| Research Article | ||
Open Vet. J.. 2025; 15(5): 2206-2217 Open Veterinary Journal, (2025), Vol. 15(5): 2026-2217 Research Article In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camelsDina E. M. Rashad1, Ahmed S. A. Sosa2, Seham F. Shehata3, Karima Gh. M. Mahmoud2 and Mohamed M. M. El-Sokary1,41Theriogenology Department, Faculty of Veterinary Medicine, Benha University, Benha, Egypt 2Animal Reproduction and Artificial Insemination Department, Veterinary Research Institute, National Research Centre, Giza, Egypt 3Department of Animal Wealth Development, Faculty of Veterinary Medicine, Benha University, Benha, Egypt 4Higher Colleges of Technology, Faculty of Health Science, Abu Dhabi, UAE *Corresponding Author: Mohamed M. M. El-Sokary. Theriogenology Department, Faculty of Veterinary Medicine, Benha University, Benha, Egypt and Higher Colleges of Technology, Faculty of Health Science, Abu Dhabi, UAE. Email: mohamed.alsokary [at] fvtm.bu.edu.eg Submitted: 26/02/2025 Revised: 02/04/2025 Accepted: 06/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Di(2-ethylhexyl) phthalate (DEHP), a commonly used plasticizer and endocrine disruptor, has been implicated in various reproductive disorders in farm animals. However, its effects on camel reproduction, particularly sperm function, have not been studied. Given the unique reproductive physiology of camels, understanding DEHP’s impact on sperm quality is crucial. Investigating these effects could provide insights into potential fertility risks and contribute to improved reproductive management strategies for camel breeding programs. Aim: This study evaluated the effects of DEHP on camel sperm quality, specifically focusing on motility, viability, mitochondrial activity, plasma membrane integrity, and sperm binding to both zona pellucida and oviduct epithelial cells. Methods: Camel spermatozoa were retrieved from the epididymis and exposed to four concentrations of DEHP (0, 10, 25, and 50 µM) to evaluate its impact on spermatozoa function. Progressive sperm movement was examined, followed by assessments of viability using SYBR 14/PI staining and mitochondrial membrane potential using Mito-Tracker Green fluorescence staining. Membrane integrity was evaluated using the Hypo-Osmotic Swelling Test. Additionally, functional competence was assessed using zona pellucida and oviduct epithelial cell binding assays. Results: DEHP exposure resulted in a dose-dependent decline in sperm functionality. Sperm motility decreased from 61.6.5% in the control group to 43% at 50 µM DEHP. Similarly, mitochondrial activity and membrane integrity significantly declined at the highest DEHP concentration (50 µM), and sperm viability exhibited a significant reduction, with sperm viability dropping from 90% in the control to 50% at 50 µM. Functional assays revealed reduced sperm binding to zona pellucida and oviduct epithelial cells at 50 µM, indicating impaired fertilization potential. Conclusion: Our findings revealed the detrimental effects of DEHP exposure on camel sperm function, highlighting its potential threat to reproductive efficiency. These results provide critical insights into the reproductive toxicity of endocrine-disrupting chemicals in livestock. Future investigations should focus on the long-term consequences of DEHP exposure, elucidating the underlying mechanisms, and developing effective mitigation strategies. Keywords: Sperm, Epididymis, DEHP, Infertility, Camel. IntroductionDromedary camels (Camelus dromedarius) are an integral part of life in countless communities inhabiting arid and semiarid regions around the world. These magnificent creatures provide not only economic sustenance through their milk, meat, and wool, but also hold deep cultural significance for these communities (Kandeel et al., 2023). Unfortunately, the reproductive health of Camelus dromedarius is increasingly threatened by various factors, including environmental contaminants. Among these threats, endocrine-disrupting chemicals, such as di(2-ethylhexyl) phthalate (DEHP), are a growing concern (Magnusson and Persson, 2015). DEHP is a ubiquitous environmental contaminant found in numerous everyday products, including plastics, personal care items, and food packaging (Yang et al., 2024). Farm animals are unwittingly exposed to DEHP through the leaching of contaminated feed and water sources from DEHP-containing plastics used in silage storage and feed troughs. Consumption of contaminated drinking and irrigation water further contributes to DEHP exposure. The inhalation of dust particles arising from animal bedding or feed can also be a route of exposure (XueXia et al., 2023). These environmental sources raise concerns regarding the potential detrimental effects of DEHP on farm animal reproduction (Schmidt et al., 2012). Exposure to DEHP can negatively impact reproductive health in various animals, including pigs, sheep, cattle, and rats (Magnusson and Persson, 2015). Studies have shown that DEHP disrupts hormonal balance, hinders sperm quality through various mechanisms, and ultimately reduces fertility potential. Additionally, DEHP exposure can decrease testosterone levels, a crucial hormone for sperm production and male sexual function (Lin et al., 2023). DEHP has been linked to alterations in testicular morphology, reduced sperm count, and abnormal sperm motility (Huang et al., 2014). Furthermore, DEHP has been shown to cause oxidative stress in male reproductive organs, DNA damage in sperm cells, and impaired fertilization potential (Hosseinzadeh et al., 2021). The epididymis is a crucial structure within the male reproductive tract where sperm undergo maturation. Evaluating sperm parameters within the epididymis provides valuable insights into their potential fertilizing ability. Additionally, the affinity of sperm to attach to the oviduct, the female reproductive tract where fertilization occurs, is essential for successful reproduction (Rashad et al., 2024). Epididymal sperm retrieval was selected to overcome the potential challenges associated with studying sperm in camel ejaculates. Moreover, studying epididymal sperm enables in vitro controlled and direct assessment of DEHP’s effects on post-testicular sperm. Although research has suggested detrimental effects of DEHP on farm animal reproduction, the specific impact on camels remains unclear. Existing knowledge focuses on hormonal disruption and sperm quality in other species. This study, for the first time, aimed to address the critical knowledge gap by investigating the impact of DEHP on the vital aspects of camel reproduction. By meticulously assessing sperm viability, morphology, DNA integrity, and binding affinity to oocytes/oviductal explants, we are attempting to elucidate the potentially detrimental effects of DEHP exposure on C. dromedarius fertility. Materials and MethodsChemicalsAll chemicals were purchased from Sigma–Aldrich unless otherwise stated. Animals and sample collectionThe testes and epididymis of mature male dromedary camels (Camelus dromedarius) aged 5–10 years were collected from a local abattoir. The collected samples were sent to the laboratory immediately to minimize the postmortem interval and to maintain optimal conditions. Experimental designTo evaluate the toxic effects of DEHP on camel epididymal sperm, four treatment groups were established with DEHP concentrations of 0 (control), 10, 25, and 50 M. DEHP, sourced from Sigma-Aldrich (CAS Number: 117-81-7), has a molecular weight of 390.56 g/mol and a molecular formula of C24H38O4. After adding DEHP, each group was incubated at 37°C for 1 hour (Khasin et al., 2020). After incubation, each treatment was evaluated for sperm motility, viability, Hypo-osmotic swelling test (HOST) reaction, mitochondrial membrane potential (MMP), Zona binding ability, and sperm oviduct binding affinity. Processing and preparation of epididymal spermA total of 44 testis with epididymis were collected. The samples were carefully separated and transported to the laboratory in chilled (approximately 4°C) physiological saline solution (0.9% NaCl) to minimize potential damage during transport. Upon arrival at the laboratory, the testes and epididymis were meticulously separated using sterile dissection techniques. The epididymides were then thoroughly rinsed with a sterile 0.9% saline solution to remove any residual blood or debris. Subsequently, the epididymal tissue was briefly immersed in 70% ethyl alcohol for surface disinfection. The epididymis was incised to allow for the collection of sperm, which were subsequently examined (Modified from Rashad et al., 2024). Sperm concentration was assessed using a hemocytometer (Neubauer chamber), and aliquots were adjusted to 20 × 106 sperm/ml for uniform exposure conditions. Pools were resuspended in 300 μl of 1× phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) and divided into 100 μl aliquots for further examination. Sperm motility and viabilitySperm from the cauda epididymis were evaluated for motility. Briefly, one drop of diluted epididymal sperm was placed on a previously warmed microscope slide, covered with a clean coverslip, and examined using a negative phase-contrast microscope (Nikon Eclipse E200, Japan) with a heated (37°C) stage at 40× magnification (Ali et al., 2014). To evaluate sperm viability, SYBR-14 (1 μM) was added to the sperm suspension and incubated for 10 minutes in the dark. Then, Propidium iodide (PI, 1 μM) was added and incubated for an additional 5 minutes (Garner and Johnson, 1995). The stained sperm were examined under a fluorescent microscope (Olympus BX51, Japan) with excitation at 488 nm and emission detection at 520 nm, observed under a 40× objective magnification. PI (appears red) stains dead sperm cells by penetrating compromised membranes. On the other hand, SYBR14 stains live sperm in green by binding to their DNA (Fig. 1).
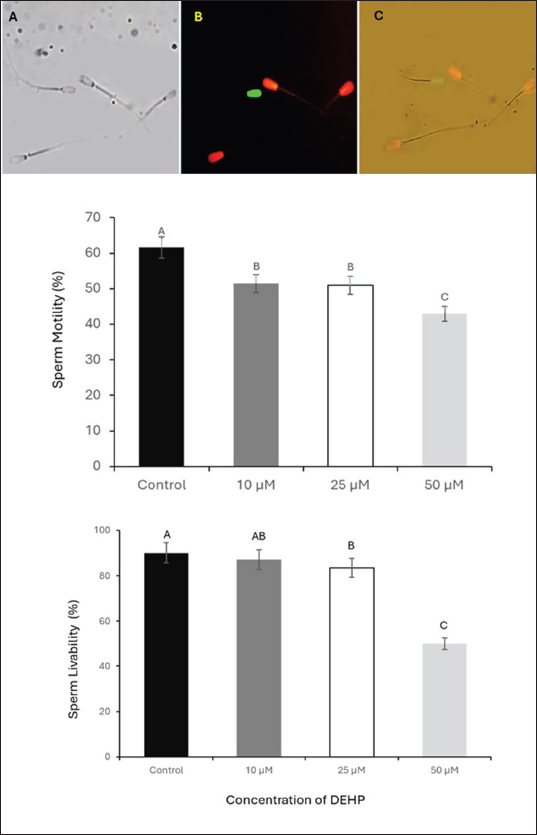
Fig. 1. Assessment of sperm motility and viability following DEHP exposure. The upper panel illustrates sperm viability using propidium iodide (PI) and SYBR 14 staining. (A) Bright-field microscopy image of spermatozoa. (B) Fluorescence microscopy image, where live spermatozoa appear green (stained with SYBR 14) and dead spermatozoa appear red (stained with PI). (C) Merged fluorescence image combining both stains scale bar=50 μm. The lower panel presents column graphs depicting the effects of different concentrations of DEHP (10, 25, and 50 μM) on sperm motility and viability percentages across experimental groups. Different letters indicate statistically significant differences among groups (p < 0.05).
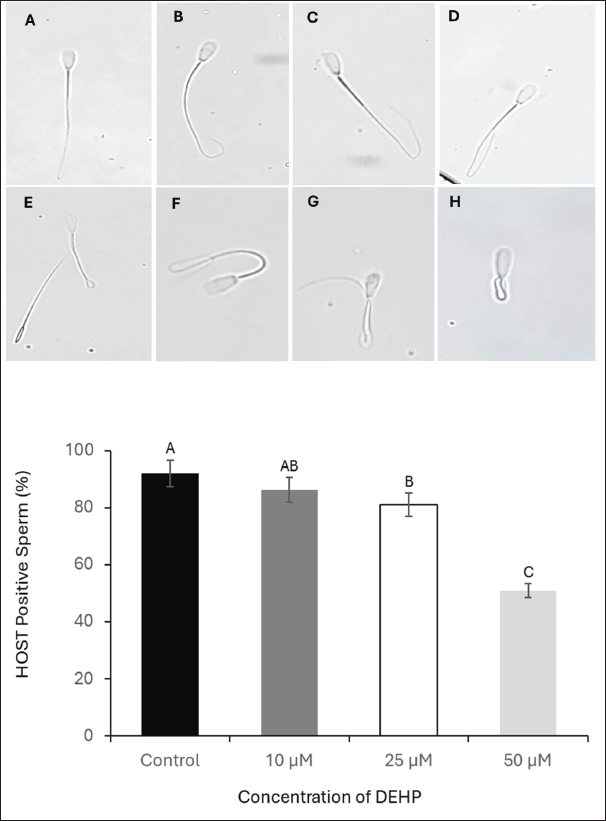
Fig. 2. Evaluation of the effects of different DEHP concentrations on sperm membrane integrity using the HOST. Images from A to H show spermatozoa classified as HOST-positive, exhibiting increasing degrees of tail coiling (×40 magnification). The lower panel presents a column graph illustrating the effect of different DEHP concentrations (10, 25, and 50 μM) on sperm membrane integrity across the experimental groups. Different letters indicate statistically significant differences among groups (p < 0.05). Assessment of sperm plasma membrane integrityThe HOS was used to evaluate the integrity of the plasma membrane of sperm. Briefly, the sperm sample (100 μl at 20 × 106 sperm/ml) was added to a prewarmed HOS reagent (900 μl) and incubated at 37°C for 60 minutes. All sperm with swollen and/or curled tails at different degrees were considered sperm with intact plasma membranes (different patterns of response shown in Fig. 2). The percentage of HOS-positive sperm was calculated for 400 sperm (Akhter et al., 2008).
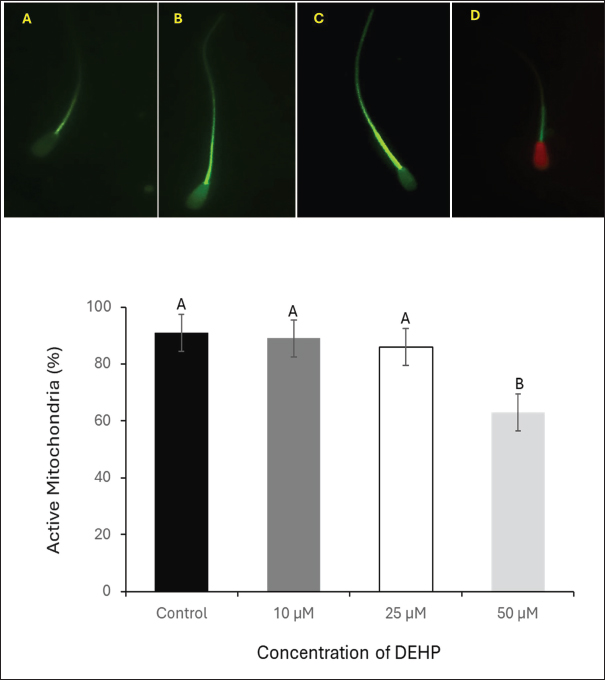
Fig. 3. Assessment of the effect of different DEHP concentrations on MMP in spermatozoa stained with Mito Tracker Green. (A) Sperm exhibiting faint fluorescence, indicating low MMP. (B) Sperm with disorganized mitochondrial staining. (C) Sperm displaying intense fluorescence, representing high MMP and mitochondrial integrity. (D) Sperm with compromised MMP, costained with Propidium Iodide (PI); scale bar=50 μm. The lower panel presents column graphs illustrating the effects of different DEHP (10, 25, and 50 μM) on sperm mitochondrial activity in the experimental groups. Different letters indicate statistically significant differences among groups (p < 0.05). Assessment of sperm mitochondrial activityThe MMP was assessed using MitoTracker Green, a dye that selectively stains active mitochondria. Sperm samples at 20 × 106 sperm/ml) were incubated with Mito Tracker Green (1 μM) for 20 minutes at 37°C in the dark. The fluorescence microscope was set with an excitation wavelength of 488 nm and an emission range of 500–540 nm. Imaging was performed using a 40× objective lens (modified from Madeja et al., 2021). Sperm with low intensity or faint fluorescence in the midpiece, indicating reduced mitochondrial activity or compromised (Low MMP). Sperm displaying disorganized mitochondria and irregular or patchy fluorescence along the midpiece, suggesting disrupted mitochondrial function. The uniform and intense fluorescence in the midpiece reflects optimal mitochondrial activity and high mitochondrial membrane potential (HMMP), as shown in Figure 3. The percentage of high MMP was calculated for 400 sperm.
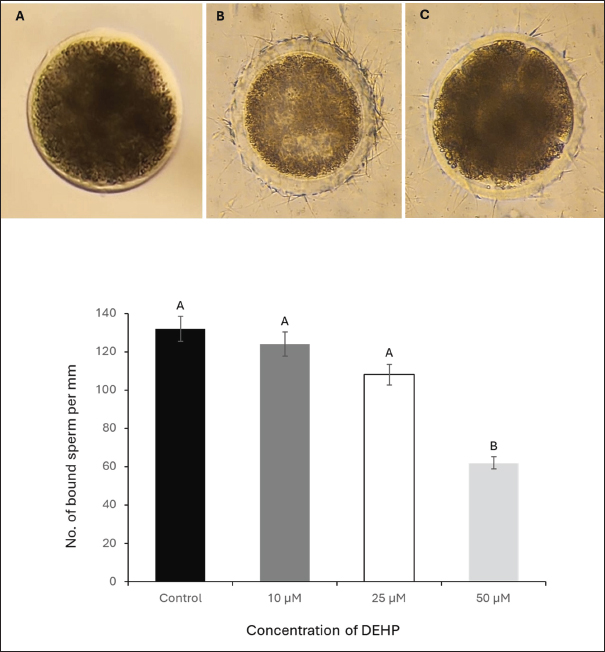
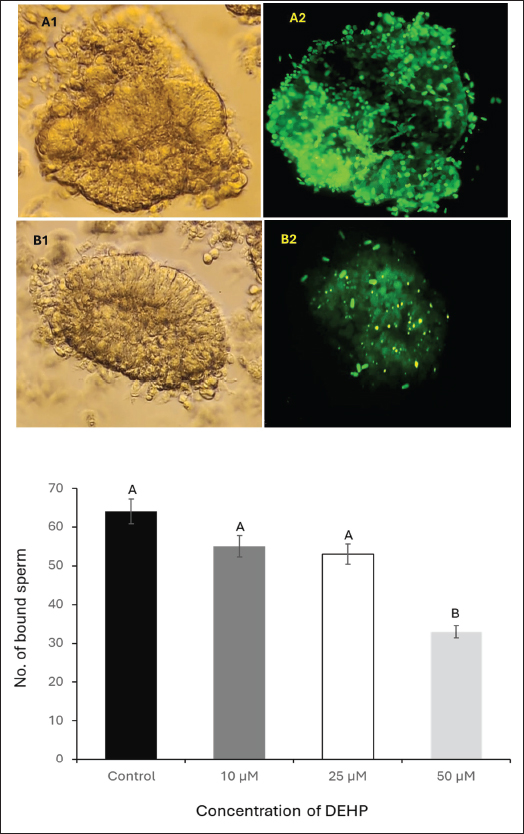
Fig. 4. Effect of different DEHP concentrations on sperm binding to the oocyte zona pellucida under different experimental conditions. (A) Zona pellucida oocyte before sperm binding. (B) The control group displayed a high number of bound sperm, indicating strong binding affinity. (C) Zona pellucida oocyte following exposure to 50 μM DEHP, showing a reduced number of bound sperm, suggesting impaired binding capacity. The column graph represents the effect of different DEHP concentrations on sperm binding ability of the zona pellucida (scale bar=50 μm. Different letters indicate statistically significant differences among groups (p < 0.05). Ovary collection and oocyte preparationA total of 50 ovaries were collected from mature, nonpregnant female camels at a local abattoir within 1–2 hours post-slaughter. Immediately after collection, ovaries were placed in prewarmed saline (PBS), supplemented with 100 IU/ml penicillin and 100 μg/ml streptomycin to reduce microbial contamination during transport. The ovaries were maintained at 37°C in an insulated container and transported to the laboratory within 2–3 hours. In the laboratory, ovaries were washed three times with sterile PBS at 37°C. Follicular fluid from 3–5 mm diameter follicles was aspirated using a 5 ml syringe needle,18-gauge. Cumulus-oocyte complexes (COCs) were selected using a stereomicroscope (Olympus SZX9, Japan) and graded according to their morphology. COCs exhibiting compact cumulus cell layers and homogeneous ooplasm were selected for maturation (Mahmoud et al., 2016). Oocyte maturationA total of 240 COCs (20 per replicate × 4 treatment groups × 3 biological replicates) were selected and subjected to in vitro maturation by culturing them in TCM-199 enhanced with 10% fetal bovine serum, 0.5 µg/ml follicle-stimulating hormone, and 1 µg/ml luteinizing hormone. The COCs were incubated at 38.5°C in a humidified incubator (Thermo Scientific Forma Series II 3110, USA) containing 5% CO2 for 24 hours. Following maturation, the oocytes were denuded by gentle pipetting after exposure to 0.1% hyaluronidase for 2–3 minutes to remove the cumulus cells. After denudation, the oocytes were washed twice in fresh HEPES-buffered TCM-199 and prepared for the sperm-oocyte zona pellucida binding assay (El-Sokary et al., 2021a). Sperm-zona binding assayFor the sperm-oocyte binding, we incubated 20 oocytes per treatment group (5 oocytes per well across 4 wells), repeating the experiment in 3 biological replicates (60 oocytes total per treatment). Denuded mature oocytes were incubated with spermatozoa from camel sperm samples. Sperm were capacitated in modified Tyrode’s albumin lactate pyruvate (TALP) medium supplemented with 10 μg/ml heparin for 30 minutes at 38.5°C in an atmosphere of 5% CO2. After capacitation, 10 µl aliquots of sperm suspension (1 × 106 sperm/ml) were added to each well containing oocytes. The gametes were co-incubated for 4 hours at 38.5°C under 5% CO2. Following incubation, unbound spermatozoa were removed via gentle washing, and the number of bound sperm on the zona pellucida of each oocyte was counted at 400× magnification (Fig. 4). The binding assay was performed in triplicate for each experimental group, and at least 20 oocytes were analyzed for each group. The results were expressed as the average number of sperm bound per oocyte (Primakoff et al., 1985). Retrieval of epithelial cells from the oviductOviducts were collected from apparently healthy pubertal female camels aged 4–8 years old (n=10), slaughtered at a local abattoir and transported to the laboratory at the Animal Reproduction and Artificial Insemination Department, Veterinary Research Institute, National Research Center, on ice. The oviduct epithelial cells were collected and prepared at the laboratory as described by Winters et al. (2018). In brief, the sheet of epithelial cells was stripped from the washed isthmus, collected in PBS, and centrifuged at 84 g for 1 minute. The pellet of cells was disaggregated using a 100-μl pipette 10 times, PBS was added, and centrifuged for 1 minute. Processed cells with TALP media were divided equally into three equal portions in100-mmm tissue culture dishes and incubated at 39°C for 90 minutes to allow cells to reaggregate. For sperm binding, spherical aggregates with a diameter of 100–150 m was selected (Fig. 5). Sperm binding to oviduct epithelial cellsThe sperm binding assay was performed as described by Winters et al. (2018). In brief, 20 oviduct cell explants were added to 20 μ sperm droplets at a final concentration of 1.6 × 106 cells/ml in triplicate. Sperm were then incubated with SYBR-14 (1 μM) for 10 minutes at room temperature in a dark room. Sperm and oviduct cell aggregates were then incubated at 39°C for 30 minutes. The free and loosely attached spermatozoa were washed out with TALP media after the incubation (Modified from El-sokary et al., 2021b). The number of spermatozoa attached to the periphery of each oviduct aggregate was counted and divided by the surface area to estimate the number of bound sperm (Fig. 5). Statistical analysisData (expressed as mean ± SE) were analyzed by ANOVA using IBM SPSS version 22. Tukey’s multiple comparison test was used to identify differences between means. Differences were considered significant at p < 0.05. Ethical approvalAll animal procedures strictly adhere to the established guidelines for animal welfare and ethical research practices. The study protocol was approved by the Ethics Committee of the College of Veterinary Medicine at Benha University (Approval Number: BUFVTM14-10-24). The experiments were carried out in the laboratories of the Animal Reproduction and Artificial Insemination Department, Veterinary Research Institute, Egypt. ResultsEffects of DEHP on sperm motilitySperm motility was assessed at four DEHP concentrations (0, 10, 25, and 50 µM). The control group exhibited the highest percentage of motile sperm at 61.6% ± 2.3%. A reduction was observed in the 10 µM group (51.5% ± 2.0%), followed by a more notable decrease in the 25 µM group (50% ± 2.8%). The most profound decrease in motility was observed in the 50 µM DEHP group, in which sperm motility dropped to 43% ± 3.5% (p < 0.01 compared to all groups). These findings indicate that sperm motility was only moderately affected by lower DEHP concentrations (10 and 25 µM), whereas the highest concentration (50 µM) led to a statistically significant reduction in motility (Fig. 1). Effects of DEHP on sperm viabilityThe effects of DEHP on sperm viability were evaluated using SYBR 14 and PI staining. The percentage of viable sperm progressively declined with increasing DEHP concentration. The control group (0 µM) exhibited the highest sperm viability at 90% ± 2.1%. A slight reduction in viability was observed in the 10 µM DEHP group, with viability decreasing to 87% ± 3.0%. However, a significant decrease in sperm viability was detected at 25 µM DEHP, in which the percentage of viable sperm decreased to 83.3% ± 2.5% (p < 0.05). The most substantial reduction occurred at the highest concentration (50 µM), where sperm viability significantly declined to 50% ± 4.1% (p < 0.01). Statistical analysis indicated that 50 µM concentration showed a significant difference when compared to both the 10 µM and 25 µM concentrations (p < 0.05). These results indicate a reduction in sperm viability, particularly evident at the 25 and 50 µM concentrations (Fig. 1).
Fig. 5. Effect of DEHP exposure on the sperm binding capacity to oviduct explants. (A1) Isthmus oviduct explant from the control group. (A2) Corresponding fluorescent microscopy image showing abundant sperm attached to the explant (dark green, SYBR 14-stained). (B1) Oviduct explant exposed to 50 μM DEHP before sperm binding. (B2) Fluorescent microscopy image showing a marked reduction in sperm attachment following DEHP exposure. Scale bar=50 μm. The lower panel presents a column graph showing the effect of different DEHP concentrations (10, 25, and 50 μM) on sperm binding capacity to the oviduct explant. Different letters indicate statistically significant differences among groups (p < 0.05). Effect of DEHP on sperm plasma membrane integrityThe integrity of the sperm plasma membrane was evaluated using the HOST. The percentage of HOST-positive sperm, indicating intact plasma membranes, progressively declined with increasing DEHP concentrations. In the control group, 92% ± 1.5% of sperm were HOST-positive, whereas exposure to 10 µM resulted in a slight decrease to 86.3% ± 2.4% of HOST-positive sperm. A more pronounced reduction in membrane integrity was observed at 25 µM DEHP, with 81% ± 2.8% HOST-positive sperm (p < 0.05 compared to the control). The most significant reduction occurred at 50 µM, where only 51% ± 3.7% of sperm remained HOST-positive (p < 0.01 compared to all other groups). The statistical analysis indicated that the control group was significantly different from the 25 µM group (p < 0.05) and the 50 µM group (p < 0.01), as shown in Figure 2. Effects of DEHP on sperm mitochondrial activitySperm MMP was evaluated by assessing the percentage of sperm with HMMP. The control group exhibited the highest percentage of sperm with HMMP at 91% ± 2.0%, followed by the 10 µM group at 89% ± 1.8% and the 25 µM group at 86% ± 2.3%. Exposure to 50 µM DEHP resulted in a marked decrease in the percentage of sperm with HMMP, which dropped significantly to 63% ± 3.5% (p < 0.01 compared to all other groups) (Fig. 3). Impact of DEHP on sperm-zona binding abilityThe effect of DEHP on sperm binding to the zona pellucida was evaluated by assessing the number of sperm bound to the oocyte. In the control group, an average of 64 ± 3.1 sperm were bound to the zona pellucida. The 10 and 25 µM groups showed slight reductions in the number of bound sperm, with 55 ± 2.7 and 53 ± 3.0 bound sperm, respectively. However, at the 50 µM DEHP concentration, the number of bound sperm significantly decreased to 33 ± 2.8 (p < 0.01) compared with the other groups (Fig. 4). DEHP disrupts sperm binding to oviduct epithelial cellsThe effect of DEHP on sperm binding to oviduct epithelial cells was assessed by measuring the number of sperm bound to oviduct explants. The control group had the highest number of bound sperm (132 ± 4.5). The 10 µM group exhibited a slight reduction to 124 ± 3.8, and the 25 µM group exhibited a more pronounced decrease to 108 ± 4.2 sperm. In contrast, the 50 µM DEHP group demonstrated a significant reduction in sperm binding, with only 62 ± 3.9 sperm bound to the oviduct explants (p < 0.01 compared to all other groups). These results suggest that DEHP at lower concentrations (10 and 25 µM) had minimal impact on sperm binding to oviduct epithelial cells, while a significant reduction in binding was observed at the highest concentration of 50 µM (Fig. 5). DiscussionEndocrine disruptors such as DEHP have been widely studied due to their adverse effects on reproduction in various species, including farm animals. DEHP, a common plasticizer, leaches into the environment, leading to widespread exposure to humans and animals. It exerts its toxic effects by mimicking or blocking hormone activity, leading to disruptions in reproductive physiology. DEHP has been shown to impair gametogenesis, reduce sperm quality, and alter reproductive tract functionality, ultimately leading to reduced fertility (Foster, 2006). Studies on livestock have demonstrated that DEHP reduces sperm motility, alters DNA integrity, and negatively impacts the oocyte’s ability to be fertilized (XueXia et al., 2023). However, to the best of our knowledge, this is the first study to evaluate the effect of DEHP on camel sperm fertility. Our study demonstrated that DEHP exposure negatively affects sperm motility in a dose-dependent manner, with a significant decline observed at the highest DEHP concentration (50 µM). The control group exhibited the highest motility (61.6%), with a moderate decrease at 10 µM (51.5%) and 25 µM (50%), before a substantial decrease at 50 µM (43%). These findings indicate that whereas lower concentrations of DEHP (10 and 25 µM) caused a gradual reduction in motility, the most severe impairment occurred at 50 µM, confirming a threshold-dependent toxic effect. The decline in sperm motility at higher DEHP concentrations may be attributed to mitochondrial dysfunction and ATP depletion, as previously reported in mammalian sperm (Huang et al., 2014). DEHP is known to induce oxidative stress, leading to lipid peroxidation and mitochondrial damage, both of which are critical factors in sperm motility impairment (Hosseinzadeh et al., 2021). However, the observed reduction in motility at 10 µM DEHP, despite unaltered mitochondrial function, suggests additional mechanisms beyond oxidative stress. DEHP may disrupt cytoskeletal components such as the axoneme, microtubules, and microfilaments, impairing flagellar movement. Furthermore, it could interfere with intracellular signaling pathways, particularly tyrosine phosphorylation, calcium homeostasis, and cAMP-dependent signaling, which are essential for sperm motility regulation (Yang et al., 2024). Although spermatozoa exhibit resilience at lower DEHP concentrations (10 and 25 µM), intrinsic antioxidant defenses may initially mitigate oxidative damage. However, at 50 µM, these protective mechanisms are overwhelmed, leading to a significant decline in motility. Sperm viability, as assessed by SYBR 14 and PI staining, showed a significant decline at the highest DEHP concentration (50 µM), with the percentage of live sperm decreasing from 90% in the control group to 50% at 50 µM. These results suggest that DEHP exerts membrane-damaging effects on sperm, leading to increased cell death (Spjuth et al., 2006). The significant difference in viability between the control and 25 µM groups suggests that sperm cells begin to experience cellular damage at this concentration and struggle to maintain homeostasis. This indicates that sperm can withstand lower levels of DEHP exposure without substantial declines in viability. This pattern suggests that camel sperm possess adaptive mechanisms that tolerate low-level stressors. The significant reduction in sperm viability at 50 µM DEHP exposure may have serious implications for reproductive outcomes and lowered fertility rates in camels exposed to DEHP. Moreover, sperm from the camel epididymis exposed to DEHP have weakened plasma membranes, specifically at the components of the tail that are responsible for sperm movement. Plasma membrane integrity is essential for maintaining the sperm’s structural integrity and its ability to undergo capacitation and acrosomal reaction, processes critical for fertilization (Spjuth et al., 2006). The alterations in membrane integrity at higher DEHP concentrations can be attributed to the disruption of lipid membrane integrity by oxidative stress (Weaver et al., 2020). The mitochondria are pivotal for generating ATP, which fuels sperm motility, and any disruption in mitochondrial function can severely impact sperm performance (Spjuth et al., 2006). Sperm MMP was significantly compromised at 50 µM DEHP, whereas lower DEHP concentrations did not induce substantial changes. Our findings are consistent with those of Li et al. (2014), who demonstrated that DEHP induces mitochondrial dysfunction, leading to reduced sperm motility and fertility. The significant reduction in MMP at the 50 µM concentration could be due to oxidative stress-induced damage to mitochondrial membranes, impairing the mitochondria’s ability to maintain membrane potential and thus ATP production (Fu et al., 2017). The interaction between sperm and the zona pellucida is a critical step in fertilization, and reduced binding can lead to fertilization failure. The sperm-zona binding assay revealed a significant reduction in sperm binding at the 50 µM concentration of DEHP, with sperm binding capacity decreasing progressively with increasing DEHP concentrations. This is consistent with research by Amjad et al. (2021), who demonstrated that DEHP impairs sperm-egg interactions by disrupting the ability of sperm to bind to the zona pellucida. This reduced binding capacity is likely a downstream effect of the compromised motility, DNA integrity, and membrane integrity of DEHP-exposed sperm. Additionally, DEHP could potentially impair the acrosome reaction, a process that enables sperm to bind the zona pellucida (Pant et al., 2008). Similarly, DEHP exposure significantly reduced sperm binding to oviduct epithelial cells. Sperm binding to the oviduct epithelium is essential for maintaining sperm viability and promoting capacitation. The process of interaction between the oviduct and sperm necessitates a sound and intact surface structure of the sperm to guarantee binding efficiency (Kölle, 2022). Disruption of this interaction, particularly at higher DEHP concentrations, could lead to reduced sperm viability in the reproductive tract, thereby decreasing the chances of successful fertilization. ConclusionThis study is the first to evaluate the effects of DEHP on camel sperm function. Our findings demonstrated that 50 µM DEHP caused the most severe impairment in sperm motility, viability, mitochondrial function, plasma membrane integrity, and the ability to bind to both zona pellucida and oviduct epithelial cells. These results highlight the potential reproductive risks associated with high-dose DEHP exposure in camels, a species with unique fertility challenges that are not yet fully understood. Further research is required to investigate potential protective strategies against DEHP-induced reproductive toxicity and to assess the long-term impact of DEHP on camel fertility. AcknowledgmentsThe authors would like to express their gratitude to Egypt, the Faculty of Veterinary Medicine, Benha University, Egypt, and the National Research Institute for their valuable support and resources during this study. Conflict of interestThe authors declare no conflict of interest. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Authors’ contributionsM.M.M.E. and A.S.A.S., D.E.M., and SFS. performed the experiments, collected the data, wrote the main manuscript text, performed critical revisions, and reviewed the manuscript. M.M.M.E. and K.G.M.M. conceptualized the work, designed the experiments, contributed to the interpretation of the data, and provided critical revisions. All authors have reviewed and approved the final manuscript. Data availabilityThe data that support the findings are available upon request from the corresponding author. ReferencesAkhter, S., Ansari, M.S., Andrabi, S., Ullah, N. and Qayyum, M. 2008. Effect of antibiotics in extender on bacterial and spermatozoal quality of cooled buffalo (Bubalus bubalis) bull semen. Reprod. Domest. Anim. 43(3), 272–278. Ali, A., Derar, R., Al-Sobayil, F., Mehana, S. and Al-Hawas, A. 2014. Impotentia generandi in male dromedary camels: clinical findings, semen characteristics, and testicular histopathology. Theriogenology 82(6), 890–896. Amjad, S., Rahman, M.S., Pang, W.K., Ryu, D.Y., Adegoke, E.O., Park, Y.J. and Pang, M.G. 2021. Effects of phthalates on the functions and fertility of mouse spermatozoa. Toxicology 454, 152746. El-Sokary, M.M.M., El-Naby, A.A.H., Hameed, A.R.A.E., Mahmoud, K.G.M. and Scholkamy, T.H. 2021a. Impact of L-carnitine supplementation on the in vitro developmental competence and cryotolerance of buffalo embryos. Vet. World. 14(12), 3164–3169. El-Sokary, M., Ibrahim, S., El-Naby, A.S., Sosa, A., Mahmoud, K. and Nawito, M. 2021b. New insights into molecular aspects of sperm-oviductal binding in Egyptian buffaloes using an in vitro model: effects of oviductal segments and media. Andrologia 53(4), e13984. Foster, P.M. 2006. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 29(1), 140–185. Fu, G., Dai, J., Zhang, D., Zhu, L., Tang, X., Zhang, L., Zhou, T., Duan, P., Quan, C., Zhang, Z., Song, S. and Shi, Y. 2017. Di(2-ethylhexyl) phthalate induces apoptosis through mitochondrial pathway in GC-2spd cells. Environ. Toxicol. 32(3), 1055–1064. Garner, D.L. and Johnson, L.A. 1995. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 53(2), 276–284. Hosseinzadeh, A., Mehrzadi, S., Siahpoosh, A., Basir, Z., Bahrami, N. and Goudarzi, M. 2021. The ameliorative effect of ellagic acid on di-(2-ethylhexyl) phthalate-induced testicular structural alterations, oxidative stress, inflammation and sperm damages in adult mice. Reprod. Biol. Endocrinol. 19, 146. Huang, L.P., Lee, C.C., Fan, J.P., Kuo, P.H., Shih, T.S. and Hsu, P.C. 2014. Urinary metabolites of di(2-ethylhexyl) phthalate relation to sperm motility, reactive oxygen species generation, and apoptosis in polyvinyl chloride workers. Int. Arch. Occup. Environ. Health. 87(6), 635–646. Kandeel, M., Morsy, M.A., Abd El-Lateef, H.M., Marzok, M., El-Beltagi, H.S., Al Khodair, K.M., Soliman, W.E., Albokhadaim, I. and Venugopala, K.N. 2023. A century of “Camel Research”: a bibliometric analysis. Front. Vet. Sci. 10, 1157667. Khasin, L.G., Della Rosa, J., Petersen, N., Moeller, J., Kriegsfeld, L.J. and Lishko, P.V. 2020. The impact of Di-2-ethylhexyl phthalate on sperm fertility. Front. Cell Dev. Biol. 8, 426. Kölle, S. 2022. Sperm-oviduct interactions: key factors for sperm survival and maintenance of sperm fertilizing capacity. Andrologia 10(5), 837–843. Li, X., Fang, E.F., Scheibye-Knudsen, M., Cui, H., Qiu, L., Li, J., He, Y., Huang, J., Bohr, V.A., Ng, T.B. and Guo, H. 2014. Di-(2-ethylhexyl) phthalate inhibits DNA replication leading to hyperPARylation, SIRT1 attenuation and mitochondrial dysfunction in the testis. Sci. Rep. 4, 6434. Lin, Y., Xu, W., Yang, L., Chen, Z., Zhai, J., Zhu, Q., Guo, Z., Wang, N., Zhang, C., Deng, H., Wang, S. and Yang, G. 2023. Mechanism of testicular injury induced by Di-ethylhexyl phthalate and its protective agents. Chem.-Biol. Interact. 381, 110575. Madeja, Z.E., Podralska, M., Nadel, A., Pszczola, M., Pawlak, P. and Rozwadowska, N. 2021. Mitochondria content and activity are crucial parameters for bull sperm quality evaluation. Antioxidants 10(8), 1204. Magnusson, U. and Persson, S. 2015. Endocrine disruptors in domestic animal reproduction: a clinical issue?. Reprod. Domest. Anim. 50 Suppl 3, 15–19. Mahmoud, K.G., El-Sokary, M.M., Kandiel, M.M., Abou El-Roos, M.E. and Sosa, G.M. 2016. Effects of cysteamine during in vitro maturation on viability and meiotic competence of vitrified buffalo oocytes. Iran. J. Vet. Res. 17(3), 165–170. Pant, N., Shukla, M., Kumar Patel, D., Shukla, Y., Mathur, N., Kumar Gupta, Y. and Saxena, D.K. 2008. Correlation of phthalate exposures with semen quality. Toxicol. Appl. Pharmacol. 231(1), 112–116. Primakoff, P., Hyatt, H. and Myles, D.G. 1985. A role for the migrating sperm surface antigen PH-20 in guinea pig sperm binding to the egg zona pellucida. J. Cell Biol. 101(6), 2239–2244. Rashad, D.E.M., Ibrahim, S., El-Sokary, M.M.M., Mahmoud, K.G.M., Abou El-Roos, M.E.A., Sosa, G.A.M. and Kandiel, M.M.M. 2024. Abundance of selected genes implicated in testicular functions in Camelus dromedarius with high and low epididymal semen quality. Biol. Reprod. 110(3), 501–508. Schmidt, J.S., Schaedlich, K., Fiandanese, N., Pocar, P. and Fischer, B. 2012. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ. Health Perspect. 120(8), 1123–1129. Spjuth, L., Ljungvall, K., Saravia, F., Lundeheim, N., Magnusson, U., Hultén, F. and Rodríguez-Martínez, H. 2006. Does exposure to di (2-ethylhexyl) phthalate in pre-pubertal boars affect semen quality post-puberty? Int. J. Androl. (Online publication date: 11-Apr-2006). 29(5), 534–542. Weaver, J.A., Beverly, B.E.J., Keshava, N., Mudipalli, A., Arzuaga, X., Cai, C., Hotchkiss, A.K., Makris, S.L. and Yost, E.E. 2020. Hazards of diethyl phthalate (DEP) exposure: a systematic review of animal toxicology studies. Environ. Int. 145, 105848. Winters, R.A., Nettenstrom, L.M., Lopez, D.G., Willenburg, K.L., Vishwanath, R., Bovin, N.V. and Miller, D.J. 2018. Effect of sorting boar spermatozoa by sex chromosomes on oviduct cell binding. Theriogenology 108, 22–28. Yang, L., Liu, X., Peng, Z., Liu, Z., Song, P., Zhou, J., Ma, K., Yu, Y. and Dong, Q. 2024. Exposure to di-2-ethylhexyl phthalate (DEHP) increases the risk of cancer. BMC Public Health. 24(1), 430. XueXia, L., YaNan, L., Zi, T., YuSheng, Z., ZeLin, W., Peng, Z., MeiNa, X. and FuJun, L. 2023. Di-2-ethylhexyl phthalate (DEHP) exposure induces sperm quality and functional defects in mice. Chemosphere 312(Pt 1), 137216. | ||
| How to Cite this Article |
| Pubmed Style Rashad DEM, Sosa ASA, Shehata SF, Mahmoud KGM, El-sokary MMM. In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camels. Open Vet. J.. 2025; 15(5): 2206-2217. doi:10.5455/OVJ.2025.v15.i5.38 Web Style Rashad DEM, Sosa ASA, Shehata SF, Mahmoud KGM, El-sokary MMM. In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camels. https://www.openveterinaryjournal.com/?mno=244704 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.38 AMA (American Medical Association) Style Rashad DEM, Sosa ASA, Shehata SF, Mahmoud KGM, El-sokary MMM. In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camels. Open Vet. J.. 2025; 15(5): 2206-2217. doi:10.5455/OVJ.2025.v15.i5.38 Vancouver/ICMJE Style Rashad DEM, Sosa ASA, Shehata SF, Mahmoud KGM, El-sokary MMM. In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camels. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2206-2217. doi:10.5455/OVJ.2025.v15.i5.38 Harvard Style Rashad, D. E. M., Sosa, . A. S. A., Shehata, . S. F., Mahmoud, . K. G. M. & El-sokary, . M. M. M. (2025) In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camels. Open Vet. J., 15 (5), 2206-2217. doi:10.5455/OVJ.2025.v15.i5.38 Turabian Style Rashad, Dina E. M., Ahmed S. A. Sosa, Seham F. Shehata, Karima Gh. M. Mahmoud, and Mohamed M. M. El-sokary. 2025. In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camels. Open Veterinary Journal, 15 (5), 2206-2217. doi:10.5455/OVJ.2025.v15.i5.38 Chicago Style Rashad, Dina E. M., Ahmed S. A. Sosa, Seham F. Shehata, Karima Gh. M. Mahmoud, and Mohamed M. M. El-sokary. "In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camels." Open Veterinary Journal 15 (2025), 2206-2217. doi:10.5455/OVJ.2025.v15.i5.38 MLA (The Modern Language Association) Style Rashad, Dina E. M., Ahmed S. A. Sosa, Seham F. Shehata, Karima Gh. M. Mahmoud, and Mohamed M. M. El-sokary. "In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camels." Open Veterinary Journal 15.5 (2025), 2206-2217. Print. doi:10.5455/OVJ.2025.v15.i5.38 APA (American Psychological Association) Style Rashad, D. E. M., Sosa, . A. S. A., Shehata, . S. F., Mahmoud, . K. G. M. & El-sokary, . M. M. M. (2025) In vitro assessment of di(2-ethylhexyl) phthalate effect on epididymal sperm quality in dromedary camels. Open Veterinary Journal, 15 (5), 2206-2217. doi:10.5455/OVJ.2025.v15.i5.38 |