| Research Article | ||
Open Vet. J.. 2025; 15(4): 1823-1835 Open Veterinary Journal, (2025), Vol. 15(4): 1823-1835 Research Article The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male ratsRana Ramadan1,2*, El-Said El-Sherbini1, Gehad Elsayed1 and Mohamed El-Adl11Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt 2Department of Basic Veterinary Science, Faculty of Veterinary Medicine, Delta University for Science and Technology, Mansoura, Egypt *Corresponding Author: Rana Ramadan. Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt; Department of Basic Veterinary Science Faculty of Veterinary Medicine Delta University for Science and Technology, Mansoura, Egypt. Email: ranar6152 [at] gmail.com Submitted: 24/02/2025 Accepted: 06/04/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
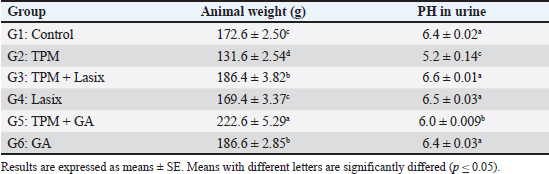
AbstractBackground: Topiramate (TPM) is commonly used for migraine prophylaxis; nonetheless, it is linked to nephrotoxicity, which mostly results from oxidative stress and inflammatory mechanisms. Aim: This study aimed to assess the preventive effects of gallic acid (GA) and furosemide (Lasix) against renal damage caused by TPM in rats. Methods: Sixty male albino rats were categorized into six groups: control, TPM-only, TPM + Lasix, Lasix-only, TPM + GA, and GA-only. The investigation lasted 60 days, evaluating renal function, oxidative stress indicators, and histological and immunohistochemical alterations. Results: The TPM-treated group exhibited considerable renal impairment, as indicated by increased levels of creatinine, urea, blood urea nitrogen, and interleukin (IL)-6, as well as reduced activity of antioxidant enzymes [catalase (CAT), superoxide dismutase]. Histopathological examination revealed tubular necrosis and inflammation, while immunohistochemistry results showed elevated expression of caspase3 and IL-6. The co-administration of GA or Lasix mitigated these effects. The TPM + GA group exhibited improved kidney function, less oxidative stress, and enhanced histological structure, underscoring GA’s powerful antioxidant and anti-inflammatory characteristics. Likewise, Lasix exerted protective effects by alleviating TPM-induced kidney injury. Conclusion: These data highlight the therapeutic efficacy of GA and Lasix against TPM-induced nephrotoxicity, indicating their clinical relevance in addressing drug-induced kidney damage. Keywords: TPM, Gallic acid, Lasix, Renal damage, Anti-inflammatory, Antioxidant. IntroductionThe kidneys are susceptible to medication poisoning because they serve as the principal organs for drug excretion and are repeatedly exposed to possible poisons (El Makawy et al., 2022). Numerous studies have indicated that most major antiepileptic medications (AEDs) can induce renal impairment. The renal toxicity of antiepileptic drugs can lead to kidney failure and may result from the formation of reactive toxic metabolites or the onset of immunoallergic reactions (Mahmoud et al., 2020). Topiramate (TPM) is a sulfamate-substituted monosaccharide with a broad mechanism of action and has been validated for its efficacy in individuals with episodic or chronic migraines (Dinkelacker et al., 2022). TPM is an effective and well-accepted prophylactic treatment for migraines in adults and adolescents (Jia et al., 2021). It is posited to mitigate migraines by obstructing sodium channels, augmenting c-aminobutyric acid-induced chloride flux, and inhibiting glutamate and carbonic anhydrase enzymes, which engage with various ion channels by either amplifying ion channel activity, as seen in gamma-aminobutyric acid type A receptors, or diminishing ion channel activity, as observed in voltage-activated sodium (Na+) channels, ionized calcium (Ca2+) channels, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptors (Borowicz-Reutt, 2022). The mechanism of renal toxicity associated with AEDs remains ambiguous, but it may involve disruption of the mitochondrial beta-oxidation of fatty acids (Steele et al., 2024). Impaired beta-oxidation of antiepileptic drugs may contribute to kidney damage (Liparoti et al., 2022). TPM induces mixed renal tubular acidosis by inhibiting carbonic anhydrase in renal tubules, resulting in systemic metabolic acidosis characterized by decreased plasma bicarbonate levels and acidic urine pH, accompanied by low urine citrate concentrations. These metabolic alterations culminate in the production of calcium phosphate stones (Damba et al., 2022; Pelzman et al., 2022; Wagner et al., 2023; Åžahin et al., 2024). This study aimed to investigate the toxicity of TPM in rat renal tissue. Additionally, biochemical and histological experiments were conducted to determine the effect of TPM on the kidneys of rats. Gallic acid (3,4,5-trihydroxybenzoic acid, GA) is a naturally occurring compound found in red wine, green tea, strawberries, pineapples, bananas, lemons, gallnuts, sumac, witch hazel, tea leaves, oak bark, and apple peels (Eslamifar et al., 2021). GA, a potent chelating agent, safeguards human cells and tissues from oxidative stress through its biological actions, which encompass antioxidant and anti-inflammatory properties (Hadidi et al., 2024). It not only safeguards the integrity of the plasma membrane but enhances the regeneration and reparative capabilities of the liver and kidneys. Furthermore, it has demonstrated properties as an antiallergic, antimutagenic, anti-inflammatory, and anticarcinogenic agent, as well as a potent natural antioxidant (Ms et al., 2022). GA suppresses melanogenesis, potentially due to its capacity to scavenge reactive oxygen species (Ashrafizadeh et al., 2021). Lasix, also known as furosemide, is a diuretic that inhibits electrolyte transport receptors in the thick ascending limb of the loop of Henle. Furosemide promotes the elimination of Na+ and water by inhibiting the Na–potassium (K)–2 chloride (Cl) co-transporter in the thick ascending limb, thereby reducing oxygen consumption facilitated by Na–K-ATPase. Fluid overload is independently associated with increased mortality and worse outcomes. Consequently, Lasix was used in this investigation to mitigate the detrimental effects of TPM (Dilken et al., 2023). For all the previous reasons, this investigation was undertaken to examine the ameliorative role of GA and/or Lasix on the renal tissue of TPM-treated rats by examining their effects on various renal chemical parameters. The antioxidant effect against TPM was also measured by evaluating the malondialdehyde (MDA) level as well as catalase (CAT) and superoxide dismutase (SOD) activities. Additionally, immunohistochemical analysis for caspase 3 and interleukin (IL)-6 was conducted in the renal tissue of treated rats. Materials and MethodsAnimalsA total of 60 mature male albino rats with weights ranging from 180 to 200 g were obtained from the animal facility at the Faculty of Veterinary Medicine, Mansoura University. The rats were housed under regulated environmental conditions with a half-day light-dark cycle, room temperature of 22°C ± 4°C, and humidity levels of 5% ± 10%. Throughout the 7-day acclimation phase, rats were provided with unlimited access to water and a commercial pellet meal. This research complied with all Institutional and National Guidelines for the ethical treatment and experimentation of animals, in accordance with the regulations established by the Mansoura University-Animal Care and Use Committee (MC-ACUC) (Ph. D/66). Experimental designThe 60 rats were sorted into six groups each having 10 rats each, for the assessment of GA and/or Lasix influence on nephrotoxicity connected to treatment with TPM. The rats were fed a standard control pellet diet in addition to water for 1 week as the acclimation phase. For the following 60 days, the first group (the placebo control group) was fed only a normal control diet without any medication. The second group, designated TPM, received an oral dosage of TPM (18 mg/kg body weight) (BAL Pharma Limited, Bangalore, Karnataka) through a stomach tube. The third group received TPM (18 mg/kg) orally in conjunction with Lasix (5 mg/kg/24 hours) orally, whereas the fourth group received only Lasix (5 mg/kg/24 hours) I/M. The fifth group received oral TPM (18 mg/kg) and oral GA (50 mg/kg/24 hours) orally. The sixth group received GA (50 mg/kg/24 hours) orally. After a 60-day experimental period, the final body weights of the rats were assessed to determine changes in weight. The rats in the four groups were anesthetized with 20 mg kg of thiopental sodium for blood sample collection via the medial canthus of the eye using capillary tubes for biochemical analysis. Subsequently, the animals were euthanized, and the kidneys were collected for further biochemical, immunohistochemical, and histopathological analyses. Blood and renal tissue samplesFollowing the collection of blood samples, the samples were centrifuged at 3,000 rpm for 15 minutes, after which the serum was separated and stored at –20°C until the evaluation of renal function markers. The left kidneys were segmented into two portions: 50 mg of fresh tissue was employed for the analysis of pro-inflammatory cytokines, including serum IL-6, electrolyte concentrations, ionized and total calcium (Ca), sodium (Na), phosphorus (P), and potassium (K), along with biomarkers indicative of kidney injury. The residual tissue from the left kidney was maintained in normal saline, then homogenized in cold potassium phosphate buffer (PH 7.5), and the supernatant was extracted and frozen at –20°C for the evaluation of CAT, SOD, and MDA concentrations. The right kidney was removed and stored in 10% formalin for histological assessment and immunohistochemical analysis of caspase-3 and IL-6. Urine collectionUrine specimens were collected to evaluate nephrolithiasis and other renal functions. We measured PH in urine in each group. Each rat was accommodated in an individual metabolic cage engineered to segregate urine and feces to prevent contamination. Twenty-four urine samples were obtained on the 60th day of the trial. To maintain the integrity of the samples and inhibit bacterial proliferation, a drop of strong hydrochloric acid was added immediately after collection. The specimens were preserved at 4°C prior to analysis. Biochemical analysisKidney function tests were conducted using the acquired serum samples to measure creatinine and urea levels by kinetic assay. The alteration in the absorbance of alkaline picric acid following the introduction of serum samples was utilized for the assessment of serum creatinine concentrations (Friedman and Young, 1989). Furthermore, urea levels were assessed using the activity of the urease enzyme, which facilitated the reaction of released ammonia with alpha-ketoglutarate to produce glutamate, while the reduction in nicotinamide adenine dinucleotide levels was measured via ultraviolet spectroscopy (Tiffany et al., 1972). Serum blood urea nitrogen (BUN) and albumin were determined using enzymatic colorimetric kits (Spinreact, Girona, Spain) using the manufacturing instructions. Creatinine clearance provides an estimate of the volume of plasma cleared of creatinine per unit of time. It was measured by the concentration of creatinine in a 24-hour urine sample and the corresponding serum creatinine level, along with the urine volume, the clearance rate can be calculated. The albumin-to-creatinine ratio (A/C ratio) was measured using a colorimetric reaction such as the Jaffé reaction. The activity of renal CAT was assessed using the method of Cohen et al. (1970), wherein the generated hydrogen peroxide interacted with a potassium permanganate solution (0.01N KMNO4), and the residual permanganate was quantified at 480 nm. The activity of renal SOD was assessed using the methodology of Nishikimi et al. (1972), who evaluated the reduction capacity of superoxide radicals against nitroblue tetrazolium using phenazine methosulfate as a mediator. The concentrations of MDA, an indicator of lipid peroxidation in renal tissues, were quantified colorimetrically using thiobarbituric acid (Ohkawa et al., 1979). Neutrophil gelatinase-associated lipocalin (NGAL), Cystatin C, and estimated glomerular filtration rate (eGFR) are key biomarkers used to assess kidney injury and function. NGAL was measured using the Human NGAL ELISA Kit (BioPorto Diagnostics). Cystatin C levels were measured using the DiaSys Cystatin C FS Kit. eGFR was calculated using the equation chronic kidney disease- epidemiology collaboration. Histopathological and immune histochemistry analysisThe excised kidney tissue was first preserved in a 4% paraformaldehyde solution and then fixed in paraffin for sectioning. Glass slides were created from the paraffin-embedded tissues. Xylene was used for deparaffinization to eliminate the paraffin. A 3% hydrogen peroxide solution was used to inhibit endogenous peroxidase activity. The slides underwent pepsin treatment at 42°C for 5 minutes to amplify the signal. Thereafter, they were incubated overnight at 4°C with mouse anti-caspase and IL-6 antibodies sourced from Abcam Limited, UK. The detection system was executed using peroxidase/3,3′-diaminobenzidine (Collins, 2018). The image analysis was categorized into four staining levels: negatively stained (0%–10%), mildly stained (10%–25%), moderately stained (26%–50%), and strongly stained (51%–100%) (Jammal et al., 2015). Paraffin sections were immunostained using antibodies against caspase-3, IL-6 receptor, and control, followed by secondary antibody incubation and 3,3’-diaminobenzidine staining. Tissue sections were counterstained with hematoxylin, dehydrated, and mounted for analysis. Statistical analysisStatistical variances in the mean biochemical variables between control and diseased rats were assessed using one-way ANOVA, followed by post hoc Tukey’s test. Normality was evaluated using the Shapiro–Wilk test. Statistical analyses were performed using GraphPad Prism for Windows version 5.0 (GraphPad Software, Inc., San Diego, CA). A significance threshold of p < 0.05 was established for all tests (Kheira et al., 2019). Ethical approvalThis study adheres to national and international regulations. The present work was approved by the research ethics council of Mansoura University (MU-ACUC, Ph. D 66). ResultsFinal weight of rats and PH in urineThe animal weight of rats in the TPM + GA group (G5) showed a significant increase (222.6 ± 5.29ᵃ) compared with the control group (172.6 ± 2.50ᶜ). In contrast, the weight of rats treated with TPM alone (G2) exhibited a notable decrease (131.6 ± 2.54ᵈ). Rats treated with TPM combined with Lasix (G3) and those treated with GA alone (G6) showed similar weights (186.4 ± 3.82ᵇ and 186.6 ± 2.85ᵇ, respectively), both higher than the control group. However, rats treated with Lasix alone (G4) exhibited weights (169.4 ± 3.37ᶜ) comparable to those of the control group but lower than those of the other treatment groups (Table 1). The urine pH in the TPM group (5.2) is acidic, indicating a significant reduction in pH compared with the control (6.4). The combination of TPM with Lasix (6.6) and Lasix alone (6.5) resulted in a slightly more alkaline pH, suggesting a potential buffering or diuretic effect of Lasix. Similarly, the TPM + GA (6.0) and GA alone (6.4) groups showed minimal pH variation, with GA appearing to maintain a near-neutral pH. These findings suggest that TPM administration leads to urine acidification, whereas LASIX and GA may counteract this effect to some extent (Table 1). Table 1. Effects of GA and/or Lasix on TPM-induced renal impairment in animals and urine.
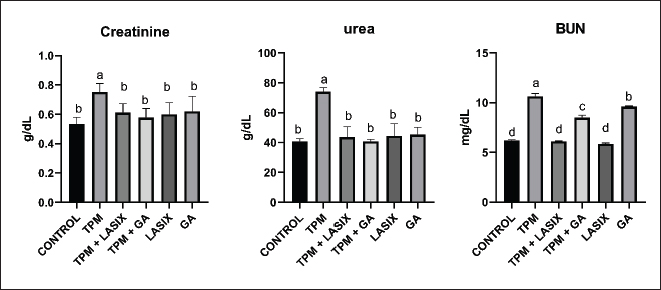
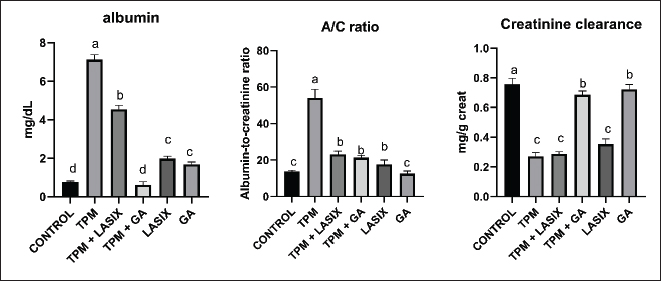
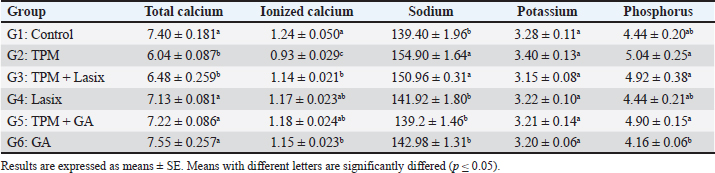
Fig. 1. Serum biochemical parameters of rats intoxicated with TPM and supplemented with GA and/or Lasix. A) Creatinine, B) urea, and C) BUN. Results was expressed as means ± SE. Means with different letters are significantly differed (p ≤ 0.05). Biochemical parameters of serum and renal tissue homogenatesThe markers of renal function were significantly different between the groups. The TPM-treated group (G2) exhibited elevated levels of creatinine (Fig. 1A), BUN (Fig. 1B), and urea (Fig. 1C), indicating significant renal impairment. Conversely, the TPM + GA group (G5) exhibited a considerable increase in creatinine, BUN, and urea levels compared with the control group (G1). The TPM + Lasix group (G3) and the Lasix-only group (G4) exhibited considerable enhancement, with creatinine and urea levels akin to the control group, but differing BUN levels. The GA-only group (G6) exhibited a rise in BUN levels while maintaining creatinine and urea levels comparable to the control group. The TPM group (G2) exhibited increased albumin levels (Fig. 2A) and A/C (Fig. 2B) ratio, yet the lowest creatinine clearance (Fig. 2C), signifying significant renal impairment. The TPM + Lasix group (G3) exhibited a moderate decrease in albumin levels and the A/C ratio, along with a marginal enhancement in creatinine clearance. The Lasix group (G4) had reduced albumin levels and enhanced creatinine clearance while sustaining a high A/C ratio. The TPM + GA group (G5) exhibited notable enhancement characterized by decreased albumin levels, moderate creatinine clearance, and an A/C ratio. Conversely, the GA group (G6) had the lowest A/C ratio, comparable albumin levels, and creatinine clearance, suggesting a protective effect similar to that of the control group (G1). The TPM group (G2) exhibited a significant decrease in total calcium and ionized calcium levels despite the presence of increased salt and phosphorus. Conversely, the TPM + GA group (G5) exhibited enhanced total calcium and ionized calcium levels, as well as adjusted salt and phosphorus levels, akin to the control group (G1). Likewise, the Lasix-only group (G4) and GA-only group (G6) had normal total calcium and ionized calcium levels, with sodium and potassium levels aligning with those of the control group. The TPM + Lasix group (G3) exhibited a slight increase in calcium levels, although salt and phosphorus levels persisted at elevated concentrations. The potassium levels exhibited no significant fluctuations among the groups, remaining constant and equivalent to the control (Table 2).
Fig. 2. Serum biochemical parameters of rats intoxicated with TPM and supplemented with GA and/or Lasix. A) Albumin, B) A/C ratio, and C) creatinine clearance. Results are expressed as means ± SE. Means with different letters are significantly differed (p ≤ 0.05). Table 2. Effects of GA and/or Lasix on TPM-induced renal impairment and electrolyte levels.
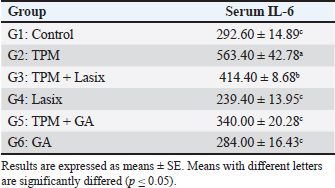
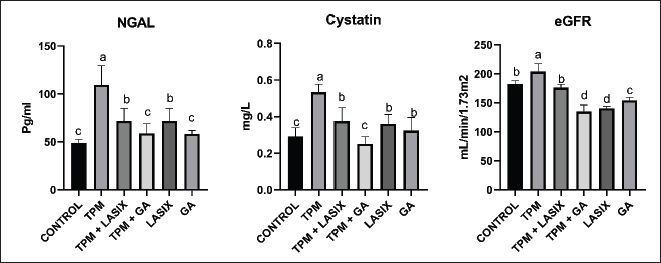
The TPM group (G2) exhibited a substantial elevation in IL-6 levels, indicating considerable inflammation. Coadministration of TPM and Lasix (G3) decreased IL-6 levels, indicating moderate enhancement. Conversely, the TPM + GA group (G5) exhibited a further decrease in IL-6 levels, approaching control levels. The Lasix-only group (G4) and GA-only group (G6) groups sustained similar IL-6 levels as the control group, demonstrating their protective effects against inflammation (Table 3). The TPM-treated group (G2) exhibited a significant elevation in NGAL (Fig. 3A) and Cystatin C (Fig. 3B), coupled with the highest eGFR value (Fig. 3C), signifying severe renal impairment. Coadministration of TPM and Lasix (G3) markedly diminished NGAL and Cystatin C concentrations, accompanied by a slight reduction in eGFR. The TPM + GA group (G5) had similar NGAL levels and somewhat reduced cystatin C levels, although they had the lowest eGFR value. The GA-only group (G6) exhibited notable improvement with decreased NGAL, moderate cystatin C levels, and improved eGFR. The Lasix-only group (G4) had the lowest NGAL and Cystatin C levels but a decreased eGFR, comparable to the control levels. Table 3. Effects of GA and/or Lasix on TPM-induced renal impairment and serum IL-6 levels.
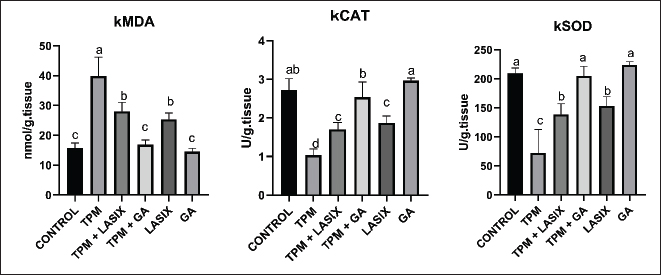
The TPM-treated group (G2) exhibited increased levels of kidney MDA (kMDA) (Fig. 4A), signifying increased oxidative stress, and the lowest kidney CAT (kCAT) (Fig. 4B) and kidney SOD (kSOD), (Fig. 4C) levels showed compromised antioxidant activity. Coadministration of TPM and Lasix (G3) decreased kMDA levels and partially reinstated kCAT and kSOD activity. The TPM + GA group (G5) exhibited enhanced outcomes with diminished kMDA, elevated kCAT, and increased kSOD levels. The GA-only group (G6) exhibited the most efficacious antioxidant profile, with the lowest kMDA levels, highest kCAT activity, and largest kSOD activity, exceeding that of the control group. The Lasix-only group (G4) had lower kMDA levels and antioxidant activity than the control group, indicating its protective effects.
Fig. 3. Tissue biochemical parameters of rats intoxicated with TPM and supplemented with GA and/or Lasix. A) NGAL, B) cystatin C, and C) eGFR. Results was expressed as means ± SE. Means with different letters are significantly differed (p ≤ 0.05).
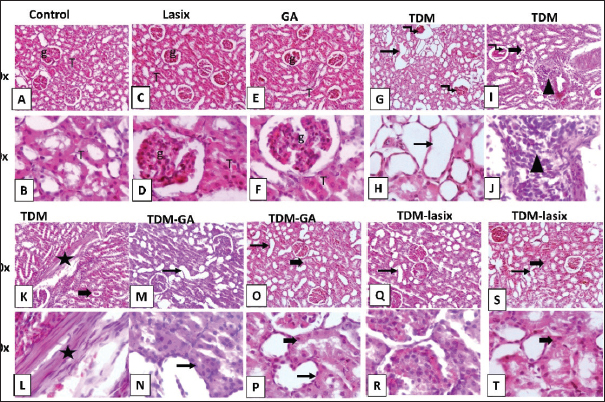
Fig. 4. Renal oxidant and antioxidant defense markers of rats intoxicated with TPM and supplemented with GA and/or Lasix. A) kMDA, B) kCAT, and C) kSOD. Results was expressed as means ± SE. Means with different letters are significantly differed (p ≤ 0.05). Histopathology of renal tissues intoxicated with TPM supplemented with GA and/or LasixThe control group showed normal histological architecture of renal tubules and glomerulus (Fig. 5A and B). The Lasix group showed a normal appearance of renal parenchyma (Fig. 5C and D). The GA group showed a normal histological appearance of tubular and glomerular architecture (Fig. 5E and F). TPM showed glomerular tuft shrinkage with severe tubular ectasia lined with attenuated epithelium (Fig. 5G and H). TPM showed severe focal coalescing perivascular aggregations of extensive mixed cellular infiltrates including abundant lymphoplasmacytic cells widely separated ectatic tubules with intraluminal hyaline cast (Fig. 5I and J). TPM showed dense interstitial fibrosis admixed with few RBCs, edema, and separated diffuse necrotic tubules with intraluminal cast (Fig. 5G and L). TPM + GA showed mild to moderate tubular ectasia with mild tubular necrosis (Fig. 5M–P). TPM-Lasix showed mild tubular ectasia and necrosis (Fig. 5Q–T).
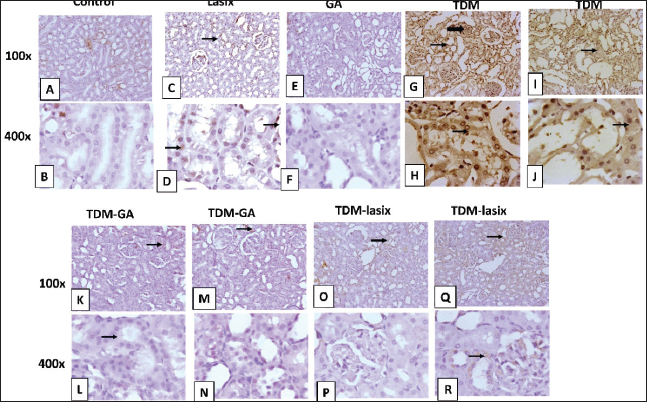
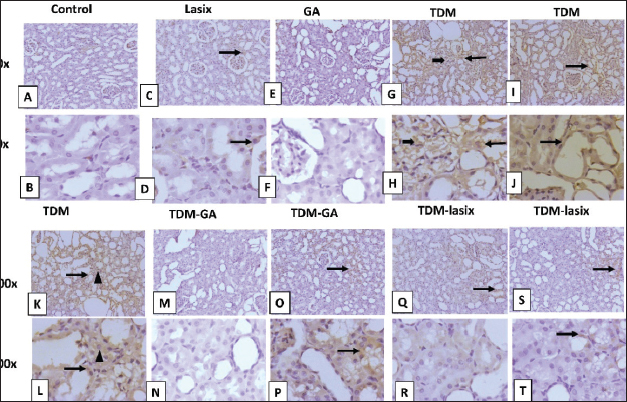
Fig. 5. Representative photomicrograph of renal tissue from different treatment groups. A,B) Control group. C,D) Lasix group. E,F) GA group. G,H) TPM group. I,J) TPM group. K,L) TPM group. M–P) TPM + GA group. Q–T) TPM-Lasix group. G=glomerulus, T=tubules, thin arrow=ectatic tubules, thick arrow=tubular necrosis with intraluminal cast, arrowhead=inflammation, star=fibrosis, twisted arrow=glomerular shrinkage. Image magnification=100×, 400×. Immunohistochemical analysis of renal tissues intoxicated with TPM supplemented with GA and/or LasixIn Figure 6A and B, the control group shows negative to faint immunopositive stained tubular epithelial cells caspase In Figure 6C and D, the Lasix group shows few faint immunostained tubular epithelial cells. In Figure 6E and F, the GA group shows negative to few immunostained renal tubules (Fig. 6). The TPM group shows high positive staining in ectatic tubular epithelial cells and glomerular tuft. In Figure 6K–N, TPM + GA shows negative to few immunopositive stained tubular epithelial cells. In Figure 6O–R, TPM + Lasix shows weak cytoplasmic expression in tubular epithelial cells. In Figure 7A and B, the control group is negative for a few immunopositive-stained tubular epithelial and interstitial cells of IL-6. In Figure 7C and D, the lasix group shows negative to few faint immunostained tubular epithelial cells. In Figure 7E and F, the GA group is negative for a few immunostained renal tubules. Figure 7G–L, TPM group showing moderate to high positive staining in ectatic tubular epithelial cells and glomerular tufts with little positivity in interstitial inflammatory cells. In Figure 7M–P, TPM + GA showing negative to mild immunopositive stained tubular epithelial cells. In Figure 7Q–T, TPM + Lasix shows weak immunoexpression of IL6 in tubular epithelial cells. DiscussionTPM, a commonly used AED, has exhibited preventive properties against several forms of organ damage, including injuries associated with oxidative stress. GA, a natural antioxidant, has demonstrated potential preventive effects in mitigating renal damage. This study aimed to assess the cumulative effects of TPM, GA, and Lasix on renal function in rats experiencing TPM-induced renal impairment. Results demonstrate substantial discrepancies in animal weight among the experimental groups. Rats administered TPM in conjunction with GA (G5) exhibited significant weight gain relative to the control group. The weight reduction noted in the TPM group (G2) corresponds with the findings of Varzandeh et al. (2024), who highlighted TPM’s catabolic effects on muscular tissue, facilitating weight loss. The mild weight gains in the TPM + Lasix group (G3) may result from Lasix’s diuretic-induced fluid control, which offsets TPM-induced weight reduction. The interaction between diuretic and metabolic effects requires further investigation.
Fig. 6. Representative IHC of caspase 3 expression in renal sections of different treatment groups. A,B) Control group. C,D) Lasix group. E,F) GA group. G–J) TPM group. K–N) TPM + GA group. O–R) TPM + Lasix group. Thin arrow=positive tubular epithelium; thick arrow=positive glomerular tufts. Image magnification=100×, 400×. Moreover, the preventive properties of GA against drug-induced toxicity have been thoroughly demonstrated. The TPM-treated group exhibited considerable increases in creatinine, BUN, and urea levels in the TPM-treated group (G2), indicating renal impairment. This is consistent with the findings of El Makawy et al. (2022), who indicated that TPM causes nephrotoxicity, as seen by elevated renal indicators and histological alterations. The renal protection noted in the GA-treated group (G5) supports the findings of Asci et al. (2017), who demonstrated that GA mitigates methotrexate-induced nephrotoxicity, considerably lowering creatinine and urea levels. Gholamine et al. (2021) corroborated these findings, indicating that GA mitigates sodium arsenite-induced renal and hepatic damage via its robust antioxidant capabilities. Research conducted by Nouri et al. (2021) and Waly et al. (2022) substantiated GA’s nephroprotective properties, illustrating its capacity to mitigate the effects of hazardous substances such as paraquat and tartrazine that impair renal function. Our results demonstrate notable enhancements in albumin and A/C ratios in the GA-treated cohorts. Significantly, TPM + GA (G5) treatment substantially decreased albumin levels and enhanced creatinine clearance, indicating improved renal function. The capacity of GA to safeguard glomerular function was further corroborated by Elghouizi et al. (2022), who illustrated its effectiveness in diminishing proteinuria and enhancing renal biomarkers in animal models of acute kidney damage. The decrease in the A/C ratio noted in G5 highlights GA’s function in maintaining glomerular integrity and averting albuminuria, which is consistent with the findings of Azab et al. (2017). They demonstrated that GA mitigated glomerular injury and restored renal function in nephrotoxic animals. Additionally, Gholamine et al. (2021) elucidated GA’s nephroprotective properties against renal damage induced by sodium arsenite and paraquat. These studies revealed elevated albumin levels and reduced oxidative stress indicators, associated with improved renal clearance. The enhancements in creatinine clearance shown in G5 align with the findings of Alejolowo et al. (2024), who identified GA’s capacity to regulate oxidative and inflammatory responses, consequently improving renal filtration efficacy. Likewise, research conducted by Doğan et al. (2022) and Elmileegy et al. (2023) emphasized the function of GA in mitigating liver toxicity generated by cisplatin and uranyl acetate, respectively, via its antioxidative and cytoprotective mechanisms. The TPM-treated group (G2) exhibited diminished total calcium and increased salt levels, indicating an electrolyte imbalance and renal impairment. These results correspond with those of Asci et al. (2017), who illustrated the nephroprotective function of GA in alleviating oxidative stress and restoring renal tubular injury. Furthermore, Elghouizi et al. (2022) emphasized the antioxidant and anti-inflammatory characteristics of GA, which are crucial for preventing electrolyte imbalances and glomerular impairment. Collectively, these data highlight the potential of GA in mitigating TPM-induced renal impairment. GA enhances calcium and phosphorus balance, as observed by Bouasla et al. (2021).
Fig. 7. Representative IHC of IL-6 expression in renal sections of different treatment groups. A,B) Control group. C,D) Lasix group. E,F) GA group. G–L) TPM group. M–P) TPM + GA group. Q–T) TPM + Lasix group. Thin arrow=positive tubular epithelial cells; thick arrow=positive glomerular tufts; arrowhead=positive inflammatory cells. Image magnification=100×, 400×. IL-6 is a proinflammatory cytokine that increases in concentration following tissue injury or infection. It can be used as an indicator of systemic inflammation. Our results illustrate a notable increase in IL-6 levels in the TPM-treated group (G2). Increased IL-6 levels signify systemic inflammation, indicating the proinflammatory properties of TPM. This discovery corroborates the work of Arab et al. (2023), which revealed that TPM induces the release of proinflammatory cytokines, specifically IL-6, through the activation of oxidative stress pathways. TPM has been demonstrated to regulate the non-obese diabetic-, left renal resection-, and pyrin domain-containing protein 3 inflammasome and AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) pathways, leading to heightened inflammation. Increased IL-6 levelscauseo tissue damage and intensify oxidative stress, potentially worsening preexisting pathological diseases. The cotreatment group administered GA in conjunction with TPM (G5) exhibited markedly reduced IL-6 levels. This illustrates the powerful anti-inflammatory properties of GA, which function by regulating inflammatory pathways and inhibiting excessive production of proinflammatory cytokines. The proinflammatory characteristics of TPM were thoroughly studied. El Makawy et al. (2022) discovered that mice administered TPM exhibited increased levels of IL-6 and tumor necrosis factor-alpha (TNF-α), indicating systemic inflammation and hepatorenal damage. In a colitis model, Varzandeh et al. (2024) found that TPM dramatically increased IL-6 levels, leading to elevated oxidative stress and tissue damage. The combined data indicate that although TPM is therapeutically advantageous in specific situations, it may provoke detrimental proinflammatory consequences via its interaction with oxidative stress pathways. GA has continuously demonstrated its ability to alleviate inflammation by decreasing IL-6 and other proinflammatory mediator levels. Nouri et al. (2021) established that GA decreased IL-6 and TNF-α levels in paraquat-induced kidney damage, highlighting its nephroprotective and anti-inflammatory properties. Moreover, Gholamine et al. (2021) also noted reduced IL-6 levels in rats exhibiting sodium arsenite-induced toxicity were administered GA, thereby further corroborating its anti-inflammatory characteristics. These findings underscore GA’s capacity to mitigate oxidative stress and inflammatory pathways, making it a potential therapeutic agent for systemic inflammation. The molecular mechanisms by which GA exerts its anti-inflammatory effects are thoroughly characterized. It obstructs the activation of the nuclear factor kappa-light-chain-enhancer of activated B cells pathway, a principal regulator of inflammatory reactions, thus diminishing the expression of pro-inflammatory cytokines, including IL-6, TNF-α, and IL-1β. Moreover, GA decreased IL-6 levels, consistent with research by Ayazoglu Demir et al. (2023), which illustrated GA’s anti-inflammatory properties across many organs. NGAL is secreted following acute kidney injury, indicating tubular damage. Cystatin C, a protein that is readily filtered by the glomeruli, exhibits elevated levels in response to diminished renal function. eGFR is a computation derived from serum creatinine or Cystatin C levels to measure renal filtration capacity (Ahmadzadeh et al., 2022). Increased NGAL levels noted in G2 (TPM-treated group) indicate early renal tubular damage, consistent with Ahmadzadeh et al. (2022), who identified NGAL as a highly sensitive biomarker for acute kidney injury. NGAL is essential for identifying renal impairment due to its rapid increase after kidney damage. The current study indicates that elevated NGAL levels in G2 imply considerable renal stress caused by TPM, supporting the findings of El Makawy et al. (2022), which recognized the nephrotoxic potential of TPM. In the GA-treated groups (G5 and G6), NGAL levels were markedly decreased, demonstrating GA’s renoprotective properties. Cystatin C, a sensitive biomarker of GFR, was significantly higher in G2, indicating compromised renal function. The decrease in Cystatin C levels noted in the GA-treated groups (G5, G6) indicates enhanced renal filtration and less glomerular injury. Moreover, Gholamine et al. (2021) highlighted GA’s capacity to avert renal injury by inhibiting oxidative and inflammatory mediators that increase Cystatin C levels. Enhancements in eGFR in GA-treated animals (G5) underscore the functional restoration of renal filtration capacity. In G2, reduced eGFR indicates impaired kidney function resulting from TPM-induced nephrotoxicity, corroborating the findings of Kamp-Jensen et al. (2024) about TPM’s detrimental effects on renal physiology. In contrast, the improved eGFR in G5 highlights GA’s preventive function in preserving renal filtration. GA diminished these biomarkers, indicating its protective benefits, as documented by Ajibade et al. (2016) and Kotb et al. (2021). Furthermore, the enhancement in eGFR values corroborates GA’s function in reinstating glomerular filtration, in accordance with Yousuf and Vellaichamy (2015). Increased MDA levels in G2 indicate heightened lipid peroxidation, a characteristic of oxidative stress. Steele et al. (2024) recognized MDA as a reliable biomarker of oxidative damage caused by cellular membrane breakdown. The substantial decrease in MDA levels in G6 (GA-treated group) signifies GA’s powerful antioxidant capabilities, which might alleviate oxidative damage by neutralizing free radicals. These findings corroborate those of Asci et al. (2017), which emphasized GA’s function in reducing MDA levels in methotrexate-induced nephrotoxicity by neutralizing reactive oxygen species and stabilizing cellular membranes. CAT is an essential antioxidant enzyme that catalyzes the decomposition of H2O2 into water and oxygen, thus preventing oxidative damage. Decreased CAT activity in G2 indicates compromised antioxidant defenses resulting from TPM-induced oxidative stress. SOD is a crucial enzyme defense mechanism against oxidative stress, facilitating the dismutation of superoxide radicals into hydrogen peroxide. Decreased SOD activity in G2 indicates oxidative damage and compromised cellular antioxidant defenses. The significant enhancement of SOD activity in G6 underscores GA’s capacity to rejuvenate intrinsic antioxidant mechanisms. TPM-induced oxidative stress was shown by elevated MDA levels and diminished CAT and SOD activity, corroborating the findings of Sheweita et al. (2018). GA markedly reinstated antioxidant enzyme activity, as evidenced by Hashemzaei et al. (2020), which underscored GA’s effectiveness in mitigating oxidative damage in renal tissues. Caspase-3 is essential for the execution of apoptosis via the cleavage of vital cellular proteins. Elevated caspase-3 expression in G2 indicates augmented apoptotic activity, a characteristic of TPM-induced kidney injury. Caspase-8 functions as an initiating caspase in the extrinsic apoptotic pathway. Increased G2 levels indicate activation of death receptor–mediated apoptosis resulting from TPM intake. Arab et al. (2023) emphasized the analogous upregulation of cadmium-induced tissue injury, associating it with heightened oxidative stress and mitochondrial impairment. GA therapy decreased caspase-8 expression in G5, highlighting its capacity to safeguard cellular integrity by regulating apoptotic signaling pathways. Caspase-9 is a crucial starter of the intrinsic apoptotic pathway, initiated by the release of mitochondrial cytochrome c. Elevated caspase-9 levels in G2 indicate mitochondrial malfunction and oxidative stress, which is consistent with the findings of El Makawy et al. (2022), who reported that TPM-induced hepatorenal injury was associated with intrinsic apoptosis. GA therapy in G5 significantly decreased caspase-9 expression, highlighting its capacity to maintain mitochondrial membranes and inhibit cytochrome c release. The GA-treated groups (G5) exhibited enhanced renal histology characterized by decreased tubular necrosis and inflammation. This finding is consistent with the findings of Azab et al. (2017), which indicated that GA mitigates nephropathy by reestablishing redox equilibrium and stabilizing renal cell membranes. Immunohistochemical examination revealed that TPM induces upregulation of caspase-3 and IL-6 in renal tissues, corroborating the findings of Apaydın et al. (2023). GA and Lasix alleviated these alterations, diminishing caspase expression and reinstating tissue architecture. Histopathological observations of tubular necrosis and fibrosis in TPM-treated rats, as documented by Olayinka et al. (2015) and Elmorsi et al. (2023), were mitigated by GA. This study emphasized the preventive role of GA in mitigating TPM-induced renal damage, as evidenced by the enhancement of biochemical, histological, and immunohistological markers. Additional research is necessary to investigate its medicinal potential. ConclusionThe results of this study demonstrated the beneficial anti-inflammatory and antioxidant effects of GA and Lasix in mitigating kidney damage induced by TPM administration, indicating their potential utility in reducing renal injury. AcknowledgmentsNone. FundingThe current manuscript did not receive any funding aid or grants from any organizations. Conflict of interestThe authors would like to disclose the absence of any conflict of interest. Authors’ contributionsAll authors contributed equally. Data availabilityThe data in the current manuscript are available upon reasonable request. ReferencesAhmadzadeh, M., Esmaeilzadeh, Z., Khezri, M.R., Jafari, A. and Ghasemnejad-Berenji, M. 2022. The promising effect of topiramate on random-pattern skin flap survival in rats. Aesthetic Plast. Surg. 46, 2548–2555. Ajibade, T.O., Oyagbemi, A.A., Omobowale, T.O., Asenuga, E.R., Afolabi, J.M. and Adedapo, A.A. 2016. Mitigation of diazinon-induced cardiovascular and renal dysfunction by gallic acid. Interdiscip. Toxicol. 9, 66–77. Alejolowo, O., Elias, A., Eseagwu, S., Charles, N. and Osemwegie, O. 2024. Gallic acid modulates oxido-inflammatory response in acrylamide-induced hepato-renal toxicity. Toxicol. Rep. 23, e02024. Apaydın, F.G., Kalender, S., Baş, H. and Kalender, Y. 2023. Protective role of gallic acid against fenitrothion-induced hepatotoxicity and nephrotoxicity via oxidative stress, histopathological and biochemical alterations. Res. Square. 2023, 30–38. Arab, H.H., Eid, A.H., Yahia, R., Alsufyani, S.E., Ashour, A.M., El-Sheikh, A.A.K., Darwish, H.W., Saad, M.A., Al-Shorbagy, M.Y. and Masoud, M.A. 2023. Targeting autophagy, apoptosis, and SIRT1/Nrf2 axis with topiramate underlies its neuroprotective effect against cadmium-evoked cognitive deficits in rats. Pharmaceuticals (Basel). 16(9), 1214. Asci, H., Ozmen, O., Ellidag, H.Y., Aydin, B., Bas, E. and Yilmaz, N. 2017. The impact of gallic acid on the methotrexate-induced kidney damage in rats. J. Food Drug Anal. 25, 890–897. Ashrafizadeh, M., Zarrabi, A., Mirzaei, S., Hashemi, F., Samarghandian, S., Zabolian, A., Hushmandi, K., Ang, H.L., Sethi, G., Kumar, A.P., Ahn, K.S., Nabavi, N., Khan, H., Makvandi, P. and Varma, R.S. 2021. Gallic acid for cancer therapy: molecular mechanisms and boosting efficacy by nanoscopical delivery. Food Chem. Toxicol. 157, 112576. Ayazoglu Demir, E., Mentese, A., Livaoglu, A., Turkmen Alemdar, N. and Demir, S. 2023. Ameliorative effect of gallic acid on cisplatin-induced ovarian toxicity in rats. Drug Chem. Toxicol. 46, 97–103. Azab, A., Albasha, M. and Elsayed, A. 2017. Prevention of nephropathy by some natural sources of antioxidants. Yangtze Med. 1, 1–25. Borowicz-Reutt, K.K. 2022. Effects of antiarrhythmic drugs on antiepileptic drug action”A critical review of experimental findings. Int. J. Mol. Sci. 23(5), 2321–3272. Bouasla, A., Barour, C., Bouasla, I. and Messarah, M.J.P.C.J. 2021. Beneficial effects of Punica granatum L. juice and gallic acid against kidney oxidative damage caused by sodium fluoride. Pharm. Chem. J. 55, 920–928. Cohen, G., Dembiec, D. and Marcus, J. 1970. Measurement of catalase activity in tissue extracts. Anal. Biochem. 34, 30–38. Collins, T.J. 2018. ImageJ for microscopy. Biotechniques. 43(1 Suppl):25–30. Damba, J.J., Bodenstein, K., Lavin, P., Drury, J., Sekhon, H., Renoux, C., Trinh, E., Rej, S. and Greenway, K.T. 2022. Psychotropic drugs and adverse kidney effects: a systematic review of the past decade of research. CNS Drug. 36, 1049–1077. Dilken, O., Ince, C., Kapucu, A., Heeman, P.M. and Ergin, B. 2023. Furosemide exacerbated the impairment of renal function, oxygenation, and medullary damage in a rat model of renal ischemia/reperfusion-induced AKI. Intensive Care Med. Exp. 11, 25. Dinkelacker, V., Valenti, M.P. and Hirsch, E. 2022. Anti-convulsant agents: topiramate. In Neuropsychopharmacotherapy. Eds., Riederer, P., Laux, G., Nagatsu, T., Le, W. and Riederer, C. Cham, Switzerland: Springer International Publishing, pp: 3633–3647. Doğan, D., Meydan, İ. and Kömüroğlu, A.U. 2022. Protective effect of silymarin and gallic acid against cisplatin-induced nephrotoxicity and hepatotoxicity. Int. J. Clin. Pract. 2022, 6541026. El Makawy, A.I., Mabrouk, D.M., Ibrahim, F.M. and Ahmed, K.A. 2022. Genotoxic, biochemical and histopathological studies to assess the topiramate hepatorenal toxicity in mice. Drug Chem. Toxicol. 45, 103–112. Elghouizi, A., Al-Waili, N., Elmenyiy, N., Elfetri, S., Aboulghazi, A., Al-Waili, A. and Lyoussi, B. 2022. Protective effect of bee pollen in acute kidney injury, proteinuria, and crystalluria induced by ethylene glycol ingestion in rats. Sci. Rep. 12, 8351. Elmileegy, I.M.H., Waly, H.S.A., Alghriany, A.A.I., Abou Khalil, N.S., Mahmoud, S.M.M. and Negm, E.A. 2023. Gallic acid rescues uranyl acetate-induced hepatic dysfunction in rats by its antioxidant and cytoprotective potentials. BMC Complement. Med. Ther. 23, 423. Elmorsi, R.M., Kabel, A.M., El Saadany, A.A. and Abou El-Seoud, S.H. 2023. The protective effects of topiramate and spirulina against doxorubicin-induced cardiotoxicity in rats. Hum. Exp. Toxicol. 42, 9603271231198624. Eslamifar, Z., Moridnia, A., Sabbagh, S., Ghaffaripour, R., Jafaripour, L. and Behzadifard, M. 2021. Ameliorative effects of gallic acid on cisplatin-induced nephrotoxicity in rat: variations of biochemistry, histopathology, and gene expression. Biomed. Res. Int. 2021, 2195238. Friedman, R.B. and Young, D.S. 1989. Effects of disease on clinical laboratory tests. New York, NY: Columbia University Press. Gholamine, B., Houshmand, G., Hosseinzadeh, A., Kalantar, M., Mehrzadi, S. and Goudarzi, M. 2021. Gallic acid ameliorates sodium arsenite-induced renal and hepatic toxicity in rats. Drug Chem. Toxicol. 44, 341–352. Hadidi, M., Liããn-Atero, R., Tarahi, M., Christodoulou, M.C. and Aghababaei, F. 2024. The potential health benefits of gallic acid: therapeutic and food applications. Nutrients. 13, 1001. Hashemzaei, M., Tabrizian, K., Alizadeh, Z., Pasandideh, S., Rezaee, R., Mamoulakis, C., Tsatsakis, A., Skaperda, Z., Kouretas, D. and Shahraki, J. 2020. Resveratrol, curcumin and gallic acid attenuate glyoxal-induced damage to rat renal cells. Toxicol. Rep. 7, 1571–1577. Jammal, M.P., Araújo da Silva, A., Martins Filho, A., de Castro Cão, E., Adad, S.J., Murta, E. F.C. and Nomelini, R.S. 2015. Immunohistochemical staining of tumor necrosis factor-α and interleukin-10 in benign and malignant ovarian neoplasms. Oncol. Lett. 9, 979–983. Jia, G., Wang, X., Lv, H., Nonyane, M.S.C., Hou, H., Ma, L., Shan, P. and Wu, X. 2021. The efficacy and safety of antiepileptics in the prophylaxis of pediatric migraine: the meta-analysis of randomized controlled trials. Transl. Pediatr. 10, 1779–1791. Kamp-Jensen, C., Donslund, L.N., Styrishave, B., Jensen, R.H. and Westgate, C.S.J. 2024. Exposure to topiramate and acetazolamide causes endocrine disrupting effects in female rats during estrus. Toxicol. Appl. Pharmacol. 486, 116919. Kheira, H.S., El-Sayed, S.A.E.-S., Elsayed, G.R. and Rizk, M.A. 2019. Dietary flaxseed oil inhibits kidney NF-kappa B activation and pro-inflammatory cytokine expression in cisplatin-treated rats. Comp. Clin. Pathol. 28, 349–357. Kotb, E.S., Serag, W.M., Elshaarawy, R.F., Hafez, H.S. and Elkhayat, Z. 2021. The protective role of gallic acid in cisplatin nephrotoxicity. Front. Sci. Res. Technol. 2, 48–52. Liparoti, G., Burchiani, B., Mencaroni, E., Tripodi, D., Di Cara, G. and Verrotti, A. 2022. Individualizing doses of antiepileptic drugs. Expert Opin. Drug Metab. Toxicol. 18, 219–233. Mahmoud, S.H., Zhou, X.Y. and Ahmed, S.N. 2020. Managing the patient with epilepsy and renal impairment. Seizure. 76, 143–152. Ms, S., Desai, S., Shethi, J. and Shah, H. 2022. Gallic acid: a review on its spectrum of pharmacological activities. Int. J. Pharm. Biol. Sci. 12(1), 2321–3272. Nishikimi, M., Rao, N.A. and Yagi, K. 1972. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854. Nouri, A., Heibati, F. and Heidarian, E. 2021. Gallic acid exerts anti-inflammatory, anti-oxidative stress, and nephroprotective effects against paraquat-induced renal injury in male rats. Naunyn Schmiedebergs Arch. Pharmacol. 394, 1–9. Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. Olayinka, E.T., Ore, A., Ola, O.S. and Adeyemo, O.A. 2015. Ameliorative effect of gallic acid on cyclophosphamide-induced oxidative injury and hepatic dysfunction in rats. Med. Sci. Basel 3, 78–92. Pelzman, D.L., Kazi, E., Jackman, S.V. and Semins, M.J. 2022. Urinary metabolic disturbances during topiramate use and their reversibility following drug cessation. Urology. 165, 139–143. Şahin, S.H., Küçük, O. and Tütüncüler, B. 2024. The relationship between anti-seizures medications and metabolic acidosis in craniotomy operations: is topiramate or zonisamide the cause of metabolic acidosis? BMC Anesthesiol. 24, 296. Sheweita, S.A., Almasmari, A.A. and El-Banna, S.G. 2018. Tramadol-induced hepato- and nephrotoxicity in rats: role of curcumin and gallic acid as antioxidants. PLoS One 13, e0202110. Steele, J.W., Krishnan, V. and Finnell, R.H. 2024. Mechanisms of neurodevelopmental toxicity of topiramate. Crit. Rev. Toxicol. 54, 465–475. Tiffany, T., Jansen, J., Burtis, C., Overton, J. and Scott, C. 1972. Enzymatic kinetic rate and end-point analyses of substrate, by use of a GeMSAEC fast analyzer. Clin. Chem. 18, 829–840. Varzandeh, R., Khezri, M.R., Esmaeilzadeh, Z., Jafari, A. and Ghasemnejad-Berenji, M. 2024. Protective effects of topiramate on acetic acid-induced colitis in rats through the inhibition of oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 397, 1141–1149. Wagner, C.A., Unwin, R., Lopez-Garcia, S.C., Kleta, R., Bockenhauer, D. and Walsh, S. 2023. The pathophysiology of distal renal tubular acidosis. Nat. Rev. Nephrol. 19, 384–400. Waly, H., El-Arab, R., Saleh, S., Saleh, M. and Al-Salahy, M. 2022. The protective effect of gallic acid on tartrazine-induced renotoxicity: Redox potential and morphological study. Toxicol. Rep. 1, 191–213. Yousuf, M.J. and Vellaichamy, E. 2015. Protective activity of gallic acid against glyoxal-induced renal fibrosis in experimental rats. Toxicol. Rep. 2, 1246–1254. | ||
| How to Cite this Article |
| Pubmed Style Ramadan R, El-sherbini E, Elsayed G, El-adl M. The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male rats. Open Vet. J.. 2025; 15(4): 1823-1835. doi:10.5455/OVJ.2025.v15.i4.35 Web Style Ramadan R, El-sherbini E, Elsayed G, El-adl M. The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male rats. https://www.openveterinaryjournal.com/?mno=249146 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.35 AMA (American Medical Association) Style Ramadan R, El-sherbini E, Elsayed G, El-adl M. The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male rats. Open Vet. J.. 2025; 15(4): 1823-1835. doi:10.5455/OVJ.2025.v15.i4.35 Vancouver/ICMJE Style Ramadan R, El-sherbini E, Elsayed G, El-adl M. The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male rats. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1823-1835. doi:10.5455/OVJ.2025.v15.i4.35 Harvard Style Ramadan, R., El-sherbini, . E., Elsayed, . G. & El-adl, . M. (2025) The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male rats. Open Vet. J., 15 (4), 1823-1835. doi:10.5455/OVJ.2025.v15.i4.35 Turabian Style Ramadan, Rana, El-said El-sherbini, Gehad Elsayed, and Mohamed El-adl. 2025. The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male rats. Open Veterinary Journal, 15 (4), 1823-1835. doi:10.5455/OVJ.2025.v15.i4.35 Chicago Style Ramadan, Rana, El-said El-sherbini, Gehad Elsayed, and Mohamed El-adl. "The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male rats." Open Veterinary Journal 15 (2025), 1823-1835. doi:10.5455/OVJ.2025.v15.i4.35 MLA (The Modern Language Association) Style Ramadan, Rana, El-said El-sherbini, Gehad Elsayed, and Mohamed El-adl. "The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male rats." Open Veterinary Journal 15.4 (2025), 1823-1835. Print. doi:10.5455/OVJ.2025.v15.i4.35 APA (American Psychological Association) Style Ramadan, R., El-sherbini, . E., Elsayed, . G. & El-adl, . M. (2025) The ameliorative effect of gallic acid and/or Lasix on topiramate-induced renal impairment in male rats. Open Veterinary Journal, 15 (4), 1823-1835. doi:10.5455/OVJ.2025.v15.i4.35 |