| Review Article | ||
Open Vet. J.. 2025; 15(8): 3448-3458 Open Veterinary Journal, (2025), Vol. 15(8): 3448-3458 Review Article Organic acids supplementation in poultry nutrition: A reviewVasko Gerzilov1, Pavlina Hristakieva2*1Department of Animal Science, Faculty of Agronomy, Agricultural University, Plovdiv, Bulgaria 2Agricultural Academy, Agricultural Institute, Stara Zagora, Bulgaria *Corresponding Author: Pavlina Hristakieva. Agricultural Academy, Agricultural Institute, Stara Zagora, Bulgaria. Email: poly_31 [at] abv.bg Submitted: 21/03/2025 Revised: 04/07/2025 Accepted: 18/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
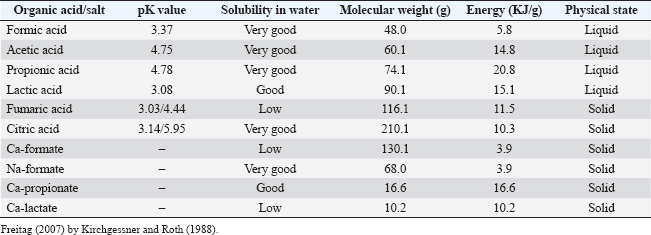
ABSTRACTThe poultry industry is continually exploring new feed additives to enhance poultry productivity and health. Since the European Union’s (EU) 2006 ban on antibiotics as growth promoters, alternative methods have become essential to support health and growth in livestock production. In response, various strategies have been developed to replace nutritional antibiotics, aiming to combat antibiotic resistance and manage diseases that would otherwise require antibiotic intervention. One promising alternative is the incorporation of organic acids (OAs) and their salts as feed additives in poultry farming. OAs improve feed palatability, reduce pH levels in the gastrointestinal tract of birds, activate digestive enzymes, and inhibit the growth of pathogenic microorganisms while preserving beneficial microflora. These acids enhance metabolism, improve feed digestibility, and accelerate growth. Additionally, OAs support overall bird health, a key factor affecting productivity traits and, consequently, the economic performance and profitability of poultry farming. This article examines the potential applications of OAs in poultry nutrition. Keywords: Antibiotic alternatives, Growth performance, Egg production, Organic acids. IntroductionIn 2006, the European Commission (EC) banned antibiotics as growth promoters in animal feed under EU Regulation No. 1831/2003, leading to declines in productivity and increased rates of certain animal diseases. This policy shift has driven researchers to investigate alternative non-therapeutic additives—such as organic acids (OAs), enzymes, probiotics, prebiotics, herbs, essential oils, and immunostimulants—to support health and productivity in poultry (Suresh et al., 2018; Lalev et al., 2020; Lalev et al., 2022a, b; Mincheva et al., 2022; Ivanova et al., 2022; Hristakieva et al., 2023; Akan et al., 2025). Among these, OAs are gaining attention as effective replacements, traditionally used as preservatives to extend shelf life in perishable foods (Coban, 2020; Braïek and Smaoui, 2021). OAs are weak acids containing a carboxylic acid group (R-COOH) and are intermediates in metabolizing carbohydrates, amino acids, and lipids. Commonly employed as antimicrobial additives in animal feed, these compounds include saturated straight-chain monocarboxylic acids and their derivatives (e.g., unsaturated, hydroxyl, phenolic, and multicarboxylic acids) and are often referred to as fatty acids, volatile fatty acids, or carboxylic acids (Cherrington et al., 1991). OAs are known for their antimicrobial efficacy against pathogenic bacteria, lowering GIT pH, thereby enhancing nutrient absorption and feed efficiency (Boling et al., 2000; Lesson et al., 2005; Kim et al., 2015). Their effectiveness as antimicrobials is primarily determined by their pKa values, which typically range between 3 and 5 (Freitag, 2007; Huyghebaert et al., 2011). Table 1 summarizes the properties of selected OAs and salts commonly used in poultry farming. OAs function as antimicrobials based on various physicochemical properties, including molecular weight, pKa, and minimum inhibitory concentration, along with factors such as the nature of the target microorganism and the buffering capacity of the feed (Dittoe et al., 2018; Coban, 2020). An acid’s pKa indicates its dissociation capacity, denoting the pH at which the acid exists equally in dissociated and undissociated forms. In the undissociated state, OAs can penetrate bacterial and fungal cell walls, altering microbial metabolism. Thus, their antimicrobial efficacy is enhanced in acidic environments like the stomach and reduced at neutral pH levels, as in the intestine. OAs with higher pKa values are typically weaker acids and more effective feed preservatives because they remain largely undissociated, effectively protecting feed from microbial spoilage. Conversely, acids with lower pKa values more readily dissociate, lowering gastric pH but having a limited antimicrobial impact in the intestine (Theobald, 2018). Table 1. Properties of some acids and salts used in poultry.
This review discusses the structure, properties, mechanisms of action, biological functions, and applications of OAs in poultry nutrition. How do the OAs work?The general chemical formula of the organic acid is R-COOH (undissociated form). In this form, they can release a proton (H+), which lowers the pH of the gut. The reduction in pH inhibits the proliferation of pathogenic bacteria, such as Escherichia coli, Salmonella spp., and Campylobacter spp., while simultaneously promoting the growth of beneficial bacteria, such as lactic acid bacteria. This pH effect is not the only effect that individual acids have, but OAs also have antibacterial activity (Theobald, 2018). The antibacterial activity of OAs increases with decreasing pH and is characterized by the reduction of pH, as well as their ability to dissociate, which is determined by the pKa value of the corresponding acid and the pH of the environment. OAs are lipid soluble in the undissociated form and can enter the microbial cell (Partanen and Mroz, 1999). In undissociated form, acidic molecules can easily penetrate the microbial cell walls of gram-negative bacteria. Inside the cell, the pH is higher than the pKa, and much of the acid dissociates and releases its hydrogen ion (H+). Upon release of hydrogen ions (H+), the microbial cell expends enormous amounts of energy that lead to cell death (Dibner and Buttin, 2002; Gümrükçüoğlu, 2022). Once inside the cell, the acid releases the proton into the more alkaline environment, causing the pH in the cell to decrease. This affects microbial metabolism by inhibiting the action of important microbial enzymes and forcing the bacterial cell to use energy to release protons, leading to an intracellular accumulation of acidic anions. This accumulation depends on the difference in pH. Generally, the antimicrobial effect of OAs increases with increasing concentrations (Lucera et al., 2012; Braïek and Smaoui, 2021) OAs exert their antimicrobial effect through the water that enters the animal’s gastrointestinal tract (GIT). The pH in the digestive tract will decrease if the ingested water becomes acidified. This has a positive effect on digestion, especially in the stomach and small intestine (small bowel). The extent of pH reduction in feed and within the GIT following the addition of OAs is influenced by both the pKa values of the specific OAs and the existing pH conditions in the GIT (Kim et al., 2005). Incorporating OAs into broiler feed results in a pH decrease across various segments of the GIT. Generally, the pH reduction is more pronounced in the upper GIT sections (crop, proventriculus, and gizzard) compared to the lower GIT regions (duodenum, jejunum, ileum, and cecum) (Thompson and Hinton, 1997). OAs are absorbed across the intestinal epithelium by passive diffusion and contribute a significant amount of energy (Table 1). OAs are absorbed across the intestinal epithelium by passive diffusion, and most OAs contribute a significant amount of energy (Table 1). They, therefore, represent an alternative energy source that can be efficiently utilized by cells through their incorporation into the Krebs cycle. For example, fumaric acid, which is a four-carbon dicarboxylic acid and an intermediate metabolite in the Krebs cycle, can contribute to cellular energy supply by participating in mitochondrial metabolism, generating a moderate amount of ATP upon complete oxidation (Ryan et al., 2022). With an energy content of approximately 1340 kJ/mol, this corresponds to about 74.3 kJ for the synthesis of 1 mole of ATP, a value comparable to the energy efficiency observed during glucose degradation (Nelson and Cox, 2021). This makes fumaric acid comparable to glucose as an energy substrate. Similar values are also observed for citric acid. In contrast, acetic and propionic acids require approximately 18% and 15% more energy, respectively, to synthesize 1 mole of ATP, making them relatively less efficient in terms of energy yield (Lobley, 2001; Gaudieri et al., 2020). Acidifiers are utilized in poultry farming through three primary methods: Feed additivesAcidifiers are incorporated into poultry feed in either solid or liquid form, inhibiting bacterial growth within the feed while simultaneously lowering the pH in the birds’ GIT. Litter treatmentAcidifiers are applied to poultry litter, targeting bacteria involved in uric acid breakdown. This process reduces ammonia release, contributing to a healthier rearing environment. Water additivesAcidifiers are introduced into drinking water to reduce the GIT pH and eliminate pathogenic bacteria, thereby promoting digestive health and supporting the bird’s immune defense against harmful microbes. Some OAs used in poultry farmingPropionic acidPropionic acid (C3H6O2) is a clear liquid with a sharp, unpleasant odor, produced through the anaerobic degradation of pyruvic acid in the cytosol. Pyruvic acid, in turn, is derived from glycolysis, where one glucose molecule is converted into two molecules of pyruvic acid. Propionic acid is commonly used as a preservative in animal feed and human food, as a growth promoter, and as a feed additive, especially for poultry and pigs, either in its direct form or as an ammonium salt. It exhibits high activity against mold and yeast but is comparatively less effective against bacteria (Zha and Cohen, 2014). Acetic acidAcetic acid (CH3COOH) is a weak acid that, despite its limited dissociation in aqueous solutions, is corrosive, and its fumes can irritate the eyes and nasal passages. In household use, its 6% and 9% solutions are known as vinegar. Acetic acid is effective against yeast and bacteria but has a limited effect on molds. Abbas et al. (2011) observed an anticoccidial effect of acetic acid, added at 0.5% concentration to drinking water, against Eimeria tenella in broiler chickens. Benzoic acidBenzoic acid (C6H5COOH) is an aromatic carboxylic acid with slight water solubility, known for its antiseptic properties. It is effective against yeast and bacteria, including putrefactive bacteria, but has a lesser effect on molds. It plays a key role in reducing the prevalence of various pathogenic bacteria, including Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica (Friedman et al., 2003). Sorbic acidSorbic acid (CH3(CH)4CO2H) is a colorless solid with limited water solubility that sublimes readily. Sorbic acid and its salts—sodium sorbate, potassium sorbate, and calcium sorbate—are commonly used as antimicrobial agents in foods and beverages to prevent mold, yeast, and fungal growth. The salts are preferred over the acid form due to their greater water solubility, though the acid form is most active against microorganisms (Lück, 1990). Sorbic acid demonstrates a broad spectrum of action, being particularly effective against yeast, mold, and bacteria, surpassing the antimicrobial efficacy of propionic acid (Razavi-Rohani and Griffiths, 1999) Formic acidFormic acid (CH2O2), the simplest carboxylic acid, is highly soluble in water and many polar organic solvents, with limited solubility in hydrocarbons. It exhibits effectiveness against yeast and bacteria but is less active against mold. While beneficial against pathogenic bacteria, formic acid may cause mucosal irritation upon inhalation in its liquid form and can irritate the skin on contact. Formates, the solid salt forms, are less corrosive. Given potential health risks, handling formic acid requires adherence to safety regulations (Dibner and Buttin, 2002; Huyghebaert et al. 2011). Citric acidCitric acid (C6H8O7), a weak organic acid, functions as a natural preservative and feed additive for poultry and swine. It plays a role in carbohydrate metabolism, and although birds can synthesize vitamin C (ascorbic acid), supplemental citric acid can be beneficial, particularly under conditions that may impair endogenous vitamin C synthesis, such as stress (Islam et al., 2008, 2010, 2012). Citric acid also exhibits antimicrobial properties, helping to preserve feed and reduce bacterial pathogens (e.g., E. coli) in the GIT, ultimately promoting growth (Eidelsburger and Kirchgessner, 1994; Deepa et al., 2011). Fumaric and succinic acidsFumaric (CHCO2H) and succinic acids (C4H6O4) are used in poultry nutrition to enhance resistance, reduce post-stress impacts, and mitigate gastrointestinal and respiratory diseases. They also serve as supplemental energy sources, enhancing appetite and supporting growth in young animals (Ding et al., 2020; He et al., 2020; Waghmare et al., 2025). Influence of dietary OAs on poultry performanceMany OAs, enzymes, phytogenic compounds, probiotics, prebiotics, and other biologically active compounds are routinely incorporated into poultry feed or drinking water to promote growth, enhance feed digestibility, and improve bird health (Gerzilov et al., 2019; Lalev et al., 2020, 2022b, 2023; Petrov et al., 2022; Hristakieva et al., 2021, 2023). OAs serve as acidifiers in poultry feed and are increasingly considered viable antibiotic alternatives for enhancing nutrient digestibility and promoting productivity (Fascina et al., 2012). They support gastric proteolysis and improve protein and amino acid digestibility (Samanta et al., 2010). Their impact on broilers and laying hens is described in the following. Broiler chickensNumerous studies have demonstrated that various OAs such as fumaric (Hernández et al., 2006; Ghazala et al., 2011), formic (Hernández et al., 2006; García et al., 2007), acetic (Hernández et al., 2006), citric (Ao et al., 2009), and ascorbic (Lohakare et al., 2005) acids enhance the digestibility of crude protein (CP), crude fiber (CF), and nitrogen-free extracts (NFE) in broilers. Dietary inclusion of OAs has been shown to promote growth, feed efficiency, nutrient utilization, and pathogen inhibition (Lückstädt and Mellor, 2011; Brzoska et al., 2013; Mustafa et al., 2021; Dittoe et al., 2018). For example, Fascina et al. (2012) found that OA mixtures in broiler feed increased productivity and slaughter performance, observing increased carcass yield and higher breast meat content in diets with OAs and phytoadditives. Adil et al. (2011) noted optimal live weights in broilers fed with 3% fumaric acid supplementation. Brzoska et al. (2013) reported growth enhancements and decreased mortality in broilers given OAs at concentrations of 0.3%–0.9%, though no significant effect was found on carcass yield. Hashemi et al. (2014) documented body weight gains when a blend of OAs (including formic, phosphoric, lactic, tartaric, citric, and malic acids) was administered at 0.15%. OAs supplementation has also been linked to improved meat quality, potentially mitigating pale, soft, and exudative (PSE) conditions in broiler meat (Sugiharto et al., 2019). Studies have shown that citric acid supplementation is associated with increased weight gain (Afsharmanesh et al., 2005; Nezhad et al., 2007) and improved feed intake (Chowdhury et al., 2009; Haque et al., 2010; Nourmohammadi et al., 2010; Salgado-Tránsito et al., 2011). This resulted in a lower FCR in diets containing citric acid compared to controls. This improvement in efficiency was associated with better nutrient absorption, a decrease in intestinal pH, and inhibition of pathogenic microflora in the digestive tract. Effects on organ development were noted by Skvortsova and Gorkovenko (2017), with citric acid supplementation, promoting heart, intestinal, gizzard, and liver development in broilers. Additionally, Skvortsova (2018) found that the differentiated inclusion of citric acid in broiler diets—where the additive concentrations are adjusted according to the age or growth phase of the birds—led to a 3.2% reduction in feed costs, accompanied by a 2.8% increase in the carcass weight and an improvement in meat quality. This approach optimizes the effects of the additive by tailoring the dosage to the specific needs and physiological characteristics of the birds at different stages of their development, thereby enhancing the efficiency and economic viability of the feeding program. Contrastingly, Kopecký et al. (2012) found no significant body weight or carcass impact from diets containing acetic and citric acids (0.25% in water); however, a reduction in total mortality was observed. Laying hensThe addition of OAs such as propionic, fumaric, sorbic, and lactic acids, along with their salts, influences both egg-laying performance and egg quality (Gama et al., 2000; Yalcin et al., 2009). Research by Yesilbag and Çolpan (2006) demonstrated that OAs supplementation in the diets of Lohmann laying hens aged 24–28 weeks significantly enhanced laying performance compared to control groups, extending the overall laying period. These findings align with other studies, concluding that supplementation of OAs positively affects egg production metrics, including shell strength, yolk and albumen indices, and shell thickness (Boling et al., 2000; Gama et al., 2000). In a study by Soltan (2008), laying hens fed a basal diet supplemented with 780 ppm OAs (ProviMax®) showed a 5.77% increase in egg production compared to the control group. Lower supplementation levels (260 and 520 ppm) did not yield statistically significant changes, a result corroborated by Rahman et al. (2008). Similarly, Kadim et al. (2008) evaluated the impact of acetic acid supplementation (200, 400, and 600 ppm) on Brown Leghorn hens, observing increases in productivity of approximately 10%, 15%, and 20%, respectively, across treatment groups relative to controls. Grashorn et al. (2013) assessed the effects of OAs supplementation (SALMO-NIL DRY® at 2 kg/ton) in the diets of 30-week-old Hisex Brown hens. Findings indicated significantly improved laying intensity and feed conversion (p < 0.05) in the OAs-supplemented group. Conversely, Kaya et al. (2015) reported that an OA mixture (60% formic acid, 20% propionic acid) did not impact feed intake, laying performance, egg weight, feed conversion, or body weight, though it did improve intestinal histomorphology, except for crypt depth. Dahiya et al. (2016) further demonstrated that adding 1.5% sodium butyrate enhanced laying rates, while a 0.5% supplementation improved egg weight but reduced laying frequency. Youssef et al. (2013a) similarly found that probiotics, prebiotics, synbiotics, or OAs supplementation in laying hen diets increased egg production, egg mass, and quality. Shalaei et al. (2014) also reported that a mixture of formic, lactic, and orthophosphoric acids notably increased the egg weight in hens aged 32 to 42 weeks. Antimicrobial activity of OAs in poultryPathogenic microbes or bacteria that proliferate in the GIT can damage the intestinal villi by inducing cell proliferation, leading to thickening of the intestinal tissue. This thickening hinders nutrient absorption, ultimately resulting in reduced growth and development in animals. OAs can penetrate the cell walls of these pathogenic microbes, disrupting normal cellular functions and causing microbial cell death. The low pH created by these acids establishes a stressful environment that contributes to cellular dysfunction and inhibits bacterial growth. Numerous studies have reported reductions in pathogenic bacteria within the GIT of birds, leading to decreased morbidity and mortality when OAs are incorporated into their diets (Kazempour and Jahanian, 2017; Fouladi et al., 2018; Thi Thuy et al., 2018). For instance, Sheikh et al. (2010) observed that the use of an OAs mixture significantly reduced gram-negative bacterial counts in the guts of broiler chickens. Aydin et al. (2010) found that adding 3% citric acid to the basal diet significantly decreased coliform content in the ileum compared to the control group (p < 0.05). The inclusion of citric acid creates an acidic environment (pH 3.5 to 4.0) in the small intestine (duodenum and jejunum), where normal pH ranges around 5.5–6.5. Thus, promoting the growth of beneficial lactobacilli while inhibiting the replication of Escherichia coli, Salmonella, and other gram-negative bacteria (Chowdhury et al., 2009). In more distal parts of the intestine, such as the cecum, the pH is more alkaline and the effect of citric acid is less pronounced. Research has shown that broiler chickens fed diets containing mixtures of OAs exhibit lower levels of pathogenic bacteria, such as coliforms and clostridia, while maintaining higher populations of beneficial bacteria, such as lactobacilli, in the ileum compared to those receiving antibiotic growth promoters (Khan and Iqbal, 2015). Lückstädt and Theobald (2009) previously reported that the addition of sodium diformate (a compound of formic acid and sodium formate) to feed reduced the presence of pathogenic bacteria (e.g., Salmonella, Campylobacter, and Escherichia coli) in broiler chickens while increasing populations of lactobacilli and bifidobacteria. Paul et al. (2007) found that the organic acid salts, ammonium formate, and calcium propionate (3 g/kg feed), also significantly reduced coliform counts in broilers compared to control groups. Influence of OAs on blood biochemical parameters in poultryYesilbag and Çolpan (2006) supplemented the basal diet of laying hens with various levels (0.5%, 1.0%, and 1.5%) of an OAs mixture. Their findings indicated that supplementation with 1% and 1.5% OAs significantly increased serum total protein and albumin concentrations, while other serum parameters, including cholesterol, high-density lipoprotein (HDL), triglycerides, total lipid concentration, and alanine aminotransferase (ALT) activity, were not significantly affected. Summarized findings from Baghban-Kanani et al. (2019) and Kamal and Ragaa (2014) also reported a significant reduction in serum low-density lipoprotein (LDL) levels in a group of birds receiving acidifiers. The beneficial role of OAs in reducing the blood lipid profile may be attributed to their ability to lower microbial intracellular pH, thereby inhibiting the action of key microbial enzymes and forcing bacterial cells to expend energy to release acidic protons, which leads to intracellular accumulation of acidic anions (Kamal and Ragaa, 2014). Soltan (2008) investigated the effects of varying concentrations of an OAs mixture (0, 260, 520, and 720 ppm) in hen diets, observing a linear increase in serum calcium concentration corresponding to the levels of organic acid. Moreover, serum total protein and albumin concentrations were significantly improved (p < 0.01) in the experimental groups compared to the control group. This enhancement is likely due to the favorable intestinal environment created by the addition of OAs, which lowers the GIT’s pH, improving protein digestibility and facilitating mineral absorption. Moreover, the elevated levels of serum protein and albumin may reflect an increased availability of circulating proteins, potentially resulting from enhanced protein synthesis and nutrient absorption. Wang et al. (2009) reported significant increases (p < 0.05) in total protein and albumin levels when the diets of 36-week-old ISA Brown hens were supplemented with phenyllactic acid. In contrast, Ozek et al. (2011) found no significant difference in serum total cholesterol levels when diets were supplemented with a mixture of herbal essential oils and OAs in laying hens. Kaya et al. (2013) investigated the effects of adding a mixture of zeolite and OAs to the diet of laying hens and observed significant reductions (p < 0.05) in serum albumin and calcium levels, with no impact on serum cholesterol, total protein, or phosphorus levels. Youssef et al. (2013a, b) reported significant improvements (p < 0.05) in plasma calcium and phosphorus concentrations in 53-week-old laying hens supplemented with sodium formate during the summer season. Nourmohammadi et al. (2011) examined the effects of citric acid and microbial phytase in broiler chickens, finding significant reductions in plasma cholesterol and phosphorus concentrations with citric acid inclusion, while plasma calcium and magnesium concentrations remained unaffected. Kamal et al. (2014) studied the effects of different types of OAs (butyric, fumaric, or lactic acid) supplementation at 3% inclusion on the performance and blood biochemistry of broiler chickens. The study reported reductions in total cholesterol and serum LDL levels in the birds fed organic acid supplements compared to the basal diet. Other possible effects of OAs in poultryPrevious studies have indicated that OAs can enhance phosphorus utilization in corn-soy broiler diets (Boling et al., 2000; Esmaeilipour et al., 2011). Adil et al. (2010) found that blood serum concentrations of calcium and phosphorus were higher in broilers fed diets supplemented with OAs compared to those in the control group. This improvement may be attributed to the formation of acid anion complexes with minerals such as calcium and phosphorus, which enhances their absorption (Li et al., 1998; Kishi et al., 1999). Some researchers have also suggested that OAs may stimulate energy metabolism by serving as energy sources for epithelial cells in the GIT (Ravindran and Kornegay, 1993; Partanen and Mroz, 1999). For instance, fumaric and citric acids act as intermediates in the tricarboxylic acid cycle, while butyric acid serves as a direct energy source for epithelial cells in the GIT (Partanen and Mroz, 1999; Pryde et al., 2002) and also as an energy source for the gut microbiota. According to Hajati (2018), there are several limitations to the use of OAs in poultry nutrition. These include feed refusal due to decreased palatability, the corrosive nature of OAs to metal equipment used in poultry feeding, the development of acid resistance in bacteria when exposed to acidic environments over prolonged periods, the potential reduction in the efficacy of OAs in the presence of other antimicrobial compounds, deterioration of cleanliness in the production environment, and the buffering capacity of feed ingredients. ConclusionDietary OAs are considered promising alternatives to antibiotic growth promoters that were previously used to enhance the growth and health of poultry. Acidifying the feed lowers the pH in the GIT, which can improve nutrient utilization and inhibit pathogenic microorganisms. The effects of OAs depend on both the type of acid used and the levels of their incorporation into the diet. A review of existing studies indicates that most OAs added to poultry diets generally improve performance and health status, although some conflicting results have been reported. Further research is necessary to elucidate the mechanisms of action, optimal dosages, and the overall impact of OAs on productivity, health, and the quality of poultry products as alternatives to antibiotic growth promoters. The potential of OAs can be further enhanced through the application of modern science and technology, particularly at the molecular, biotechnological, and nanotechnological levels, to validate and expand their beneficial uses. AcknowledgmentsThe authors thank the support of the Agricultural University-Plovdiv for paying the fee for publishing the accepted article under Grant № 17-12. Authors’ contributionsVG and PH drafted the manuscript. PH revised and edited the manuscript.VG participated in critical checking of the final manuscript. PH edited the references. Both authors have read and approved the final manuscript. Conflict of interestThe authors have no conflicts of interest to declare. Data availabilityAll data are provided in the manuscript. ReferencesAbbas, R.Z., Munawar, S.H., Manzoor, Z., Iqbal Z., Khan, M.N., Saleemi, M.K., Zia, M.A. and Yousaf, A. 2011. Anticoccidial effects of acetic acid on performance and pathogenic parameters in broiler chickens challenged with Eimeria tenella. Pesq. Vet. Bras. 31(2), 99–103; doi: 10.1590/S0100-736X2011000200001 Adil, S., Banday, T., Bhat, G.A., Mir, M.S. and Rehman, M. 2010. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010, 1–7; doi: 10.4061/2010/479485 Adil, M.S., Banday, T., Bhat, G.A., Qureshi, S.D. and Wani, S.A. 2011. Effect of supplemental organic acids on growth performance and gut microbial population of broiler chicken. Livest. Res. Rural Dev. 23(1), 1–8. Afsharmanesh, M. and Pourreza, J. 2005. Effect of calcium, citric acid, ascorbic acid, vitamin D3 on the efficacy of microbial phytase in broiler starters fed wheat-based diets on performance, bone mineralization and ileal digestibility. Int. J. Poult. Sci. 4(6), 418–424. Akan, M., Diker, K.S., Özlü, S., Çelebi, M., Sariçam, İnce S., Doğan, B. and Elibol, O. 2025. Effects of diet supplemented with a blend of protected organic acids and essential oils on growth performance and cecal microbiota composition in broilers. Turk. J. Vet. Anim. Sci. 49(1), 11–18; doi: 10.55730/1300-0128.4365 Ao, T., Cantor, A.H., Pescatore, A.J., Ford, M.J., Pierce, J.L. and Dawson, K.A. 2009. Effect of enzyme supplementation and acidification of diets on nutrient digestibility and growth performance of broiler chicks. Poult. Sci. 88, 111–117; doi: 10.3382/ps.2008-00191 Aydin, A., Pekel, A.Y., Issa, G., Demirel, G. and Patterson, P.H. 2010. Effect of dietary copper, citric acid, and microbial phytase on digesta pH and ileal and carcass microbiota of broiler chickens fed a low available phosphorus diet. J. Appl. Poult. Res. 19, 422–431; doi: 10.3382/japr.2009-00123 Baghban-Kanani, P., Hosseintabar-Ghasemabad, B., Azimi-Youvalari, S., Seidavi, A., Ragni, M., Laudadio, V. and Tufarelli, V. 2019. Effects of using Artemisia annua leaves, probiotic blend, and organic acids on performance, egg quality, blood biochemistry, and antioxidant status of laying hens. J. Poult. Sci. 56(2), 120–127; doi: 10.2141/jpsa.0180050 Braïek, O.B. and Smaoui, S. 2021. Chemistry, safety, and challenges of the use of organic acids and their derivative salts in meat preservation. J. Food Qual. 2021, 6653190; doi: 10.1155/2021/6653190 Boling, S.D., Webel, D.M., Mavromichalis, I., Parsons, C.M. and Baker, D.H. 2000. The effects of citric acid on phytate phosphorus utilization in young chicks and pigs. J. Anim. Sci. 78, 682–689; doi: 10.2527/2000.783682x Brzoska, F., Sliwinski, B. and Michalik-Rutkowska, O. 2013. Effect of dietary acidifier on growth, mortality, post-slaughter parameters and meat composition of broiler chickens. Ann. Anim. Sci. 13, 85–96; doi: 10.2478/v10220-012-0061-z Cherrington, C.A., Hinton, M., Mead, G.C. and Chopra, I. 1991. Organic acids: Chemistry, antibacterial activity and practical applications. Adv. Microb. Physiol. 32, 87–108; doi: 10.1016/S0065-2911(08)60006-5 Chowdhury, R., Islam, K.M.S., Khan, M.J., Karim, M.R., Haque, M.N., Khatun, M. and Pesti, G.M. 2009. Effect of citric acid, avilamycin, and their combination on the performance, tibia ash, and immune status of broilers. Poult. Sci. 88, 1616–1622; doi: 10.3382/ps.2009-00119 Coban, H.B. 2020. Organic acids as antimicrobial food agents: applications and microbial productions. Bioprocess Biosyst. Eng. 43, 569–591; doi: 10.1007/s00449-019-02256-w Dahiya, R., Berwal, R.S., Sihag, S., Patil, C.S. and Lalit. 2016. The effect of dietary supplementation of salts of organic acid on production performance of laying hens. Vet. World, 9(12), 1478–1484; doi: 10.14202/vetworld.2016.1478-1484 Deepa, C., Jeyanthi, G. and Pand Chandrasekaran, D. 2011. Effect of phytase and citric acid supplementation on the growth performance, phosphorus, calcium and nitrogen retention on broiler chicks fed with low level of available phosphorus. Asian J. Poult. Sci. 5, 28–34; doi: 10.3923/ajpsaj.2011.28.34 Dibner, J.J. and Buttin, P. 2002. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 11, 453–463. Ding, X., Wang,J., Bai, S., Zeng, Q., Su, Z., Xuan, Y., Zhang, K. and Liu, S. 2020. Effects of dietary supplementation with fumaric acid on growth performance, nutrient digestibility, serum biochemical parameters, and intestinal morphology of broiler chickens. Poult. Sci. 99(12), 6737–6743; doi: 10.1016/j.psj.2020.09.070 Dittoe, D.K., Ricke, S.C. and Kiess, A.S. 2018. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci. 5, 216; doi: 10.3389/fvets.2018.00216 Eidelsburger, U. and Kirchgessne, R.M. 1994. Effect of organic acids and salts in the feed on fattening performance of broilers. Arch. Geflügelk. 58, 268–277. Esmaeilipour, O., Shivazad, M., Moravej, H., Aminzadeh, S., Rezaian, M. and van Krimpen, M.M. 2011. Effects of xylanase and citric acid on the performance, nutrient retention, and characteristics of gastrointestinal tract of broilers fed low-phosphorus wheat-based diets. Poult. Sci. 90, 1975–1982; doi: 10.3382/ps.2010-01264 Fascina, V.B., Sartori, J.R., Gonzales, E., Barros De Carvalho, F., Pereira De Souza, I.M.G., Polycarpo, G.V., Stradiotti, A.C. and Pelícia, V.C. 2012. Phytogenic additives and organic acids in broiler chicken diets. Rev. Bras. Zootec. 41, 2189–219. Fazayeli-Rad, A.R., Nazarizadeh, H., Vakili, M., Afzali, N. and Nourmohammadi, R. 2014. Effect of citric acid on performance, nutrient retention and tissue biogenic amine contents in breast and thigh meat from broiler chickens. J. Eur. Poult. Sci. 78, 9; doi: 10.1399/eps.2014.56 Fouladi, P., Ebrahimnezhad, Y., Aghdam, H.S., Maheri, N. and Ahmadzadeh, A. 2018. Effects of organic acids supplement on performance, egg traits, blood serum biochemical parameters and gut microflora in female Japanese quail (Coturnix coturnix Japonica). Braz. J. Poult. Sci. 20, 133–144; doi: 10.1590/1806-9061-2016-0375 Freitag, M. 2007. Organic acids and salts promote performance and health in animal husbandry. In: Acidifiers in animal nutrition, A guide for feed preservation and acidification to promote animal performance. Nottingham: Nottingham University Press, 89. Friedman, M., Henika, P.R. and Mandrell, R.E. 2003. Antibacterial activities of phenolic benzaldehydes and benzoic acids against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes and Salmonella enterica. J. Food Prot. 66, 1811–1821. Gama, N.M.S.Q., Oliveira, M.B.C., Santin, E. and Berchieri, A. 2000. Supplementation with organic acids in diet of laying hens. Ciênc. Rural, 30, 499–502; doi: 10.1590/S0103-84782000000300022 García, V., Catalá-Gregori, P., HernáNdez, F., Megías, M.D. and Madrid, J. 2007. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J. Appl. Poult. Res. 16, 555–562; doi: 10.3382/japr.2006-00116 Gaudieri, G.E., Smith, A.L. and Thomas, R.J. 2020. The metabolic fate of short-chain fatty acids. J. Clin. Biochem. 55(4), 312–318. Gerzilov, V., Boncheva, V., Alexandrova, A., Tzvetanova, E. and Georgieva, A. 2019. Influence of immunobeta® dietary supplementation on egg production and some parameters of oxidative stress in laying hens. J. Agric. Sci. Technol. 21(5), 1117–1130. Ghazala, A.A., Atta, A.M., Elkloub, K., Mustafa, M.E.L. and Shata, R.F.H. 2011. Effect of dietary supplementation of organic acids on performance, nutrient digestibility and health of broiler chicks. Int. J. Poult. Sci. 10(3), 176–184. Grashorn, M.A., Gruzauskas, R., Dauksiene, A., Raceviciute-Stupeliene, A., Zdunczyk, Z., Juskiewicz, J., Bliznikas, S., Svirmickas, G.J. and Slausgalvis, V. 2013. Influence of organic acids supplement to the diet on functioning of the digestive system in laying hens. Arch. Geflügelk. 77, 155–159. Gümrükçüoğlu, N. 2022. Antimicrobial organic acids. Res. Rev. Health Sci. 10, 148–158. Hashemi, S.R., Zulkifli, I., Davoodi, H., Bejo, M.H. and Loh, T.C. 2014. Intestinal histomorphology changes and serum biochemistry responses of broiler chickens fed herbal plant (Euphorbia hirta) and mix of acidifier. Iran J. Appl. Anim. Sci. 4(1), 95–103. Haque, M.N., Islam, K.M.S., Akbar, M.A., Chowdhury, R., Khatun, M., Karim, M.R. and Kemppainen, B.W. 2010. Effect of dietary citric acid, flavomycin and their combination on the performance, tibia ash, and immune status of broiler. Can. J. Anim. Sci. 90, 57–63; doi: 10.4141/CJAS09048 Hajati, H. 2018. Application of organic acids in poultry nutrition. Int. J. Avian Wildl. Biol. 3(4), 324‒329; doi: 10.15406/ijawb.2018.03.00114 He, Y., Ding, X., Bai, S., Zeng, Q., Su, Z., Cheng, Y., Liu, X., Wang, J. and Zhang, K. 2020. Effects of dietary fumaric acid supplementation on growth performance, serum antioxidant status, and intestinal morphology of broilers under chronic heat stress. Poult. Sci. 99(12), 6606–6614; doi: 10.1016/j.psj.2020.09.038 Hernández, F., García, V., Madrid, J., Orengo, J. and Catalá, P. 2006. Effect of formic acid on performance, digestibility, intestinal histomorphology and plasma metabolite levels of broiler chickens. Br. Poult. Sci. 47, 50–56; doi: 10.1080/00071660500475574 Hristakieva, P., Velikov, K., Mincheva, N., Ivanova, I., Lalev, M., Atanasov, A., Belorechkov, D. and Petrova, A. 2021. The black soldier fly as an alternative to soybean meal in feeding laying hens. Poultry, 6, 14–20. Hristakieva, P., Mincheva, N., Ivanova, I., Velikov, K. and Petrova, A. 2023. Evaluation of two Black soldier fly products on hens’ performance, hatchability and health traits. In: 74th Annual Meeting of the EAAP “Climate change, biodiversity and global sustainability of animal production,” Lyon, France. p: 227. Available via https://www.wageningenacademic.com/doi/book/10.3920/978-90-8686-936-7 Huyghebaert, G., Ducatelle, R. and Van Immersel, F. 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 187(2), 182–188; doi: 10.1016/j.tvjl.2010.03.003 Islam, M.Z., Khandaker, Z.H., Chowdhury, S.D. and Islam, K.M.S. 2008. Effect of citric acid and acetic acid on the performance of broilers. J. Bangladesh. Agric. Univ. 6, 315–320; doi: 10.22004/ag.econ.208308 Islam, K.M.S., Haque, M.N., Chowdhury, R., Shahin, M.S.A. and Islam, K.N. 2010. Effect of citric acid administration through water on the performance of broiler fed commercial diet. Bangladesh. J. Prog. Sci. Technol. 8, 181–184. Islam, K.M.S., Schaeublin, H., Wenk, C., Wanner, M. and Liesegang, A. 2012. Effect of dietary citric acid on the performance and mineral metabolism of broiler. J. Anim. Physiol. Anim. Nutr. 96, 808–817. Ivanova, I., Mincheva, N., Hristakieva, P., Velikov, K., Lalev, M., Atanasov, A., Belorechkov, D. and Petrova, A. 2022. The role of exogenous enzymes in avian digestion. Poultry 5, 13–18. Józefiak, D., Kaczmarek, S., Bochenek, M. and Rutkowski, A. 2007. A note on effects of benzoic acid supplementation on the performance and microbiota populations of broiler chickens. J Anim Feed Sci, 16, 252–256; doi: 10.22358/jafs/66746/2007 Józefiak, D., Kaczmarek, S. and Rutkowski A. 2010. The effects of benzoic acid supplementation on the performance of broiler chickens. J. Anim. Physiol. Anim. Nutr. 94(1), 29–34; doi: 10.1111/j.1439-0396.2008.00875.x Kadim, I.T., Al-Marzooqi, W., Mahgoub, O., Al-Jabri, A. and Al Waheebi, S.K. 2008. Effect of acetic acid supplementation on egg quality characteristics of commercial laying hen during hot season. Int. J. Poult. Sci. 7, 1015–1021. Kamal, A.M. and Ragaa, N.M. 2014. Effect of dietary supplementation of organic acid on performance and serum biochemistry of broiler chicken. Nat. Sci. 12(2), 282–286. Kaya, H., Kaya, A., Gul, M. and Celebi, S. 2013. Effect of zeolite and organic acids mixture supplementation in layers’ diet on performance, egg quality traits and some blood parameters. J. Anim. Vet. Adv. 12(6), 782–787. http://docsdrive.com/pdfs/medwelljournals/javaa/2013/782-787.pdf Kaya, A., Kaya, H., Gül, M., Apaydin, Yildirim, B. and Timurkaan, S. 2015. Effect of different levels of organic acids in the diets of hens on laying performance, egg quality criteria, blood parameters, and intestinal histomorphology. Indian J. Anim. Res. 49(5), 645–651; doi: 10.18805/ijar.5577 Kazempour, F. and Jahanian, R. 2017. Effects of different organic acids on performance, ileal microflora, and phosphorus utilization in laying hens fed diet deficient in non-phytate phosphorus. Anim. Feed. Sci. Technol. 223, 110–118; doi: 10.1016/j.anifeedsci.2016.11.006 Khan, S.H. and Iqbal, J. 2015. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 44(1), 359–369; doi: 10.1080/09712119.2015.1079527 Kishi, M., Fukay, M., Tsukamoto, Y., Nagasawa, T., Takehana, K. and Nishizawa, N. 1999. Enhancing effect of dietary vinegar on the intestinal absorption of calcium in ovariectomized rats. Biosci. Biotechnol. Biochem. 63(5), 905–910; doi: 10.1271/bbb.63.905 Kirchgessner, M. and Roth F.X. 1988. Nutritive effects of organic acids in piglet rearing and pig fattening. Übers Tierernähr. 16, 93–108. Kim, J.W., Kim, J.H. and Kil, D.Y. 2015. Dietary organic acids for broiler chickens: a review. Rev. Colomb. Cienc. Pecu. 28, 109–123. http://rccp.udea.edu.co/index.php/ojs/article/viewFile/974/1144 Kim, Y.Y., Kil, D.Y., Oh, H.K. and Han, I.K. 2005. Acidifier as an alternative material to antibiotics in animal feed. Asian-Australas. J. Anim. Sci. 18, 1048–1060. Kopecký, J., Hrnčár, C. and Weis, J. 2012. Effect of organic acids supplement on performance of broiler chickens. Sci. Pap. Anim. Sci. Biotechnol. 45(1), 51–54. Lalev, M., Hristakieva, P. and Oblakova, M. 2020. Effect of prebiotic feed supplement on the performance and carcass yield of broilers Ross. Zhivotnovadni Nauki 57(2), 37–44. Lalev, M., Hristakieva, P., Mincheva, N., Oblakova, M. and Ivanova, I. 2022a. Insect meal as alternative protein ingredient in broiler feed. Bulg. J. Agric. Sci. 28(4), 743–751. Lalev, M., Mincheva, N., Hristakieva, P., Oblakova, M. and Ivanova, I. 2022b. Performance of laying hens fed diets supplemented with probiotics and prebiotics. J. Hygienic. Eng. Des. 39, 143–148. Lassén, T.M. 2007. Acidified raw materials and acids in fur animal feed: Acid preservation of raw materials intended for fur animal feed. Workshop Nordiske Jordbrugs forskeres Foreign (NJF). Subsection for Fur Animals, 37–65. Lesson, S., Namkung, H., Antongiovanni, M. and Lee, E.H. 2005. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 84, 1418–1422; doi: 10.1093/ps/84.9.1418 Li, D., Che, X., Wang, Y., Hong, C. and Thacker, P.A. 1998. Effect of microbial phytase, vitamin D3, and citric acid on growth performance and phosphorus, nitrogen and calcium digestibility in growing swine. Anim Feed Sci Technol, 73(1–2), 173–186; doi: 10.1016/S0377-8401(98)00124-2 Lobley, G.E. 2001. Short-chain fatty acid metabolism in ruminants. Anim. Feed Sci. Technol. 90(1–2), 15–32; doi: 10.1016/S0377-8401(01)00195-8 Lohakare, J.D., Ryu, M.H., Hahn, T.W., Lee, J.K. and Chae, B.J. 2005. Effects of supplemental ascorbic acid on the performance and immunity of commercial broilers. J. Appl. Poult. Res. 14, 10–19; doi: 10.1093/japr/14.1.10 Lucera, A., Costa, C., Conte, A. and Del Nobile, M.A. 2012. Food applications of natural antimicrobial compounds. Front. Microbiol. 3, 287–301. Lück, E. 1990. Food applications of sorbic acid and its salts. Food Addit. Contam. 7(5), 711–715; doi: 10.1080/02652039009373936 Lückstädt, C. and Theobald, P. 2009. Effect of a formic acid-sodium formate premixture on Salmonella, Campylobacter and further gut microbiota in broilers. In Proceedings of 17th European Symposium on Poultry Nutrition, p 246. Lückstädt, C. and Mellor, S. 2011. The use of organic acids in animal nutrition, with special focus on dietary potassium diformate under European and Austral-Asian conditions. Recent Adv. Anim. Nutr. Aust. 18, 123–130. Mincheva, N., Hristakieva, P., Ivanova, I., Velikov, K. and Petrova, A. 2022. Black soldier fly product-specific interactions with Lactobacillus plantarum in the diet of layers hens. J. Insects Food Feed. 8(1), S41; doi: 10.3920/JIFF2022.S1 Moghadam, A.N., Pourreza, J. and Samie, A.H. 2006. Effect of different levels of citric acid on calcium and phosphorus efficiencies in broiler chicks. Pak. J. Biol. Sci. 9(4), 1250–1256; doi: 10.3923/pjbs.2006.1250.1256 Mordakin, V.N. 2006. Economic and biological characteristics of broiler chickens of the Smena-4 cross when using ascorbic, citric and fumaric acids in diets. Ph. D. Thesis, Ryazan, p: 116. Mustafa, A., Bai, S., Zeng, Q., Ding, X., Wang, J., Xuan, Y., Su, Z. and Zhang, K. 2021. Effect of organic acids on growth performance, intestinal morphology, and immunity of broiler chickens with and without coccidial challenge. AMB Express 11, 140; doi: 10.1186/s13568-021-01299-1 Nelson, D.L. and Cox M.M. 2021. Lehninger principles of biochemistry, 8th ed.). London: W.H. Freeman and Company. Nezhad, Y.E., Shivazad, M., Nazeeradl, M. and Babak M.M.S. 2007. Influence of citric acid and microbial phytase on performance and phytate utilization in broiler chicks fed a corn-soybean meal diet. J. Fac. Vet. Med. Univ. Tehran. 61(4), 407–413. Nourmohammadi, R., Mohammed, H. and Farhangfar, H. 2010. Effect of dietary acidification on some blood parameters and weekly performance of broiler chickens. J. Anim. Vet. Adv. 9, 3092–3097. Ozek, K., Wellmann, K.T., Ertekin, B. and Tarum, B. 2011. Effect of dietary herbal essential oil mixture and organic acid preparation on laying traits, gastrointestinal tract characteristics, blood parameters and immune response of laying hens in a hot summer season. J. Anim. Vet. Adv. 20, 575–586; doi: 10.22358/jafs/66216/2011 Partanen, K.H. and Mroz Z. 1999. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 12, 117–145; doi: 10.1079/095442299108728884 Paul, S.K., Halder, G., Mondal, M.K. and Samanta, G. 2007. Effect of organic acid salt on the performance and gut health of broiler chicken. J. Poult. Sci. 44, 389–395; doi: 10.2141/jpsa.44.389 Petrov, P., Lukanov, H., Gerzilov,V., Ivanova, P., Keranova, N. and Penchev I. 2022. Effect of herbal and immunomodulatory supplements on growth performance and meat quality in broilers. J. Cent. Eur. Agric. 23(3), 513–525; doi: 10.5513/JCEA01/23.3.3611 Pinchasov, Y. and Elmaliah, S. 1995. Broiler chick responses to anorectic agents: dietary acetic and propionic acids and the blood metabolites. Ann. Nutr. Metab. 39(2), 107–116; doi: 10.1159/000177850 Pirgozliev, V., Murphy, T.C., Owens, B., George, J. and McCann, M.E.E. 2008. Fumaric and sorbic acid as additives in broiler feed. Res. Vet. Sci. 84(3), 387–394; doi: 10.1016/j.rvsc.2007.06.010 Pryde, S.E., Duncan, S.H., Hold, G.L., Stewart, C.S. and Flint, H.J. 2002. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 217, 133–139; doi: 10.1111/j.1574-6968.2002.tb11467.x Rahman, M.S., Howlider, M.A.R., Mahiuddin, M. and Rahman M.M. 2008. Effect of supplementation of organic acids on laying performance, body fatness and egg quality of hens. Bangladesh. J. Anim. Sci. 37(2), 74–81. Ravindran, V. and Kornegay, E.T. 1993. Acidification of weaner pig diets: a review. J. Sci. Food Agric. 62, 313–322; doi: 10.1002/jsfa.2740620402 Razavi-Rohani, S.M. and Griffiths M.W. 1999. Antifungal effects of sodium acetate, sodium lactate and sodium propionate on Penicillium expansum and Aspergillus niger. J. Food Saf. 19(2), 117–132; doi: 10.1111/j.1745- Ryan, D.G., Yang, M., Prag, H.A., Rodriguez Blanco, G., Nikitopoulou, E., Segarra-Mondejar, M., Powell, C.A., Young, T., Burger, N., Miljkovic, J.L., Minczuk, M., Murphy, M.P., von Kriegsheim, A. and Frezza, C. 2021. Disruption of the TCA cycle reveals an ATF4-dependent integration of redox and amino acid metabolism. eLife 10, e72593; doi:10.7554/eLife.72593 Salgado-Tránsito, L.L., Del Río-García, J.C., Arjona-Román, J.L., Moreno-Martínez, E. and Méndez-Albores, A. 2011. Effect of citric acid supplemented diets on aflatoxin degradation, growth performance, and serum parameters in broiler chickens. Arch. Med. Vet. 43, 215–222. https://www.redalyc.org/articulo.oa?id=173022397003 Samanta, S., Hardar, S. and Ghosh, T.K. 2010. Comparative efficacy of an organic acid blend and bacitracin methylene disalicylate as growth promoters in broiler chickens: effects on performance, gut histology, and small intestinal milieu. Vet. Med. Int. 645–650; doi: 10.4061/2010/645150 Shalaei, M., Hosseini, S.M. and Zergani, E. 2014. Effect of different supplements on eggshell quality, some characteristics of gastrointestinal tract and performance of laying hens. Vet. Res. Forum 5, 277–286. Sheikh, A., Tufail, B., Gulam, A.B., Masood, S.M. and Manzoor, R. 2010. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 1, 1–7; doi: 10.4061/2010/479485 Skvortsova, L.N. and Osepchuk, D.V. 2016. Meat quality of broiler chickens fed prebiotic. Collection of scientific papers of the North Caucasus Research Institute of Animal Husbandry, 1(5), 125–127. Skvortsova, L.N. 2010. Application of prebiotics for growing broiler chicks. Rep. Russ. Acad. Agric. Sci. 3, 38–40. Skvortsova, L.N. and Gorkovenko L.G. 2017. Use of acidifiers in poultry. Collection of scientific papers. Available via https://cyberleninka.ru/article/n/ispolzovanie-podkisliteley-v-ptitsevodstve Skvortsova, L.N. 2018. Increasing meat productivity and meat quality of broiler chickens when using ascorbic acid in compound feeds. Agrar. Bull. Verkhnevolzhye 2(23), 51–59. Soltan, M.A. 2008. Effect of dietary organic acid supplementation on egg production, egg quality and some blood serum parameters in laying hens. Int. J. Poult. Sci. 7(6), 613–621. Sugiharto, S., Yudiarti, T., Isroli, I., Widiastuti, E., Wahyuni, H. I., Sartono, T.A., Nurwantoro, N. and Al-Baarri, A.N. 2019. Effect of dietary supplementation of formic acid, butyric acid or their combination on carcass and meat characteristics of broiler chickens. J. Indones. Trop. Anim. Agric. 44(3), 286–294; doi: 10.14710/jitaa.44.3.286-294 Suresh, G., Das, R.K., Kaur Brar, S., Rouissi, T., Avalos Ramirez, A., Chorfi, Y. and Godbout, S. 2018. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 44, 318–335; doi: 10.1080/1040841X.2017.1373062 Theobald, P. 2018. Principles of using organic acids in animal nutrition. Available via https://www.dsm.com/anh/en/feedtalks/principles-organic-acids-animal-nutrition.html (Accessed 25 June 2019). Thi Thu, N., Nguyen Thi My Phung, L., Luu Thi Ty, Nguyen Thi Hoang Bich and Thai Viet An. 2018. Effectof organic acid products on growth performance and intestine health of Tam Hoang chicken. Can. Tho. Univ. J. Sci. 54, 17–23. Thompson, J.L. and Hinton M. 1997. Antibacterial activity of formic and propionic acids in the diet of hens on Salmonellas in the crop. Br. Poult. Sci. 38, 59–65; doi: 10.1080/00071669708417941 Yalcin, S.K., Bozdemir, M.T. and Ozbas, Z.Y. 2009. A comparative study on citric acid production kinetics of two Yarrowia lipolytica strains in two different media. Indian. J. Biotechnol. 8, 408–417. Yesilbag, D. and Çolpan, I. 2006. Effects of organic acid supplemented diets on growth performance, egg production and quality and on serum parameters in laying hens. Rev. Med. Vet. 157(5), 280–284. Youssef, A.W., El-Daly, E.F., Abd El-Azeem, N.A. and El-Monairy, M.M. 2013a. Effect of sodium formate on laying hen performance, gastrointestinal tract pH and some blood components under heat stress conditions. Asian J. Poult. Sci. 7, 17–26; doi: 10.3923/ajpsa.2013.17.26 Youssef, A.W., Hassan, H.M.A., Ali, H.M. and Mohamed, M.A. 2013b. Effect of supplementation of probiotics and organic acid on layer performance and egg quality. Asian J. Poult. Sci. 7(2), 65–74; doi: 10.3923/ajpsa.2013.65.74 Vopolskaya, E.A., Kravchenko, V.V. and Skvortsova, L.N. 2016. The importance of organic acids in metabolic processes in poultry. Collection of articles based on the materials of the 71st scientific and practical conference of students based on the results of research work for 2015 year “Scientific support of the agro-industrial complex”. Krasnodar, pp. 154–157. Wang, J.P., Yoo, J.S., Lee, J.H., Zhou, T.X., Jang, H.D., Kim, H.J. and Kim, I.H. 2009. Effects of phenyl lactic acid on production performance, egg quality parameters and blood characteristics in laying hens. Appl. Poult. Res. 18, 203–209; doi: 10.3382/japr.2008-00071 Waghmare, S., Gupta, M., Bahiram, K.B., Korde, J.P., Bhat, R., Datar, Y., Rajora, P., Kadam, M., Kaore, M.M. and Kurkure, N.V. 2025. Effects of organic acid blends on the growth performance, intestinal morphology, microbiota, and serum lipid parameters of broiler chickens. Poult. Sci. 104, 104546; doi: 10.1016/j.psj.2024.104546 Zha, C. and Cohen, A.C. 2014. Effects of anti-fungal compounds on feeding behavior and nutritional ecology of tobacco budworm and painted lady butterfly larvae. Entomol. Ornithol. Herpetol. 3, 120; doi: 10.4172/2161-0983.1000120 | ||
| How to Cite this Article |
| Pubmed Style Gerzilov V, Hristakieva P. Organic acids supplementation in poultry nutrition: A review. Open Vet. J.. 2025; 15(8): 3448-3458. doi:10.5455/OVJ.2025.v15.i8.8 Web Style Gerzilov V, Hristakieva P. Organic acids supplementation in poultry nutrition: A review. https://www.openveterinaryjournal.com/?mno=248628 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.8 AMA (American Medical Association) Style Gerzilov V, Hristakieva P. Organic acids supplementation in poultry nutrition: A review. Open Vet. J.. 2025; 15(8): 3448-3458. doi:10.5455/OVJ.2025.v15.i8.8 Vancouver/ICMJE Style Gerzilov V, Hristakieva P. Organic acids supplementation in poultry nutrition: A review. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3448-3458. doi:10.5455/OVJ.2025.v15.i8.8 Harvard Style Gerzilov, V. & Hristakieva, . P. (2025) Organic acids supplementation in poultry nutrition: A review. Open Vet. J., 15 (8), 3448-3458. doi:10.5455/OVJ.2025.v15.i8.8 Turabian Style Gerzilov, Vasko, and Pavlina Hristakieva. 2025. Organic acids supplementation in poultry nutrition: A review. Open Veterinary Journal, 15 (8), 3448-3458. doi:10.5455/OVJ.2025.v15.i8.8 Chicago Style Gerzilov, Vasko, and Pavlina Hristakieva. "Organic acids supplementation in poultry nutrition: A review." Open Veterinary Journal 15 (2025), 3448-3458. doi:10.5455/OVJ.2025.v15.i8.8 MLA (The Modern Language Association) Style Gerzilov, Vasko, and Pavlina Hristakieva. "Organic acids supplementation in poultry nutrition: A review." Open Veterinary Journal 15.8 (2025), 3448-3458. Print. doi:10.5455/OVJ.2025.v15.i8.8 APA (American Psychological Association) Style Gerzilov, V. & Hristakieva, . P. (2025) Organic acids supplementation in poultry nutrition: A review. Open Veterinary Journal, 15 (8), 3448-3458. doi:10.5455/OVJ.2025.v15.i8.8 |