| Research Article | ||
Open Vet. J.. 2025; 15(8): 3703-3710 Open Veterinary Journal, (2025), Vol. 15(8): 3703-3710 Research Article Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extenderSuherni Susilowati1, Imam Mustofa1*, Zulfi Nur Amrina Rosyada2, Tri Wahyu Suprayogi1, Tatik Hernawati1, Adeyinka Oye Akintunde3, Yudit Oktanella4, Aswin Rafif Khairullah5, Ulvi Fitri Handayani6, Riza Zainuddin Ahmad5, Lili Anggraini6, Bima Putra Pratama7, Chairdin Dwi Nugraha6 and Latifah Latifah61Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Division of Animal Husbandry, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria 4Department of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 5Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 6Research Center for Animal Husbandry, National Research and Innovation Agency (BRIN), Bogor, Indonesia 7Research Center for Agroindustry, National Research and Innovation Agency (BRIN), South Tangerang, Indonesia *Corresponding Author: Imam Mustofa. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: imam.mustofa [at] fkh.unair.ac.id Submitted: 16/03/2025 Revised: 19/06/2025 Accepted: 02/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
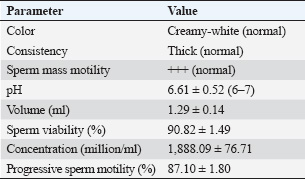
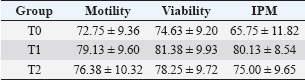
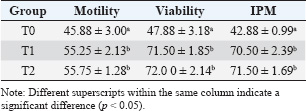
ABSTRACTBackground: One native goat species in Indonesia that faces extinction because of cross-breeding is the Kacang goat (Capra hircus). In addition to ensuring food security as a meat-producing livestock, artificial insemination of the Kacang goat could simultaneously conserve it and improve its genetic quality. Aim: The purpose of this study was to investigate the effect of the addition of epigallocatechin-3-gallate nanoparticles (EGCG) to skimmed egg yolk (SM-EY) extender on the quality of post-thawed Kacang buck spermatozoa for artificial insemination. Methods: Fresh semen of Kacang buck was diluted in SM-EY without (T0) and with the addition of 50 or 100 mg EGCG/dl extender for T1 and T2, respectively. Extended semen was frozen in liquid nitrogen (−196°C) for a week in French tiny straws with 60 million spermatozoa per straw, following a standard protocol. Six thawed replicates from each group were tested for sperm motility, viability, intact plasma membrane (IPM), malonaldehyde (MDA), DNA integrity, and chromatin density. Results: Pre-equilibration sperm motility, viability, and IPM were not significantly different among the groups (p > 0.05). Post-equilibration sperm motility, viability, and IPM were higher (p < 0.05) in the EGCG group than in the control group. In post-thawed semen, the EGCG groups showed higher sperm motility, viability, IPM, and DNA integrity and lower MDA levels (p < 0.05) than the control group. The chromatin density did not differ between the groups (p > 0.05). Conclusion: The addition of 50 or 100 mg EGCG/dl SM-EY extender significantly improved the post-thawed spermatozoa quality of Kacang buck. Keywords: Food security, DNA integrity, Extinction risk, Sperm viability, Sperm motility. IntroductionOne of the native goats of Indonesia, the kacang (Capra hircus), is widely used, particularly in rural areas, to promote nutrition and guarantee food security. The sustainability of Kacang goats, a particular local goat, must be preserved because they possess many genetic resources typical of local livestock. According to field observations, the Kacang goat population is at risk of extinction. Larger amounts were sold, particularly on specific holidays (Sujarwanta et al., 2024). If this trend persists, superior bucks will become scarce, and Kacang goats will get smaller in stature from generation to generation. Furthermore, rather than breeding pure Kacang goat breed offspring, rural farmers prefer to breed their does with imported bucks in order to produce offspring that are heavier at birth, weaning, and adulthood (Elieser et al., 2012). Goat sperm parameters crucial for semen fertility include sperm concentration, motility, morphology, and integrity of the plasma membrane and acrosome. High motility and average path velocity are correlated with increased fertility in inseminated goats (Tanga et al., 2021). Artificial insemination (AI) is probably the most important biotechnology for significant genetic improvement used in goats (Luo et al., 2019). Post-thawed spermatozoa quality is a critical factor influencing the success of AI (Oktanella et al., 2024). Sperm motility is a key factor in determining semen quality and a reliable predictor of fertilization success (Chakraborty and Saha, 2022). Kacang goat is a local Indonesian goat, whose population is decreasing because breeders prefer crossbred goats, such as female Kacang goat mated with Ettawa buck to be Peranakan Ettawa, female Kacang goat mated with Boer buck to be Boerka. Therefore, the preservation of the Kacang goat’s germplasm must be carried out. Spermatozoa’s vulnerability to reactive oxygen species (ROS) and lipid peroxidation is linked to high unsaturated acyl chains (Gautier and Aurich, 2022). Antioxidants included in extenders protect sperm by reducing oxidative stress and combating ROS (Kowalczyk, 2022). Natural plant antioxidants contain the potent antioxidant epigallocatechin-3 gallate (EGCG) (Prastiya et al., 2023). The quality of spermatozoa, particularly membrane structure and DNA quality, will decline if goat semen is maintained at freezing temperatures (Oktanella et al., 2024). The larger amounts of unsaturated fatty acids in the membrane of Kacang goat spermatozoa make them more susceptible than spermatozoa from other animals (Susilowati et al., 2021). The ROS molecular pathways are necessary for normal spermatozoa metabolism. Therefore, spermatozoa metabolism requires a concentration of ROS (Liu et al., 2019). According to Zhu et al. (2025), a tiny quantity of ROS is required to regulate certain normal sperm activity under normal physiological settings. However, increased ROS has a detrimental effect on spermatozoa survival (Zou et al., 2021). Since spermatozoa lack cytoplasmic antioxidant enzymes and have high amounts of unsaturated fatty acids in their membrane, they are really especially susceptible to oxidation (Zhang et al., 2022a). This vulnerability to oxidation has been linked to harmful effects on sperm quality and function (Zhao et al., 2023). Thus, the balance of oxidants and antioxidants determines the quality of sperm (Zhang et al., 2022b). Including NPs GTE or EGCG CNPs in skimmed egg yolk (SM-EY) extender when the sperm motility percentage is close to borderline (40%) (Mustofa et al., 2023a; Mustofa et al., 2023b). This could be the result of an antioxidant capacity that is either too powerful (CNPs EGCG) or too weak (NPs GTE), failing to achieve the physiological balance of oxidant-antioxidant. Nevertheless, it is still unclear to what degree the addition of a particular EGCG amount to the extender medium could improve the quality of the Kacang buck’s post-thawed sperm. Therefore, this study aimed to evaluate the quality of the spermatozoa extended in SM-EY, which included EGCG at 50 and 100 mg/dl concentrations in an SM-EY extender. Materials and MethodsEthical approval and research designThis long-term study was approved by Airlangga University’s Animal Care and Use Committee and is identified by reference number 520/HRECC.FODM/VII/2023. This research was conducted from February 2024 to July 2024. The study was conducted at the Artificial Insemination Center of Airlangga University, situated in Tanjung village, Gresik District, East Java, Indonesia. Experimental animalsThe male goats used were two males that were accustomed to having their fresh semen taken using an artificial vagina for student practicums. The male goats were 24.76 ± 3.23 months old with a body weight of 35.58 ± 4.71 kg. In this investigation, three two- to three-year-old Kacang bucks weighing 35–40 kg were used. The daily diet of the Kacang bucks included approximately 4 kg of forage and 3.5 kg of concentrate, which had a crude protein content of 16%–18%. In addition, they had unfettered access to potable water. Six ejaculate semen samples were collected using an artificial vagina twice a week. The semen was then frozen for additional processing. Skim milk–egg yolk extenderA 7.5-g skim milk powder (Merck 115338) weighing 7.5 g was dissolved in 75 ml of distilled water. The mixture was heated to 92°C–95°C for 10 minutes and then cooled to 26°C. After adding 2.5 ml of skim milk solution, 47.5 ml of egg yolk was mixed with 0.5 IU/ml of penicillin, and 0.5 mg/ml of streptomycin (Susilowati et al., 2022). Treatment groups T1 and T2, which received increases of 50 and 100 mg/dl EGCG, respectively, and the control group (T0), which did not receive any EGCG (Sigma-Aldrich), each received three equal portions of the aliquot. Frozen semenTwo equal volumes were created from the SM-EY extender groups. The extender of each group was divided into two parts (A and B, see the explanation about adding diluent) with the same volume. Fresh semen was added to the first part to achieve a concentration of 480 million spermatozoa/ml. The second part was added with glycerol to achieve a concentration of 32%. Furthermore, the two parts of the extender were mixed to obtain 240 million spermatozoa/ml with a glycerol concentration of 16%. Blending fresh semen with the original volume yielded 480 million spermatozoa/ml. There were 240 million spermatozoa per milliliter when glycerol was added to the second volume until it reached a 16% concentration. The semen sample was filled and sealed in 0.25 ml French straws containing 60 million sperm after cooling to 5°C for an hour. Then, the straws were kept in liquid nitrogen (−196°C) for a week after being cryogenically frozen according to the prescribed protocol (Susilowati et al., 2022). Evaluation of post-thawed sperm qualityFrozen semen straws were thawed in sterile water at 37°C for 30 seconds to evaluate different parameters. To assess chromatin density, intact plasma membrane (IPM) levels, sperm vitality, malonaldehyde (MDA) levels, progressive motility, and DNA integrity, six duplicates were used (Susilowati et al., 2022). Spermatozoa viability10 µl sample and 10 µl eosin negrosin (Sigma-Aldrich) were applied to an object glass, which was then air dried. In order to determine the percentage of viable sperm, the specimens were observed using a 400x magnification microscope with 200 spermatozoa. The heads of non-viable spermatozoa were pink, whereas those of live sperm were translucent (Susilowati et al., 2022). Spermatozoa motilityOne milliliter of 0.9% NaCl and ten microliters of homogenized sample sperm were placed on a glass slide. A microscope with warming stages set at 37°C–38°C was used to count the total motility of sperm at 400x magnification (Susilowati et al., 2022). Spermatozoa IPM1 ml of this hypoosmotic solution was incubated with 0.1 ml of semen for 30 minutes at 37°C. 7.35 g of Sigma-Aldrich sodium citrate and 13.52 g of fructose were diluted in 1 l of distilled water to form a hypoosmotic solution. A 400× Olympus BX-53 light microscope (Tokyo, Japan) was used to assess the IPM of 100 sperm. The tails of sperm with damaged membranes were straight, whereas those of sperm with IPM were curled (Susilowati et al., 2022). Malondialdehyde levelsThiobarbituric acid (Sigma-Aldrich) technique was used to measure the MDA levels in the semen samples. MDA kits (0–8 µg/ml), 50 µl of distilled water, and 100 µl of 20% trichloroacetic acid were added to the semen sample. 100 μl of 1% sodium thiobarbiturate, 250 µl of 1N HCl, and another homogenization were added after these combinations had been homogenized for 30 seconds. After centrifuging the mixture for 10 minutes at 28°C and letting the supernatant cool to room temperature, the mixture was incubated for 30 minutes at 100°C in a water bath. A Thermo Fisher Scientific spectrophotometer was used to detect color absorption at a wavelength of 533 nm. Sample absorbance data were extrapolated against the standard MDA curve to determine the MDA levels (ng/ml) (Susilowati et al., 2022). Chromatin densityThe spermatozoa chromatin density was measured using Toluidine Blue staining (Vidal and Mello, 2019). A glass object was smeared with one drop of semen, which was then allowed to air dry before being fixed in 96% ethanol-acetone (1:1) for 30 minutes at 4°C. After fixing, the preparations were allowed to air dry before being hydrolyzed for 5 minutes at 4°C in 0.1 N HCl. The preparations were mixed with 0.05% Toluidine Blue dye for 10 minutes, following three rinses with distilled water. The colored preparations were cleaned twice with xylol, rinsed once more with distilled water, and dehydrated twice with t-butanol (Pourmasumi et al., 2019). The preparations were examined using a 400x magnification Olympus BX-53 microscope. Spermatozoa with dense chromatin have bright blue heads, whereas those with less dense chromatin have blue heads (Agudo-Rios et al., 2023). Analysis was performed on 100 spermatozoa. Data analysisBased on the spermatozoa’s vitality, motility, IPM, MDA levels, DNA integrity, and chromatin density, the quality of the post-thawing Kacang buck spermatozoa was evaluated. The data were analyzed using one-way analysis of variance, and then the Tukey honestly significant difference test was performed at a 95% level of significance. SPSS Version 23 was utilized for statistical analysis. ResultAssessment of fresh semen of Kacang bucksThe spermatic feasibility of fresh semen from Kacang buck was evaluated first. Macroscopic and microscopic analyses were performed after semen collection. The macroscopic analysis included measurements of consistency, pH, color, and volume. A microscope was used to assess spermatozoa mass motility, spermatozoa progressive motility, concentration, and viability. Table 1 displays the assessment results of Kacang bucks’ fresh semen. The addition of EGCG affects the quality of spermatozoa from Kacang bucks both before and after equilibration (5°C)The results of the study show that there was no significant difference (p > 0.05) in sperm motility, viability, or pre-equilibration (before the equilibration stage) at 5°C between the EGCG-added groups at T1 and T2 and the group that did not receive EGCG as a control (T0) (Table 2). In contrast to the control group (T0), which did not receive any EGCG supplementation, the addition of EGCG to groups T1 and T2 produced noticeably higher levels (p < 0.05) of IPM, sperm viability, and motility of post-equilibration (5°C) (Table 3). Table 1. Macroscopic and microscopic characteristics of Kacang buck fresh semen.
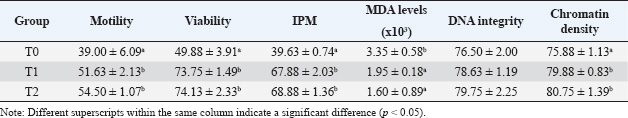
Quality of post-thawed spermatozoa of Kacang bucks according to the presence of EGCGMDA levels were decreased, and post-thawed spermatozoa quality was significantly (p < 0.05) improved when EGCG was introduced to the extender (T1 and T2) as opposed to when it was not (T0). Table 4 demonstrates that the optimal sperm viability, motility, DNA integrity, and IPM were obtained when EGCG was added at T2. This difference was significant for T0 (p < 0.05), but not for T1. Therefore, spermatozoa with the highest levels of viability, motility, IPM, and DNA integrity had the best quality (p < 0.05) when EGCG 100 mg/dl EGCG extender was added. Nevertheless, Table 4 shows that the chromatin density in groups T1 and T2 was not significantly different (p > 0.05), and both were higher (p < 0.05) than in T0. Additionally, EGCG-100 mg/dl supplemented extender media had the lowest MDA levels (p < 0.05) compared with T1 and T0 (Table 4). Statistical significance (p < 0.05) demonstrated that the addition of EGCG to the extender significantly increased total sperm motility following freezing. Both EGCG-based therapies (T1 and T2) demonstrated a total motility of more than 30%, which qualified them for AI. Table 2. The Kacang buck spermatozoa’s motility, viability, and percentage of intact plasma membrane (IPM) in egg yolk milk extender with or without EGCG at pre-equilibration (5°C).
Table 3. Kacang buck spermatozoa motility, viability, and IPM (%) in egg yolk milk extender with or without EGCG after post-equilibration (5°C, 1 hour).
Table 4. Quality of post-thawed Kacang buck spermatozoa in egg yolk milk extender with added EGCG.
DiscussionBecause of its particular structure, activity, and vulnerability to oxidative stress, a spermatozoon is different from other cells (Aitken and Drevet, 2020; Rosyada et al., 2023). Additionally, sperm ROS levels and MDA in such physiological settings were linked, as evidenced by the considerable deterioration in semen quality measures during long-term incubation and cryopreservation of spermatozoa (Qamar et al., 2023). Furthermore, when they freeze and thaw, ROS molecules seriously harm things (Peris-Frau et al., 2020). Because the freezing and thawing cycle can damage Kacang buck spermatozoa, an exogenous antioxidant must be added to the extender medium. According to the current study, EGCG increased a number of microscopic indicators (including chromatin density, DNA integrity, sperm motility, viability, and IPM) and decreased MDA levels when added to the extender medium. These findings imply that EGCG can reduce Kacang buck spermatozoa’s oxidative stress index. Semen quality was lowest in the T0 group both before and after thawing and after equilibration. Sperm had the lowest IPM, motility, and viability levels, and their chromatin density and DNA integrity were also reduced. Furthermore, the sperm had the highest MDA concentrations. Given that freezing semen produces a cold shock in sperm, this makes it is reasonable. The sperm membrane becomes less stable when the cold shock alters the ratio of unsaturated fatty acids and decreases cholesterol (Huang et al., 2018). Each molecule of docosahexaenoic acid in sperm cell membranes has six double bonds, which peroxidize when ROS levels rise. MDA is one of the products of lipid peroxidation (Ahmed et al., 2020). ROS Free radicals have the ability to alter nuclear and mitochondrial DNA and peroxidize lipids. Interchain disulfide bridges created by freezing in protamine-rich chromatin may impact sperm DNA (Juan et al., 2021). EGCG decreases DNA damage, protein carbonylation, and lipid peroxidation, thereby increasing sperm motility and acrosome integrity (Ahmed et al., 2020). EGCG in the extender increased post-thawed semen quality to a specific level. Although antioxidants were unable to completely offset the increased ROS from freeze-thaw, the T1 group’s semen quality was significantly better than that of the control group’s (p < 0.05). Sperm generate antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, and catalase. Nevertheless, a very small quantity of sperm cytoplasm prevented the enzymes from moving to another part of the sperm (Bibov et al., 2018). The consequences of oxidative stress include compromised sperm plasma integrity and reduced sperm motility. Through the action of catechin, EGCG may improve sperm quality by lowering the production of ROS (Rahman et al., 2018). Catechin improves the characteristics of bovine spermatozoa during cryopreservation. In this study, the equilibration process (before freezing) was performed at a temperature of 5°C. EGCG served as a promising protectant for hypothermic liquid preservation. EGCG could effectively eliminate excessive ROS (Wang et al., 2025). In addition to the freezing process, EGCG treatment can reduce oxidative stress caused by excessive ROS formation and maintain normal sperm function associated with ROS (Zhang et al., 2020). In buffalo semen cryopreservation, the addition of EGCG to the extender reduced lipid peroxidation and increased the levels of seminal plasma antioxidant enzymes activities (glutathione peroxidase, superoxide dismutase) compared with the control (Ahmed et al., 2020). The T2 group exhibited the lowest MDA concentrations and the maximum post-thaw motility, viability, chromatin density, and DNA integrity. According to the study findings, the Kacang breed’s buck ejaculate’s average motility of over 70% satisfies the requirements for AI (INSA, 2014). These results are consistent with those of Tvrda et al. (2019), who found that catechin increases the motility of bovine spermatozoa under cryopreservation. Freezing-induced sperm damage, often associated with oxidative stress, can result in regulated cell death. Oxidative stress can trigger various forms of regulated cell death, such as ferroptosis (Hai et al., 2025) or apoptosis (Liu et al., 2025). In buffalo semen freezing, Ahmed et al. (2020) demonstrated that apoptosis-like changes (%) in spermatozoa were significantly decreased in EGCG addition than control. EGCG-100 mg/dl (T2) in an extender medium enhanced vitality and reduced apoptotic spermatozoa in comparison to the control. Similar findings were made by Ahmed et al. (2022), who discovered that rosemary aqueous extract in a cryo-diluent solution enhanced buck spermatozoa that were alive but decreased dead ones. Thus, these findings show that the ratio of dead to surviving spermatozoa can be altered by adding EGCG-100 mg/dl (T2) to an extender media, potentially because of its antioxidant properties. Furthermore, one of the most important semen quality factors influencing the mammalian spermatozoa fertilization is the plasma membrane integrity (Tanga et al., 2021). The main organelle of spermatozoa, the mitochondria, produces ROS. Spermatozoa solely create and synthesize ATP in their mitochondria. Therefore, this organelle deficiency interferes with the system that produces ATP (Bucci et al., 2023). Mitochondria activate the motility apparatus and maintain energy balance. Antioxidants added to extender media can help preserve the structural and functional traits of spermatozoa from stallions, rams, and buffalo bulls while also shielding them from damage caused by cryopreservation (Motlagh et al., 2014; Filho et al., 2017; Ahmed et al., 2020). Similar results from Tvrda et al. (2019) showed that the flavonoid epicatechin increases the mitochondrial activity of bovine spermatozoa during the cryopreservation procedure. Therefore, it has been proposed that EGCG has strong antioxidant qualities that lessen oxidative stress caused by the spermatozoa’s freeze-thaw cycle, thereby mitigating the negative consequences of cryopreservation. The lipid content of the plasmalemma of spermatozoa is degraded by oxidative stress, which also produces ROS (Sanocka and Kurpisz, 2004). The antioxidant defense system of spermatozoa is destroyed by the increasing production of ROS (Agarwal et al., 2021). Additionally, there is a close connection between DNA fragmentation and ROS (Tvrda et al., 2019). Measurements of sperm DNA fragmentation are strongly linked with the likelihood of conception (Rosyada et al., 2020). Since spermatozoa and ovum DNA will join to form a zygote during fertilization, spermatozoa DNA integrity is crucial (Barati et al., 2020). The spermatozoa population’s high proportion of DNA integrity is a prerequisite for the successful development of the zygote, embryo, and fetus to develop successfully (Kumaresan et al., 2020). Our earlier research demonstrated that the freeze-thaw cycle of Kacang buck semen without the addition of an antioxidant extender reduced the integrity of the sperm DNA (Mustofa et al., 2021; Susilowati et al., 2022). Addition of substances that act as antioxidants into the extender, such as green tea extract, antioxidant Simmental bull seminal plasma protein, or insulin-like growth factor-1 complex of Simmental bull seminal plasma (Susilowati et al., 2020; Mustofa et al., 2021; Susilowati et al., 2021). In contrast to the control, extenders enriched with EGCG significantly increased the GPx and SOD concentrations of buffalo bull spermatozoa following freezing, according to Ahmed et al. (2020). Furthermore, the addition of EGCG to extenders dramatically decreased DNA fragmentation after buffalo spermatozoa were thawed. Chromatin quality is one metric used to assess sperm quality. Condensed chromatin causes the sperm head to be more hydrodynamic, allowing it to migrate more easily and reach the egg cell more quickly for the fertilization process (Asmarinah et al., 2016). The makeup of the protamines and histones in sperm determines the quality of their chromatin (Agudo-Rios et al., 2023). At least 85% protamines and 15% histones are present in mature sperm. The composition of protamine, which is higher than that of histones, influences the process of packing sperm DNA into a denser form. Male infertility is linked to sperm with less than ideal chromatin density (Dutta et al., 2019; Moritz and Hammoud, 2022). Sperm’s thick chromatin shields the paternal genome in the nucleus from DNA-damaging substances, including mutagens and nuclease enzymes (Pardede et al., 2021). Fertility was increased in post-thawed semen with greater chromatin integrity (Madrid-Bury et al., 2005). Following the changes in chromatin density caused by freeze-thaw, sperm’s capacity to fertilize decreased (Tamburrino et al., 2023). Reduced density of chromatin in frozen-thawed sperm causes the sperm cell nucleus to become elastic, the sperm head to become asymmetrical, and ultimately disrupts the motility of spermatozoa (Kusumawati et al., 2023). Nonetheless, in this investigation, the chromatin density of post-thawed semen frozen with or without EGCG addition was comparable. Thus, in this case, it is crucial to have a sufficient supply of antioxidants like EGCG since they can prevent ROS from harming Kacang Buck spermatozoa. The quality of Kacang buck spermatozoa after thawing might be significantly improved by adding exogenous antioxidants, like EGCG, to an extender media. ConclusionIn conclusion, the addition of 50 or 100 mg/dl of EGCG to an extender medium improved the post-equilibration and post-thaw microscopic characteristics and the ability of Kacang buck spermatozoa to reproduce while lowering oxidative stress indicators. Adding antioxidants like EGCG to extension media creates a new research topic that could be used in the future. AcknowledgmentThe authors would like to thank Dikky Eka Mandala Putranto, DMV, M.Sc, the Chairman of the Insemination Center, Airlangga University; Subchan Aziz, and Agus Purwanto for their technical support. Conflict of interestThe authors declare no conflict of interest. FundingThis study was funded by Sumber dana Direktorat Riset, Teknologi dan Pengabdian Kepada Masyarakat (DRTPM) (grant number: 1273/UN3.LPPM/PT.01.03/2023. Data availabilityAll data supporting the findings of this study are available within the manuscript, and no additional data sources are required. Author’s contributionsLaboratory work and data collection: SS, IM, and TH. Field sampling: ARK, ZNAR, CDN, and LL. Data analysis and manuscript writing: AOA, TWS, LA, and UFH. Research concept: RZA, YO, and BPP. All authors have read, revised, and approved the final version of the manuscript. ReferencesAgarwal, A., Leisegang, K., Majzoub, A., Henkel, R., Finelli, R., Selvam, M.K.P., Tadros, N., Parekh, N., Ko, E.Y., Cho, C.L., Arafa, M., Alves, M.G., Oliveira, P.F., Alvarez, J.G. and Shah, R. 2021. Utility of antioxidants in the treatment of male infertility: clinical guidelines based on a systematic review and analysis of evidence. World J. Mens. Health 39(2), 233–290. Agudo-Rios, C., Sanchez-Rodriguez, A., Idrovo, I.I.D., Laborda-Gomariz, J.Á., Soler, A.J., Teves, M.E. and Roldan, E.R.S. 2023. Sperm chromatin status and DNA fragmentation in mouse species with divergent mating systems. Int. J. Mol. Sci. 24(21), 15954. Ahmed, H., Jahan, S., Alam, I., Ullah, F. and Ijaz, M.U. 2022. The evaluation of rosemary (Rosmarinus officinalis) leaf extract inclusion in freezing medium on quality parameters of buffalo bull spermatozoa. Cryo Letters 43(2), 91–98. Ahmed, H., Jahan, S., Khan, A., Khan, L., Ullah, H., Riaz, M., Ullah, K. and Ullah, F. 2020. Supplementation of l-tryptophan (an aromatic amino acid) in tris citric acid extender enhances post-thaw progressive motility, plasmalemma, mitochondrial membrane potential, acrosome, and DNA integrities, and in vivo fertility rate of buffalo (Bubalus bubalis) bull spermatozoa. Cryobiology 92(1), 117–123. Ahmed, H., Jahan, S., Riaz, M., Khan, B.T. and Ijaz, M.U. 2020. Epigallocatechin-3-gallate (EGCG) addition as an antioxidant in a cryo-diluent media improves microscopic parameters, and fertility potential, and alleviates oxidative stress parameters of buffalo spermatozoa. Cryobiology 97(1), 101–109. Aitken, R.J. and Drevet, J.R. 2020. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: a two-edged sword. Antioxidants (Basel) 9(2), 111. Asmarinah, Syauqy, A., Umar, L.A., Lestari, S.W., Mansyur, E., Hestiantoro, A. and Paradowszka-Dogan, A. 2016. Sperm chromatin maturity and integrity correlated to zygote development in ICSI program. Syst. Biol. Reprod. Med. 62(5), 309–316. Barati, E., Nikzad, H. and Karimian, M. 2020. Oxidative stress and male infertility: current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 77(1), 93–113. Bibov, M.Y., Kuzmin, A.V., Alexandrova, A.A., Chistyakov, V.A., Dobaeva, N.M. and Kundupyan, O.L. 2018. Role of the reactive oxygen species induced DNA damage in human sperm dysfunction. AME Med. J. 3(1), 19. Bucci, D., Spinaci, M., Bustamante-Filho, I.C. and Nesci, S. 2023. The sperm mitochondria: clues and challenges. Anim. Reprod. 19(4), e20220131. Chakraborty, S. and Saha, S. 2022. Understanding sperm motility mechanisms and the implication of sperm surface molecules in promoting motility. Middle East Fertil. Soc. J. 27(1), 4. Dutta, S., Majzoub, A. and Agarwal, A. 2019. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J. Urol. 17(2), 87–97. Elieser, S., Sumadi, Budisatria, G.S. and Subandriyo. 2012. Productivity comparison between boer and Kacang goat dam. J. Indones. Trop. Anim. Agric. 37(1), 15–21. Filho, J.S., Corcini, C.D., Santos, F.C.C., Anciuti, A.N., Gatti, N.L.S., Anastacio, E., Mielke, R., Nogueira, C.E.W., Curcio, B.R. and Junior, V.A.S. 2017. Quercetin in equine frozen semen. Cryo Letters 38(4), 299–304. Gautier, C. and Aurich, C. 2022. “Fine feathers make fine birds”—The mammalian sperm plasma membrane lipid composition and effects on assisted reproduction. Anim. Reprod. Sci. 246(1), 106884. Güvenç, M., Cellat, M., Gökçek, İ., Yavaş, İ. and Özsoy, Ş.Y. 2019. Effects of thymol and carvacrol on sperm quality and oxidant/antioxidant balance in rats. Arch. Physiol. Biochem. 125(5), 396–403. Hai, E., Li, B., Song, Y., Zhang, J. and Zhang, J. 2025. Ferroptosis emerges as the predominant form of regulated cell death in goat sperm cryopreservation. J. Anim. Sci. Biotechnol. 16(1), 26. Huang, Z., Pang, Y., Hao, H., Du, W., Zhao, X. and Zhu, H. 2018. Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian-Australas. J. Anim. Sci. 31(9), 1420–1430. INSA (Indonesian National Standard Agency). 2014. Frozen Semen–Part 3: goat and sheep. Jakarta. Retrieved September 4, 2022, Available via http://bibit.ditjenpkh.pertanian.go.id/sites/ Juan, C.A., de la Lastra, J.M.P., Plou, F.J. and Pérez-Lebeña, E. 2021. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22(9), 4642. Kowalczyk, A. 2022. The role of the natural antioxidant mechanism in sperm cells. Reprod. Sci. 29(5), 1387–1394. Kumaresan, A., Das Gupta, M., Datta, T.K. and Morrell, J.M. 2020. Sperm DNA integrity and male fertility in farm animals: a review. Front. Vet. Sci. 7(1), 321. Kusumawati, A., Satrio, F.A., Indriastuti, R., Rosyada, Z.N.A., Pardede, B.P., Agil, M. and Purwantara, B. 2023. Sperm head morphology alterations associated with chromatin instability and lack of protamine abundance in frozen-thawed sperm of Indonesian local bulls. Animals (Basel) 13(15), 2433. Liu, F., Dai, J., Gao, J., He, M., Xu, J., Wu, C., Zhang, S., Zhu, X. and Sun, L. 2025. BGP-15 improves quality of goat sperm by mitigating oxidative stress during cryopreservation. Cryobiology 119(1), 105232. Liu, T., Han, Y., Zhou, T., Zhang, R., Chen, H., Chen, S. and Zhao, H. 2019. Mechanisms of ROS-induced mitochondria-dependent apoptosis underlying liquid storage of goat spermatozoa. Aging (Albany NY) 11(18), 7880–7898. Luo, J., Wang, W. and Sun, S. 2019. Research advances in reproduction for dairy goats. Asian-Australas. J. Anim. Sci. 32(8), 1284–1295. Madrid-Bury, N., Pérez-Gutiérrez, J.F., Pérez-Garnelo, S., Moreira, P., Sanjuanbenito, B.P., Gutiérrez-Adán, A. and de la Fuente Martínez, J. 2005. Relationship between non-return rate and chromatin condensation of deep frozen bull spermatozoa. Theriogenology 64(2), 232–241. Moritz, L. and Hammoud, S.S. 2022. The art of packaging the sperm genome: molecular and structural basis of the histone-to-protamine exchange. Front. Endocrinol. (Lausanne) 13(1), 895502. Motlagh, M.K., Sharafi, M., Zhandi, M., Mohammadi-Sangcheshmeh, A., Shakeri, M., Soleimani, M. and Zeinoaldini, S. 2014. Antioxidant effect of rosemary (Rosmarinus officinalis L.) extract in soybean lecithin-based semen extender following freeze-thawing process of ram sperm. Cryobiology 69(2), 217–222. Mustofa, I., Susilowati, S., Suprayogi, T.W., Akintunde, A.O., Oktanella, Y. and Purwanto, D.A. 2023a. Epigallocatechin-3-gallate chitosan nanoparticles in an extender improve the antioxidant capacity and post-thawed quality of Kacang goat semen. F1000Res. 12(1), 32. Mustofa, I., Susilowati, S., Suprayogi, T.W., Oktanella, Y., Purwanto, D.A. and Akintunde, A.O. 2023b. Combination of nanoparticle green tea extract in tris-egg yolk extender and 39°C thawing temperatures improve the sperm quality of post-thawed Kacang goat semen. Anim. Reprod. 19(4), e20220025. Mustofa, I., Susilowati, S., Wurlina, W., Hernawati, T. and Oktanella, Y. 2021. Green tea extract increases the quality and reduced DNA mutation of post-thawed Kacang buck sperm. Heliyon 7(3), e06372. Oktanella, Y., Mustofa, I., Susilowati, S., Hendrawan, V. and Hernawati, T. 2024. Effect of cryopreservation duration time on post-thawing sperms’ characteristics of goat semen. J. Vet. 25 (2), 175–185. Pardede, B.P., Maulana, T., Kaiin, E.M., Agil, M., Karja, N.W.K., Sumantri, C. and Supriatna, I. 2021. The potential of sperm bovine protamine as a protein marker of semen production and quality at the National Artificial Insemination Center of Indonesia. Vet. World 14(9), 2473–2481 Peris-Frau, P., Soler, A.J., Iniesta-Cuerda, M., Martín-Maestro, A., Sánchez-Ajofrín, I., Medina-Chávez, D.A., Fernández-Santos, M.R., García-Álvarez, O., Maroto-Morales, A., Montoro, V. and Garde, J.J. 2020. Sperm cryodamage in ruminants: understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci. 21(8), 2781. Pourmasumi, S., Khoradmehr, A., Rahiminia, T., Sabeti, P., Talebi, A.R. and Ghasemzadeh, J. 2019. Evaluation of sperm chromatin integrity using aniline blue and toluidine blue staining in infertile and normozoospermic men. J. Reprod. Infertil. 20(2), 95–101. Prastiya, R.A., Suprayogi, T.W., Debora, A.E., Wijayanti, A., Amalia, A., Sulistyowati, D. and Nugroho, A.P. 2023. Green tea extract addition into a Tris-based egg yolk extender improves Bali bull sperm quality. Anim. Biosci. 36(2), 209–217. Qamar, A.Y., Naveed, M.I., Raza, S., Fang, X., Roy, P.K., Bang, S., Tanga, B.M., Saadeldin, I.M., Lee, S. and Cho, J. 2023. Role of antioxidants in fertility preservation of sperm—a narrative review. Anim. Biosci. 36(3), 385–403. Rahman, S.U., Huang, Y., Zhu, L., Feng, S., Khan, I.M., Wu, J., Li, Y. and Wang, X. 2018. Therapeutic role of green tea polyphenols in improving fertility: a review. Nutrients 10(7), 834. Rosyada, Z.N.A., Pardede, B.P., Kaiin, E.M., Tumbelaka, L.I.T.A., Solihin, D.D., Purwantara, B. and Ulum, M.F. 2023. Identification of heat shock protein70-2 and protamine-1 mRNA, proteins, and analyses of their association with fertility using frozen-thawed sperm in Madura bulls. Anim. Biosci. 36(12), 1796–1805. Rosyada, Z.N.A., Ulum, M.F., Tumbelaka, L.I.T.A. and Purwantara, B. 2020. Sperm protein markers for Holstein bull fertility at national artificial insemination centers in Indonesia. Vet. World 13(5), 947–955. Sanocka, D. and Kurpisz, M. 2004. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2(1), 12. Sujarwanta, R.O., Afidah, U., Suryanto, E., Rusman, Triyannanto, E. and Hoffman, L.C. 2024. Review: goat and sheep meat production in Indonesia. Sustainability 16(11), 4448. Susilowati, S., Mustofa, I., Wurlina, W., Hernawati, T., Oktanella, Y., Soeharsono, S. and Purwanto, D.A. 2022. Green tea extract in the extender improved the post-thawed semen quality and decreased amino acid mutation of Kacang buck sperm. Vet. Sci. 9(8), 403. Susilowati, S., Mustofa, I., Wurlina, W., Triana, I.N., Utama, S. and Rimayanti, R. 2021. Effect of insulin-like growth factor-1 complex of Simmental bull seminal plasma on post-thawed Kacang buck semen fertility. Vet. World 14(8), 2073–2084. Susilowati, S., Triana, I.N., Sardjito, T., Suprayogi, T.W., Wurlina, W. and Mustofa, I. 2020. Effect of Simmental bull seminal plasma protein in egg yolk-citrate extender on Kacang buck semen fertility. Cryobiology 2020; 97(1), 20–27. Tamburrino, L., Traini, G., Marcellini, A., Vignozzi, L., Baldi, E. and Marchiani, S. 2023. Cryopreservation of human spermatozoa: functional, molecular and clinical aspects. Int. J. Mol. Sci. 24(5), 4656. Tanga, B.M., Qamar, A.Y., Raza, S., Bang, S., Fang, X., Yoon, K. and Cho, J. 2021. Semen evaluation: methodological advancements in sperm quality-specific fertility assessment—a review. Anim. Biosci. 34(8), 1253–1270. Tvrda, E., Straka, P., Galbavy, D. and Ivanic, P. 2019. Epicatechin provides antioxidant protection to bovine spermatozoa subjected to induced oxidative stress. Molecules 24(18), 3226. Vidal, B.C. and Mello, M.L.S. 2019. Toluidine blue staining for cell and tissue biology applications. Acta. Histochem. 121(2), 101–112. Wang, L., Xiong, M., Li, S., Ma, S., Jiang, S., Wang, H., Zhang, J. and Li, X. 2025. Beneficial effects of EGCG on boar sperm quality during liquid storage at 4°C are mediated by DRD2 receptor. Theriogenology 234(1), 174–185. Zhang, W., Min, L., Li, Y., Lang, Y., Hoque, S. A. M., Adetunji, A. O. and Zhu, Z. 2022a. beneficial effect of proline supplementation on goat spermatozoa quality during cryopreservation. Animals 12(19), 2626. Zhang, X., Hu, Z.T., Li, Y., Li, Y.X., Xian, M., Guo, S.M. and Hu, J.H. 2022b. Effect of astragalus polysaccharides on the cryopreservation of goat semen. Theriogenology 193(1), 47–57. Zhang, Y., Lin, H., Liu, C., Huang, J. and Liu, Z. 2020. A review for physiological activities of EGCG and the role in improving fertility in humans/mammals. Biomed. Pharmacother. 127(1), 110186. Zhao, J., Meng, P., Jin, M., Ma, X., Ma, H., Yang, H., Chen, Y., Zhang, J., Zhang, Y., Luo, Y. and Liu, J. 2023. Combined addition of L-carnitine and L-proline improves cryopreservation of dairy goat semen. Anim. Reprod. Sci. 257(1), 107325. Zhu, Z., Li, W., Ding, K., Bastawy, E.M., Kamel, A.M., Kou, X. and Min, L. 2025. Ellagic acid maintains post-thaw goat sperm quality via protecting mitochondrial function from ROS damage. Cryobiology 119(1), 105231, Zou, J., Wei, L., Li, D., Zhang, Y., Wang, G., Zhang, L., Cao, P., Yang, S. and Li, G. 2021. Effect of glutathione on sperm quality in Guanzhong dairy goat sperm during cryopreservation. Front. Vet. Sci. 8(1), 771440. | ||
| How to Cite this Article |
| Pubmed Style Susilowati S, Mustofa I, Rosyada ZNA, Suprayogi TW, Hernawati T, Akintunde AO, Oktanella Y, Khairullah AR, Handayani UF, Ahmad RZ, Anggraini L, Pratama BP, Nugraha CD, Latifah L. Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extender. Open Vet. J.. 2025; 15(8): 3703-3710. doi:10.5455/OVJ.2025.v15.i8.34 Web Style Susilowati S, Mustofa I, Rosyada ZNA, Suprayogi TW, Hernawati T, Akintunde AO, Oktanella Y, Khairullah AR, Handayani UF, Ahmad RZ, Anggraini L, Pratama BP, Nugraha CD, Latifah L. Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extender. https://www.openveterinaryjournal.com/?mno=247688 [Access: January 26, 2026]. doi:10.5455/OVJ.2025.v15.i8.34 AMA (American Medical Association) Style Susilowati S, Mustofa I, Rosyada ZNA, Suprayogi TW, Hernawati T, Akintunde AO, Oktanella Y, Khairullah AR, Handayani UF, Ahmad RZ, Anggraini L, Pratama BP, Nugraha CD, Latifah L. Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extender. Open Vet. J.. 2025; 15(8): 3703-3710. doi:10.5455/OVJ.2025.v15.i8.34 Vancouver/ICMJE Style Susilowati S, Mustofa I, Rosyada ZNA, Suprayogi TW, Hernawati T, Akintunde AO, Oktanella Y, Khairullah AR, Handayani UF, Ahmad RZ, Anggraini L, Pratama BP, Nugraha CD, Latifah L. Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extender. Open Vet. J.. (2025), [cited January 26, 2026]; 15(8): 3703-3710. doi:10.5455/OVJ.2025.v15.i8.34 Harvard Style Susilowati, S., Mustofa, . I., Rosyada, . Z. N. A., Suprayogi, . T. W., Hernawati, . T., Akintunde, . A. O., Oktanella, . Y., Khairullah, . A. R., Handayani, . U. F., Ahmad, . R. Z., Anggraini, . L., Pratama, . B. P., Nugraha, . C. D. & Latifah, . L. (2025) Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extender. Open Vet. J., 15 (8), 3703-3710. doi:10.5455/OVJ.2025.v15.i8.34 Turabian Style Susilowati, Suherni, Imam Mustofa, Zulfi Nur Amrina Rosyada, Tri Wahyu Suprayogi, Tatik Hernawati, Adeyinka Oye Akintunde, Yudit Oktanella, Aswin Rafif Khairullah, Ulvi Fitri Handayani, Riza Zainuddin Ahmad, Lili Anggraini, Bima Putra Pratama, Chairdin Dwi Nugraha, and Latifah Latifah. 2025. Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extender. Open Veterinary Journal, 15 (8), 3703-3710. doi:10.5455/OVJ.2025.v15.i8.34 Chicago Style Susilowati, Suherni, Imam Mustofa, Zulfi Nur Amrina Rosyada, Tri Wahyu Suprayogi, Tatik Hernawati, Adeyinka Oye Akintunde, Yudit Oktanella, Aswin Rafif Khairullah, Ulvi Fitri Handayani, Riza Zainuddin Ahmad, Lili Anggraini, Bima Putra Pratama, Chairdin Dwi Nugraha, and Latifah Latifah. "Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extender." Open Veterinary Journal 15 (2025), 3703-3710. doi:10.5455/OVJ.2025.v15.i8.34 MLA (The Modern Language Association) Style Susilowati, Suherni, Imam Mustofa, Zulfi Nur Amrina Rosyada, Tri Wahyu Suprayogi, Tatik Hernawati, Adeyinka Oye Akintunde, Yudit Oktanella, Aswin Rafif Khairullah, Ulvi Fitri Handayani, Riza Zainuddin Ahmad, Lili Anggraini, Bima Putra Pratama, Chairdin Dwi Nugraha, and Latifah Latifah. "Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extender." Open Veterinary Journal 15.8 (2025), 3703-3710. Print. doi:10.5455/OVJ.2025.v15.i8.34 APA (American Psychological Association) Style Susilowati, S., Mustofa, . I., Rosyada, . Z. N. A., Suprayogi, . T. W., Hernawati, . T., Akintunde, . A. O., Oktanella, . Y., Khairullah, . A. R., Handayani, . U. F., Ahmad, . R. Z., Anggraini, . L., Pratama, . B. P., Nugraha, . C. D. & Latifah, . L. (2025) Reduction of post-thawed sperm quality of Kacang goat using the addition of epigallocatechin-3-gallate into the skim milk–egg yolk extender. Open Veterinary Journal, 15 (8), 3703-3710. doi:10.5455/OVJ.2025.v15.i8.34 |