| Research Article | ||
Open Vet. J.. 2025; 15(8): 3862-3870 Open Veterinary Journal, (2025), Vol. 15(8): 3862-3870 Research Article Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, IndonesiaIrfan Alias Kendek1, Aswin Rafif Khairullah2, Mustofa Helmi Effendi3, Freshinta Jellia Wibisono4, Wiwiek Tyasningsih5*, Emmanuel Nnabuike Ugbo6, Budiastuti Budiastuti7, Nurhusien Yimer Degu8, Ikechukwu Benjamin Moses6, Riza Zainuddin Ahmad2, Sheila Marty Yanestria4, Dea Anita Ariani Kurniasih9, Ricadonna Raissa10 and Saifur Rehman111Master Program in Veterinary Science and Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Wijaya Kusuma Surabaya, Surabaya, Indonesia 5Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 6Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 7Study Program of Pharmacy Science, Faculty of Health Science, Universitas Muhammadiyah Surabaya, Surabaya, Indonesia 8Department of Veterinary Sciences, School of Medicine, IMU University, Kuala Lumpur, Malaysia 9Research Center for Public Health and Nutrition, National Research and Innovation Agency (BRIN), Bogor, Indonesia 10Department of Pharmacology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 11Department of Pathobiology, Faculty of Veterinary and Animal Sciences, Gomal University, Dera Ismail Khan, Pakistan *Corresponding Author:: Wiwiek Tyasningsih. Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: wiwiek-t [at] fkh.unair.ac.id Submitted: 23/03/2025 Revised: 15/06/2025 Accepted: 02/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
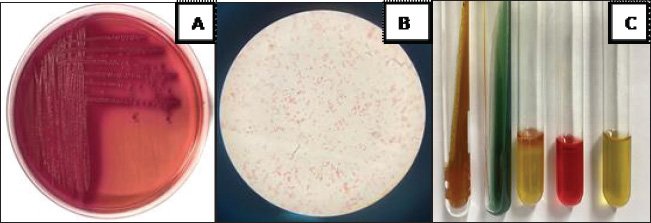
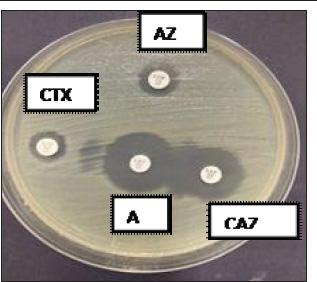
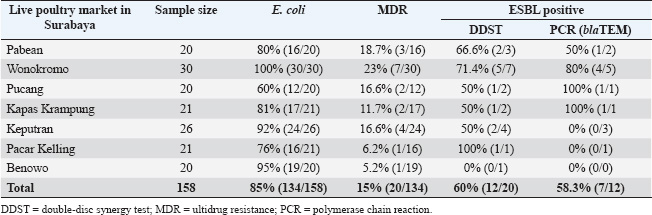
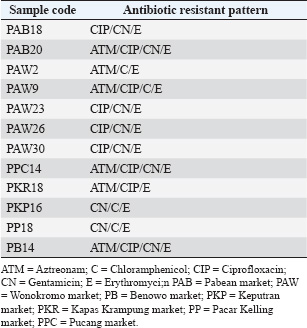
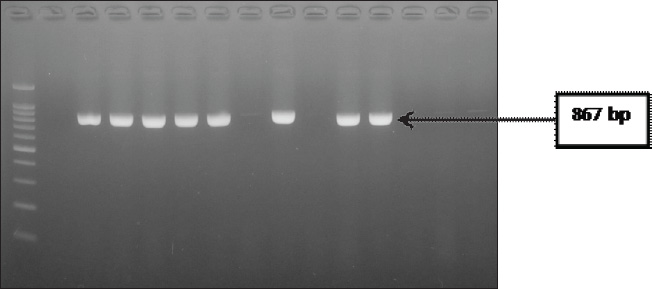
ABSTRACTBackground: Escherichia coli bacteria are a normal flora in the digestive tract of animals and humans. However, some E. coli isolates are pathogenic, causing disease in humans and animals. Escherichia coli is one of the extended-spectrum β-lactamase (ESBL)-producing bacteria responsible for increasing antibiotic resistance. ESBL is a β-lactamase enzyme that can hydrolyze penicillin, first-, second-, and third-generation cephalosporins, and aztreonam. Aim: This study aimed to identify ESBL-producing E. coli bacteria in duck cloacal swab samples taken from seven live poultry markets in Surabaya. Methods: A total of 158 duck cloacal swab samples were obtained, isolated, and identified with 85% (134/158) positive for E. coli bacteria using Mac Conkey Agar media, Gram staining, and then continued with biochemical tests as follows: Triple Sugar Iron Agar, Methyl red, Sulfide Indole Motility, Simmons Citrate Agar, and Voges–Proskauer. Results: ESBL detection using a double-disc synergy test (DDST) showed that 60% (12/20) of patients were positive for ESBL. Confirmation of the polymerase chain reaction test confirmed that 58.3% (7/12) of samples were positive for the blaTEM gene, with different percentages of positivity in each market. Conclusion: This study provides an important contribution to understanding E. coli as one of the ESBL-producing bacteria in live poultry markets, as well as the importance of monitoring and controlling the incidence of antibiotic resistance in food safety of animal origin. Keywords: Ducks, E. coli, blaTEM, ESBL, Public health. IntroductionAntibiotic resistance is a serious threat to public health worldwide. Exposure to antibiotics of various classes can cause cross-resistance, and antibiotic resistance genes can spread among bacteria through horizontal gene transfer (Khairullah et al., 2019; Yanestria et al., 2022). The use of antibiotics as prophylaxis, anabolic, and metaphylaxis causes antibiotic resistance. Mączyńska et al. (2023) reported that a nationwide assessment of antibiotic resistance revealed that the prevalence of multidrug resistance (MDR) with markers of Escherichia coli and Klebsiella pneumoniae that produce extended-spectrum β-lactamase (ESBL) varied between 50% and 82%. The source of resistance of pathogenic E. coli strains can be the contaminated environment, food, and water (Pradika et al., 2019; Liu et al., 2024). Escherichia coli is a Gram-negative bacterium that is included in the normal flora of the poultry digestive tract, but E. coli can turn into pathogenic bacteria (Putri et al., 2023). Escherichia coli can cause the spread of antibiotic resistance to various classes of broad-spectrum antibiotics, such as gentamicin, aztreonam, chloramphenicol, erythromycin, and ciprofloxacin to various classes of antibiotics (Effendi et al., 2021a). The development of MDR, in which bacteria become resistant to three or more different classes of antibiotics, is triggered by E. coli bacteria that consistently exhibit resistance. The ESBL enzyme is produced by E. coli bacteria as one of their resistance strategies (Putra et al., 2020). ESBL-producing E. coli is one of the health threats and as an indicator of the epidemiological survey of antibiotic resistance with the One Health approach (Gay et al., 2023). Exposure to ESBL-infected individuals, eating tainted meat, excrement in the environment, and human-to-animal transmission are some of the ways that ESBL-producing E. coli bacteria can spread (Miltgen et al. 2022; Widodo et al., 2023). This resistance is due to the transfer of plasmids containing the ESBL enzyme, which can hydrolyze antibiotics of the third-generation cephalosporins, penicillin, and monobactam (aztreonam) group (Husna et al., 2023). The ESBL-producing bacteria have three main genes, including the blaTEM, sulfhydryl variable (SHV), and blaCTX-M genes (Gundran et al., 2019). blaTEM genes are ESBLs that can be encoded by plasmids through transfer. The worldwide spread of blaTEM continues to grow. These genes have been detected in the environment, animals, and humans, and approaches are needed to control the problem (Badr et al., 2022). Ducks may harbor harmful bacteria that can infect humans and cause zoonotic illnesses (Na et al., 2019). The incidence of blaTEM genes in ESBL-producing E. coli bacteria in ducks from Thailand was 36.6% (Tansawai et al., 2019); 4.26% of wild ducks in Germany (Dreyer et al., 2022); 4.1% of ducks in Korea (Na et al., 2019); and one in 10 isolates of E. coli contained blaTEM genes in the Surabaya live market (Prayudi et al., 2023). Duck is one of the most frequently consumed food products in Surabaya, Indonesia. The increasing consumption of duck meat in Indonesia is a food safety concern. However, information on duck food safety is still limited in the world, including Indonesia. In addition, antibiotic resistance has increased in poultry. Therefore, this study is expected to provide information on E. coli antibiotic resistance and determine the molecular characteristics of ESBL-producing E. coli bacteria. Materials and MethodsEthical approvalThe Research Ethics Commission of the Faculty of Veterinary Medicine at Wijaya Kusuma University in Surabaya, Indonesia, granted approval for the study of animal ethics (ethics number: 139-KKE-2023). Isolation and identification of E. coli strainsSamples were taken from seven live poultry markets in Surabaya, East Java, Indonesia: Pabean, Wonokromo, Pucang, Kapas Krampung, Keputran, Pacar Kelling, and Benowo markets. A total of 158 duck cloacal swab samples were taken in this study using a sterile cotton swab (Onemed, Indonesia). Cloacal swabs were inserted into sterile tubes containing Buffer Peptone Water media (HiMedia, India). and brought using a thermobox at 4°C to the laboratory for further analysis (Yanestria et al., 2022). Samples were isolated with MacConkey Agar media (HIMEDIA MH081) and then incubated at 37°C for 24 hours. Escherichia coli bacteria were identified morphologically with a Gram stain (Kendek et al., 2024). This was followed by physiological biochemical tests using Simmons Citrate Agar (SCA) (HiMedia M099), Triple Sugar Iron Agar (TSIA) media (HIMEDIA M021), and IMVIC media, such as Methyl red (MR) (Merck; 105712), Sulfide Indole Motility (SIM) (HiMedia M181), and Voges–Proskauer (VP) (Merck; 105712) (Effendi et al., 2021b; Kendek et al., 2024). MDR testThe resistance test was performed using the Kirby–Bauer method, one colony of E. coli bacteria from MacConkey Agar (MCA) media was taken, mixed into a sterile physiological NaCl solution containing 8 ml in a reaction tube, and adjusted to the McFarland 0.5 standard (1.5 x 108 CFU/ml). Then, cultured using a sterile cotton swab on Mueller Hinton Agar (MHA) media, and then disks were placed on MHA media using antibiotics in this study in the form of Aztreonam (30 µg), Chloramphenicol (30 µg), Gentamicin (10 µg), Ciprofloxacin (5 µg), and Erythromycin (5 µg). After incubation for 24 hours at 37°C, after 24 hours, the diameter of the inhibition zone was measured using a caliper in mm according to the Clinical Laboratory Standards Institute standard (CLSI, 2020; Putra et al., 2020). Escherichia coli is classified as MDR if it is resistant to at least ≥3 classes of antibiotics tested. Confirmation of ESBL using double-disc synergy testDouble-disk synergy test (DDST) was performed on Mueller–Hinton Agar medium (Merck, 105437) with antibiotic disks of ceftazidime (CAZ 30 µg), cefotaxime (CTX 30 µg), amoxycillin-clavulanic (AMC 30 µg), and azithromycin (AZM 30 µg) placed on the confirmation medium in parallel. Escherichia coli that are confirmed positive for DDST are isolates that show a pattern of resistance or decreased sensitivity to one or more of the four antibiotic discs (Wibisono et al., 2021). Characteristics of the blaTEM gene using polymerase chain reactionBacteria ESBL-positive E. coli colonies were then confirmed by molecular polymerase chain reaction (PCR) test to identify the presence of the blaTEM gene. DNA extraction was performed using a QIAamp® DNA kit (QIAGEN, Germany). blaTEM forward primer: TAAAATTCTTGAAGACGAAA and reverse primer: GACAGTTACCAATGCTTAATC with a length of 867 bp using the gene. Conditions for PCR denaturation were as follows: 94°C at 30 seconds, annealing for 52°C at 30 seconds, extension for 72°C at 30 seconds, and extended extension for 72°C at 5 minutes. This reaction was run for 30 rounds and amplified using PCR. Next, a 2% agarose gel was used for electrophoresis to visualize the amplicon (Ferreira et al., 2011). ResultsBased on the results of research conducted on duck cloaca swabs, it shows that of the 158 samples, 85% (134/158) were positively identified with E. coli and the negative ones were 15% (24/158). The macroscopic morphology of E. coli bacteria on MCA media shows the results of pink colonies, small round, separate, and irregular (Fig. 1A). Further Gram staining was performed to determine the morphology of E. coli bacterial cells with a short rod shape (cocobasil) in red (Fig. 1B). Physiological identification of E. coli bacteria using media such as TSIA, SCA, SIM, MR, and VP (Fig. 1C). Positive results on TSIA media are characterized by positive (acid/acid) both at the base (butt) and slope (slant); there are gas and negative H2S. SCA test on E. coli bacteria is negative, which is indicated by the absence of green color changes in the media because E. coli bacteria do not produce citrate as a carbon source. The SIM media test shows positive results. A positive indole and positive motility test is characterized by the spread of bacteria in the puncture area and a negative sulfide. MR test results show a red color change after adding 0.5% MR reagent. VP test results of E. coli bacteria on VP are negative. According to the DDST findings of ESBL-producing E. coli bacteria derived from duck cloacal swabs at the Surabaya city live market, 60% of the isolates tested positive (12/20) (Fig. 2). The DDST test results are characterized by an inhibition zone between the two disks, indicating a positive ESBL. The results of positive ESBL-producing bacteria showed that ESBL-positive bacteria were found with an inhibition zone of more than 5 mm between the diameter of the cephalosporin disc and the combination of the cephalosporin-clavulanate disc (CLSI, 2020). Based on the results of testing the incidence rate of E. coli ESBL in MDR isolates (Table 1), the highest incidence came from Wonokromo market at 23% (7/30), Pabean market at 18.7% (3/16), Pucang market at 16.6% (2/12), Keputran market at 16.6% (4/24), Kapas Krampung market at 11.7% (2/17), Pacar Kelling market at 6.2% (1/16), and Benowo market at 5.2% (1/19). Escherichia coli bacteria that have MDR properties and E. coli as ESBL producers amounted to 60%% (12/20). blaTEM gene confirmation showed a positive result of 58.3% (7/12) using the PCR test. Phenotypic patterns of MDR of ESBL-producing E. coli isolates from duck cloak swabs in Surabaya’s live market showed that the incidence of ESBL as shown in Table 2. The E. coli isolates with the highest to lowest resistance pattern were CIP/CN/E with four samples (4/12; 33%), followed by ATM/CIP/CN/E with three samples (3/12; 25%), CN/C/E with (2/12; 16.6%), ATM/CIP/C/E with one sample (1/12; 8.3%), ATM/C/E with one sample (1/12; 8.3%), and ATM/CIP/E with one sample (1/12; 8.3%). The results of the confirmation test of the ESBL-producing blaTEM gene from E. coli bacteria showed that 7 of the 12 E. coli bacterial isolates produced ESBL derived from the DDST test, so that a positive band was seen at 867 bp, resulting in (7/12) 58.3% of E. coli isolates that had blaTEM virulence encoding genes in this study (Fig. 3).
Fig. 1. Isolation results of Escherichia coli bacteria on MCA (A), Gram staining (B), and Biochemical test (C).
Fig. 2. DDST findings indicate synergy between antibiotics. DiscussionEscherichia coli bacteria are a family of Enterobacteriaceae that are normal flora and pathogens in the poultry world (Wibisono et al., 2021). Traditional methods of selling ducks in the live market result in a lack of biosecurity and hygienic practices by market employees (Prayudi et al., 2023). Escherichia coli is normally found in the digestive tract and then excreted through the cloaca of ducks, both pathogenic and non-pathogenic (Kendek et al., 2024). After the duck is infected with E. coli bacteria in the digestive system, it has a high level of resistance to several antibiotics (Kissinga et al., 2018; Na et al., 2019). Isolation of E. coli bacteria using MCA media characterized by pink, small, round, separate, and irregular colonies (Wibisono et al., 2022; Bayoumi et al., 2023). Gram staining is performed to determine the morphology of E. coli bacterial cells characterized by a short rod shape (cocobasil) and red (Prayudi et al., 2023; Putri et al., 2023). Biochemical test results are characterized by acid/acid at both the base (butt) and slope (slant), which is characterized by positive gas and negative H2S due to the ability of E. coli to ferment lactose, glucose, and sucrose (Mazumder et al., 2023). The results of the SCA test are negative, which is indicated by no color change because E. coli bacteria do not produce citrate as a carbon source (Prayudi et al., 2023). The SIM test shows a positive indole result if a red ring is formed after adding Kovacs reagent and motile looks like the spread of bacteria in the puncture area (Kendek et al., 2024). Positive results for the MR test after adding 1% MR reagent show a red color change, while if negative, it remains yellow, the MR test on E. coli bacteria is positive (+). Positive VP test results are characterized by a change in color to brownish red, while if the results are negative (−) no color change occurs (Putri et al., 2023; Kendek et al., 2024). According to the isolation and identification study findings, 85% (134/158) of the positive samples included E. coli bacteria extracted from duck cloacal swabs from live poultry markets in Surabaya. These results may differ due to various isolation methods, sanitary and hygienic practices, other livestock and live poultry market management practices, and geographical location (Chowdhury et al., 2020). This result compares to 12/29 (41%) in Zimbabwe (Dube and Mbanga, 2018); China 15,6% (Li et al., 2023); in Mesir 40/120 (16,0%) (Darwish et al., 2015); 32% (32/100) of samples from cloacal swabs of ducks in Surabaya traditional markets (Prayudi et al., 2023), but lower than the previous study in Tanzania 91% duck cloacal swab (Kissinga et al., 2018). Antibiotic and MDR in E. coli bacteria can be intensified by the poultry industry’s careless use of antibiotics (Yanestria et al., 2022). Based on the results of research conducted on E. coli bacteria that have MDR properties of 15% (20/134). The results of this research are lower than in Blitar 85.63% (Wibisono et al., 2020); in China 100% (44/44) (Yassin et al., 2017); in Tanzania 38.5% (Kissinga et al., 2018). ESBL-producing bacteria, E. coli, are known to be found in food-producing animals. These bacteria can be transmitted from animals to humans and may result in zoonotic illnesses (Dreyer et al., 2022; Widodo et al., 2022). MDR has the ability of bacteria to transfer genetically (Khairullah et al., 2022), which carries resistance properties from one bacterium to another through genetic mutation, horizontally in the form of conjugation, transduction, and transformation (Yunita et al., 2020), thus causing the incidence of MDR to become more severe (Wibisono et al., 2020; Effendi et al., 2021b). The results of testing ESBL-producing E. coli bacteria showed a result of 60% (12/20). This result was lower than that in Sadat city (72.3%) (Bayoumi et al., 2023), but lower than the study in Pasuruan (9.14%) (Yanestria et al., 2022); in Thailand (43.3%) (Ueda et al., 2015); in Vietnam (42.6%) (Rahman et al., 2021); Pakistan (14%) (Rodroo et al., 2021); Washington of (11%), and in Spanyol (20.7%) (Shah et al., 2020; Martínez-álvarez et al., 2022), while in the country of Nigeria (4.6%) (Aworh et al., 2021). The creation of β-lactamase enzymes is a process by which resistance to β-lactam antibiotics develops, particularly in Gram-negative bacteria (Hussain et al., 2021). This enzyme can break down the β-lactam ring, rendering the antibiotic useless (Tooke et al., 2019). DDST uses several antibiotics, including cefotaxime (CTX-M), ceftazidime (CAZ), and Amoxicillin-Clavulanic acid (AMC) (Guo et al., 2019). The results showed a form of synergy with the inhibition of β-lactam antibiotics disks as an ESBL technique (Prayudi et al., 2023). Transmission can occur through a number of pathways, including tainted meat ingestion, contact with patients or people infected with ESBL-producing bacteria, and an environment polluted with excrement harboring ESBL-producing E. coli (Kendek et al., 2024). Table 1. Incidence rate of ESBL Escherichia coli in MDR isolates.
Table 2. Phenotypic pattern of MDR of ESBL-producing Escherichia coli isolates.
According to research by Yanestria et al. (2022), information related to the incidence of ESBL-producing E. coli in Indonesian poultry farms is still limited. The Temoniera gene (blaTEM), SHV gene, and Cefotaxime (blaCTX-M) are the main genes of E. coli that produce ESBL (Mgaya et al., 2021). Escherichia coli that can produce ESBLs can spread in the farm environment through poultry feces and food product contamination. In addition, poultry in the market may spread E. coli that can produce ESBL. blaTEM-type genes can survive better in the environment because they have plasmids as growth factors. Antibiotics used in agriculture, aquaculture, and waste disposal systems are another cause of resistance transmission (Li et al., 2023). PCR testing using the blaTEM gene showed results with a percentage of 58.3% (7/12) positive samples from seven live poultry markets in Surabaya. The blaTEM genotype test confirmation of each market has a different percentage, including the Pabean market by 50% (1/2), Wonokromo market by 80% (4/5), Pucang and Kapas Krampung markets by 100% (1/1), Keputran market 0% (0/3), Pacar Kelling 0% (0/1), and Benowo market by 0% (0/0). The negative results from the Keputran, Pacar Kelling, and Benowo markets could be due to the presence of other β-lactamase-producing genes, such as blaCTX and SHV (Prayudi et al., 2023). This result is lower than the 58.9% (33/56) in Africa (Badr et al., 2022); in Blitar of 70% (Effendi et al., 2021b); in Bangladesh by 90.48% (Islam et al., 2022); but lower than in Pakistan by 45.5% (Ilyas et al., 2021); in Surabaya by 32% (32/100) (Prayudi et al., 2023). Resistance genes can spread through live poultry markets. This agrees with the research of Effendi et al. (2021a) which shows that ESBL-producing E. coli bacteria are very dangerous if contaminated in food of animal origin. In addition, ESBL-producing E. coli is at risk of spreading resistance genes from humans to animals or vice versa, posing a potential danger to public health worldwide (Islam et al., 2023). The discovery of the blaTEM gene in poultry samples from several live poultry markets in Surabaya, Indonesia, is a matter of concern and requires immediate action to prevent the development of antibiotic resistance. The presence of E. coli that produces ESBL enzymes must be understood and treated as a threat to public health (Faridah et al., 2023). Additionally, this could be a new MDR issue that spreads and puts public health at risk (Khairullah et al., 2023). Multidrug-resistant bacteria can drive poultry meat production through nearby pollutant sources in poultry waste. A clean environment can be maintained to prevent the widespread spread of contamination, and this is especially important in areas close to live poultry markets. Future research should look into other risk factors for the spread of MDR E. coli, such as the usage of antibiotics and the general management of chicken farms. More comprehensive wastewater treatment techniques must be developed immediately in order to prevent the transmission and increase the prevalence of MDR E. coli, particularly in live poultry markets where excellent hygiene must be ensured. The government should take the necessary steps and raise public awareness of the value of sanitation and hygiene in order to stop a significant rise in the incidence of ESBL E. coli (Yanestria et al., 2022).
Fig. 3. PCR result confirmation test of the ESBL-producing blaTEM gene was positive in the 867-bp band. ConclusionIn conclusion, sampling was conducted in seven live poultry markets in Surabaya with 158 duck cloacal swab samples, resulting in 58.3% of multidrug-resistant E. coli, and found ESBL-producing E. coli (7/12) isolates that have genes encoding blaTEM virulence in this study, so these results are categorized as having a high incidence rate. Limited awareness among farmers and low public concern regarding the safety, sanitation, and biosecurity of food products of animal origin, as well as the need for appropriate use of antibiotics in poultry and veterinary supervision. AcknowledgmentsThe authors thank Universitas Airlangga. Author’s contributionsIAK, ARK, and MHE: Conceived, designed, and coordinated the study. FJW, WT, and BB: Designed data collection tools, supervised the field sample and data collection, and performed laboratory work and data entry. ENU, IBM, and NYD: Validation, supervision, and formal analysis. RZA and DAAK: Contributed reagents, materials, and analysis tools. RR, SR, and SMY: Carried out the statistical analysis and interpretation and participated in the preparation of the manuscript. All authors have read, reviewed, and approved the final version of the manuscript. Conflict of interestThe authors declare no conflict of interest. FundingThis study was partly supported by The International Research Consortium, Lembaga Penelitian dan Pengabdian Masyarakat, Universitas Airlangga, Surabaya, Indonesia in the year 2024 with grant number: 171/UN3.LPPM/PT.01.03/2024. Data availabilityAll data are available in the revised manuscript. ReferencesAworh, M.K., Kwaga, J.K.P., Hendriksen, R.S., Okolocha, E.C. and Thakur, S. 2021. Genetic relatedness of multidrug resistant Escherichia coli isolated from humans, chickens and poultry environments. Antimicrob. Resist. Infect. Control 10(1), 1–13. Badr, H., Reda, R.M., Hagag, N.M., Kamel, E., Elnomrosy, S.M., Mansour, A.I., Shahein, M.A., Ali, S.F. and Ali, H.R. 2022. Multidrug-resistant and genetic characterization of extended-spectrum beta-lactamase-producing E. coli recovered from chickens and humans in Egypt. Animals (Basel) 12(3), 346. Bayoumi, A., Zidan, S., Sakr, M.A., ElMashtouly, A. and Hadad, G. 2023. Prevalence of extended spectrum Β-lactamase (ESBL) producing Escherichia coli and molecular characterization of ESBL, carbapenemases, and blacmy2 genes in broilers and humans at Menoufia Governorate, Egypt. J. Curr. Vet. Res. 5(2), 179–195. Chowdhury, S., Azziz-Baumgartner, E., Kile, J.C., Hoque, M.A., Rahman, M.Z., Hossain, M.E., Ghosh, P.K., Ahmed, S.S.U., Kennedy, E.D., Sturm-Ramirez, K. and Gurley, E.S. 2020. Association of biosecurity and hygiene practices with environmental contamination with influenza A viruses in live bird markets, Bangladesh. Emerg. Infect. Dis. 26(9), 2087–2096. Clinical and Laboratory Standards Institute (CLSI). 2020. Performance standards for antimicrobial susceptibility testing, 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. Darwish, W.S., Eldin, W.F.S. and Eldesoky, K.I. 2015. Prevalence, molecular characterization and antibiotic susceptibility of Escherichia Coli isolated from duck meat and giblets. J. Food Saf. 35(3), 410–415. Dreyer, S., Globig, A., Bachmann, L., Schütz, A.K., Schaufler, K. and Homeier-Bachmann, T. 2022. Longitudinal study on extended-spectrum beta-lactamase-E. coli in sentinel mallard ducks in an important baltic stop-over site for migratory ducks in Germany. Microorganisms 10(10), 1968. Dube, N. and Mbanga, J. 2018. Molecular characterization and antibiotic resistance patterns of avian fecal Escherichia coli from turkeys, geese, and ducks. Vet. World 11(6), 859–867. Effendi, M.H., Tyasningsih, W., Yurianti, Y.A., Rahmahani, J., Harijani, N. and Plumeriastuti, H. 2021a. Presence of multidrug resistance (MDR) and extended-spectrum beta-lactamase (ESBL) of Escherichia coli isolated from cloacal swab of broilers in several wet markets in Surabaya, Indonesia. Biodiversitas 22(1), 304–310. Effendi, M.H., Wibisono, F.J., Witaningrum, A.M. and Permatasari, D.A. 2021b. Identification of BlaTEM and BlaSHV genes of extended spectrum beta lactamase (ESBL) producing Escherichia coli from broilers chicken in Blitar, Indonesia. Syst. Rev. Pharm. 12(1), 976–981. Faridah, H.D., Wibisono, F.M., Wibisono, F.J., Nisa, N., Fatimah, F., Effendi, M.H., Ugbo, E.N., Khairullah, A.R., Kurniawan, S.C. and Silaen, O.S.M. 2023. Prevalence of the blaCTX-M and blaTEM genes among extended-spectrum beta lactamase-producing Escherichia coli isolated from broiler chickens in Indonesia. J. Vet. Res. 67(2), 179–186. Ferreira, C.M., Ferreira, W.A., Almeida, N.C., Naveca, F.G. and Barbosa, M.D. 2011. Extended-spectrum beta-lactamase-producing bacteria isolated from hematologic patients in Manaus, State of Amazonas, Brazil. Braz. J. Microbiol. 42(3), 1076–1084. Gay, N., Rabenandrasana, M.A.N., Panandiniaina, H.P., Rakotoninidrina, M.F., Ramahatafandry, I.T., Enouf, V., Roger, F., Collard, J.M., Cardinale, E., Rieux, A. and Loire, E. 2023. One Health compartment analysis of ESBL-producing Escherichia coli reveals multiple transmission events in a rural area of Madagascar. J. Antimicrob. Chemother. 78(8), 1848–1858. Gundran, R.S., Cardenio, P.A., Villanueva, M.A., Sison, F.B., Benigno, C.C., Kreausukon, K., Pichpol, D. and Punyapornwithaya, V. 2019. Prevalence and distribution of blaCTX-M, blaSHV, blaTEM genes in extended- spectrum β- lactamase- producing E. coli isolates from broiler farms in the Philippines. BMC Vet. Res. 15(1), 227. Guo, S., Tay, M.Y.F., Aung, K.T., Seow, K.L.G., Ng, L.C., Purbojati, R.W., Drautz-Moses, D.I., Schuster, S.C. and Schlundt, J. 2019. Phenotypic and genotypic characterization of antimicrobial resistant Escherichia coli isolated from ready-to-eat food in Singapore using disk diffusion, broth microdilution and whole genome sequencing methods. Food Control 99(1), 89–97. Husna, A., Rahman, M.M., Badruzzaman, A.T.M., Sikder, M.H., Islam, M.R., Rahman, M.T., Alam, J. and Ashour, H.M. 2023. Extended-spectrum β-lactamases (ESBL): challenges and opportunities. Biomedicines 11(11), 2937. Hussain, H.I., Aqib, A.I., Seleem, M.N., Shabbir, M.A., Hao, H., Iqbal, Z., Kulyar, M.F., Zaheer, T. and Li, K. 2021. Genetic basis of molecular mechanisms in β-lactam resistant gram-negative bacteria. Microb. Pathog. 158(1), 105040. Ilyas, S., Rasool, M.H., Arshed, M.J., Qamar, M.U., Aslam, B., Almatroudi, A. and Khurshid, M. 2021. The Escherichia coli sequence type 131 harboring extended-spectrum beta-lactamases and carbapenemases genes from poultry birds. Infect. Drug Resist. 14(1), 805–813. Islam, M.S., Rahman, A.M.M.T., Hassan, J. and Rahman, M.T. 2023. Extended-spectrum beta-lactamase in Escherichia coli isolated from humans, animals, and environments in Bangladesh: a One Health perspective systematic review and meta-analysis. One Health 16(1), 100526. Islam, M.S., Sobur, M.A., Rahman, S., Ballah, F.M., Ievy, S., Siddique, M.P., Rahman, M., Kafi, M.A. and Rahman, M.T. 2022. Detection of blaTEM, blaCTX-M, blaCMY, and blaSHV genes among extended-spectrum beta-lactamase-producing Escherichia coli isolated from migratory birds travelling to Bangladesh. Microb. Ecol. 83(4), 942–950. Kendek, I.A., Putri, M.F.R., Wibisono, F.J., Effendi, M.H., Tyasningsih, W., Ugbo, E.N. and Agumah, N.B. 2024. Molecular detection of hlyF gene on multidrug resistance of avian pathogenic Escherichia coli isolated from ducks on wet markets of Surabaya, Indonesia. Biodiversitas 25(3), 1246–1253. Khairullah, A.R., Kurniawan, S.C., Effendi, M.H., Sudjarwo, S.A., Ramandinianto, S.C., Widodo, A., Riwu, K.H.P., Silaen, O.S.M. and Rehman, S. 2023. A review of new emerging livestock-associated methicillin-resistant Staphylococcus aureus from pig farms. Vet. World 16(1), 46–58. Khairullah, A.R., Raharjo, D., Rahmahani, J., Suwarno, Tyasningsih, W. and Harijani, N. 2019. Antibiotics resistant at Staphylococcus aureus and Streptococcus sp isolated from bovine mastitis in Karangploso, East Java, Indonesia. Indian J. Forensic Med. Toxicol. 13(4), 439–444. Khairullah, A.R., Rehman, S., Sudjarwo, S.A., Effendi, M.H., Ramandinianto, S.C., Gololodo, M.A., Widodo, A., Riwu, K.H.P. and Kurniawati, D.A. 2022. Detection of mecA gene and methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk and risk factors from farms in Probolinggo, Indonesia. F1000Res. 11(1), 722. Kissinga, H.D., Mwombeki, F., Said, K., Katakweba, A.A.S., Nonga, H.E. and Muhairwa, A.P. 2018. Antibiotic susceptibilities of indicator bacteria Escherichia coli and Enterococci spp. isolated from ducks in Morogoro Municipality, Tanzania. BMC Res. Notes 11(1), 87. Li, G., Li, X., Hu, J., Pan, Y., Ma, Z., Zhang, L., Xiong, W., Zeng, D. and Zeng, Z. 2023. Molecular epidemiology and transmission of rmtB-positive Escherichia coli among ducks and environment. Poult. Sci. 102(5), 102579. Liu, C., Sun, S., Sun, Y., Li, X., Gu, W., Luo, Y., Wang, N. and Wang, Q. 2024. Antibiotic resistance of Escherichia coli isolated from food and clinical environment in China from 2001 to 2020. Sci. Total Environ. 939(1), 173498. Mączyńska, B., Frej-Mądrzak, M., Sarowska, J., Woronowicz, K., Choroszy-Król, I. and Jama-Kmiecik, A. 2023. Evolution of antibiotic resistance in Escherichia coli and Klebsiella pneumoniae clinical isolates in a multi-profile hospital over 5 years (2017-2021). J. Clin. Med. 12(6), 2414. Martínez-Álvarez, S., Sanz, S., Olarte, C., Hidalgo-Sanz, R., Carvalho, I., Fernández-Fernández, R., Campaña-Burguet, A., Latorre-Fernández, J., Zarazaga, M. and Torres, C. 2022. Antimicrobial resistance in Escherichia coli from the broiler farm environment, with detection of SHV-12-producing isolates. Antibiotics (Basel) 11(4), 444. Mazumder, R., Hussain, A., Rahman, M.M., Phelan, J.E., Campino, S., Abdullah, A., Clark, T.G. and Mondal, D. 2023. Genomic and functional portrait of multidrug-resistant, hydrogen sulfide (H2S)-producing variants of Escherichia coli. Front. Microbiol. 14(1), 1206757. Mgaya, F.X., Matee, M.I., Muhairwa, A.P. and Hoza, A.S. 2021. Occurrence of multidrug resistant Escherichia coli in raw meat and cloaca swabs in poultry processed in slaughter slabs in Dar es Salaam, Tanzania. Antibiotics (Basel) 10(4), 343. Miltgen, G., Martak, D., Valot, B., Kamus, L., Garrigos, T., Verchere, G., Gbaguidi-Haore, H., Cimon, C.B., Ramiandrisoa, M., Picot, S., Lignereux, A., Masson, G., Jaffar-Bandjee, M.C., Belmonte, O., Cardinale, E., Hocquet, D., Mavingui, P. and Bertrand, X. 2022. One Health compartmental analysis of ESBL-producing Escherichia coli on Reunion Island reveals partitioning between humans and livestock. J. Antimicrob. Chemother. 77(5), 1254–1262. Na, S.H., Moon, D.C., Choi, M.J., Oh, S.J., Jung, D.Y., Sung, E.J., Kang, H.Y., Hyun, B.H. and Lim, S.K. 2019. Antimicrobial resistance and molecular characterization of extended-spectrum β-lactamase-producing Escherichia coli isolated from ducks in South Korea. Foodborne Pathog. Dis. 16(12), 799–806. Pradika, A.Y., Chusniati, S., Purnama, M.T.E., Effendi, M.H., Yudhana, A. and Wibawati, P.A. 2019. Total test of Escherichia coli on fresh cow milk at dairy farmer cooperative (KPSP) Karyo Ngremboko Purwoharjo Banyuwangi. J. Med. Vet. 2(1), 1–6. Prayudi, S.K.A., Effendi, M.H., Lukiswanto, B.S., Az Zah-Ra, R.L., Benjamin, M.I., Kurniawan, S.C., Khairullah, A.R., Silaen, O.S.M., Lisnanti, E.F., Baihaqi, Z.A., Widodo, A. and Riwu, K.H.P. 2023. Detection of genes on Escherichia coli producing extended spectrum β-lactamase isolated from the small intestine of ducks in traditional markets Surabaya City, Indonesia. J. Adv. Vet. Res. 13(8), 1600–1608. Putra, A.R.S., Effendi, M.H. and Kurniawan, F. 2020. Investigation of extended spectrum beta-lactamase (ESBL) producing Escherichia coli by vitek-2 on dairy cows in Surabaya, Indonesia. Biochem. Cell. Arch. 20(2), 6773–6777. Putri, M.F.R., Kendek, I.A., Wibisono, F.J., Effendi, M.H., Rahardjo, D., Tyasningsih, W. and Ugbo, E.N. 2023. Molecular detection of iron gene on multidrug resistant avian fecal Escherichia coli isolated from broiler on traditional markets, Surabaya, Indonesia. Biodiversitas 24(12), 6454–6460. Rahman, S.U, Muhammad, N., Ali, T., Saddique, U., Ahmad, S., Shafiq, M. and Han, B. 2021. Genotypic characterization of multidrug resistant Escherichia coli isolates reveals co-existence of ESBL- and carbapenemase-encoding genes linked to ISCR1. Vet. Ital. 57(4), 275–285. Rodroo, J., Intanon, M., Kreausukon, K., Kongkaew, A., Bender, J. and Awaiwanont, N. 2021. Occurance of extended-spectrum beta-bactamase producing Escherichia coli in broiler farm workers and the farm environment in Chiang Mai-Lumphun, Thiland. Vet. Integr. Sci. 19(1), 23–35. Shah, D.H., Board, M.M., Crespo, R., Guard, J., Paul, N.C. and Faux, C. 2020. The occurrence of Salmonella, extended-spectrum β-lactamase producing Escherichia coli and carbapenem resistant non-fermenting Gram-negative bacteria in a backyard poultry flock environment. Zoonoses Public Health 67(6), 742–753. Tansawai, U., Walsh, T.R. and Niumsup, P.R. 2019. Extended spectrum ß-lactamase-producing Escherichia coli among backyard poultry farms, farmers, and environments in Thailand. Poult. Sci. 98(6), 2622–2631. Tooke, C.L., Hinchliffe, P., Bragginton, E.C., Colenso, C.K., Hirvonen, V.H.A., Takebayashi, Y. and Spencer, J. 2019. β-lactamases and β-lactamase inhibitors in the 21st century. J. Mol. Biol. 431(18), 3472–3500. Ueda, S., Ngan, B.T., Huong, B.T., Hirai, I., Tuyen, le D. and Yamamoto, Y. 2015. Limited transmission of BlaCTX-M-9-type-positive Escherichia coli between humans and poultry in Vietnam. Antimicrob. Agents Chemother. 59(6), 3574–3577. Wibisono, F.J., Effendi, M.H. and Wibisono, F.M. 2022. Occurrence, antimicrobial resistance, and potential zoonosis risk of avian pathogenic Escherichia coli in Indonesia: a review. Int. J. One Health 8(2), 76–85. Wibisono, F.J., Sumiarto, B., Untari, T., Effendi, M.H., Permatasari, D.A. and Witaningrum, A.M. 2020. Prevalence and risk factor analysis of multidrug resistant Escherichia coli bacteria in commercial chicken in Blitar District. J. Trop. Anim. Vet. Sci. 10(1), 15–22. Wibisono, F.J., Sumiarto, B., Untari, T., Effendi, M.H., Permatasari, D.A. and Witaningrum, A.M. 2021. Molecular identification of CTX gene of extended spectrum betalactamases (ESBL) producing Escherichia coli on layer chicken in Blitar, Indonesia. J. Anim. Plant Sci. 31(4), 954–959. Widodo, A., Lamid, M., Effendi, M.H., Khailrullah, A.R., Kurniawan, S.C., Silaen, O.S.M., Riwu, K.H.P., Yustinasari, L.R., Afnani, D.A., Dameanti, F.N.A.E.P. and Ramandinianto, S.C. 2023. Antimicrobial resistance characteristics of multidrug resistance and extended-spectrum beta-lactamase producing Escherichia coli from several dairy farms in Probolinggo, Indonesia. Biodiversitas 24(1), 215–221. Widodo, A., Lamid, M., Effendi, M.H., Khailrullah, A.R., Riwu, K.H.P., Yustinasari, L.R., Kurniawan, S.C., Ansori, A.N.M., Silaen, O.S.M. and Dameanti, F.N.A.E.P. 2022. Antibiotic sensitivity profile of multidrug-resistant (MDR) Escherichia coli isolated from dairy cow’s milk in Probolinggo, Indonesia. Biodiversitas 23(10), 4971–4976. Yanestria, S.M., Dameanti, F.N.A.E.P., Musayannah, B.G., Pratama, J.W.A., Witaningrum, A.M., Effendi, M.H. and Ugbo, E.N. 2022. Antibiotic resistance pattern of Extended-Spectrum β-Lactamase (ESBL) producing Escherichia coli isolated from broiler farm environment in Pasuruan district, Indonesia. Biodiversitas 23(9), 4460–4465. Yassin, A.K., Gong, J., Kelly, P., Lu, G., Guardabassi, L., Wei, L., Han, X., Qiu, H., Price, S., Cheng, D. and Wang, C. 2017. Antimicrobial resistance in clinical Escherichia coli isolates from poultry and livestock, China. PLoS One 12(9), e0185326. Yunita, M.N., Effendi, M.H., Rahmaniar, R.P., Arifah, S. and Yanestria, S.M. 2020. Identification of spa gene for strain typing of methicillin resistant Staphylococcus aureus (MRSA) isolated from nasal swab of dogs. Biochem. Cell. Arch. 20(1), 2999–3004. | ||
| How to Cite this Article |
| Pubmed Style Kendek IA, Khairullah AR, Effendi MH, Wibisono FJ, Tyasningsih W, Ugbo EN, Budiastuti B, Degu NY, Moses IB, Ahmad RZ, Yanestria SM, Kurniasih DAA, Raissa R, Rehman S. Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, Indonesia. Open Vet. J.. 2025; 15(8): 3862-3870. doi:10.5455/OVJ.2025.v15.i8.52 Web Style Kendek IA, Khairullah AR, Effendi MH, Wibisono FJ, Tyasningsih W, Ugbo EN, Budiastuti B, Degu NY, Moses IB, Ahmad RZ, Yanestria SM, Kurniasih DAA, Raissa R, Rehman S. Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, Indonesia. https://www.openveterinaryjournal.com/?mno=248839 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.52 AMA (American Medical Association) Style Kendek IA, Khairullah AR, Effendi MH, Wibisono FJ, Tyasningsih W, Ugbo EN, Budiastuti B, Degu NY, Moses IB, Ahmad RZ, Yanestria SM, Kurniasih DAA, Raissa R, Rehman S. Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, Indonesia. Open Vet. J.. 2025; 15(8): 3862-3870. doi:10.5455/OVJ.2025.v15.i8.52 Vancouver/ICMJE Style Kendek IA, Khairullah AR, Effendi MH, Wibisono FJ, Tyasningsih W, Ugbo EN, Budiastuti B, Degu NY, Moses IB, Ahmad RZ, Yanestria SM, Kurniasih DAA, Raissa R, Rehman S. Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, Indonesia. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3862-3870. doi:10.5455/OVJ.2025.v15.i8.52 Harvard Style Kendek, I. A., Khairullah, . A. R., Effendi, . M. H., Wibisono, . F. J., Tyasningsih, . W., Ugbo, . E. N., Budiastuti, . B., Degu, . N. Y., Moses, . I. B., Ahmad, . R. Z., Yanestria, . S. M., Kurniasih, . D. A. A., Raissa, . R. & Rehman, . S. (2025) Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, Indonesia. Open Vet. J., 15 (8), 3862-3870. doi:10.5455/OVJ.2025.v15.i8.52 Turabian Style Kendek, Irfan Alias, Aswin Rafif Khairullah, Mustofa Helmi Effendi, Freshinta Jellia Wibisono, Wiwiek Tyasningsih, Emmanuel Nnabuike Ugbo, Budiastuti Budiastuti, Nurhusien Yimer Degu, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Sheila Marty Yanestria, Dea Anita Ariani Kurniasih, Ricadonna Raissa, and Saifur Rehman. 2025. Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, Indonesia. Open Veterinary Journal, 15 (8), 3862-3870. doi:10.5455/OVJ.2025.v15.i8.52 Chicago Style Kendek, Irfan Alias, Aswin Rafif Khairullah, Mustofa Helmi Effendi, Freshinta Jellia Wibisono, Wiwiek Tyasningsih, Emmanuel Nnabuike Ugbo, Budiastuti Budiastuti, Nurhusien Yimer Degu, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Sheila Marty Yanestria, Dea Anita Ariani Kurniasih, Ricadonna Raissa, and Saifur Rehman. "Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, Indonesia." Open Veterinary Journal 15 (2025), 3862-3870. doi:10.5455/OVJ.2025.v15.i8.52 MLA (The Modern Language Association) Style Kendek, Irfan Alias, Aswin Rafif Khairullah, Mustofa Helmi Effendi, Freshinta Jellia Wibisono, Wiwiek Tyasningsih, Emmanuel Nnabuike Ugbo, Budiastuti Budiastuti, Nurhusien Yimer Degu, Ikechukwu Benjamin Moses, Riza Zainuddin Ahmad, Sheila Marty Yanestria, Dea Anita Ariani Kurniasih, Ricadonna Raissa, and Saifur Rehman. "Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, Indonesia." Open Veterinary Journal 15.8 (2025), 3862-3870. Print. doi:10.5455/OVJ.2025.v15.i8.52 APA (American Psychological Association) Style Kendek, I. A., Khairullah, . A. R., Effendi, . M. H., Wibisono, . F. J., Tyasningsih, . W., Ugbo, . E. N., Budiastuti, . B., Degu, . N. Y., Moses, . I. B., Ahmad, . R. Z., Yanestria, . S. M., Kurniasih, . D. A. A., Raissa, . R. & Rehman, . S. (2025) Detection of the blaTEM gene on multidrug-resistant Escherichia coli producing extended spectrum β-lactamase from ducks in live poultry markets in Surabaya, Indonesia. Open Veterinary Journal, 15 (8), 3862-3870. doi:10.5455/OVJ.2025.v15.i8.52 |