| Research Article | ||
Open Vet. J.. 2025; 15(8): 3684-3692 Open Veterinary Journal, (2025), Vol. 15(8): 3684-3692 Research Article High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, IndonesiaAnjani Marisa Kartikasari1, Aditya Yudhana2*, Ryanka Edila3, Fransiska Okta Zania4, Audina Putri Geraldine4, April Hari Wardhana5 and Ulfiani Fauzia Hanafi61Department of Animal Diseases and Veterinary Public Health, Faculty of Medicine, Universitas Hasanuddin, Makassar, Indonesia 2Department of Health and Life Sciences, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia 3Doctoral Program of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Profession Program of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Research Center for Veterinary Science, National Research and Innovation Agency, Cibinong, Indonesia 6College of Veterinary Medicine, Kangwon National University, Chuncheon, Republic of Korea *Corresponding Author: Aditya Yudhana, Department of Health and Life Sciences, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia. Email: adityayudhana [at] fkh.unair.ac.id Submitted: 17/03/2025 Revised: 15/06/2025 Accepted: 03/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
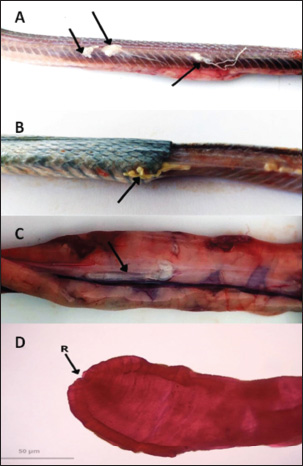
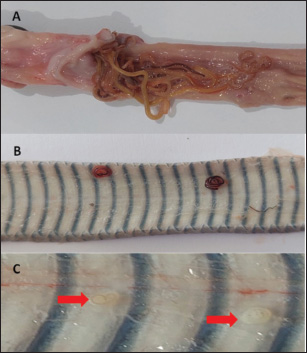
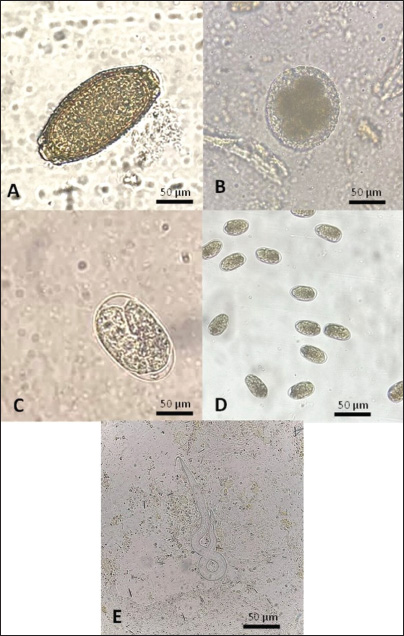
ABSTRACTBackground: Wild-caught Javanese keelback water snakes (Fowlea melanzostus) are at a significant risk of parasitic infections, including various helminths, with potential public health implications. However, comprehensive data on helminth infection prevalence and diversity in this species remain limited. Aim: This study aimed to investigate the prevalence and diversity of parasitic helminths in wild-caught Javanese keelback water snakes from Mojokerto City, Indonesia. Methods: A total of 23 snakes were collected, euthanized, and examined for parasitic helminths. The helminth stages were identified using microscopic fecal examination and carmine staining methods. Results: Helminth infections were detected in 95.65% (22/23) of the examined snakes. A total of six helminth taxa were identified, including four nematode genera (Ophidascaris spp., Rhabdias spp., Physaloptera spp., and Capillaria spp.), one nematode order (Oxyurida), and one cestode genus (Spirometra spp.). Conclusion: This study highlights the role of Javanese keelback water snake in parasitic disease transmission within ecosystems and underscores the importance of identifying parasite biodiversity in wild-caught reptiles to control illegal reptile trade and mitigate public health risks, including zoonotic potential. Keywords: Fowlea melanzostus, Helminths, Neglected disease, Parasites, Wildlife. IntroductionThe Javanese keelback water snake (Fowlea melanzostus) is a semiaquatic snake species that inhabits ponds, rivers, and reservoirs. Water snakes typically prey on frogs, rodents, fish, and small birds (Bharati, 2018). The use of snakes today extends beyond being show animals, food, medicine, and raw materials for factories but also as pets (Telnoni et al., 2016). While several reptile species sold as pets are bred in captivity, others are taken from the wild environment, generally known as wild-caught reptiles, including F. melanzostus. The maintenance of wild-caught water snakes carries the risk of disease infection and plays a role in the spread of various parasites, some of which can be zoonotic (Nardoni et al., 2008). Exotic reptiles originating from the wild are frequently infected with various pathogenic parasites, including zoonotic ones. Various parasitic helminths in snake species have been reported worldwide. In the Philippines, Oxyrhabdium leporinum snakes were reported to have a 100% prevalence of nematodes from the genus Kalicephalus (Kuzmin et al., 2013), while in Thailand, a 60% prevalence of Kalicephalus spp. infection was reported in 35 Siamese Cobra snakes (Naja kaouthia) (Vasaruchapong et al., 2017). Infections caused by cestodes have been reported, such as Bothridium pithonis infecting Python sebae snakes in Georgia (Murvanidze et al., 2013). Cestoda zoonosis from the genus Spirometra infects Pantherophis obsoletus and Coluber constrictor snakes in the United States (Kuchta et al., 2021). In Myanmar and Thailand, infections were reported in Ptyas korros snakes (67%) and Zaocys dhumnades snakes (55%) (Jongthawin et al., 2014; Liu et al., 2022). Previous helminth studies in Indonesia have reported infections, including Physaloptera sp. in Javan Spitting Cobra Snakes (Edila et al., 2023). Infections of Kalicephalus spp. and Ophidascaris spp. were reported in 13% and 33% of 30 reticulated pythons, respectively (Telnoni et al., 2016). In another case reported in Bali, Indonesia, 15 cobra snakes (Naja sputatrix) were infected with Kalicephalus spp. (20.01% prevalence) and Capillaria spp. (6.67% prevalence) (Sismami, 2014). Infections of the acanthocephalan phylum have also been reported in Naja sputatrix and Ahaetulla prasina snakes (Yudhana et al., 2023; Edila et al., 2024). Spirometra larval infections (spargana) have been reported in 68% of Ptyas mucosus snakes with an incidence rate of 56.70% of cobra snakes (Naja sputatrix) (Pranashinta et al., 2017; Yudhana et al., 2020). To the best of our knowledge, there is no further report specifically on Javanese keelback water snakes (F. melanzostus) from other regions in Indonesia, although many reptile wholesale markets still exist, particularly in East Java Province, predominately in Mojokerto City, Indonesia. Additionally, there are no certified captive breeders for Javanese keelback water snake in Indonesia, indicating that all individuals kept as exotic pets are sourced from the wild. We hypothesize that wild-caught snakes have a higher risk of parasitic infections, particularly from various helminths, including zoonotic species, due to the lack of proper health screenings and their consumption of diverse live prey that may serve as intermediate hosts in helminth life cycles (Rizal and Rahmawati, 2021). Therefore, it is necessary to strengthen public awareness, particularly among veterinarians and exotic animal lovers who keep Javanese keelback water snakes as pets and frequently have direct contact with parasitic agents. This study aims to investigate the diversity and zoonotic potential of helminth parasites in wild-caught Javanese keelback water snakes (F. melanzostus), providing a scientific reference for diagnostic efforts and preventive measures against zoonotic diseases originating from this species. Materials and MethodsStudy location and samplingThe sample size was determined based on the number of individuals available during the study period, as F. melanzostus is not commonly bred in captivity in Indonesia. All specimens were obtained opportunistically from wild-caught individuals submitted to our institution for health assessments or other research purposes. Due to ethical considerations and the limited availability of these species, we were unable to increase the sample size. Although this may constrain the generalizability of our findings, the study provided important baseline data on parasitic infections in this snake species. The snakes were collected from the wild by local collectors in three villages: Surodinawan, Pulorejo, and Meri, located in Mojokerto City, East Java, Indonesia (longitude 112.434084; latitude –7.472638). The sampling sites were characterized by lowland habitats with a mix of agricultural land, residential areas, and small freshwater bodies such as irrigation canals and fish ponds, indicating moderate levels of human activity and potential contact between snakes and anthropogenic environments. The collection period spanned from September to October 2022. Javanese keelback water snakes (F. melanzostus) exhibited a range of dorsal coloration, varying from yellowish-brown to greenish-brown or gray. They typically featured a distinct black or dark brown stripe beneath the eye, along with an oblique stripe extending from behind the eye, which was yellowish-gray and darker than the surrounding body coloration. The ventral surface was yellowish-gray with black-edged scales, while the chin and throat were a creamy yellowish color (Vogel and David, 2012). Examination and collection of the helminthThe presence of parasitic helminths in Javanese keelback water snakes was examined according to the methods described by Yudhana et al. (2020). Snakes were euthanized using a combination of ketamine and xylazine, weighed, and skinned. Macroscopic examination of the muscles, visceral organs, and subcutaneous tissues was conducted to detect adult helminths or larvae. The helminths were carefully extracted from the muscles, visceral organs, or subcutaneous tissues and placed in a Petri dish containing physiological saline to observe their response and movement. The number of helminths collected from each infected water snake was carefully counted to calculate the parasitic helminth infection intensity rate. Fecal examinationFecal examination was conducted to detect parasitic worm eggs using native, sedimentation, and flotation methods. Feces were collected during observation of the digestive tract of each snake. The parasite testing procedure was initiated by spreading the feces onto a glass slide. A drop of water was then added, stirred, and covered with a cover slip. The sedimentation method involved filtering the fecal suspension and centrifuging it for 2–5 minutes at 1,500 rpm. The supernatant was discarded, and the sediment was retained for microscopic examination. The sediment was then stirred and placed onto a glass slide. The flotation method involved adding a saturated sugar solution to the centrifuged sample, placing a cover slip on top, and allowing it to sit for 1–2 minutes before transferring it to a glass slide for examination under a microscope with 40× and 100× magnification (Wolf et al., 2014). Semichen-acetic carmine staining was performedThe staining method was performed to aid in identification and preserve helminth preparations for longevity. The helminth staining and identification method followed the previous reference by Rataj et al. (2011). The helminths intended for preparation with semichen-acetic carmine staining were either fresh specimens or specimens preserved in a 10% formaldehyde solution. Helminths collected from the muscles, visceral organs, or subcutaneous tissues were sandwiched between two glass slides, tied with a string, and then immersed in 5% glycerin alcohol for 24 hours. This was followed by a fixation process using 70% alcohol for 5 minutes, followed by transfer into a diluted carmine solution and left for approximately 8 hours, depending on the thickness of the helminth’s cuticle. After fixation, the helminths were transferred to acid alcohol for 2 minutes and then to an alkaline alcohol solution for 20 minutes. Subsequently, multilevel dehydration was performed with alcohol, starting with 70% alcohol for 5 minutes, followed by 85% alcohol for 5 minutes, and 95% alcohol for 5 minutes. The helminths were then mounted in Hung’s I solution for 20 minutes, removed from the solution, placed on a clean glass slide, dripped with sufficient Hung’s II solution, and covered with a glass slip cover. The final step involved drying the preparation in an incubator at 37°C, followed by cooling at room temperature. The identification of the adult stage of helminths or larvae was conducted using a light microscope with 40× and 100× magnification. Ethical approvalThis study was reviewed and approved by the Animal Care and Use Committee of the Faculty of Veterinary Medicine, Universitas Airlangga, Indonesia (No. 2.KEH.141.10.2022.). ResultsHelminth infections were detected in 95.65% (22/23) of the snakes examined, with 91.30% (21/23) naturally infected with a cestode and 95.65% (22/23) naturally infected with nematodes. The only cestode genus identified in this study was Spirometra spp., which was found exclusively in its plerocercoid (larval) stage. These larvae were primarily detected through macroscopic examination, with infections localized in the muscle (105 plerocercoids), subcutaneous tissues (11 plerocercoids), and visceral organs (23 plerocercoids) (Fig. 1A–C). Photomicrographs of the plerocercoid following carmine staining, highlighting the morphology of the anterior region, including the scolex, are presented in Figure 1D. Additionally, several nematode taxa were identified, including Ophidascaris spp., Rhabdias spp., and a nematode belonging to the order Oxyurida, with their localization in the stomach, subcutaneous tissue, and fecal samples. Ophidascaris spp. adults were found in the stomach and feces (Fig. 2A), Rhabdias spp. were found in both subcutaneous tissue and visceral organs (Fig. 2B), and Oxyurida nematodes were found in the subcutaneous layer (Fig. 2C). Fecal examinations also revealed eggs from Ophidascaris spp., Physaloptera spp., Capillaria spp., and Rhabdias spp. (Fig. 3A–E). In this study, fecal examinations revealed a high prevalence 95,65% (22/23) of nematodes, highlighting the significant helminthic infection burden in F. melanzostus.
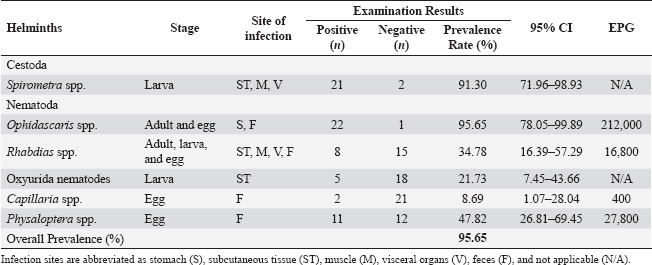
Fig. 1. A. Plerocercoid from Fowlea melanzostus in muscles; B. Plerocercoid in subcutaneous tissues; C. Plerocercoid in intestinal organs, D. Photomicrographs of plerocercoid from Fowlea melanzostus, anterior end showing the scolex (Images A, B, and C were taken macroscopically, while image D was taken under 100x magnification). The overall prevalence data and distribution by helminth species, developmental stage, infection site, and eggs per gram (EPG) are detailed in Table 1. Ophidascaris spp. exhibited the highest EPG (212,000), indicating a significant parasitic load in most hosts. Physaloptera spp. and Rhabdias spp. also showed considerable infection intensity, with EPG values of 27,800 and 16,800, respectively. Capillaria spp. were detected exclusively through fecal examination, presenting a lower prevalence (8.69%) and EPG value (400). Oxyurida nematodes were observed in larval form within subcutaneous tissue but were not quantified by EPG because of the absence of detection at the egg stage.
Fig. 2. Macroscopic appearance of nematodes in Fowlea melanzostus A. Ophidascaris spp. in the stomach, B. Rhabdias spp. in subcutaneous tissue, C. A nematode belonging to the order Oxyurida in subcutaneous tissue (red arrow). DiscussionThe prevalence of parasitic infections in Javanese keelback water snakes (F. melanzostus) from Mojokerto City, Indonesia, indicates that cestode and nematode species are susceptible hosts. The study revealed a 91.30% prevalence of Spirometra spp. infections, which aligns with previous findings of cestode infestations in semi-aquatic snakes and is higher than the infection rates reported in previous studies (Jongthawin et al., 2014; Yudhana et al., 2021b). Larva migration indicates that Spirometra spp. is primarily localized in muscle tissue, with lower occurrences in subcutaneous tissue and visceral organs, suggesting a pattern of larval migration and tissue predilection toward high muscle-density areas. In studies conducted in the Indonesian region, cases of Spirometra spp. plerocercoid tapeworm infections have also been reported in Ptyas mucosus snakes in Sidoarjo, Indonesia, with a prevalence of 68% (Pranashinta et al., 2017). The prevalence of spargana infections in Dendrelaphis pictus snakes was recorded at 50.85% in Mojokerto, Indonesia, while Naja sputatrix snakes showed an infection rate of 56.70% (Yudhana et al., 2019; Fransiska, 2020). Meanwhile, in Trimeresurus insularis snakes from Banyuwangi, Indonesia, the prevalence of spargana reached 100% among 43 snake samples (Yudhana et al., 2020a). Similar to our findings, previous studies have frequently noted the predominance of spargana in muscle tissue, suggesting a potential predilection of plerocercoids for high muscle-density areas. Previous studies in Indonesia have mostly focused on parasitological examinations. Infections with Spirometra spp. have been reported in various snake species occupying distinct habitats, including Naja sputatrix (terrestrial), Dendrelaphis pictus (arboreal), and Trimeresurus insularis (arboreal), suggesting that transmission can occur across different ecological niches. These findings support the notion that several snake hosts can contribute to the maintenance of Spirometra’s life cycle. Potential intermediate or paratenic hosts vary depending on habitat, such as frogs and lizards in terrestrial and arboreal environments, and fish or amphibians in semiaquatic habitats like those occupied by F. melanzostus. Spirometra spp. infection causes sparganosis, a zoonotic disease that affects reptiles and humans who consume raw or undercooked intermediate hosts, such as amphibians, fish, and birds (Wiwanitkit, 2005). This highlights the significant zoonotic risk posed by Spirometra spp. in regions where wild-caught snakes, such as F. melanzostus, are still consumed as food. In snakes, the clinical manifestations of sparganosis include weakness, anemia, malnutrition, gastrointestinal ulceration, and inflammation, which are associated with tissue damage caused by larval migration (Yudhana et al., 2021a). Human sparganosis symptoms depend on larval migration and tissue invasion sites, commonly presenting as subcutaneous nodules, localized pain, and edema. More severe cases involve ocular or cerebral infection, leading to vision problems or neurological deficits (Wiwanitkit, 2005; Liu et al., 2022). Transmission occurs mainly through the ingestion of raw or undercooked meat from intermediate or paratenic hosts, use of raw flesh as poultices, or contact of open wounds with contaminated water. Since F. melanzostus is occasionally consumed in traditional settings and harvested from the wild, it serves as a paratenic host, posing a notable zoonotic threat, especially where public awareness and food safety are limited. Nematode infections, on the other hand, were recorded in 95.65% of the examined snakes, with Ophidascaris spp., Rhabdias spp., and Oxyurida nematodes being the most prevalent genera. This elevated prevalence is likely linked to the feeding habits of snakes, which involve intermediate hosts such as amphibians and fish, thereby promoting parasite transmission (Mendoza et al., 2020; Kuchta et al., 2021). Furthermore, the discovery of nematode eggs in fecal samples emphasizes the ongoing transmission cycle within the environment, enhancing the risk of reinfection not only in F. melanzostus but also in other susceptible hosts such as amphibians, fish, birds, small mammals, and other reptiles (Herman et al., 2009; Choe et al., 2016). To our knowledge, nematode infections in water snakes are rarely reported, with only a few documented cases in the region. One such report from Thailand, a country geographically close to Indonesia, described the infection of Tanqua siamensis in the rainbow water snake (Enhydris enhydris) (Charoennitiwat et al., 2024). Another report involved the same snake species as in the present study (F. melanzostus), but the sample was collected from Banyuwangi City, Indonesia, and showed infection with the nematode Ophidascaris spp. (Putri et al., 2024).
Fig. 3. Nematode eggs were observed under a binocular microscope at 400x magnification. A=Capillaria spp.; B=Ophidascaris spp.; C=Rhabdias spp.; D=Physaloptera spp.; E=Rhabdias sp. larva identified in fecal sample from Fowlea melanzostus. The identification of Ophidascaris spp. larvae in the stomach and intestines suggests direct ingestion of infected prey, aligning with a previous study on Ophidascaris spp. infections in Burmese python (Python molurus bivittatus), which have been associated with severe gastrointestinal pathology (Zhao et al., 2021). Additionally, the detection of Physaloptera spp., Capillaria spp., and Rhabdias spp. eggs in fecal samples indicates the continuous shedding of parasites into the environment, further contributing to environmental contamination and supporting the continuation of the transmission cycle in susceptible snake populations (de Oliveira et al., 2019). Recent findings have highlighted the zoonotic potential of Ophidascaris spp., particularly after the first documented case of neural larva migrans in a human caused by Ophidascaris robertsi, a species typically found in Australian carpet python (Morelia spilota) (Hossain et al., 2023). Notably, F. melanzostus, the species investigated in this study, is still used for meat consumption in certain local food vendors in Indonesia. Although this species is smaller in size compared to pythons, its high infection rate with zoonotic nematodes, particularly Ophidascaris spp., raises concern. Considering that transmission occurs via ingestion of embryonated eggs (fecal–oral route), the handling or consumption of inadequately cooked wild-caught snake meat may pose a risk for zoonotic transmission (Hossain et al., 2023). Table 1. Helminths in Fowlea melanzostus: stages, infection sites, examination results, and eggs per gram (EPG).
The clinical impact of these parasitic infections on F. melanzostus is significant, particularly concerning overall health. Nematode infestations, notably by Ophidascaris spp., have been linked to gastritis, intestinal obstruction, and necrotic enteritis, consistent with previous reports on snake parasitism (Taylor et al., 2015). Additionally, Rhabdias spp. can migrate into the pulmonary system, potentially causing respiratory distress and further compromising snake health (Mihalca et al., 2010). Because of the chronic nature of these infections, affected snakes may suffer long-term negative effects on growth, reproduction, and survival, which could ultimately influence population dynamics. The environmental conditions in Mojokerto City, Indonesia, significantly contribute to the high parasite burden observed in F. melanzostus. The region’s tropical climate, characterized by elevated humidity and the presence of river ecosystems, provides an optimal environment for parasite development and transmission (Marguno et al., 2007). The abundance of intermediate hosts, including amphibians, fish, and rodents, commonly inhabiting wetland areas, facilitates the continuation of cestode and nematode life cycles (Kusuma et al., 2010). Notably, the zoonotic tapeworm Spirometra spp. had been detected with high prevalence in this study from wild-caught snake species which frequently sold as exotic pet and culinary purposes (Yudhana et al., 2021b). A comprehensive understanding of these ecological factors is crucial for developing effective parasite control strategies and ensuring the conservation of snake populations within their natural habitats. The high prevalence of parasitic infections in F. melanzostus has important implications for its conservation and public health. As reservoir hosts, these snakes contribute to the persistence of zoonotic parasites, posing risks to other wildlife and humans who handle or consume infected snakes (Kuchta et al., 2021). Public health efforts should prioritize community education on the dangers of undercooked reptile meat consumption and promote proper hygiene to reduce transmission (Kim et al., 2018). At the same time, conservation strategies should also focus on protecting wetland habitats, as ecosystem stability helps regulate parasite dynamics and reduce infection rates in wild snake populations (Yudhana et al., 2020c). This study was conducted as an exploratory survey to document helminth infections in F. melanzostus, a reptilian host with limited parasitological information and little representation in previous research. Due to restricted resources and funding, species-level identification and molecular characterization were not performed at this stage. Nevertheless, the study provides valuable baseline data and documents previously unreported parasitic associations, providing important insights into the host–parasite ecology of this understudied species. Further research is needed to explore the genetic diversity of these parasites, as identifying strain variations may offer insights into their virulence and potential adaptation to F. melanzostus as a susceptible host. Future studies should also incorporate larger sample sizes from multiple locations across Java Island, where this snake species is distributed, to enhance representativeness and allow for regional comparisons. ConclusionThis study reveals a high prevalence of helminth infection in the Javanese keelback water snake (F. melanzostus), underscoring its ecological role of this species as a susceptible host for parasitic nematodes and cestodes. Notably, two identified genera, Spirometra spp. and Ophidascaris spp., are recognized as zoonotic parasites with the potential to infect humans through oral transmission, primarily through the consumption of raw or undercooked snakes. Furthermore, these findings emphasize the importance of continuous parasitological surveillance in wild reptiles, particularly those inhabiting areas with close human interaction, to assess potential public health risks and inform future control strategies against zoonotic helminthiases. AcknowledgmentAll authors sincerely thank the Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Banyuwangi Campus, Indonesia, for providing the necessary facilities for sample analysis. We also extend our gratitude to the Research Group for Animal Biomedical and Conservation, Universitas Airlangga, for their support during the laboratory work. Special thanks to the Parasitina team for their valuable contribution to fieldwork and sample collection. Conflict of interestThe authors declare no conflicts of interest. FundingThis study was partly supported by the Penelitian Internal Universitas Airlangga, Indonesia, in 2022 (Grant Number: 251/UN3/2022). Author’s contributionsAMK, AY, and RE: Conceptualization and study design. APG and FOZ: Collected samples. AY, APG, and FOZ: Performed the laboratory procedures. AY and AHW: Interpreted the data. AMK and RE: Original draft writing. UFH: Edited the final manuscript. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityAll references are open-access, so data can be obtained from the online web. ReferencesBharati, K. 2018. Snake, snakebite and its management. Indian Sci. Cruis. 32(6), 46; doi: 10.24906/isc/2018/v32/i6/179818 Bimi, L., Odamtten, F.K., Anto, F. and Tetteh, A.K. 2021. Ophidascaris sp. in an African Rock Python (Python sebae) in Ghana: a case report. J. Med. Microbiol. Infect. Dis. 9(2), 103–107; doi: 10.52547/JoMMID.9.2.103 Charoennitiwat, V., Thaenkham, U., Tongpon, S., Chaisiri, K., Laoungbua, P., Tawan, T., Kanjanapruthipong, T., Ampawong, S., Chan, A.H.E. and Ratnarathorn, N. 2024. A new nematode species, Tanqua siamensis sp. nov. (Nematoda: Gnathostomatidae) in the rainbow water snake, Enhydris enhydris, from Thailand. Parasitology 151(8), 821–831; doi: 10.1017/S0031182024000908 Choe, S., Lim, J., Kim, H., Kim, Y., Kim, H., Lee, D. and Eom, K.S. 2016. Three nematode species recovered from terrestrial snakes in Republic of Korea. Korean J. Parasitol. 54(2), 205; doi: 10.3347/kjp.2016.54.2.205 de Oliveira, M.C., Lima, V.F., Pinto, C.L.D.M., da Silva, É.G., Teles, D.A., Ferreira-Silva, C. and Almeida, W.D.O. 2019. New record of Physaloptera sp. (Nematoda: Physalopteridae) parasitizing Philodryas nattereri (Ophidia: Dipsadidae) in Brazil. Notes. 12, 1031–1034. Edila, R., Effendi, M.H., Suwanti, L.T., Kwon, H.K., Yudhana, A., Wardhana, A.H. and Alhada, A. 2024. A comprehensive study on the occurrence rate and morphology characteristics of the acanthocephalan parasite in Javan spitting cobra (Naja sputatrix) in Sidoarjo, Indonesia. Biodiversitas. 25(2), 210; doi: 10.13057/biodiv/d250210 Edila, R., Yudhana, A. and Praja, R.N. 2023. First report of Physaloptera (Nematoda: Physalopteridae) parasitizing Javan spitting cobra snake (Naja sputatrix) in Indonesia. AIP Conference Proceedings. AIP Publishing; doi: 10.1063/5.0115940 Fransiska, E.M. 2020. Identifikasi dan prevalensi cacing pita Spirometra sp. pada ular kobra (Naja sputatrix) di Kabupaten Banyuwangi. Undergraduate thesis, Universitas Airlangga, Airlangga. Herman, J.S. and Chiodini, P.L. 2009. Gnathostomiasis, another emerging imported disease. Clin. Microbiol. Rev 22(3), 484–492; doi: 10.1128/cmr.00003-09 Herman, J.S., Wall, E.C., van-Tulleken, C., Godfrey-Faussett, P., Bailey, R.L. and Chiodini, P.L. 2009. Gnathostomiasis acquired by British tourists in Botswana. Emerg. Infect. Dis. 15(4), 594; doi: 10.3201/1504.081646 Hossain, M.E., Kennedy, K.J., Wilson, H.L., Spratt, D., Koehler, A., Gasser, R.B., Šlapeta, J., Hawkins, C.A., Bandi, H.P. and Senanayake, S.N. 2023. Human neural larva migrans caused by ophidascaris robertsi ascarid. Emerg. Infect. Dis. 29(9), 1900; doi: 10.3201/eid2909.230351 Jongthawin, J., Intapan, P.M., Sanpool, O., Sadaow, L., Laymanivong, S., Thanchomnang, T. and Maleewong, W. 2014. Molecular evidence of Spirometra erinaceieuropaei infection in Ptyas korros from Lao PDR and Thailand and frogs Hoplobatrachus rugulosus from Myanmar. Southeast Asian J. Trop. Med. Public Health. 45(6), 1271. Kim, J.G., Ahn, C.S., Sohn, W.M., Nawa, Y. and Kong, Y. 2018. Human sparganosis in Korea. J. Korean Med. Sci. 33(44), 273; doi: 10.3346/jkms.2018.33.e273 Kuchta, R., Kołodziej-Sobocińska, M., Brabec, J., Młocicki, D., Sałamatin, R. and Scholz, T. 2021. Sparganosis (Spirometra) in Europe in the molecular era. Clin. Infect. Dis. 72(5), 882–890; doi: 10.1093/cid/ciaa1036 Kusuma, K.I., Eprilurahman, R. and Vogel, G. 2010. First record of Xenochrophis melanzostus (Gravenhorst, 1807) on Bali Island, Indonesia. Hamadryad. 35(1), 113–115. Kuzmin, Y., Kinsella, J.M., Tkach, V.V. and Bush, S.E. 2013. New species of Kalicephalus (Nematoda: Diaphanocephalidae) from a snake, Oxyrhabdium leporinum, on Luzon Island, Philippines. Comp. Parasitol. 80(2), 240–246 Liu, W., Gong, T., Chen, S., Liu, Q., Zhou, H., He, J., Wu, Y., Li, F. and Liu, Y. 2022. Epidemiology, diagnosis, and prevention of sparganosis in Asia. Animals 12(12), 1578; doi: 10.3390/ani12121578 Mendoza-Roldan, J.A., Modry, D., & Otranto, D. 2020. Zoonotic parasites of reptiles a crawling threat. Trends Parasitol. 36(8), 677–687; doi: 10.1016/j.pt.2020.04.014 Mihalca, A.D., Micl˘uş, V. and Lefkaditis, M. 2010. Pulmonary lesions caused by the nematode Rhabdias fuscovenosa in a grass snake, Natrix natrix. J. Wildl. Dis. 46(2), 678–681; doi: 10.7589/0090-3558-46.2.678 Murvanidze, L., Lomidze, T. and Nikolaishvili, K. 2013. Morphological and biochemical investigation of the Bothridium pithonis Blainville, 1824 (Cestoda: Diphyllobothriidae). Bull. Georgian Natl. Acad. Sci. 7(1), 100–104. Nardoni, S., Papini, R., Marucci, G.M. and Mancianti, F. 2008. Survey on the fungal flora of the cloaca of healthy pet reptiles. Rev Med. Vet. 159, 159–165. Pranashinta, G.T., Suwanti, L.T., Koesdarto, S. and Poetranto, E.D. 2017. Spirometra in Ptyas mucosus snake in Sidoarjo, Indonesia. KnE Life Sci. 114, 34–40; doi: 10.18502/kls.v3i6.1104 Putri, E.S., Yudhana, A., Prastiya, R.A., Yunita, M.N., Agustono, B. and Wibawati, PA. 2024. First report of Ophidascaris spp. (Class: Nematode) infection in wild Javanese keelback water snake (Fowlea melanzostus) in Banyuwangi. J. Parasite Sci. 8(1), 27–30. doi:10.20473/jops.v8i1.54578. Rataj, V.A., Lindtner-Knific, R., Vlahovic, K., Mavri, U. and Dovc, A. 2011. Parasites in pet reptiles. Acta Vet. Scand. 53(33), 1–20; doi: 10.1186/1751-0147-53-33 Rizal, S. and Rahmawati, R.A. 2021. Pathogenic organisms in Varanidae and their potential as zoonotic diseases. Wartazoa 31(2), 97–107 Sismami, D.A., Oka, I.B. and Dharmawan, N.S. 2014. Infeksi cacing pada ular kobra (Naja sputatrix) di Bali. J. Veteriner. 15(3), 401–405. Telnoni, F.R., Oka, I. and Widyastuti, S.K. 2016. Prevalensi infeksi cacing nematoda pada ular Python reticulatus yang dipelihara pecinta ular di Denpasar. Indones. Med. Vet. 5(2), 104–112. Vasaruchapong, T., Laoungbua, P., Tawan, T. and Chanhome, L. 2017. The survey of internal parasites of consumed-Siamese cobra (Naja kaouthia) in Thailand. Vet. Parasitol. Reg. Stud. Reports. 9, 88–92; doi: 10.1016/j.vprsr.2017.06.005 Vogel, G. and David, P. 2012. A revision of the species group of Xenochrophis piscator (Squamata: Natricidae). Zootaxa 3473(1), 1–60; doi: 10.11646/zootaxa.3473.1.1 Wiwanitkit, V. 2005. A review of human sparganosis in Thailand. Int. J. Infect. Dis. 9(6), 312–316; doi: 10.1016/j.ijid.2004.08.003 Wolf, D., Vrhovec, M.G., Failing, K., Rossier, C., Hermosilla, C. and Pantchev, N. 2014. Diagnosis of gastrointestinal parasites in reptiles: comparison of two coprological methods. Acta Vet. Scand. 56(1), 1–13. Yudhana, A., Kartikasari, A.M., Edila, R., Praja, R.N., Hamonangan, J.M., Wardhana, A.H., Mufasirin, M. and Koesdarto, S. 2024. Molecular detection of zoonotic Spirometra (Cestoda: Diphyllobothriidae) in Javan-spitting cobra (Naja sputatrix) snakes in Indonesia. Biodiversitas 25(12), 1221; doi: 10.13057/biodiv/d251221 Yudhana, A., Praja, R. N., Pratiwi, A., Kartikasari, A. M. 2021b. Sparganosis in wild-caught Javanese keelback water snakes (Fowlea melanzostus) from Indonesia. J. Vet. Parasitol. 35(1), 56–63; doi: 10.5958/0974-0813.2021.00010.3 Yudhana, A., Praja, R.N. and Edila, R. 2023. First report of acanthocephalan parasite in wild-caught Asian vine snake (Ahaetulla prasina) in Indonesia. Vet. World. 16(2), 317; doi: 10.14202/vetworld.2023.317-321 Yudhana, A., Praja, R.N. and Kartikasari, AM. 2021. Sparganosis (Spirometra spp.) in Asian Water Monitor (Varanus salvator): a medical implications for veterinarians, breeders, and consumers. Vet World. 14(9), 2482; doi: 10.14202/vetworld.2021.2482-2487 Yudhana, A., Praja, R.N. and Supriyanto, A. 2019. The medical relevance of Spirometra tapeworm infection in Indonesian bronzeback snakes (Dendrelaphis pictus): a neglected zoonotic disease. Vet World. 12(6), 844; doi: 10.14202/vetworld.2019.844-848 Yudhana, A., Praja, R.N., Yunita, M.N., Wardhana, D.K. and Fikri, F. 2020a. Prevalence of Spirometra in white-lipped green pit viper (Trimeresurus insularis) in Banyuwangi City, Indonesia. J. Vet. Parasitol. 34(1), 12–16; doi: 10.5958/0974-0813.2020.00003.0 Yudhana, A., Praja, R.N., Yunita, M.N. and Wardhana, D.K. 2020b. Molecular evidence of Spirometra erinaceieuropaei in Asian wild frogs (Rana rugulosa) from Banyuwangi city, Indonesia. J. Worlds Poult. Res. 10(2), 170–174; doi: 10.36380/scil.2020.wvj22 Yudhana, A., Praja, R.N., Wardhana, D.K., Yunita, M.N., Fikri, F., Fransiska, E.M. and Ismail, W.A. 2020c. Public health relevance of sparganosis in Javan spitting cobra snakes (Naja sputatrix): a neglected zoonotic disease in Indonesia. Indian J. Public Health Res. Dev. 11, 3. Zhao, Q., Abuzeid, A. M., He, L., Zhuang, T., Li, X., Liu, J. and Li, G. 2021. The mitochondrial genome sequence analysis of Ophidascaris baylisi from the Burmese python (Python molurus bivittatus). Parasitol. Int. 85, 102–434. doi:10.1016/j.parint.2021.102434 | ||
| How to Cite this Article |
| Pubmed Style Kartikasari AM, Yudhana A, Edila R, Zania FO, Geraldine AP, Wardhana AH, Hanaf UF. High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, Indonesia. Open Vet. J.. 2025; 15(8): 3684-3692. doi:10.5455/OVJ.2025.v15.i8.32 Web Style Kartikasari AM, Yudhana A, Edila R, Zania FO, Geraldine AP, Wardhana AH, Hanaf UF. High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, Indonesia. https://www.openveterinaryjournal.com/?mno=247795 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.32 AMA (American Medical Association) Style Kartikasari AM, Yudhana A, Edila R, Zania FO, Geraldine AP, Wardhana AH, Hanaf UF. High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, Indonesia. Open Vet. J.. 2025; 15(8): 3684-3692. doi:10.5455/OVJ.2025.v15.i8.32 Vancouver/ICMJE Style Kartikasari AM, Yudhana A, Edila R, Zania FO, Geraldine AP, Wardhana AH, Hanaf UF. High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, Indonesia. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3684-3692. doi:10.5455/OVJ.2025.v15.i8.32 Harvard Style Kartikasari, A. M., Yudhana, . A., Edila, . R., Zania, . F. O., Geraldine, . A. P., Wardhana, . A. H. & Hanaf, . U. F. (2025) High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, Indonesia. Open Vet. J., 15 (8), 3684-3692. doi:10.5455/OVJ.2025.v15.i8.32 Turabian Style Kartikasari, Anjani Marisa, Aditya Yudhana, Ryanka Edila, Fransiska Okta Zania, Audina Putri Geraldine, April Hari Wardhana, and Ulfiani Fauzia Hanaf. 2025. High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, Indonesia. Open Veterinary Journal, 15 (8), 3684-3692. doi:10.5455/OVJ.2025.v15.i8.32 Chicago Style Kartikasari, Anjani Marisa, Aditya Yudhana, Ryanka Edila, Fransiska Okta Zania, Audina Putri Geraldine, April Hari Wardhana, and Ulfiani Fauzia Hanaf. "High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, Indonesia." Open Veterinary Journal 15 (2025), 3684-3692. doi:10.5455/OVJ.2025.v15.i8.32 MLA (The Modern Language Association) Style Kartikasari, Anjani Marisa, Aditya Yudhana, Ryanka Edila, Fransiska Okta Zania, Audina Putri Geraldine, April Hari Wardhana, and Ulfiani Fauzia Hanaf. "High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, Indonesia." Open Veterinary Journal 15.8 (2025), 3684-3692. Print. doi:10.5455/OVJ.2025.v15.i8.32 APA (American Psychological Association) Style Kartikasari, A. M., Yudhana, . A., Edila, . R., Zania, . F. O., Geraldine, . A. P., Wardhana, . A. H. & Hanaf, . U. F. (2025) High prevalence of Spirometra spp. and nematode infections in Fowlea melanzostus from Mojokerto City, Indonesia. Open Veterinary Journal, 15 (8), 3684-3692. doi:10.5455/OVJ.2025.v15.i8.32 |