| Research Article | ||
Open Vet. J.. 2025; 15(5): 2251-2258 Open Veterinary Journal, (2025), Vol. 15(5): 2251-2258 Research Article Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysisKosei Nakajima1,2.3,*, Daichi Sugiyama1, Akiharu Miyahara1 and Karen Bannai11Division of Orthopedic Surgery, Faculty of Veterinary Medicine, Imabari Campus, Okayama University of Science, Ehime, Japan 2Division of Translational Research, Exploratory Oncology Research and Clinical Trial Center, National Cancer Center, Tokyo, Japan 3Consultation service of Skeletal Tumors for Animals (CoSTA), Ehime, Japan *Corresponding Author: Kosei Nakajima, DVM PhD. Division of Orthopedic Surgery, Faculty of Veterinary Medicine, Imabari Campus, Okayama University of Science, 1-3 Ikoinooka, Imabari, Ehime, 794-8555, Japan. Email: k-nakajima [at] ous.ac.jp Submitted: 27/02/2025 Revised: 14/04/2025 Accepted: 21/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Pathological fractures result from abnormal bone remodeling caused by local bone lesions, such as malignant tumors, compromising bone strength. Tumor-derived factors disrupt the balance between bone resorption and formation, leading to osteolytic and osteosclerotic lesions that weaken bone integrity. While surgical treatments, including limb amputation and limb-sparing surgery, are commonly used, the therapeutic efficacy of fracture repair remains unclear due to limited evidence. Aim: This study characterized pathological fractures and evaluated survival time and functional prognoses following surgical repair in dogs. The goal was to provide robust evidence to inform clinical decision-making and improve treatment outcomes. Methods: A systematic review and meta-analysis were conducted following a PRISMA-compliant approach. MEDLINE (PubMed) and Google Scholar were used to search for relevant studies published from database inception to March 1, 2025. Data from past reports were integrated with cases from our institution, comprising 70 dogs with pathological fractures. Survival and prognosis were analyzed using the Kaplan–Meier method, and outcomes between adjuvant chemotherapy were compared using the log-rank test. Results: Osteosarcoma was the most common cause of pathological fractures (n=55; 78.6%), followed by multiple myeloma, undifferentiated sarcoma, and bone metastases. Surgical stabilization was the most common therapeutic intervention (n=40; 57.1%), with plate stabilization being the most frequently used technique (n=20; 28.6%). The median time to lameness recurrence was 163 days (95% confidence interval [CI]: 130–510 days), while the median survival time with osteosarcoma was 292 days (95% CI: 163–518 days). The subgroup analysis revealed no significant difference in survival between patients who received adjuvant therapy (radiation therapy or chemotherapy) and those who did not (p =0.675). Clinical and statistical heterogeneities were not assessed due to the integration of case reports and case series. Conclusion: Surgical stabilization of pathological fractures resulting from osteosarcoma should be considered a palliative treatment option for cases in which amputation is declined by the owner or in dogs with advanced disease, including metastatic lesions. With appropriate patient selection, this approach may represent a viable third-line treatment following limb-sparing surgery and amputation. Keywords: Fracture repair, Meta-analysis, Osteosarcoma, Palliative treatment, Pathological fracture. IntroductionPathological fractures result from abnormal bone remodeling and are commonly associated with skeletal tumors, including primary and metastatic bone tumors. In normal bone metabolism, bone resorption and formation occur via a balanced process known as coupling. However, tumor-derived secretory factors disrupt this balance in the bone microenvironment, leading to abnormal remodeling (Nakajima et al., 2016a; Nakajima and Raz, 2020; Nakajima et al., 2021). As a result, tumor-induced bone tissue becomes mechanically fragile and unable to withstand external forces effectively. Even minor stresses can induce high stress concentrations at the defective site, increasing the risk of pathological fractures (Nakajima et al., 2016b). Bone tumors can manifest as osteolytic and osteosclerotic lesions, both of which contribute to compromised bone integrity. Bisphosphonates, which induce osteoclast apoptosis, are commonly used to counteract excessive local bone resorption. Surgical treatments for pathological fractures typically involve either amputation or limb-sparing reconstruction of the affected area while preserving as much of the normal bone and spine integrity as possible (van Ee et al., 1989; Heidner et al., 1991; Pernell et al., 1992; Montgomery et al., 1993; Emmerson and Muir, 1997; Cooley and Waters, 1998; Chauvet et al., 1999; Emmerson and Pead, 1999; Banks et al., 2003; Cabassu and Moissonnier, 2007; Bhandal and Boston, 2011; Boston et al., 2011a; Boston et al., 2011b; Boston et al., 2017; Choate and Arnold, 2011; Covey et al., 2014; Hayes et al., 2020; Melilli, 2020). However, due to the limited number of published case reports, the therapeutic efficacy of pathological fracture repair remains unclear, and no comprehensive review on treatment outcomes exists. Clinical decision-making should be based on robust evidence rather than individual case studies. While single studies have inherent limitations, a meta-analysis provides a statistical approach to synthesizing results, offering a more precise and comprehensive assessment of treatment efficacy. By integrating multiple studies, a meta-analysis increases the overall sample size and enhances the strength of the evidence (Page et al., 2021). This large-scale cohort study aims to characterize pathological fractures and address the clinical question of survival time and functional prognosis following surgical repair using meta-analysis. Materials and MethodsTo ensure research quality, we employed a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) compliant protocol to enhance the credibility and confidence of the review methodology. The protocol for this systematic review and meta-analysis has been previously published (Page et al., 2021). Systematic reviewWe surveyed existing studies to address the clinical question: What is the survival duration or functional prognosis following surgical repair of pathological fractures? To identify eligible cases, we reviewed the medical records (2018–2025) of dogs with pathological fractures. Dogs with imaging evidence of a pathological fracture—including radiography, computed tomography, and magnetic resonance imaging—and a confirmed bone biopsy were included. Additionally, eligible canine patients were identified through a comprehensive search of publications on MEDLINE (PubMed) using the search terms “Pathologic fracture,” “Pathological fracture,” and “dog” on May 23, 2023. A manual search of Google Scholar was also conducted on June 30, 2023, to identify additional publications. Finally, the database was updated on March 1, 2025. Screening and selectionTwo veterinary clinical researchers of small animals (K.N., D.S.) independently reviewed the titles and abstracts of the first 92 records during the initial screening. Studies involving clinical research on human patients, traumatic fractures, or in vivo/in vitro molecular analyses, among others, were excluded. Discrepancies were resolved through discussions until a consensus was reached. Subsequently, the researchers independently examined the titles and abstracts of all retrieved articles. In cases of disagreement, consensus on the full screening of articles was achieved through discussion. After removing duplicates, we reviewed 29 full-text documents. For the second screening, both researchers independently evaluated the full-text articles for inclusion. Disagreements were again resolved through discussion to determine inclusion or exclusion. The inclusion criteria comprised bona fide case reports or case series documenting pathologic fractures in dogs. Eleven studies were excluded through the second screening from the review, with the main reason being that pathological fractures resulted from complications of local stereotactic radiotherapy. Risk of bias assessmentThe two researchers independently assessed the risk of bias for each evidence certainty domain in the studies included in the meta-analyses. The final database comprised 18 studies and 70 dogs, as summarized in the PRISMA flow diagram (Fig. 1). Meta-analysisAmong the 18 studies comprising 70 dogs, we analyzed all identified surgical interventions for pathological fractures. We collected data on study characteristics (author, year, and source of publication), patient characteristics (breed, diagnosis, and fracture location), intervention details (treatment type and implant used), adjuvant therapies (duration, dose, timing, and mode of delivery), functional prognosis, and survival time. Studies were excluded from the meta-analysis if they contained missing or unclear critical information. The primary outcome measures were functional prognosis and survival time following fracture repair. The functional prognosis was defined as the number of days from fracture repair until the onset of lameness, as evaluated by orthopedic veterinarians. Survival time was defined as the number of days from diagnosis to natural death or euthanasia. Information on the date and cause of death or euthanasia was obtained through telephone interviews with referring veterinarians and medical record reviews. A standardized data extraction form was developed, and both reviewers extracted data from the 18 eligible studies. The included studies for the meta-analysis were summarized in Supplementary Table 1 for reproducibility. The extracted data were compared, and discrepancies were resolved through discussion. The reviewers then entered the data into statistical software and independently verified accuracy through double-checking.
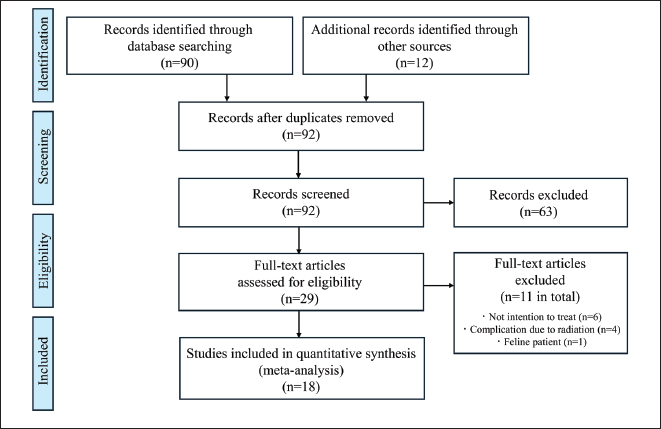
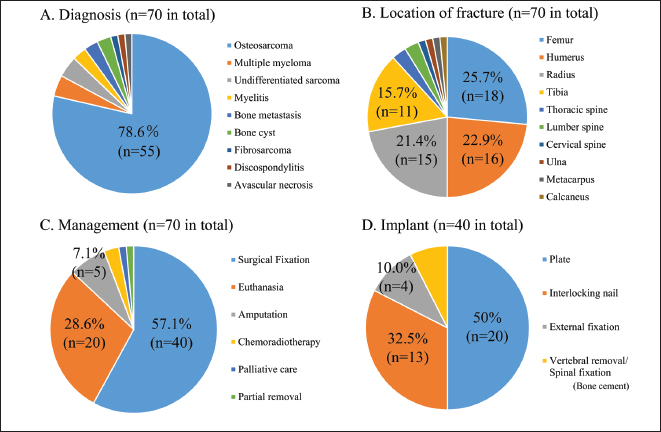
Fig. 1. PRISMA flow diagram. Statistical analysisFor all 70 dogs, the diagnosis, fracture location, treatment type, implant used for stabilization, and adjuvant therapy data were summarized using standard descriptive statistics. Survival time and functional prognosis were analyzed using the Kaplan–Meier method. For the meta-analysis, statistical evaluation of the subgroup analysis comparing postoperative adjuvant chemotherapy (presence vs. absence) was conducted using the log-rank test. Differences were considered statistically significant at p< 0.05. Statistical graphics, including pie charts and Kaplan–Meier curves, were generated to illustrate the descriptive analysis, survival time, and functional prognosis for each group. All statistical analyses were performed using EZR software (Kanda, 2013), based on the R Project for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria). Ethical approvalNot needed for this study. ResultsPatients and fracture characteristicsSeventy dogs met the inclusion criteria. Osteosarcoma was the most common cause of pathological fractures (n=55; 78.6%), followed by multiple myeloma (n=3; 4.3%), undifferentiated sarcoma (n=3; 4.3%), bone metastases from prostate carcinoma and transitional cell carcinoma (n=2; 2.9%), bone cyst (n=2; 2.9%), osteomyelitis (n=2; 2.9%), fibrosarcoma (n=1; 1.4%), discospondylitis (n=1; 1.4%), and avascular necrosis (n=1; 1.4%) (Fig. 2A). Pathological fractures most commonly occurred in the appendicular skeleton, including the femur (n=18; 25.7%; classification: proximal, n=8; diaphyseal, n=5; distal, n=5), humerus (n=16; 22.9%; classification: proximal, n=9; diaphyseal, n=1; distal, n=6), radius (n=15; 21.4%; all distal), and tibia (n=11; 15.7%; all distal). Less frequently affected sites included the thoracic spine (n=2; 2.9%, vertebral body), lumbar spine (n=2; 2.9%, vertebral body), cervical spine (n=1; 1.4%, vertebral body), ulna (n=1; 1.4%, distal), metacarpus (n=1; 1.4%), and calcaneus (n=1; 1.4%) (Fig. 2B). Among the breeds, Rottweilers (n=16), Greyhounds (n=6), mixed-breed dogs (n=5), German Shepherds (n=5), Golden Retrievers (n=4), and Labrador Retrievers (n=4) were most affected by pathological fractures. Therapeutic interventionsThe most common therapeutic intervention was surgical stabilization (n=40; 57.1%), followed by euthanasia (n=20; 28.6%), amputation (n=5; 7.1%), chemoradiotherapy (n=2; 2.9%), palliative care (n=1; 1.4%), and partial removal (n=1; 1.4%) (Fig. 2C). The surgical techniques used included plate stabilization (n=20; including arthrodesis, 28.6%), followed by interlocking nails (n=13; 18.6%), external fixation (n=4; 5.7%), and spinal fixation (n=3; 4.3%) (Fig. 2D). The mean follow-up duration was 302 days.
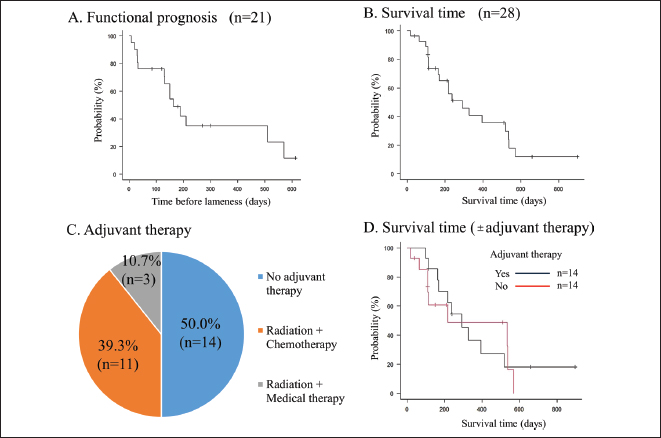
Fig. 2. Descriptive analysis results. Clinical data from 70 cases are summarized using pie charts. (A) The cause of the pathological fracture, (B) the affected location, (C) clinical management, and (D) the type of implant used at the time of surgery. Functional prognosis and complicationsThe long-term postoperative follow-up of functional prognosis was available for 21 patients, with a median time to lameness occurrence of 163 days (95% confidence interval (CI): 130–510 days) (Fig. 3A). Subgroup analysis of implant types revealed no significant differences between plates and interlocking nails (p=0.415). Postoperative complications included local infections (n=4), local recurrence (n=3), implant fractures (n=2), and implant-induced fractures (n=1). Survival analysisFor osteosarcoma cases, long-term follow-up was possible in 28 patients, with a median survival time of 292 days (95% CI: 163–518 days) (Fig. 3B). Of these, 14 received adjuvant therapy (radiation therapy with or without chemotherapy, including carboplatin, doxorubicin, and cisplatin), while the other 14 received no treatment (n=14) (Fig. 3C). Subgroup analysis showed no significant differences in survival between the adjuvant therapy and non-therapy groups (p=0.675) (Fig. 3D). DiscussionPrevious retrospective studies have reported that the median survival time for canine osteosarcoma is 4 to 6 months with amputation alone (Mauldin et al., 1988; Straw et al., 1991) and approximately 12 months with amputation and chemotherapy (Morello et al., 2011). In terms of life expectancy, survival following pathological fracture repair is shorter than that observed with amputation and chemotherapy. These findings suggest that repair surgery for pathological fractures, particularly in load-bearing bones of the extremities, may serve as a palliative treatment option for owners who decline amputation, as well as for dogs with progressive diseases, including metastatic lesions. Additionally, repair surgery may be considered in cases of malignant progression of localized disease, which can lead to poor pain control. In such cases, repair surgery for pathological fractures can be considered as a surgical option. In the present study, multiple patients were euthanized after experiencing pathological fractures. These findings suggest that palliative treatment for pathological fractures may be beneficial, as it allows for moderate gait function and offers a relatively promising survival time. The absence of significant differences between cohorts with or without adjuvant therapy may be attributed to type II error.
Fig. 3. (A) Kaplan–Meier curves illustrate functional prognosis after surgery, defined as the number of days from fracture repair until lameness is observed, as evaluated by orthopedic veterinarians. Tick marks indicate censoring events, such as euthanasia or natural death. (B) Kaplan–Meier curves depicting survival analysis for patients with osteosarcoma, with tick marks indicating censoring events, such as euthanasia. (C) Pie chart showing subgroup analysis results comparing cohorts with or without adjuvant therapy. (D) Kaplan–Meier curves comparing functional prognosis between patients who received adjuvant therapy (black line) and those who did not (red line). A discrepancy was observed between the median time to lameness and the median survival time. This indicates that pain management is essential for maintaining quality of life during the months when limb function is impaired. Pain control strategies may include the use of nonsteroidal anti-inflammatory drugs, opioids, bisphosphonates, and novel analgesics. In this regard, we are currently developing a new drug candidate that targets osteoclast precursor cell fusion to suppress abnormal bone remodeling (unpublished data). This therapy may improve the prognosis of canine patients. Regarding complications, the risk of infection is negligible in tumor-associated orthopedic surgeries, such as limb-sparing procedures, as reported in previous studies (Morello et al., 2001; Morello et al., 2003; Ehrhart, 2005; Liptak et al., 2006; Hodge et al., 2011). The use of currently available silver-coated implants—featuring an ultrathin antimicrobial coating with pure elemental silver particles embedded in a biocompatible plasma polymer—may be necessary to mitigate infection-related complications in future studies (Azab et al., 2016; Engel et al., 2024). The potential for bone healing following surgical stabilization of pathological fractures remains a subject of ongoing debate. Hayes et al reported a case of complete bone regeneration after plate fixation of a pathological femoral fracture caused by sarcoma (Hayes et al., 2020). Progressive bone healing in such cases is likely influenced by a complex interplay of anatomical, mechanical, and biological factors, including regional variations in vascular supply (Shah et al., 2025a; Shah et al., 2025b). To preserve periosteal and intraosseous vascularity, we advocate the use of minimally invasive osteosynthesis with interlocking nailing or minimally invasive plate osteosynthesis. This study has some limitations. Due to the nature of the meta-analysis, follow-up data were limited. In addition to our cases, we incorporated clinical information from published case reports and case series, some of which lacked follow-up data, although a high percentage of our cases included follow-up data. Furthermore, because case reports and case series were integrated, clinical and statistical heterogeneity were not assessed. Clinical heterogeneity arises from variations in patient backgrounds, diagnostic tests, and treatments across studies. In contrast, statistical heterogeneity reflects the degree to which it is reasonable to assume that the effect sizes from all studies represent the same population effects. Additionally, reporting bias may have influenced the findings, as studies with positive results are more likely to be published than those with negative results (Stern and Simes, 1997). In conclusion, this study demonstrates the clinical efficacy of repair in pathological fractures, highlighting the functional prognosis and survival time following the procedure. Reduction and stabilization surgery for pathological fractures may serve as a third treatment option in addition to limb-sparing surgery and amputation. AcknowledgmentsWe thank Dr Daniel A Degner, DVM, Diplomate ACVS (small animal), Animal Surgical Center of Michigan, 5045 Miller Rd, Flint, MI 48507, USA for the data presentation. FundingThis research received no specific grant. Authors’ contributionsConceptualization: K.N.; Data curation: K.N., D.S.; Formal analysis: K.N., D.S.; Investigation: K.N., D.S.; Methodology: K.N.; Visualization: K.N., D.S.; Writing-original draft: K.N.; Writing - review & editing: D.S., M.A., K.B. Conflict of interestThe authors declare no conflict of interest. Data availabilityThe datasets used and analyzed in the current study are available from the corresponding author upon reasonable request. ReferencesAzab, M.A., Allen, M.J. and Daniels, J.B. 2016. Evaluation of a silver-impregnated coating to inhibit colonization of orthopaedic implants by biofilm forming methicillin-resistant Staphylococcus pseudintermedius. Vet. Comp. Orthop. Traumatol. 29, 347–350. Banks, T., Langova, V. and Straw, R. 2003. Repair of three pathologic fractures in a dog with multiple myeloma. Aust. Vet. Practit. 33, 98–102. Bhandal, J. and Boston, S.E. 2011. Pathologic fracture in dogs with suspected or confirmed osteosarcoma. Vet. Surg. 40, 423–430. Boston, S.E., Bacon, N.J., Culp, W.T., Bhandal, J., Bruce, C., Cavanaugh, R.P., Hamilton, M.H., Lincoln, J.D., Liptak, J.M. and Scharvogel, S. 2011a. Outcome after repair of a sarcoma-related pathologic fracture in dogs: a Veterinary Society of Surgical Oncology Retrospective Study. Vet. Surg. 40, 431–437. Boston, S.E., Barry, M. and O’Sullivan, M.L. 2011b. Transtumoral plating as a novel method for palliative limb spare and thromboembolism in a dog with a distal radial primary bone tumor. Can. Vet. J. 52, 650–655. Boston, S.E., Vinayak, A., Lu, X., Larue, S., Bacon, N.J., Bleedorn, J.A., Souza, C.H.M. and Ehrhart, N.P. 2017. Outcome and complications in dogs with appendicular primary bone tumors treated with stereotactic radiotherapy and concurrent surgical stabilization. Vet. Surg. 46, 829–837. Cabassu, J. and Moissonnier, P. 2007. Surgical treatment of a vertebral fracture associated with a haematogenous osteomyelitis in a dog. Vet. Comp. Orthop. Traumatol. 20, 227–230. Chauvet, A.E., Hogge, G.S., Sandin, J.A. and Lipsitz, D. 1999. Vertebrectomy, bone allograft fusion, and antitumor vaccination for the treatment of vertebral fibrosarcoma in a dog. Vet. Surg. 28, 480–488. Choate, C.J. and Arnold, G.A. 2011. Elbow arthrodesis following a pathological fracture in a dog with bilateral humeral bone cysts. Vet. Comp. Orthop. Traumatol. 24, 398–401. Cooley, D.M. and Waters, D.J. 1998. Skeletal metastasis as the initial clinical manifestation of metastatic carcinoma in 19 dogs. J. Vet. Intern. Med. 12, 288–293. Covey, J.L., Farese, J.P., Bacon, N.J., Schallberger, S.P., Amsellem, P., Cavanaugh, R.P. and Milner, R.J. 2014. Stereotactic radiosurgery and fracture fixation in 6 dogs with appendicular osteosarcoma. Vet. Surg. 43, 174–181. Ehrhart, N. 2005. Longitudinal bone transport for treatment of primary bone tumors in dogs: technique description and outcome in 9 dogs. Vet. Surg. 34, 24–34. Emmerson, T.D. and Muir, P. 1997. Pathological fracture of distal femora in a young dog. Vet. Rec. 141, 551–552. Emmerson, T.D. and Pead, M.J. 1999. Pathological fracture of the femur secondary to haematogenous osteomyelitis in a weimaraner. J. Small Anim. Pract. 40, 233–235. Engel, D.M., McCoy, A.M. and Robbins, M.A. 2024. Silver-coated tibial plateau leveling osteotomy implants do not improve surgical site infection rates over noncoated implants in a randomized trial in 73 canines. J. Am. Vet. Med. Assoc. 262, 1–7. Hayes, M.A., Jemilo, S., Muir, P., Sullivan, R. and Bleedorn, J.A. 2020. Pathologic fracture healing after femoral limb salvage in a dog. Aust. Vet. J. 98, 84–89. Heidner, G.L., Page, R.L., McEntee, M.C., Dodge, R.K. and Thrall, D.E. 1991. Treatment of canine appendicular osteosarcoma using cobalt 60 radiation and intraarterial cisplatin. J. Vet. Intern. Med. 5, 313–316. Hodge, S.C., Degner, D., Walshaw, R. and Teunissen, B. 2011. Vascularized ulnar bone grafts for limb-sparing surgery for the treatment of distal radial osteosarcoma. J. Am. Anim. Hosp. Assoc. 47, 98–111. Kanda, Y. 2013. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48, 452–458. Liptak, J.M., Dernell, W.S., Ehrhart, N., Lafferty, M.H., Monteith, G.J. and Withrow, S.J. 2006. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: a prospective clinical comparison of two different limb-sparing techniques. Vet. Surg. 35, 518–533. Mauldin, G.N., Matus, R.E., Withrow, S.J. and Patnaik, A.K. 1988. Canine osteosarcoma. Treatment by amputation versus amputation and adjuvant chemotherapy using doxorubicin and cisplatin. Jou J. Vet. Intern. Med. 2, 177–180. Melilli, A. 2020. Uncommon skeletal metastasis secondary to transitional cell carcinoma. Open Vet. J. 9, 313–316. Montgomery, R.D., Finn, S. and Cooper, R. 1993. What is your diagnosis? Congenital elbow luxation with avascular necrosis of the femoral head, pathologic fracture of the femoral neck and bilateral medial luxation of the patellas in a dog. J. Am. Vet. Med. Assoc. 203, 1535–1536. Morello, E., Buracco, P., Martano, M., Peirone, B., Capurro, C., Valazza, A., Cotto, D., Ferracini, R. and Sora, M. 2001. Bone allografts and adjuvant cisplatin for the treatment of canine appendicular osteosarcoma in 18 dogs. J. Small Anim. Pract. 42, 61–66. Morello, E., Martano, M. and Buracco, P. 2011. Biology, diagnosis and treatment of canine appendicular osteosarcoma: similarities and differences with human osteosarcoma. Vet. J.189, 268–277. Morello, E., Vasconi, E., Martano, M., Peirone, B. and Buracco, P. 2003. Pasteurized tumoral autograft and adjuvant chemotherapy for the treatment of canine distal radial osteosarcoma: 13 cases. Vet. Surg. 32, 539–544. Nakajima, K. and Raz, A. 2020. Autocrine motility factor and its receptor expression in musculoskeletal tumors. J. Bone Oncol. 24, 100318. Nakajima, K., Kho, D.H., Yanagawa, T., Harazono, Y., Hogan, V., Chen, W., Ali-Fehmi, R., Mehra, R. and Raz, A. 2016a. Galectin-3 Cleavage Alters Bone Remodeling: Different Outcomes in Breast and Prostate Cancer Skeletal Metastasis. Cancer Res. 76, 1391–1402. Nakajima, K., Kho, D.H., Yanagawa, T., Zimel, M., Heath, E., Hogan, V. and Raz, A. 2016b. Galectin-3 in bone tumor microenvironment: a beacon for individual skeletal metastasis management. Cancer Metastasis Rev. 35, 333–346. Nakajima, K., Kidani, T. and Miura, H. 2021. Molecular profiling of bone remodeling occurring in musculoskeletal tumors. J. Orthop. Res. 39, 1402–1410. Page, M.J., Moher, D., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D., Shamseer, L., Tetzlaff, J.M., Akl, E.A., Brennan, S.E., Chou, R., Glanville, J., Grimshaw, J.M., Hróbjartsson, A., Lalu, M.M., Li, T., Loder, E.W., Mayo-Wilson, E., McDonald, S., McGuinness, L.A., Stewart, L.A., Thomas, J., Tricco, A.C., Welch, V.A., Whiting, P. and McKenzie, J.E. 2021. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372, n160. Pernell, R.T., Dunstan, R.W. and DeCamp, C.E. 1992. Aneurysmal bone cyst in a six-month-old dog. J. Am. Vet. Med. Assoc. 201, 1897–1899. Shah, M.A.A., Tang, W., Ma, Y., Zhe, W., Yu, S.B. and Sui, H.J. 2025a. Regional distribution of microvasculature within the canine femoral head: a study of plastinated tissue histology and 3D reconstruction. J. Orthopaed. Surg. Res. 20, 170. Shah, M.A.A., Tang, W., Zhang, J.H., Chen, C., Wang, J.W., Lü, S.J., Yu, X.T., Zhang, Z.J., Li, C., Yu, S.B. and Sui, H.J. 2025b. Microvasculature and trabecular bone in beagle proximal femur: Microstructural insights. Ann. Anat. 258, 152368. Stern, J.M. and Simes, R.J. 1997. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ (Clin. Res. Ed.) 315, 640–645. Straw, R.C., Withrow, S.J., Richter, S.L., Powers, B.E., Klein, M.K., Postorino, N.C., LaRue, S.M., Ogilvie, G.K., Vail, D.M., Morrison, W.B. and Dickinson, G.K. 1991. Amputation and cisplatin for treatment of canine osteosarcoma. J. Vet. Intern. Med. 5, 205–210. van Ee, R.T., Selcer, R.R., Toal, R. and Walker, M. 1989. What is your diagnosis? Lysis of the vertebral body of C7, with a pathologic compression fracture and compression of the spinal cord at the level of C7. J. Am. Vet. Med. Assoc. 194, 285–286. Supplementary Table 1. The 18 studies in the table were included in this meta-analysis.
| ||
| How to Cite this Article |
| Pubmed Style Nakajima K, Sugiyama D, Miyahara A, Bannai K. Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis. Open Vet. J.. 2025; 15(5): 2251-2258. doi:10.5455/OVJ.2025.v15.i5.42 Web Style Nakajima K, Sugiyama D, Miyahara A, Bannai K. Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis. https://www.openveterinaryjournal.com/?mno=244890 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.42 AMA (American Medical Association) Style Nakajima K, Sugiyama D, Miyahara A, Bannai K. Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis. Open Vet. J.. 2025; 15(5): 2251-2258. doi:10.5455/OVJ.2025.v15.i5.42 Vancouver/ICMJE Style Nakajima K, Sugiyama D, Miyahara A, Bannai K. Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2251-2258. doi:10.5455/OVJ.2025.v15.i5.42 Harvard Style Nakajima, K., Sugiyama, . D., Miyahara, . A. & Bannai, . K. (2025) Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis. Open Vet. J., 15 (5), 2251-2258. doi:10.5455/OVJ.2025.v15.i5.42 Turabian Style Nakajima, Kosei, Daichi Sugiyama, Akiharu Miyahara, and Karen Bannai. 2025. Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis. Open Veterinary Journal, 15 (5), 2251-2258. doi:10.5455/OVJ.2025.v15.i5.42 Chicago Style Nakajima, Kosei, Daichi Sugiyama, Akiharu Miyahara, and Karen Bannai. "Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis." Open Veterinary Journal 15 (2025), 2251-2258. doi:10.5455/OVJ.2025.v15.i5.42 MLA (The Modern Language Association) Style Nakajima, Kosei, Daichi Sugiyama, Akiharu Miyahara, and Karen Bannai. "Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis." Open Veterinary Journal 15.5 (2025), 2251-2258. Print. doi:10.5455/OVJ.2025.v15.i5.42 APA (American Psychological Association) Style Nakajima, K., Sugiyama, . D., Miyahara, . A. & Bannai, . K. (2025) Survival and functional outcomes following surgical repair of pathological fractures in dogs: A meta-analysis. Open Veterinary Journal, 15 (5), 2251-2258. doi:10.5455/OVJ.2025.v15.i5.42 |