1020C2D2, Midea Refrigeration Co., Hefei, China). The film thickness was measured using a micrometer (IP54, Sanwen Measuring Instrument Co., Zhuhai, China). A colorimeter (LS173, Linshang Technology Co., Ltd., Shenzhen, China) was used to record the color values of the films. MethodsPigment extraction and purification AHO extraction AHO were extracted from purple cabbage leaves according to the method of (He, et al. 2023) with minor modifications. Briefly, fresh purple cabbage leaves were dried in a forced air oven at 60°C for 24 hours and then pulverized into powder. Purple cabbage powder (50 g) was extracted in 500 ml of 70% (v/v) ethanol solution (pH 3.0, adjusted with acetic acid) at 50°C for 30 minutes with continuous stirring. The resulting extract was filtered, and the extraction process was repeated twice to maximize yield. The combined filtrates were centrifuged at 7,000 rpm for 20 minutes at 4°C, and the supernatant containing the crude anthocyanin was collected and purified. AHO content determination The total AHO content was determined using the pH differential method with minor modifications (Giusti and Wrolstad, 2001). AHO solutions were prepared by diluting extracts in two different buffers. A potassium chloride-hydrochloric acid buffer (pH 1.0) and a sodium acetate-acetic acid buffer (pH 4.5). Absorbance was measured at 520 and 700 nm using a UV-Vis spectrophotometer with distilled water as the blank. The anthocyanin content was calculated using the following formula:
where A=(A520 nm”A700 nm) pH 1.0 – (A520 nm”A700 nm) pH 4.5, DF=Dilution Factor, MW=Molecular weight of cyanidin-3-glucoside (449.2 g/mol ), ε=Molar absorptivity of cyanidin-3-glucoside (26900 L mol-1 cm-1), and L=Path length (1 cm). BTA extraction and content determination BTA was extracted from the peels of red dragon fruit following a previous method with slight modification (Pichayajittipong and Thaiudom, 2014). BTA content was assessed in red dragon fruit peel extracts prepared as follows: Peels were cut into approximately 2–3 cm2 pieces, homogenized in a juice extractor, and mixed with distilled water (1:5 w/v, pH 5). The mixture was extracted in a 30°C water bath for 60 minutes, centrifuged (8,000 rpm, 15 minutes), and the supernatant was analyzed for absorbance at 535 nm using a UV-Vis spectrophotometer. The BTA concentration was calculated using the Lambert-Beer Law. The BTA yield was conducted using the following equation:
where A=Absorbance at 538 nm, MW=Molecular weight of betanin (550 g/mol), DF=Dilution factor, ε=Molar absorptivity of betanin (65,000 l mol-1 cm-1), L=Path length (1 cm), V= total volume of the extract, M=Mass of dragon fruit peel (g), and Y=BTA Yield, in mg/100 g. Macroporous resin purification The macroporous resins AB-8 and HPD-100A were used for the purification of AHO and BTA, respectively, based on previous studies (Xu et al., 2024b). Prior to use, the resins were washed with 95% ethanol followed by distilled water to remove impurities. For AHO purification, the crude AHO extract (50 ml) was subjected to adsorption using 8 g of HPD-100A resin with stirring at 180 rpm and 28°C for 1 hour. The mixture was filtered, and the adsorbed AHO was eluted using 70% ethanol (pH 3.0) with shaking for 1 hour under the same conditions and recovered by evaporation at 40°C. For BTA purification, a column was packed with AB-8 resin, and the crude BTA extract was loaded into the column. After the equilibrium of resin absorption, the resin was washed with 60% ethanol, and the elutes were measured at a wavelength of 535 nm. BTA was recovered from the collected eluates after evaporation at 40°C. To preserve the concentrated solution, it was first frozen at –4°C for 24 hours and then lyophilized using a freeze dryer. The purity of the resulting AHO and BTA powders was calculated according to previously described methods (Liu et al., 2023). Indicator film preparation Indicator films were prepared based on the method of (Liu et al., 2023) with slight modifications. PVA (12 g) was dissolved in 150 ml of distilled water at 85°C with magnetic stirring at 300 rpm for 2 hours. CMC-Na (3 g) was dissolved in another 150 ml of distilled water at 75°C and stirred at 300 rpm for 30 minutes, mixed with PVA in a 1:1 ratio by weight, and stirred for 30 minutes; then, different pigment combinations were added. Two separate formulations with pigment ratios AHO:BTA at 1:3 and 3:1 were prepared by mixing 0.75 g of AHO with 0.25 g of BTA for 1:3 and 0.25 g of AHO with 0.75 g of BTA for 3:1 ratio and mixing into the PVA/CMC solution, respectively. The resulting solutions were degassed using a sonicator. To prepare the films, 15 ml of each film-forming solution was cast onto 990-mm-diameter glass Petri dishes and dried at 40°C in an oven for 24 hours. After drying, the films were peeled from the dishes and stored in desiccators for subsequent use. Colorimetric properties of AHO/BTA solutions at different pH values The sensitivity of the AHO/BTA solutions (both ratios of 1:3 and 3:1) to different pH levels was evaluated by measuring their UV-Vis spectra in various buffer solutions. The pH sensitivity of the AHO/BTA solution (0.25 g/50 ml) was assessed by adding 1 ml of the solution to 10 ml of buffer solutions ranging from pH 2.0 to 11.0 (Abedi-Firoozjah et al., 2022). UV-Vis spectra were then recorded from 400 to 700 nm. Characterization of indicator films Film thickness The film thickness was measured using a micrometer at three different points on each film, and the average thickness was calculated. Mechanical properties The mechanical properties, specifically tensile strength (TS) and elongation at break (EAB), were determined using a texture analyzer. Film samples were prepared as 5 cm × 2 cm strips, and the analysis was performed with an initial grip separation of 33 mm and a crosshead speed of 50 mm/minute. TS and EAB were calculated using the following equations:
In these equations, the variables are defined as follows: L represents the breaking load (N), p represents the sample width (mm), q represents the sample thickness (mm), A represents the stretched length at break (mm), and A0 represents the initial length (mm). Antioxidant activity The antioxidant activity of the films was measured using the DPPH radical scavenging assay (Brand-Williams et al., 1995). The film was immersed in 10-ml of methanol at 25°C for 1 hour in the dark. Then, 5 ml of the immersion solution was mixed with 1 ml of a 100 μM DPPH solution. The absorbance was measured at 517 nm after 1 hour of incubation in the dark using a UV-Vis spectrophotometer. A methanol and DPPH solution was used as the control. The DPPH radical scavenging activity (%) was calculated as follows:
Here, A0 is the absorbance of the control solution, and A1 is the absorbance of the film sample. Response to volatile ammonia An ammonia solution was used to assess the response of the indicator films to volatile ammonia (Fang et al., 2017). Ten milliliters of an 8 mM ammonia solution were placed in a 90-mm Petri dish. The film (2.5 cm × 2.5 cm) was adhered to the lid, approximately 1 cm above the solution, and color change was monitored over a period of 30 minutes. The color parameters of the samples were recorded by recording photographs with a digital camera every 3 minutes under the same light source. Sensitivity to ammonia was calculated using the following equation:
Here, S is the sensitivity to ammonia, R1, G1, and B1 are the initial red, green, and blue values, respectively, and R2, G2, and B2 are the final values after exposure to ammonia. Application of indicator films for meat freshness monitoring Meat sample preparation Small pieces (5–6 g) of beef, fish, lamb, and pork, each containing both lean and fat components, were placed in 35-mm Petri dishes. Indicator films (2.5 cm × 2.5 cm) were placed on the inner surfaces of the Petri dish lids. The Petri dishes were then sealed with parafilm and stored at ambient temperature (approx. 25°C) or refrigerated temperature (4°C). Total volatile basic nitrogen measurement The TVB-N content was measured using the semi-micro Kjeldahl method according to the national standard method (GB 5009.228-2016) (National Health and Family Planning Commission of the People’s Republic of China, 2016). A homogenized sample (8 g) and 40 ml distilled water was added, and a few drops of a mixed indicator solution (1 g/l Methyl red solution in ethanol and 5 g/l bromocresol green solution in ethanol at 1:5 ratio) were added to a receiving bottle containing 10 ml of boric acid solution (20 g/l). After introducing 10 ml of the solution and 5 ml of a 4-g/l magnesium oxide suspension into the reaction chamber, the sample was distilled for an initial 5 minutes, followed by repositioning of the receiver to finalize the distillation. Finally, the receiving solution was titrated with 0.01 M HCl until the endpoint (a shift from green to violet color). TVB-N content was calculated using the following equation:
where X=TVB-N content (mg/100 g), V1=Volume of HCl used for sample titration (ml), V2=Volume of HCl used for blank titration (ml), C=Concentration of the HCl (mol/l), m=Mass of the sample (g), V=Volume of the filtrate (10 ml), and V0=Total volume of the sample (40 ml). Measurement of pH Eight grams of meat were mixed with 40 ml of distilled water using a homogenizer. After 30 minutes, the pH was measured using a digital pH meter after calibration. Data analysisAll experiments were conducted in triplicate, and data are presented as the mean ± standard deviation. Statistical analysis, including one-way ANOVA and Student’s t-tests, was performed using Microsoft Excel 2021, with p < 0.05 considered statistically significant. Ethical approvalNot needed for this study. ResultsFilm thickness and mechanical propertiesThe thicknesses of the indicator films and their mechanical properties, as quantified by TS and EAB, are presented in Table 1. The AHO:BTA (1:3) film had an average thickness of 0.13 ± 0.02 mm, slightly greater than that of the AHO:BTA (3:1) film, which was 0.11 ± 0.01 mm. The AHO:BTA (1:3) film demonstrated a higher TS of 31.26 ± 1.2 MPa and EAB of 31.93% ± 1.5%, whereas the AHO:BTA (3:1) film exhibited a lower TS of 19.41 ± 0.9 MPa and a significantly higher EAB of 66.80% ± 2.1%. Antioxidant capacity of indicator filmsThe antioxidant capacity of the indicator films, as measured by the DPPH radical scavenging assay, is shown in Table 1. The AHO-rich (3:1) film exhibited a significantly higher (p < 0.05) DPPH radical scavenging rate of 85.45% ± 2.5% compared to the AHO:BTA (1:3) film, which exhibited 80.0% ± 2.1%. Table 1. Thickness, mechanical properties, antioxidant capacity, and ammonia sensitivity of indicator films.
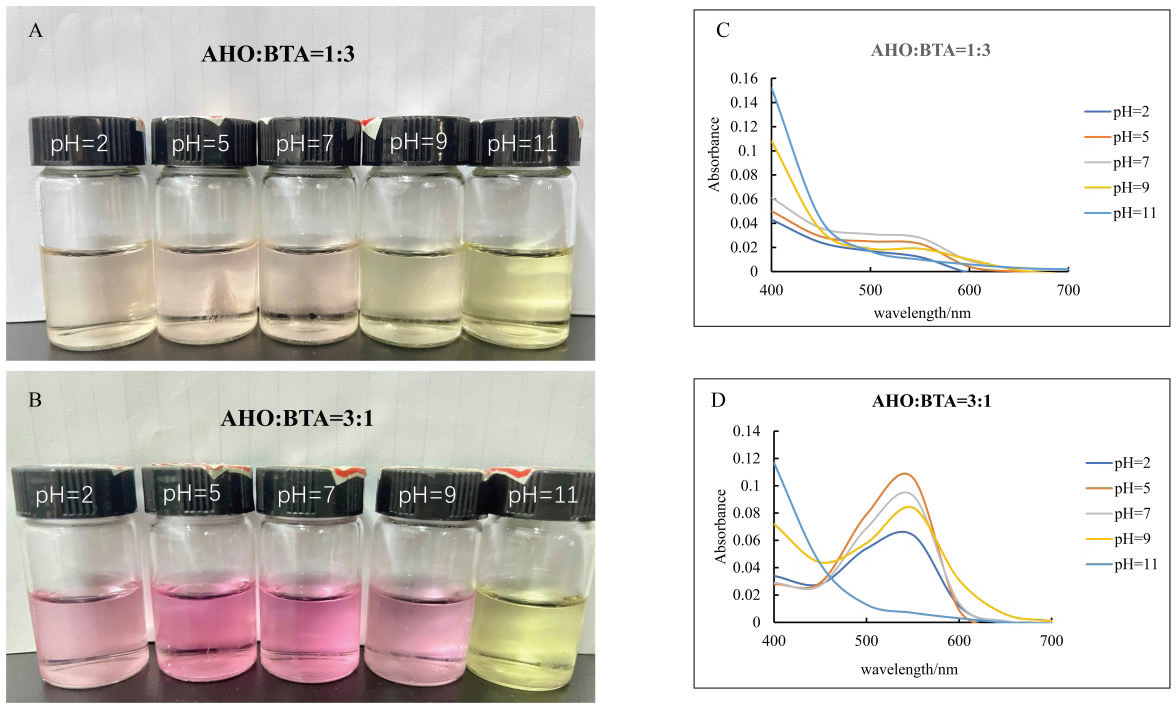
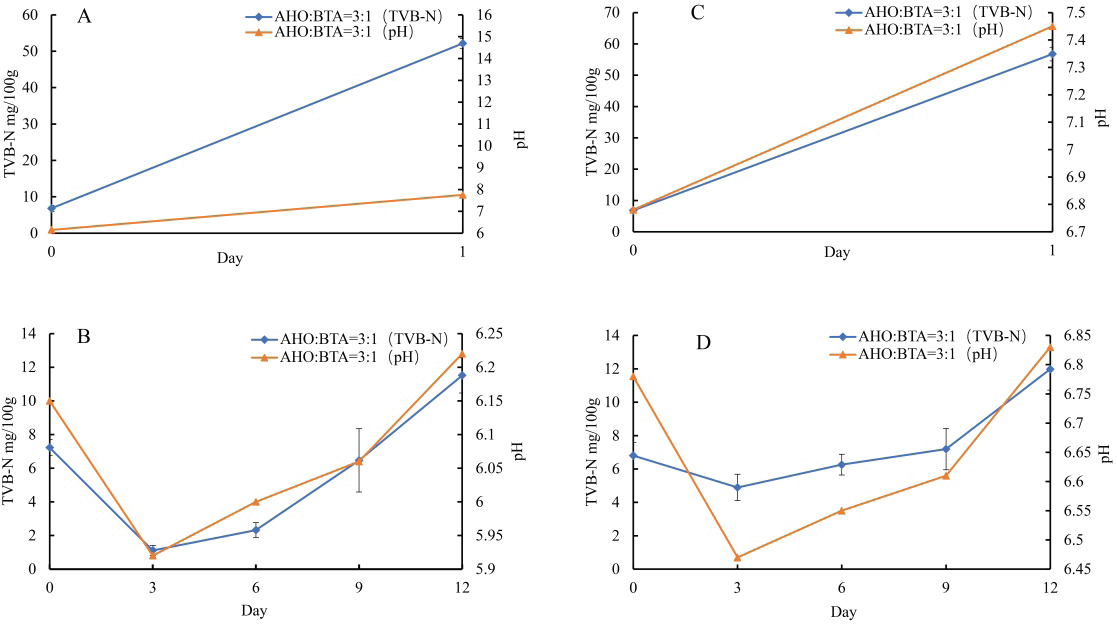
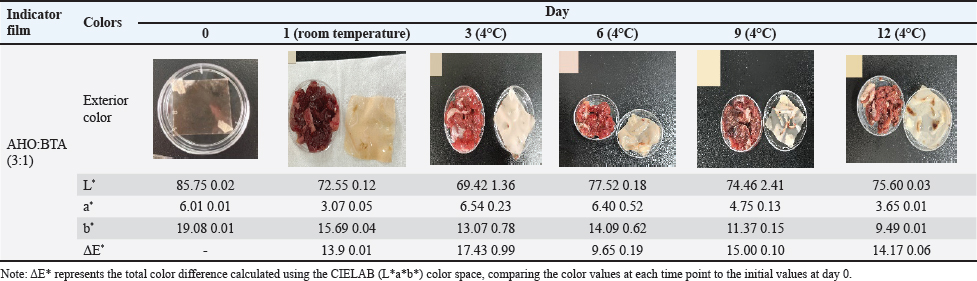
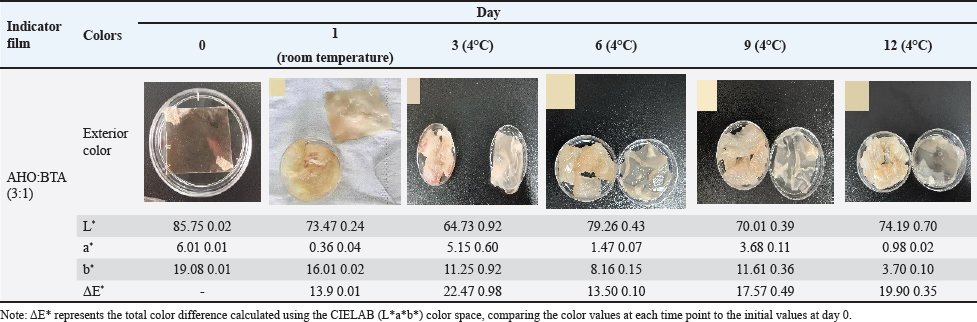
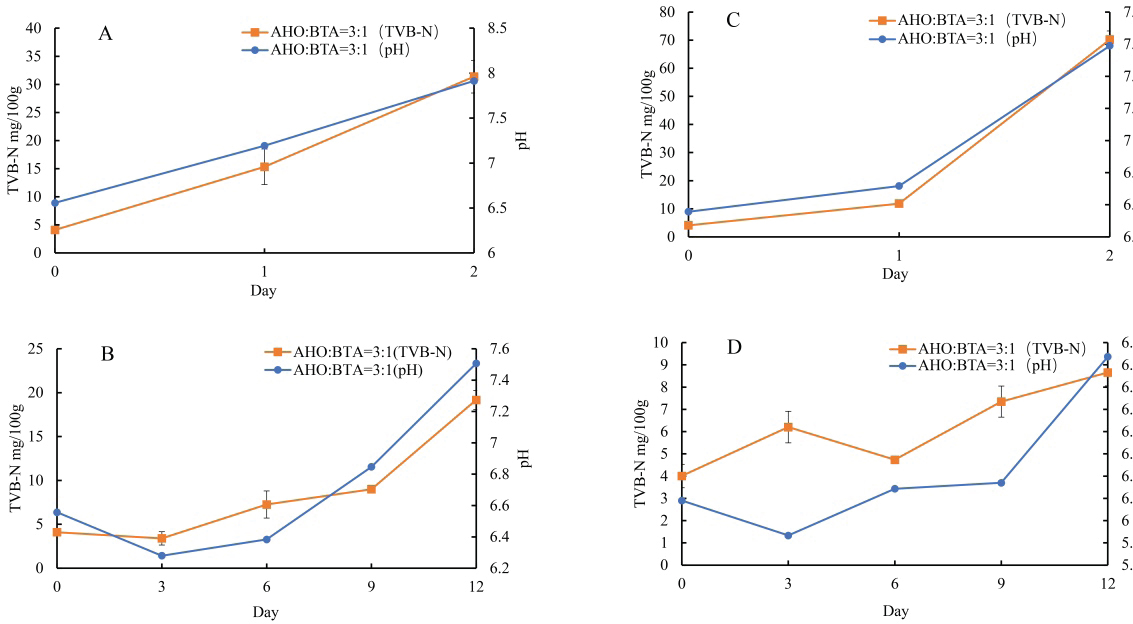
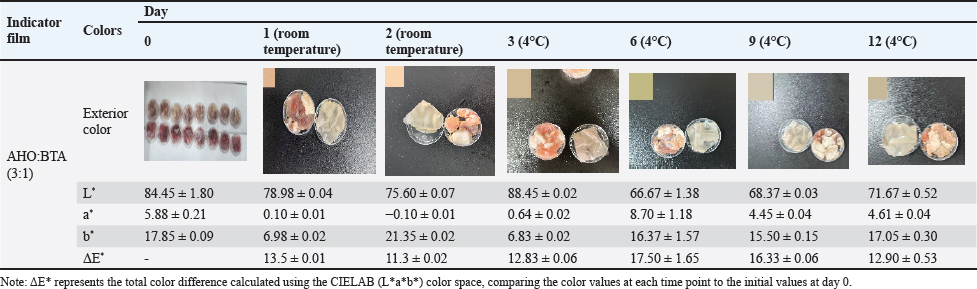
Fig. 1. A: Color change of AHO:BTA (1:3) in different buffer solutions (pH=2.0, 5.0, 7.0, 9.0 and 11.0); B: Color change of AHO:BTA (3:1) in different buffer solutions (pH=2.0, 5.0, 7.0, 9.0 and 11.0); C: UV-Vis spectra of AHO/ BTA(1:3) solutions at different pH values; D: UV-Vis spectra of AHO/BTA(3:1) solutions at different pH values. Response of indicator films to volatile ammoniaThe sensitivities of the indicator films to volatile ammonia are presented in Table 1. The AHO:BTA (1:3) film showed a higher S value (5.86%) than the AHO:BTA (3:1) film, which showed an S value of 5.43%. Colorimetric response of AHO/BTA solutions at different pH valuesThe colorimetric response of the extracted AHO/BTA solutions at various pH levels was examined using UV-Vis spectroscopy. Both the extracted solutions with 1:3 and 3:1 ratios showed similar color trends over the tested pH range. At pH values of 2.0, 5.0, and 7.0, both solutions presented a reddish color although the color intensity varied. For the AHO:BTA (1:3) extracted solution, the color turned pink and deepened with increasing the pH (Fig. 1A). However, the 3:1 extracted solution exhibited an intense red color with varying intensity over the same pH range (Fig. 1B). At pH values of 9.0 and 11.0, both solutions exhibited a transition toward yellow and orange colors. The UV-Vis spectra of the extracted AHO/BTA solutions revealed significant changes in the absorbance peaks at different pH values (Fig. 1C and D). At pH 2.0, a characteristic peak at approximately 400 nm was observed in both solutions. This peak intensity decreased as the pH increased. As the pH increased from 2.0 to 9.0, a shift was observed, where a second peak appeared between 500 and 550 nm. Application of indicator films to meat freshness monitoringBeef At ambient temperature, the indicator film showed an increase in pH within the first day to 7.75 ± 0.07 (Fig. 2A). The TVB-N content also increased to 52.14 ± 1.7 mg/100 g (AHO:BTA 3:1). Concomitantly, the color difference (ΔΕ*) was observed to be 13.9 ± 0.01 for the 3:1 film. Under refrigerated conditions, initially, there was a decrease in pH and TVB-N between days 0 and 3 for the films, followed by a gradual increase until day 12 (Fig. 2B). The ΔΕ* value for the films initially increased and then decreased by day 6 before rising again in the later stages of the study (Table 2). Fish At ambient temperature, the films exhibited significant changes within the first day. The corresponding ΔΕ* values was 13.9 ± 0.01. The pH value reached 7.45 ± 0.06 with TVB-N levels of 56.76 ± 1.9 mg/100 g (Fig. 2C). Under refrigerated conditions (4°C), distinct changes in pH, TVB-N, and ΔΕ* were observed throughout the 12-day storage period. The pH initially decreased until day 3 and subsequently increased, reaching 6.83 for AHO:BTA (3:1) (Fig. 2D). A continuous increase in ΔΕ* was observed under refrigeration conditions. The results show that by day 12 the TVB-N values surpassed the 20 mg/100 g threshold (Table 3).
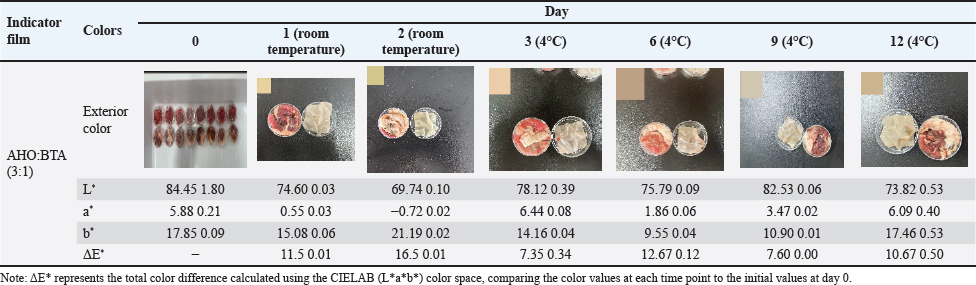
Fig. 2. A: Changes in TVB-N content and pH value of beef stored at room temperature for 1 day; B: Changes in TVB-N content and pH value of beef stored at 4°C for 12 days; C: Changes in TVB-N content and pH value of fish stored at room temperature for 1 day; Changes in TVB-N content and pH value of fish stored at 4°C for 12 days. Lamb At ambient temperature, within the first day, the pH increased to 7.19 ± 0.06 with TVB-N content increasing to 15.34 ± 0.7 mg/100 g (Fig. 3A). Color difference values were found to be 16.5 ± 0.01 within two days for the AHO:BTA (3:1) films (Table 4). Under refrigerated storage, an initial decrease in pH occurs until day 3, followed by an increase till day 12 (Fig. 3B). A gradual increase in ΔΕ* over time was observed (Table 4). The films exceeded the limit for TVB-N content after 12 days of refrigeration. Pork At ambient temperature, the pH values of the AHO:BTA (3:1) films increased to 6.72 ± 0.03 on the first day and 7.59 ± 0.05 on the second day, respectively (Fig. 3C). The corresponding TVB-N values increased to 7.95 ± 0.5 mg/100 g and 70.22 ± 0.4 mg/100 g, respectively. The color difference values (ΔΕ*) decreased from 13.5 ± 0.01 to 11.3 ± 0.02, respectively (Table 5). Under refrigerated conditions (4°C), the data presented in Table 5 reveal an initial rise in ΔΕ* values that peaks on day 6 and then decreases until day 12. In the first 6 days, the pH increased gradually before rising sharply thereafter, whereas TVB-N followed a similar trend. The TVB-N value did not exceed the threshold of 15 mg/100 g until day 12 (Fig. 3D). DiscussionFilm thickness and mechanical properties The slight variation in film thickness observed between the AHO:BTA (1:3) and (3:1) formulations can be directly attributed to differences in their solid component ratios. Specifically, the higher BTA concentration in the 1:3 formulation contributed to a slightly thicker film matrix (Liu et al., 2023), as evidenced by its average thickness of 0.13 mm, compared to the 0.11 mm for the 3:1 film. This increase in solid content directly impacted the film’s mechanical strength. Intriguingly, our results showed that increasing the BTA concentration within the films led to a pronounced increase in the tensile strength. This phenomenon likely arises from the enhanced stability of BTA during the film-forming process, which minimizes the occurrence of pigment separation during drying, creating a more cohesive and robust structure, as supported by a tensile strength of 31.26 MPa for the 1:3 film compared with 19.41 MPa for the 3:1 film. In contrast, the higher EAB observed in the AHO-rich films suggests that AHO, due to its smaller molecular size and improved compatibility with the film matrix (Jiang, 2021), leads to a more flexible structure with an EAB of 66.80% compared to 31.93% for the BTA-rich film. Taken together, these findings underscore that the AHO:BTA ratio offers a route to tailoring the mechanical properties of films for various applications, with the 1:3 film exhibiting superior strength and the 3:1 film exhibiting greater flexibility. Table 2. Color change of indicator films for monitoring beef freshness.
Table 3. Color changes in indicator films when applied to monitoring fish freshness.
Fig. 3. A: Changes in TVB-N content and pH of lamb during storage at room temperature for 2 days; B: Changes in TVB-N content and pH of lamp during storage at 4°C for 12 days; C: Changes in TVB-N content and pH of pork during storage at room temperature for 2 days; D: Changes in TVB-N content and pH of pork during storage at 4°C for 12 days. Antioxidant capacity of indicator films A notable difference in antioxidant capacity, as measured using the DPPH radical scavenging assay, was observed between the AHO-rich (3:1) and BTA-rich (1:3) indicator film formulations. The AHO-rich film exhibited a significantly higher DPPH scavenging rate (85.45%), as compared to the 80% of the BTA-rich films. This difference can be directly attributed to the superior antioxidant properties of AHO (Lee et al., 2015). The higher scavenging ability of AHO stems from its rich composition of multiple hydroxyl groups on its phenolic rings, which facilitate the donation of hydrogen atoms for stabilizing free radicals (Ullah et al., 2019). Consequently, the inclusion of AHO not only imparts antioxidant activity and suggests that these prepared films have the potential to effectively extend the shelf life of packaged meats by mitigating the effects of oxidative degradation. Response of indicator films to volatile ammonia The sensitivity of the indicator films to volatile ammonia, an important indicator of meat spoilage, also differed depending on the pigment ratio. The AHO:BTA (1:3) film showed higher sensitivity (5.86%) than the AHO:BTA (3:1) film (5.43%). This indicates that the BTA is more susceptible to the alkaline environment caused by ammonia exposure, resulting in a greater colorimetric response. Furthermore, a higher BTA content seems to facilitate more robust interactions between the film components, leading to increased ammonia absorption. This enhanced interaction is likely due to the polar nature of BTA molecules, which enables them to interact more readily with polar ammonia molecules through dipole–dipole interactions (Dey et al., 2022). Further studies are warranted to better elucidate this mechanism and explore its full potential as an ammonia sensor. Colorimetric response of AHO/BTA solutions at different pH valuesThe observed colorimetric response of the extracted AHO/BTA solutions at different pH levels is consistent with the structural transformations that these natural pigments undergo. The changes in the color and absorbance peaks confirmed their suitability as pH-sensitive indicators. The color transition from reddish to pink and then yellow/orange with increasing pH corresponds to the known behavior of anthocyanins and betacyanins, in which anthocyanins exhibit a red color under acidic conditions, transitioning to a quinoidal base and a chalcone structure with increased pH (Zhao et al., 2023). This color change is indicative of a change in the chemical form of the molecules. The greater color change exhibited by the AHO:BTA (3:1) samples relative to their 1:3 counterparts made them more sensitive. Given these findings, the AHO:BTA (3:1) film was logically selected for subsequent meat freshness monitoring studies. Table 4. Color changes in the indicator films when applied to monitoring lamb freshness.
Table 5. Color changes in indicator films when applied to monitoring pork freshness.
Application of indicator films for meat freshness monitoringBeef The application of our colorimetric films in the monitoring of beef freshness produced results consistent with meat spoilage dynamics. At ambient temperature, the observed increase in pH and TVB-N levels, accompanied by a noticeable change in color over the first day, accurately reflect the early signs of meat deterioration. Under refrigerated conditions, the initial decrease in pH and TVB-N within the first 3 days was followed by a subsequent increase, indicating reduced microbial activity and a shift toward anaerobic metabolism, which initially produces acidic byproducts (Kameník and Dušková, 2016), eventually leading to further spoilage and increased TVB-N levels. These results demonstrated a clear and significant correlation between TVB-N and pH values, further validating our approach for real-time spoilage monitoring, as supported by previous research (Alejo-Armijo et al., 2019). Fish The performance of the indicator films in monitoring tilapia freshness demonstrated similar trends to those observed for beef. At ambient temperature, the rapid changes in film color and the increase in pH and TVB-N levels within the first day mirrored the fast rate of spoilage observed in fish at room temperature. Under refrigeration, the films again exhibited an initial decrease in pH, followed by an increase with prolonged storage, similar to the trends seen in beef. A continuous change in color (ΔE*) coupled with the surpassing of the 20 mg/100 g TVB-N threshold by day 12 underscores that the films can serve as a practical and effective means to detect fish spoilage. The congruence of these findings with the underlying spoilage mechanisms (anaerobic metabolism is initially favored with a subsequent increase due to microbial activity) further emphasizes the potential of our indicator films to be used across different meat types (Kameník and Dušková, 2016). Lamb In the context of lamb freshness monitoring, our indicator films exhibited a response that closely aligned with the expected degradation pattern. The significant increase in pH values with increasing TVB-N content within the first 24 hours of ambient storage indicates rapid spoilage. In the case of refrigeration, an initial decrease in pH followed by subsequent increases indicates a complex interplay between meat metabolism and microbial activity (Kameník and Dušková, 2016). The increasing values over time further confirm this trend, indicating continuous degradation during the storage period. The films exceeded the 15 mg/100 g limit of TVB-N content, suggesting their capacity to successfully detect spoilage. These results highlight the ability of the proposed method to effectively track the degradation process in lamb. Pork The application of the films on pork samples revealed a unique spoilage pattern compared with other meats in this study. The increase in pH and TVB-N at ambient temperature are consistent with spoilage, although, under refrigeration, The intricate interplay of colorimetric changes, TVB-N levels, and pH values displayed a consistent pattern, with color difference initially increasing until day 6 before declining until day 12. This result, together with the lower rate of TVB-N accumulation throughout the 12-day refrigeration period, suggests that pork exhibits a slower degradation rate under refrigerated conditions, a trend reported in earlier research (Manalo and Gabriel, 2020). However, it is worth noting that the TVB-N value did not cross the threshold of 15 mg/100 g on day 12, further suggesting that pork spoils more slowly. ConclusionThis study successfully developed and evaluated a novel colorimetric indicator film combining AHO and BTA within a PVA/CMC-Na matrix for real-time monitoring of meat freshness. The film’s mechanical strength, antioxidant capacity, and sensitivity to volatile ammonia were characterized, demonstrating that the AHO:BTA ratio significantly influenced its properties. Notably, the films exhibited distinct colorimetric changes in response to pH shifts and increasing spoilage in various meats, correlating well with established indicators like TVB-N. The results demonstrate the potential of these natural pigment-based films as effective and nontoxic tools for monitoring meat freshness, offering a visually accessible and reliable method for ensuring food safety and quality. The AHO:BTA (3:1) film was identified as a particularly effective indicator; however, further refinement of film composition and thickness, as well as testing with more diverse meat varieties, could enhance its practical application in food packaging. AcknowledgmentsNone. Conflict of interestThe authors have not declared any conflict of interest. FundingThis work was financially supported by the Science and Technology Project of Zhaoqing University (FW202306; QN202444); the Zhaoqing University High-level Project Cultivation Program (GCCZK202411), and the Training Program of the College Students Sci-Tech Innovation of the Guangdong Province (X202310580150,$ 202410580021). Authors’ contributionJin-Kui Ma: Conceptualization, Methodology, Investigation, Writing”original draft, Funding acquisition. Qian-Ru Lin: Formal analysis, Visualization. Zhi-Yao Liu: Methodology, Formal analysis. Jia-Min Wang: Formal analysis, Visualization, Writing”review & editing. Xin Peng: Resources, Writing–review & editing. Yu-Tong Lin: Investigation, Formal analysis. Li-Zhen Nie: Resources, Visualization, Project administration. Xiao-Chen Huang: Resources, Formal analysis, Project administration, Funding acquisition. Data AvailabilityThe original data presented in this study are included in the manuscript. ReferencesAbedi-Firoozjah, R., Yousefi, S., Heydari, M., Seyedfatehi, F., Jafarzadeh, S., Mohammadi, R., Rouhi, M. and Garavand, F. 2022. Application of red cabbage anthocyanins as Ph-sensitive pigments in smart food packaging and sensors. Polymers, 14, 1629m Alejo-Armijo, A., Parola, A. J. and Pina, F. 2019. Ph-dependent multistate system generated by a synthetic furanoflavylium compound: an ancestor of the anthocyanin multistate of chemical species. Acs Omega, 4, 4091–4100. Alizadeh-Sani, M., Mohammadian, E., Rhim, J.W. and Jafari, S.M. 2020. Ph-sensitive (Halochromic) smart packaging films based on natural food colorants for the monitoring of food quality and safety. Trends Food Sci. Technol. 105, 93–144. Azman, N., Khairul, W.M. and Sarbon, N. 2022. A comprehensive review on biocompatible film sensor containing natural extract: active/intelligent food packaging. Food Control 141, 109189. Brand-Williams, W., Cuvelier, M.E. and Berset, C.J.L.F.S. 1995. Use of a free radical method to evaluate antioxidant activity. Lwt-Food Sci. Technol. 28, 25–30. Chen, H.Z., Zhang, M., Bhandari, B. and Yang, C.H. 2020. Novel Ph-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 100, 105438. Dey, D., Hemachandran, H., Doss, G.P., Priyadarshini, R. and Siva, R. 2022. Accumulation of betacyanin in Hylocereus undatus rind: pigment stability analysis and its role in xanthine oxidase inhibition. Phytomed. Plus 2, 100197. Fang, Z., Zhao, Y., Warner, R.D. and Johnson, S.K. 2017. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 61, 60–71. Ghaani, M., Cozzolino, C.A., Castelli, G. and Farris, S. 2016. An overview of the intelligent packaging technologies in the food sector. Trends Food Sci. Technol. 51, 1–11. Giusti, M. and Wrolstad, R. 2001. Anthocyanins. Characterization and measurement with uv-visible spectroscopy. Curr. Protocols Food Anal. Chem. 1, 1–13. He, Y. Preparation and application research of anthocyanin active intelligent packaging film. M.S., Zhejiang Gongshang University, Hangzhou, China, 2023. Jay, J.M., Loessner, M.J. and Golden, D.A. 2008. Modern Food Microbiology. Berlin, Germany: Springer Science & Business Media. Jiang, Q. Preparation and application of chitosan/anthocyanin smart tag. M.S, Wuhan University Of Technology, Wuhan, China, 2021. Kameník, J. and Dukovã, M. 2016. Lactic acid bacteria and their role in the meat processing. Theory Pract. Meat Process. 1, 25–31. Kanha, N., Osiriphun, S., Rakariyatham, K., Klangpetch, W. and Laokuldilok, T. 2022. On-package indicator films based on natural pigments and polysaccharides for monitoring food quality: a review. J. Sci. Food Agricul. 102, 6804–6823. Kossyvaki, D., Contardi, M., Athanassiou, A. and Fragouli, D. 2022. Colorimetric Indicators based on anthocyanin polymer composites: a review. Polymers 14, 4129. Lee, S.G., Vance, T.M., Nam, T.G., Kim, D.O., Koo, S.I. and Chun, O.K. 2015. Contribution of anthocyanin composition to total antioxidant capacity of berries. Plant Foods Human Nutr. 70, 427–432. Lim, T.W., Lim, C.J., Liow, C.A., Ong, S.T., Lim, L.H., Pui, L.P., Tan, C.P. and Ho, C.W. 2022. Studies on the storage stability of betacyanins from fermented red dragon fruit (Hylocereus Polyrhizus) drink imparted by xanthan gum and carboxymethyl cellulose. Food Chem. 393, 133404. Liu, D., Zhang, C., Pu, Y., Chen, S., Li, H. and Zhong, Y. 2023. Novel colorimetric films based on polyvinyl alcohol/sodium carboxymethyl cellulose doped with anthocyanins and betacyanins to monitor pork freshness. Food Chem 404, 134426. Majid, I., Nayik, G.A., Dar, S.M. and Nanda, V. 2018. Novel food packaging technologies: innovations and future prospective. J. Saudi Soc. Agricul. Sci. 17, 454–462. Manalo, M.R. and Gabriel, A.A. 2020. Changes in the physicochemical and microbiological properties of pork and chicken meats at ambient storage condition. Sci. J. Meat Technol. 61, 44–53. Marin, E., Rojas, J. and Ciro, Y. 2014. A review of polyvinyl alcohol derivatives: promising materials for pharmaceutical and biomedical applications. Afr. J. Pharm. Pharmacol. 8, 674–684. Mcmullen, R.L., Ozkan, S. and Gillece, T.J.C. 2022. Physicochemical properties of cellulose ethers. Cosmetics, 9, 52. Mittal, A., Garg, S., Premi, A. and Giri, A.S. 2021. Synthesis of polyvinyl alcohol/modified starch-based biodegradable nanocomposite films reinforced with starch nanocrystals for packaging applications. Polymers Polymer Compos. 29, 405–416. Mohammadian, E., Alizadeh-Sani, M. and Jafari, S.M. 2020. Smart monitoring of gas/temperature changes within food packaging based on natural colorants. Comprehen Rev Food SciFood Saf 19, 2885–2931. National Health And Family Planning Commission Of The People’s Republic Of China 2016. Gb 5009.228-2016 National Food Safety Standard-Determination Of Volatile Basic Nitrogen In Foods. Beijing, China: China Standards Press. Pichayajittipong, P. and Thaiudom, S. 2014. Optimum condition of beta-cyanin colorant production from red dragon fruit (Hylocercus Polyrhizus) peels using response surface methodology. Chiang Mai Unive. J. Nat. Sci. 13, 483–496. Singh, P., Wani, A.A., Saengerlaub, S. and Langowski, H.C. 2011. Understanding critical factors for the quality and shelf-life of map fresh meat: a review. Crit. Rev. Food Sci. Nutr. 51, 146–177. Ullah, R., Khan, M., Shah, S.A., Saeed, K. and Kim, M.O. 2019. Natural antioxidant anthocyanins”a hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients 11, 1195. Wrolstad, R.E., Durst, R.W. and Lee, J. 2005. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 16, 423–428. Xu, M., Fang, D., Kimatu, B.M., Lyu, L., Wu, W., Cao, F. and Li, W. 2024. Recent advances in anthocyanin-based films and its application in sustainable intelligent food packaging: a review. Food Control. 162, 110431. Xu, X., Jiang, Y., Yeo, Q. X. and Zhou, W. 2024b. Purification and characterization of betacyanin monomers from Hylocereus Polyrhizus peel: a comparative study of their antioxidant and antidiabetic activities with mechanistic insights. Food Chem. 451, 139467. Yadav, S., Tiwari, K.S., Gupta, C., Tiwari, M.K., Khan, A. and Sonkar, S.P. 2023. A brief review on natural dyes, pigments: recent advances and future perspectives. Results Chem. 5, 100733. Zhao, Y., Gao, L., Wang, J., Xue, Z., Zhang, M., Ma, X., Wang, G. and Lv, S. 2023. Preparation and application of Ph-sensitive film containing anthocyanins extracted from Lycium Ruthenicum Murr. Materials 16, 3828. Zhou, X., Yu, X., Xie, F., Fan, Y., Xu, X., Qi, J., Xiong, G., Gao, X. and Zhang, F. 2021. Ph-responsive double-layer indicator films based on Konjac Glucomannan/Camellia oil and Carrageenan/Anthocyanin/Curcumin for monitoring meat freshness. Food Hydrocoll. 118, 106695. | ||
| How to Cite this Article |
| Pubmed Style Ma J, Lin Q, Liu Z, Wang J, Peng X, Lin Y, Nie L, Huang X. Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meats. Open Vet. J.. 2025; 15(4): 1836-1847. doi:10.5455/OVJ.2025.v15.i4.36 Web Style Ma J, Lin Q, Liu Z, Wang J, Peng X, Lin Y, Nie L, Huang X. Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meats. https://www.openveterinaryjournal.com/?mno=249148 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.36 AMA (American Medical Association) Style Ma J, Lin Q, Liu Z, Wang J, Peng X, Lin Y, Nie L, Huang X. Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meats. Open Vet. J.. 2025; 15(4): 1836-1847. doi:10.5455/OVJ.2025.v15.i4.36 Vancouver/ICMJE Style Ma J, Lin Q, Liu Z, Wang J, Peng X, Lin Y, Nie L, Huang X. Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meats. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1836-1847. doi:10.5455/OVJ.2025.v15.i4.36 Harvard Style Ma, J., Lin, . Q., Liu, . Z., Wang, . J., Peng, . X., Lin, . Y., Nie, . L. & Huang, . X. (2025) Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meats. Open Vet. J., 15 (4), 1836-1847. doi:10.5455/OVJ.2025.v15.i4.36 Turabian Style Ma, Jin-kui, Qian-ru Lin, Zhi-yao Liu, Jia-min Wang, Xin Peng, Yu-tong Lin, Li-zhen Nie, and Xiao-chen Huang. 2025. Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meats. Open Veterinary Journal, 15 (4), 1836-1847. doi:10.5455/OVJ.2025.v15.i4.36 Chicago Style Ma, Jin-kui, Qian-ru Lin, Zhi-yao Liu, Jia-min Wang, Xin Peng, Yu-tong Lin, Li-zhen Nie, and Xiao-chen Huang. "Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meats." Open Veterinary Journal 15 (2025), 1836-1847. doi:10.5455/OVJ.2025.v15.i4.36 MLA (The Modern Language Association) Style Ma, Jin-kui, Qian-ru Lin, Zhi-yao Liu, Jia-min Wang, Xin Peng, Yu-tong Lin, Li-zhen Nie, and Xiao-chen Huang. "Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meats." Open Veterinary Journal 15.4 (2025), 1836-1847. Print. doi:10.5455/OVJ.2025.v15.i4.36 APA (American Psychological Association) Style Ma, J., Lin, . Q., Liu, . Z., Wang, . J., Peng, . X., Lin, . Y., Nie, . L. & Huang, . X. (2025) Development and evaluation of anthocyanin-betacyanin colorimetric films for real-time monitoring of the freshness of different meats. Open Veterinary Journal, 15 (4), 1836-1847. doi:10.5455/OVJ.2025.v15.i4.36 |