| Research Article | ||
Open Vet. J.. 2025; 15(4): 1790-1797 Open Veterinary Journal, (2025), Vol. 15(4): 1790-1797 Research Article Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigationFawiziah Khalaf Alharbi*Department of Biology, College of Science, Buraydah, Qassim University, Buraydah, Saudi Arabia. *Corresponding Author: Fawiziah Khalaf Alharbi, Department of Biology, College of Science, Buraydah, Qassim University, Buraydah, Saudi Arabia. Email: hrbief [at] qu.edu.sa Submitted: 12/02/2025 Accepted: 06/04/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
AbstractBackground: One of the primary concerns of the pharmaceutical industry is the potential for liver damage caused by drugs. Diclofenac is a member of the family of nonsteroidall anti-inflammatory medications, which are known for their anti-inflammatory, antipyretic, and analgesic effects. Although it is commonly prescribed, liver damage is one of the primary risks associated with this medication. Therefore, it is necessary to use a prospective therapeutic medication that can counteract the cytotoxic impact of diclofenac. Humans have utilized natural items as a means of obtaining sustenance, maintaining their health, and treating a wide variety of illnesses. Antioxidant, anti-inflammatory, and liver-protective properties are associated with silymarin, which is a flavonoid chemical derived from the seeds of the Silybum marianum plant. Aim: This study aimed to evaluate the possible hepatoprotective effect of silymarin against diclofenac-induced liver injury. Methods: Forty Wistar rats were used in the current investigation and grouped randomly into four equal groups. The first group (G1) was kept as a control group. G2 received intraperitoneal injections of diclofenac (50 mg/kg. BW/day) for 5 days. G3 received oral silymarin for 5 days (200 mg/kg BW/day) dissolved in distal water. G4 received diclofenac plus silymarin with the same previously mentioned doses for 5 days. The animals were collected and subjected to histopathological examination. Results: The diclofenac-treated group showed several pathological changes within the hepatic parenchyma, including diffuse coagulative and hydropic degenerations with general disorganization of the hepatic cords, severe dilatation and congestion of the central vein and portal blood vessels, severe sinusoidal dilatation and congestions accompanied with severe pressure atrophy of the hepatic cords, and severe inflammatory cell infiltrations. Meanwhile, the diclofenac plus silymarin-treated group showed a significant good effect, where silymarin ameliorated almost all histopathological lesions induced after diclofenac treatment as mitigating the degenerative changes of hepatocytes, minimizing inflammatory cell infiltrations, and normal organized hepatic rays with normal sinusoids in between were distinguished. Conclusion: Silymarin has a significant hepatoprotective effect against diclofenac-induced hepatotoxicity through neutralization of all histopathological injuries. Keywords: Silymarin, Diclofenac, Hepatotoxicity, Hepatoprotective, Histopathological IntroductionInduced liver damage by drugs is a major concern about doctors and drug companies alike (Labbe et al., 2008). Most pharmaceuticals prescribed are nonsteroidall anti-inflammatory drugs (NSAIDs), which are known for their anti-inflammatory, analgesic, and antipyretic properties. However, because they are harmful to the liver, several of these drugs have been taken off the market (Masubuchi et al., 1998; Boelsterli, 2013). The analgesic, antipyretic, and antiinflammatory properties of diclofenac are well known. It belongs to the class of NSAIDs (Ertekin et al., 2019). All types of muscle pain, such as rheumatoid arthritis, osteoarthritis, spondylosis, and ankylosing spondylitis, are pain-related illnesses that can be alleviated by treatment (Klomjit and Ungprasert, 2022). These goals are accomplished by preventing the production of physiological and inflammatory prostaglandins (Ertekin et al., 2019). Hepatotoxicity ranks high among the most serious adverse effects of this drug, while some patients may experience persistent gastrointestinal and renal toxicity (Todd and Sorkin, 1988; Klomjit and Ungprasert, 2022). An NSAID’s principal mode of action is blocking the cyclooxygenase (COX) enzyme, which is responsible for converting arachidonic acid to prostaglandin. Pain, fever, and inflammation are all reduced as a result (Moore et al., 2015). Nevertheless, various studies have shown that Diclofenac-induced hepatotoxicity can be partially explained by factors such as mitochondrial injury (Siu et al., 2008; Adeyemi and Olayaki, 2018), oxidative stress (Galati et al., 2002), and changes in covalent protein integrity caused by reactive metabolites (Gil et al., 1995). Diaclofenac has been included in the FDA’s black box warning list because of concerns regarding its mitochondrial liabilities, hepatotoxicity, and cardiovascular toxicity (Dykens and Will, 2007). Ensuring patient safety and effective inflammation and pain management requires an understanding of this risk and the implementation of strategies to mitigate it. Therefore, a potential therapeutic agent that blocks diclofenac’s capacity to activate any of the pathogenic pathways could be used to prevent or reverse the drug’s cytotoxic effects. Natural commodities are used by humans for food production, health maintenance, and the treatment of various diseases (Dillard and German, 2000). Research conducted by Surai (2015) and Yassin et al. (2022) indicated that silymarin, a chemical derived from plants, has multiple protective effects. This compound possesses a wide range of beneficial properties, including antioxidant, anti-inflammatory, antiviral, antifibrotic, immunomodulatory, and liver-protective effects. The polyphenolic flavonoid silymarin is extracted from milk thistle seed (Silybum marianum), which belongs to Asteraceae family (Sherif and Al-Gayyar, 2013). A powerful free radical scavenger, silymarin shields liver cells from deleterious consequences of oxidative stress (Teschke, 2018). To accomplish this, it boosts glutathione production, which in turn enhances the liver’s antioxidant capacity and inhibits the activity of several reactive oxygen species (ROS), as stated by Esmaeil et al. (2017). Additionally, research has demonstrated that silymarin can inhibit the growth of skin and liver cancer cells (Gu et al., 2015). The purpose of this study was to determine if silymarin can prevent liver damage caused by diclofenac. Materials and methodsChemicalsDiclofenac sodium was obtained from the El-Nasr Pharmaceutical Chemical Company, Egypt. Silymarin (80% purity) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Animals and housingForty adult male Wistar albino rats, all apparently healthy, were used in this study. The rats were from the animal unit of the Veterinary Medicine Faculty of Zagazig University in Egypt. The rats had an average age of 3 months and weighed 170 ± 20 grams. With balanced feed and water available at all times, the animals were housed in transparent polypropylene cages with 10 rats per cage in typical laboratory settings. All procedures used in the experiments were approved by the National Research Center, Egypt’s Ethical Committee, and all regulations on the treatment of laboratory animals were followed. Study design and treatmentThere were 40 rats in total, with 10 animals in each of the four groups: The first group, G1, consisted of rats that served as controls and were fed a standard rat diet without the addition of any drugs, such as diclofenac or silymarin.The second group, G2, rats were given 50 mg/kg BW/day of diclofenac via intraperitoneal (i.p.) injections for five consecutive days. Following the guidelines of Giridharan et al. (2017), the dosage of Diclofenac was administered. The third group (G3) was given 200 mg/kg BW/day of Silymarin dissolved in distal water orally for 5 days. Kropacova et al. (1998) and Zaidi and Mahboob (2017) were followed when administering the Silymarin dosage. The fourth group (G4) received diclofenac (50 mg/kg. BW/day, i.p.) and Silymarin (200 mg/kg. BW/day, orally) dissolved in distal water for a duration of 5 days. Histopathological evaluationsThe experiment ended with the anesthesia-induced euthanasia of the animals. The liver was exposed during animal dissection. Quickly after that, the livers were cut into small pieces for histological examination, and those portions were kept in 10% neutral buffered formalin for 48 h. Paraffin blocks were made by dehydrating specimens in escalating ethanol grades, cleaning them with xylene, infiltrating them with soft melted paraffin in a hot-air oven, and finally embedding them in firm paraffin wax. Before being placed on glass slides, the paraffin blocks were sectioned transversely using a rotatory microtome to achieve a thickness of 4–5 μm. Regular histological examinations were conducted on the obtained sections using hematoxylin and eosin (H&E) staining (Suvarna et al., 2018). Ethical approvalThe Institutional Animal Care and Use Committee of Zagazig University in Egypt assessed and approved this study protocol, assigning it the approval number (ZU”IACUC; No. ZU-IACUC/ 2 / F/ 1 /2025). ResultsThe livers of adult male Wistar albino rats in the control group (G1) were found to be normal, with no abnormalities detected through histopathology. The hepatic parenchyma primarily consists of many typical hepatic lobules. The central vein (Fig. 1a) and portal triad (Fig. 1b) form the center and peripheral boundaries of each lobule, respectively. Hepatocytes, which are the primary cells in each lobule, have a variety of nuclei, including single, spherical, central, and euchromatic, as well as some binucleated cells. Their morphology was irregular and polygonal. Hepatocytes were guided to the outer edges of each lobule by a series of radiations that extended dorsally from the central vein. The hepatic rays that supplied the hepatocytes also contained intact hepatic sinusoids with intact lining epithelium (Fig. 1b). It seemed as though the branches of the bile duct, lymph vessel, portal vein, hepatic artery, nerves, and their lining epithelium were all in good health inside the portal triad (Fig.1c).On the other hand, histological analysis of liver samples taken from the G2; Diclofenac treated group revealed the presence of diffuse coagulative necrotic cells, which had a ghostly appearance, enlarged hepatocytes, hypereosinophilic cytoplasm, and nuclear disappearance (Fig. 2a). The hallmarks of ballooning degeneration, a type of diffuse hydropic degeneration, are enlarged and inflated hepatocytes, pale cytoplasm, tiny pyknotic nuclei, and overall disarray of the hepatic cords (Fig. 2b). The hallmark of this enlargement degeneration was the abundance of vacuoles within the cytoplasm, which could vary in size and shape (Fig. 2b). Figure 2c shows that the central vein was severely dilated and congested (full of red blood cells), and Figure 2d shows that in some areas of the vein, the lining epithelium had severely degenerated, allowing lining cells to slough into the lumen. Hepatic cord pressure atrophy and significant sinusoidal dilatation with congestion were noted (Fig. 2e). In addition, a significant enlargement of the portal blood vessels, acute bleeding, and extravasated red blood cells were observed among the hepatocytes (Fig. 2f, g). In addition to bile duct hyperplasia and extensive vacuolations of the ductal lining epithelium, the portal triad showed significant congestion and blood vessel dilatation (Fig. 2h). Figure 2i shows that many coagulative necrotic hepatocytes encircled severely dilated and crowded blood vessels inside the portal triad, as well as severe inflammatory cell infiltrations, primarily lymphocytes. The G3; silymarin-treated group showed no abnormalities in their livers and had normal, intact hepatic parenchyma (Fig. 3a). Hepatocytes, which are normally arranged as hepatic rays or cords with intact hepatic sinusoids among the cords with intact lining epithelium, were found inside the hepatic lobules. These cells had an irregular polygonal shape and a single central spherical euchromatic nucleus (Fig. 3b). The portal triad also showed normal, unbroken blood vessels with normal lining epithelium (Fig. 3c). In comparison to the G2; Diclofenac treated group, the G4; Diclofenac plus Silymarin group demonstrated significantly better liver function, with hepatic parenchyma that appeared normal, with intact hepatocytes, vasculature, and obvious hepatic cords; and normal tissue architecture and cellular details devoid of abnormalities (Fig. 4a, b). The hepatic parenchyma revealed only individual coagulative necrotic cells and mild hydropic degenerative alterations of hepatocytes in certain sections that were investigated (Fig. 4c). Normal structured hepatic rays encircle minor degenerative alterations of the central vein lining epithelium (Fig. 4d) and mild-to-moderate congestion (Fig. 4e). Additionally, it was noted that there was mild inflammatory cell infiltration between the hepatocytes (Fig. 4f) and around the blood vessels within the portal triad (Fig. 4g). Also noted were mild sinusoidal dilatations, mild congestions, and the absence of pressure atrophy in the hepatic cords (Fig. 4h). DiscussionThe pharmaceutical sector is concerned about drug-induced liver damage (Labbe et al., 2008). A member of the NSAID family, diclofenac, is frequently prescribed due to its anti-inflammatory, antipyretic, and analgesic (painkilling) effects. However, it is important to note that diclofenac can cause liver toxicity (Todd and Sorkin, 1988; Masubuchi et al., 1998; Boelsterli, 2013). According to Aljuhani et al. (2019), diclofenac can cause acute poisoning if taken in large doses too quickly or multiple times in a short period. Therefore, it is important to employ a prospective medicinal substance that can avert or undo the cytotoxic effects of diclofenac. Both humans and animals have long relied on natural products for a wide range of needs, including sustenance, health care, and illness treatment. According to Ramezannezhad et al. (2019), silymarin, a flavonoid component derived from seeds of the Silybum marianum plant, has antioxidant and anti-inflammatory properties.Histological examination of the liver in the G2; Diclofenac treated group showed several abnormalities, including diffuse coagulative necrosis, diffuse hydropic degeneration (ballooning degeneration), swollen and enlarged hepatocytes, small pyknotic nuclei, and pale cytoplasm. The hepatic cords were generally disorganized, with central vein dilatation and congestion as well as severe sinusoidal dilatation and congestion. There was also severe pressure atrophy of the hepatic cords, as well as inflammatory cell infiltration (primarily lymphocytes) around severely dilated and congested blood vessels. Furthermore, hyperplasia of the bile ducts was noted within the portal triad. Aydin et al. (2003) discovered that rats that were given diclofenac had liver abnormalities, such as cloudy swelling, hydropic degeneration of hepatocytes, focal dilatation of the sinusoidal and vena centralis bile ducts, enlarged periportal areas with mononuclear cell infiltration, hyperemia, and dose-dependent proliferation of fibrous tissues and focal necrosis. These findings agree with our own findings. Further research conducted by Sagãstegui-Guarniz William Antonio et al. (2020) in rats revealed that the administration of diclofenac resulted in the development of liver lesions. An aberrant arrangement of the hepatic cord in relation to the central vein is one of these diseases. Other lesions included chromatin condensation or pyknosis, fatty alterations, and degenerative and necrotic hepatocytes. Furthermore, a study conducted by Ramezannezhad et al. (2019) revealed that a group administered 50 mg/kg diclofenac intraperitoneally (i.p.) exhibited histological abnormalities and infiltration of mononuclear cells, which indicated liver toxicity. This was in contrast to the group that served as the control. Furthermore, Tabari et al. (2024) discovered that the group given diclofenac experienced a considerable increase in immune cell infiltration as well as a decline in fatty hepatocytes. Further evidence that diclofenac administration effectively co-induced liver injury was provided by the altered blood biochemical indicators of liver function, serum levels of liver enzyme activity, and liver tissue histology. Additional evidence supporting a role for oxidative stress in diclofenac-induced kidney and liver damage has been found in the liver and kidney tissues of diclofenac-intoxicated mice, where levels of malondialdehyde (MDA) are increased and levels of antioxidant protection enzymes, superoxide dismutase (SOD), and catalase (CAT) are decreased (Gãmez-Lechãn et al., 2003; Fattori et al., 2017; Al-Dossari et al., 2020; Aboubakr et al., 2021; Heidarian and Nouri, 2021; Basist et al., 2022). As a result of oxidative stress-induced cellular damage, it is highly probable that the liver and kidneys have lost both their functional and structural integrity (Alabi and Akomolafe, 2020; Al-Dossari et al., 2020). Additionally, Heidarian and Nouri (2021) found that the control group had significantly lower serum levels of liver enzymes in comparison to the diclofenac treatment group. This was discovered by comparing the two groups at the same time. Based on this information, diclofenac has hepatotoxic potential, causing damage to the membranes of hepatic tissues and enzyme release into the circulation. Compared with the liver of the group that was just given diclofenac, the liver of the group that was given both diclofenac and silymarin performed significantly better. Within the first group, the hepatic parenchyma appeared to be normal, with normal tissue architecture and cellular details. There were no abnormalities, and the hepatocytes, vasculature, and hepatic cords were all in their original state. On the other hand, silymarin relieved diclofenac-induced histopathological damage, which is comparable to the degenerative modifications that occur in hepatocytes. Moreover, it reduces the inflammatory response and immune cell infiltration. Furthermore, the hepatic cords did not exhibit any signs of pressure atrophy, and the hepatic radiographs were presented in a well-organized manner, with typical sinusoids located in the middle. In addition, these findings were in complete agreement with those of El-Kot et al. (2023), who observed that the administration of silymarin resulted in the recovery of hepatocyte and lobule architecture, the presence of eosinophilic granules in the cytoplasm of hepatocytes, the restoration of the arrangement of the hepatic cord as a whole, and the presence of intervening blood sinusoids along with some blood congestion in the portal artery branch and nuclei of cellular infiltrate in the portal triad stroma. The groups treated with silymarin showed a clear decrease in inflammatory cell infiltration, which led to a noticeable improvement and recovery toward normality for nearly all of the liver histological lesions (Ramezannezhad et al., 2019). This was compared with the groups exposed to diclofenac. The group given 100 mg/kg silymarin experienced a reduction in the number of inflammatory cells, whereas the group given 200 mg/kg silymarin experienced a higher reduction. In addition, the researcher observed that silymarin administration increased glutathione peroxidase (GPx), SOD, intracellular glutathione (GSH), and CAT. Furthermore, the researcher observed a decrease in total bilirubin, alanine aminotransferase (AST), nitrite content, alanine aminotransferase (ALT), MDA, serum tumor necrosis factor-α (TNF-α), and TNF-α gene expression. These findings provide evidence of the antioxidant and anti-inflammatory properties of silymarin. The release of the cytokine TNF-α occurs both in inflammatory environments and during the migration of macrophages. According to Tracey (2002), TNF-α causes oxidative stress, which worsens liver cell damage. In addition, Heidarian and Nouri (2021) demonstrated that silymarin administration at doses of 100 or 200 mg/kg counteracted the effects of diclofenac on the systemic levels of AST, ALT, ALP, and total bilirubin. He postulated that silymarin prevented the leakage of these enzymes into the plasma by protecting the structural integrity of hepatocyte membranes. In addition, prior research has indicated that silymarin has antioxidant and anti-inflammatory effects, which may explain why these liver enzyme levels were lower in the group that received silymarin (García-Ramírez et al. 2018, Zhang et al. 2018). Heidarian and Nouri (2021) further showed that silymarin has antioxidant properties and can reduce oxidative stress by restoring SOD and CAT levels in rats treated with silymarin.
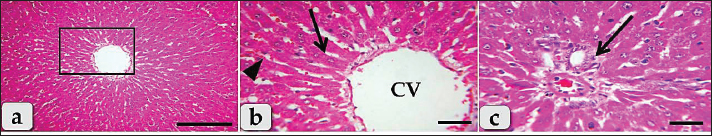
Fig. 1. Photomicrographs of the liver of the G1; control group, (a) showing normal, intact hepatic parenchyma without any abnormalities. (b) Higher magnification of the inset box in fig. a showing intact hepatic parenchyma with normal hepatocytes of an irregular polygonal shaped cells with single, central spherical nucleus, and intact hepatic cords (arrow), intact hepatic sinusoids with intact lining epithelium (arrow head), intact central vein (CV) with normal lining epithelium. (c) showing normal and intact portal blood vessels (arrow) of portal triad. Staining: H&E. Scale bars: All=30 μm, except a=200 μm.
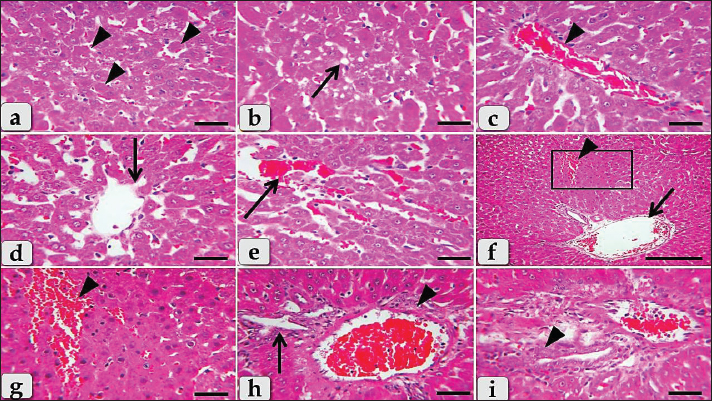
Fig. 2. Photomicrographs of the liver of G2 (Diclofenac treated group), (a) Diffuse coagulative necrotic cells characterized by enlarged cells, hypereosinophilic cytoplasm with nuclear disappearance (arrowheads). (b) Showing diffuse hydropic degenerations (ballooning degeneration) characterized with formation of vacuoles of variable shapes and sizes within the hepatocytes cytoplasm with sever disorganization of the hepatic cords (arrow). (c) Showing severe dilatation and congestion of the central vein (arrow head). (d) Showing severe degenerative changes of the central vein lining epithelium (arrow). (e) Sever sinusoidal dilatation with congestions (arrow) accompanied with severe pressure atrophy of hepatic cords. (f) Showing severe hemorrhage; extravasated RBCs (arrowhead) in between hepatocytes beside sever portal blood vessels dilatation (arrow). (g) Higher magnification of the inset box in fig. f showing the wide area of hemorrhage (arrow head). (h) Showing severe dilatation and congestion of portal blood vessels (arrow head) with bile duct hyperplasia (arrow) accompanied with sever vacuolations of the ductal lining epithelium. (i) Showing severe inflammatory cells infiltrations of mainly lymphocytes surrounding blood vessels within the portal triad (arrow head). Staining: H&E. Scale bars: All=30 μm, except f=200 μm.
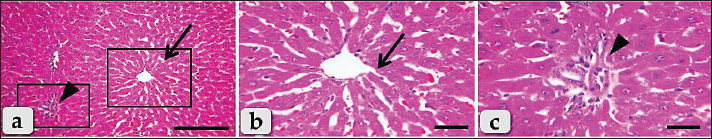
Fig. 3. Photomicrographs of the liver of G3 (Silymarin treated group), (a) showing normal, intact hepatic parenchyma without any abnormalities with normal central vein surrounded with intact hepatic rays (arrow) with normal intact blood vessels of portal triad (arrow head). (b and c) Higher magnification of the inset boxes in fig. a showing the same; intact hepatic parenchyma with normal and intact hepatic cords (arrow), intact central vein with normal lining epithelium of fig. b. and intact portal blood vessels (arrow head) of portal triad (fig. c). Staining: H&E. Scale bars: All=30 μm, except a=200 μm.
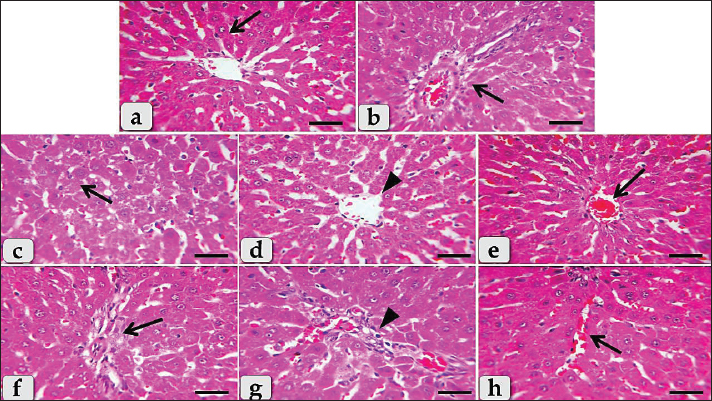
Fig. 4. Photomicrographs of the liver of G4 (Diclofenac plus Silymarin treated group), (a) showing normal, intact hepatic parenchyma with normal central vein surrounded with intact hepatic rays (arrow). (b) showing normal, intact blood vessels of portal triad (arrow). (c) Showing very few or individual coagulative necrotic cells within the hepatic parenchyma (arrow) with mild to moderate hydropic degenerative changes of hepatocytes. (d) Showing mild degenerative changes of the central vein lining epithelium (arrowhead) surrounded with normal organized hepatic rays. (e) Showing mild to moderate congestion of the central vein (arrow). (f) Showing mild inflammatory cells infiltrations of mainly lymphocytes in between the hepatocytes within the hepatic parenchyma (arrow). (g) Showing mild inflammatory cells infiltrations of mainly lymphocytes surrounding blood vessels within the portal triad (arrow head). (h) showing mild sinusoidal dilatation with congestions (arrow) accompanied with no any pressure atrophy of hepatic cords. Staining: H&E. Scale bars: All=30 μm. ConclusionWe conclude that silymarin has a significant hepatoprotective effect against diclofenac-induced liver toxicity in male Wistar rats through the neutralization and improvement of all histopathological injuries. AcknowledgmentThe author would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University, Saudi Arabia for financial support (QU-APC-2025). Conflict of interestNo competing interests. Data availabilityAll data generated during the current study are included in this manuscript. ReferencesAboubakr, M., Abdelkader, A., Habotta, O.A., Adel, N., Emam, M.A., Abdelhiee, E.Y., Shanab, O., Shoghy, K., Elnoury, H., Soliman, M.M. and Ibrahim, S.F. 2021. Cefepime and diclofenac sodium combined treatment-potentiated multiple organ injury: role of oxidative damage and disrupted lipid metabolism. J. Biochem. Mol. Toxicol. 35(12), e22929. Adeyemi, W.J. and Olayaki, L.A. 2018. Diclofenac–induced hepatotoxicity: low dose of omega-3 fatty acids have more protective effects. Toxicol. Rep. 5, 90–95. Alabi, Q.K. and Akomolafe, R.O., 2020. Kolaviron diminishes diclofenac-induced liver and kidney toxicity in wistar rats via suppressing inflammatory events, upregulating antioxidant defenses, and improving hematological indices. Dose-Response 18(1), 1559325819899256. Al-Dossari, M.H., Fadda, L.M., Attia, H.A., Hasan, I.H. and Mahmoud, A.M. 2020. Curcumin and selenium prevent lipopolysaccharide/diclofenac-induced liver injury by suppressing inflammation and oxidative stress. Biol. Trace Elem. Res. 196, 173–183. Aljuhani, N., Elkablawy, M.A., Elbadawy, H.M., Alahmadi, A.M., Aloufi, A.M., Farsi, S.H., Alhubayshi, B.S., Alhejaili, S.S., Alhejaili, J.M. and Abdel-Halim, O.B. 2019. Protective effects of Ajwa date extract against tissue damage induced by acute diclofenac toxicity. J. Taibah Univ. Med. Sci. 14(6), 553–559. Aydin, G., Gökãimen, A., ã–ncã, M., ãicek, E., Karahan, N. and Gökalp, O. 2003. Histopathologic changes in liver and renal tissues induced by different doses of diclofenac sodium in rats. Turk. J. Vet. Anim. Sci. 27(5), 1131–1140. Basist, P., Khan, M.U., Jan, B., Gaurav, Khan, M.A., Parveen, R. and Ahmad, S. 2022. Metabolite profiling and nephroprotective Potential of the Zea mays L. Silk Extract against Diclofenac-Induced Nephrotoxicity in Wistar Rats. ACS omega 7(41), 36519–36534. Boelsterli, U.A. 2013. Mechanisms underlying the hepatotoxicity of nonsteroidal antiinflammatory drugs. In: Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam, Netherlands: Elsevier 343–367. Dillard, C.J. and German, J.B. 2000. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 80, 1744–1756. Dykens, J.A. and Will, Y. 2007. The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today 12(17–18), 777–785. El-Kot, S.M., Wanas, W., Hafez, A.M., Mahmoud, N.A., Tolba, A.M., Younis, A.H., Sayed, G.E. and Abdelwahab, H.E. 2023. Effect of silymarin on the relative gene expressions of some inflammatory cytokines in the liver of CCl4-intoxicated male rats. Sci. Rep. 13(1), 15245. Ertekin, T., Bilir, A., Aslan, E., Koca, B., Turamanlar, O., Ertekin, A. and Albay, S. 2019. The effect of diclofenac sodium on neural tube development in the early stage of chick embryos. Folia Morphol. 78(2), 307–313. Esmaeil, N., Anaraki, S.B., Gharagozloo, M. and Moayedi, B. 2017. Silymarin impacts on immune system as an immunomodulator: One key for many locks. Int. Immunopharmacol. 50, 194–201. Fattori, V., Borghi, S.M., Guazelli, C.F., Giroldo, A.C., Crespigio, J., Bussmann, A.J., Coelho-Silva, L., Ludwig, N.G., Mazzuco, T.L., Casagrande, R. and Verri Jr, W.A. 2017. Vinpocetine reduces diclofenac-induced acute kidney injury through inhibition of oxidative stress, apoptosis, cytokine production, and NF-κB activation in mice. Pharmacol. Res. 120, 10–22. Galati, G., Tafazoli, S., Sabzevari, O., Chan, T.S. and O’Brien, P.J. 2002. Idiosyncratic NSAID drug induced oxidative stress. Chem. Biol. Interact. 142(1–2), 25–41. García-Ramírez, M., Turch, M., Simã-Servat, O., Hernãndez, C. and Simã, R. 2018. Silymarin prevents diabetes-induced hyperpermeability in human retinal endothelial cells. Endocrinol. Diabetes Nutr. 65(4), 200–205. Gil, M.L., Ramirez, M.C., Terencio, M.C. and Castell, J. 1995. Immunochemical detection of protein adducts in cultured human hepatocytes exposed to diclofenac. Biochim. Biophys. Acta 1272(3), 140–146. Giridharan, R., Lavinya, U. and Sabina, E.P. 2017. Suppressive effect of Spirulina fusiformis on diclofenac-induced hepato-renal injury and gastrointestinal ulcer in Wistar albino rats: a biochemical and histological approach. Biomed. Pharmacother. 88, 11–18. Gãmez-Lechãn, M.J., Ponsoda, X., O’Connor, E., Donato, T., Castell, J.V. and Jover, R. 2003. Diclofenac induces apoptosis in hepatocytes by alteration of mitochondrial function and generation of ROS. Biochem. Pharmacol. 66(11), 2155–2167. Gu, H.R., Park, S.C., Choi, S.J., Lee, J.C., Kim, Y.C., Han, C.J., Kim, J., Yang, K.Y., Kim, Y.J., Noh, G.Y. and No, S.H. 2015. Combined treatment with silibinin and either sorafenib or gefitinib enhances their growth-inhibiting effects in hepatocellular carcinoma cells. Clin. Mol. Hepatol. 21(1), 49. Heidarian, E. and Nouri, A. 2021. Hepatoprotective effects of silymarin against diclofenac-induced liver toxicity in male rats based on biochemical parameters and histological study. Arch. Physiol. Biochem. 127(2), 112–118. Klomjit, N. and Ungprasert, P. 2022. Acute kidney injury associated with non-steroidal anti-inflammatory drugs. Eur. J. Intern. Med. 101, 21–28. Kropacova, K., Misurova, E. and Hakova, H. 1998. Protective and therapeutic effect of silymarin on the development of latent liver damage. Radiats. Biol. Radioecol. 38(3), 411–415. Labbe, G., Pessayre, D., and Fromenty, B. 2008. Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam. Clin. Pharmacol. 22(4), 335–353. Masubuchi, Y., Saito, H. and Horie, T. 1998. Structural requirements for the hepatotoxicity of nonsteroidal anti-inflammatory drugs in isolated rat hepatocytes. J. Pharmacol. Exp. Ther. 287(1), 208–213. Moore, N., Pollack, C. and Butkerait, P. 2015. Adverse drug reactions and drug–drug interactions with over-the-counter NSAIDs. Ther. Clin. Risk. Manag. 11, 1061–1075. Ramezannezhad, P., Nouri, A. and Heidarian, E. 2019. Silymarin mitigates diclofenac-induced liver toxicity through inhibition of inflammation and oxidative stress in male rats. J. Herbmed Pharmacol. 8(3), 231–237. Sagãstegui-Guarniz William Antonio, W.A., Silva-Correa, C.R., Torre, V.L., Víctor, E., Cruzado-Razco, J.L., Calderãn-Peãa, A.A., Aspajo-Villalaz, C.L., Gamarra-Sãnchez, C.D., Ruiz-Reyes, S.G. and Chãvez-Flores, J.E. 2020. Hepatoprotective and nephroprotective activity of Artemisia absinthium L. on diclofenac-induced toxicity in rats. Pharm. J. 12(5), 1032–41. Sherif, I.O. and Al-Gayyar, M.M. 2013. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur. Cytokine Netw. 24(3), 114–121. Siu, W.P., Pun, P.B.L., Latchoumycandane, C. and Boelsterli, U.A. 2008. Bax-mediated mitochondrial outer membrane permeabilization (MOMP), distinct from the mitochondrial permeability transition, is a key mechanism in diclofenac-induced hepatocyte injury: Multiple protective roles of cyclosporin A. Toxicol. Appl. Pharmacol. 227(3), 451–461. Surai, P.F. 2015. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants 4(1), 204–247. Suvarna, S.K., Layton, C. and Bancroft, J.D. 2018. Bancroft’s theory and practice of histological techniques, 8th ed. New York, NY: Churchill Livingstone, 83–92. Tabari, M.A., Houshyar, M., Araghi, A., Mirzakhani, N., Crescenzo, G., Cardone, R. and Zizzadoro, C. 2024. Nephroprotective and hepatoprotective effects of lemongrass essential oil and citral on diclofenac-induced toxicity in mice. Biomed. Pharmacother. 180, 117541. Teschke, R. 2018. Alcoholic liver disease: Alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines 6(4), 106. Todd, P.A. and Sorkin, E.M. 1988. Diclofenac sodium: a reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 35, 244–285. Tracey, KJ. 2002. The inflammatory reflex. Nature 420(6917), 853–859. Yassin, N.Y., AbouZid, S.F., El-Kalaawy, A.M., Ali, T.M., Almehmadi, M.M. and Ahmed, O.M. 2022. Silybum marianum total extract, silymarin and silibinin abate hepatocarcinogenesis and hepatocellular carcinoma growth via modulation of the HGF/c-Met, Wnt/β-catenin, and PI3K/Akt/mTOR signaling pathways. Biomed. Pharmacother. 145, 112409. Zaidi, S.N.F. and Mahboob, T. 2017. Prevention of liver cirrhosis by Silymarin. Pak. J. Pharm. Sci. 30(4), 1203–1211. Zhang, Q., Xu, F., Li, Y., Zheng, M., Xi, X. and Han, C. 2018. Silybum marianum seeds oil attenuates CCl4-induced hepatic fibrosis via regulation of inflammatory response and oxidative stress. Curr Nutr Food Sci. 14(3), 197–203. | ||
| How to Cite this Article |
| Pubmed Style Fawiziah Khalaf Alharbi. Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation. Open Vet. J.. 2025; 15(4): 1790-1797. doi:10.5455/OVJ.2025.v15.i4.31 Web Style Fawiziah Khalaf Alharbi. Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation. https://www.openveterinaryjournal.com/?mno=247108 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.31 AMA (American Medical Association) Style Fawiziah Khalaf Alharbi. Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation. Open Vet. J.. 2025; 15(4): 1790-1797. doi:10.5455/OVJ.2025.v15.i4.31 Vancouver/ICMJE Style Fawiziah Khalaf Alharbi. Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1790-1797. doi:10.5455/OVJ.2025.v15.i4.31 Harvard Style Fawiziah Khalaf Alharbi (2025) Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation. Open Vet. J., 15 (4), 1790-1797. doi:10.5455/OVJ.2025.v15.i4.31 Turabian Style Fawiziah Khalaf Alharbi. 2025. Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation. Open Veterinary Journal, 15 (4), 1790-1797. doi:10.5455/OVJ.2025.v15.i4.31 Chicago Style Fawiziah Khalaf Alharbi. "Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation." Open Veterinary Journal 15 (2025), 1790-1797. doi:10.5455/OVJ.2025.v15.i4.31 MLA (The Modern Language Association) Style Fawiziah Khalaf Alharbi. "Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation." Open Veterinary Journal 15.4 (2025), 1790-1797. Print. doi:10.5455/OVJ.2025.v15.i4.31 APA (American Psychological Association) Style Fawiziah Khalaf Alharbi (2025) Silymarin alleviates diclofenac-induced hepatotoxicity in male Wistar rats: Histopathological investigation. Open Veterinary Journal, 15 (4), 1790-1797. doi:10.5455/OVJ.2025.v15.i4.31 |