| Research Article | ||
Open Vet. J.. 2025; 15(9): 4470-4481
Open Veterinary Journal, (2025), Vol. 15(9): 4470-4481 Research Article Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathyFranca Borella1*, Elena Benvenuti2, Alessio Pierini3, Antonio Borrelli1, Federica Cagnasso1, Renato Zanatta1, Veronica Marchetti3 and Paola Gianella11Department of Veterinary Sciences, University of Turin, Grugliasco, Italy 2Associazione Professionale Endovet Group, Roma, Italy 3Department of Veterinary Sciences, University of Pisa, Via Livornese Lato Monte, Pisa, Italy *Corresponding Author: Franca Borella, Department of Veterinary Sciences, University of Turin, Grugliasco, Italy. Email: franca.borella [at] unito.it Submitted: 25/02/2025 Revised: 18/07/2025 Accepted: 08/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
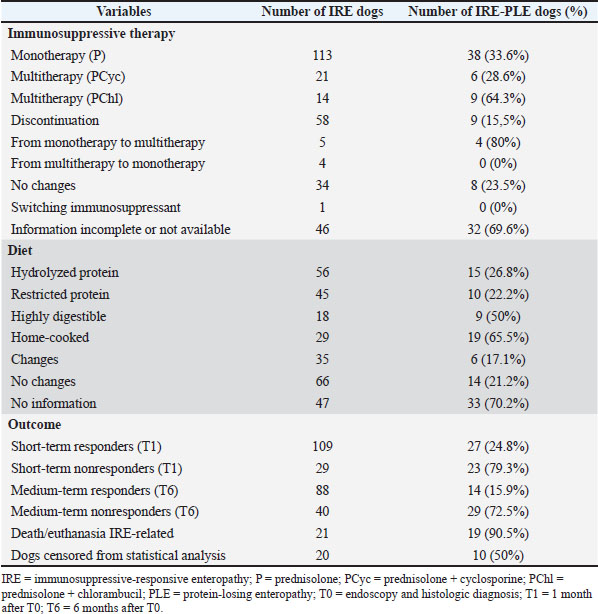
AbstractBackground: Chronic intestinal inflammation in dogs is common worldwide; however, it can be very difficult to manage and predict clinical response to different treatment protocols. Aim: This retrospective analysis included 148 dogs with chronic intestinal inflammation with the aim of describing different treatment protocols and their impact on clinical response and identifying potential predictors of treatment failure. Methods: Of the 148 dogs, 53 were classified as having presumptive inflammatory protein-losing enteropathy (IRE-PLE). The factors associated with treatment failure were also analyzed. Clinical severity (CCECAI), serum albumin concentration, type of immunosuppressive therapy (prednisolone vs prednisolone in combination with cyclosporine or chlorambucil), type of diet (hydrolyzed protein, restricted antigen, highly digestible, restricted fat), and cobalamin supplementation were evaluated at diagnosis (T0) and after 1 (T1) and 6 (T6) months. CCECAI at T1 and T6 were used to evaluate the short- and medium-term response, respectively. Results: A total of 113 dogs (33.6% IRE-PLE) received prednisolone. Twenty-one dogs (28.6% IRE-PLE) received prednisolone and cyclosporine, whereas 14 dogs (64.3% IRE-PLE) received prednisolone and chlorambucil. Ninety-five (41% IRE-PLE) received cobalamin supplementation. Hydrolyzed protein and restricted antigen diets were most commonly prescribed. At T1 and T6, 79% and 59.5% of dogs were responders (24.8% and 15.9% IRE-PLE), and 21% and 27% (79.3% and 72.5% IRE-PLE) were nonresponders, respectively. The median CCECAI scores were 2 (IQR 4) and 0 (IQR 2) at T1 and T6, respectively. Conclusion: Most dogs showed favorable clinical outcomes, although a subset did not respond to treatment at short- and medium-term follow-up. Most deaths or euthanasia occurred within 1 month of diagnosis. The therapeutic response was not influenced by the type of immunosuppressive protocol, but rather by the presence of hypoalbuminemia, hypocobalaminemia, and the need for a home-cooked restricted-fat diet. Keywords: Canine, Diet, Follow-up, IBD, Therapy. IntroductionThe criteria for diagnosing chronic enteropathies in dogs are the presence of gastrointestinal signs lasting 3 or more weeks, the presence of non-neoplastic gastrointestinal inflammation, as evidenced by histological examination, and the exclusion of other diseases causing the gastrointestinal signs (Washabau et al., 2010; Allenspach et al., 2016; Dandrieux, 2016). The response to therapy is used to retrospectively subclassify chronic enteropathies as food-responsive (FRE), antibiotic-responsive (ARE), referred to as microbiota-related modulation-responsive enteropathy, and immunosuppressant-responsive (IRE). The term non-responsive enteropathy (NRE) is used for dogs that do not respond to immunosuppressants (Craven et al., 2004; García-Sancho et al., 2007; Schreiner et al., 2008; Allenspach et al., 2016; Dandrieux, 2016; Erdmann and Heilmann, 2017; Heilmann and Steiner, 2018; Dandrieux and Mansfield, 2019; Cerquetella et al., 2020; Dupouy-Manescau et al., 2024). Protein-losing enteropathy (PLE) is a challenging syndrome characterized by chronic gastrointestinal signs and loss of proteins through the gastrointestinal tract (Craven and Washabau, 2019; Allenspach and Iennarella-Servantez, 2021; Green and Kathrani, 2022; Jergens and Heilmann, 2022). FRE, IRE, NRE, intestinal lymphangiectasia, and neoplasia are common causes of PLE in dogs (Craven and Washabau, 2019; Wennogle et al., 2021). Glucocorticoids (prednisone, prednisolone, and budesonide) are commonly used as first-line medical treatment alone or in combination with other immunosuppressive medications (cyclosporine, chlorambucil). A stepwise approach is often employed (Allenspach et al., 2016; Dandrieux, 2016; Erdmann and Heilmann, 2017; Cerquetella et al., 2020). Mycophenolate mofetil is generally not a first-line choice due to potential gastrointestinal side effects, while scarce information is available for azathioprine (Willard et al., 2000; Craven et al., 2004; Münster et al., 2006; Dandrieux et al., 2013; Allenspach et al., 2016; Swann et al., 2019). Several parameters, such as disease severity, patient size, drug costs, and side effects, influence the therapeutic choice. However, the ideal immunosuppressant protocol for inducing and maintaining long-term remission in dogs with IRE has not been determined yet (Jergens and Simpson, 2012). Overall, prednisolone, budesonide, and cyclosporine seem adequate for short-term (< 6 months) control (Allenspach et al., 2006, 2007, 2016; Dye et al., 2013). Immunosuppressants have also been widely used in dogs with PLE, although doubts about their immune-mediated etiology have been recently raised (Dossin and Lavoué, 2011; Craven and Washabau, 2019). However, a recent retrospective study concluded that PLE dogs with higher clinical activity scores were less likely to respond to dietary monotherapy (Nagata et al., 2020). Diet has several effects on the intestinal microbiome and metabolome; therefore, its role in achieving remission is important in all subtypes of CE (Martínez-López et al., 2021; Rhimi et al., 2022; Vecchiato et al., 2023). Recent research suggests that dietary modifications, regardless of antigen restriction or supplementation, can result in prompt and sustained recovery (Simpson et al., 2023). To date, the choice, customized for each patient and subtype of CE, falls between highly digestible, limited ingredient/novel protein, ultra-low-fat and low-fat, and hydrolyzed diets (Kathrani, 2021). Some dogs with PLE benefit from lifelong high-protein, low-fat diets (Okanishi et al., 2014; Rudinsky et al., 2017; Nagata et al., 2020), and a low dietary compliance was recently found in PLE dogs that relapsed compared to dogs that remained in remission (Green and Kathrani, 2022). However, no markers can currently predict food-responsiveness in dogs with PLE (Craven and Washabau, 2019), even though the CCECAI scores of FRE-PLE dogs were lower than those of IRE-PLE dogs (Nagata et al., 2020). To the authors’ knowledge, scarce information on short- and medium-term responses to different treatment protocols is available. Therefore, the aims of this retrospective study were to (1) describe short- and medium-term response to different immunosuppressive and dietary approaches in a cohort of dogs with IRE and to (2) identify potential factors associated with the lack of short- and medium-term clinical response. Materials and MethodsA search of the electronic medical records at the Veterinary Teaching Hospitals of the Universities of Pisa and Turin was conducted to identify dogs with a diagnosis of IRE from September 2018 to September 2022. The owners provided consent to use the clinical data for scientific purposes. DiagnosisA diagnosis of IRE was made if the following criteria were met: (1) gastrointestinal signs lasting 3 or more weeks; (2) exclusion of other intestinal and extra-intestinal diseases causing similar gastrointestinal signs; (3) incomplete or non-sustained remission of clinical signs while on at least two subsequent dietary trials (highly digestible, hydrolyzed protein, restricted antigen, or low-fat diets); (4) incomplete or non-sustained remission of clinical signs after microbial manipulation treatment strategies (prebiotics, probiotics, synbiotics, postbiotics, fecal microbiota transplantation and antibiotics); (5) histopathological evidence of benign gastrointestinal mucosal inflammation with or without lymphangiectasia on multiple biopsies collected from stomach, duodenum, and, when available, ileum and colon, by endoscopy; and (6) clinical improvement after an immunosuppressive monotherapy (prednisolone) or multitherapy (prednisolone combined with cyclosporine or chlorambucil) (Dandrieux, 2016; Dandrieux and Mansfield, 2019). To ensure an accurate diagnosis, the following analyses were requested: fecal testing for parasites, including Giardia antigen test, a comprehensive hematobiochemical panel including pre- and postprandial bile acids, serum trypsin-like immunoreactivity, canine pancreatic lipase, serum basal cortisol or ACTH stimulation test, serum folate and cobalamin concentrations, and urinalysis, including the evaluation of urine protein-to-creatinine ratio. In addition, all dogs were required to have an abdominal ultrasound examination and, for those that failed the dietary and microbial manipulation trials, a digestive endoscopic examination to confirm and characterize the gastrointestinal inflammation, before starting the immunosuppressive medication. Gastrointestinal biopsies were analyzed according to the histopathological guidelines of the World Small Animal Veterinary Association’s Gastrointestinal Standardization Group (Washabau et al., 2010). Dogs with IRE and concurrent hypoalbuminemia (≤ 2.0 g/dl) of presumptive gastrointestinal origin were subclassified as having PLE. Hypoalbuminemia was considered of presumptive gastrointestinal origin based on the absence of clinically relevant proteinuria (negative urine dipstick test result or urine protein to creatinine ratio <0.5), and clinically relevant hepatic disease (normal pre- and post-prandial bile acid concentrations or normal synthetic liver function and enzyme activity). At T0 (time of endoscopy and histologic diagnosis), the following information was recorded: gender, age, breed, chronic canine enteropathy clinical activity index (CCECAI) score, serum albumin concentration, type of immunosuppressive treatment, type of diet, and cobalamin supplementation. The CCECAI score was obtained by evaluating serum albumin levels, in addition to the presence or absence of peripheral edema and peritoneal fluid, and the owner’s assessment of appetite, vomiting, activity level, defecation, weight loss, and pruritus (Allenspach et al., 2007). TherapyImmunosuppressive therapy consisted of prednisolone (0.5–1 mg/kg every 12 hours, PO) alone or combined with cyclosporine (5 mg/kg every 24 hours, PO) or chlorambucil (2–4 mg/m2 every 24 hours, PO) at the discretion of the clinician. Anti-inflammatory/immunosuppressive therapy was categorized as follows: monotherapy (prednisolone) or multitherapy (prednisolone in combination with cyclosporine or chlorambucil). Supplementation with parenteral/oral cobalamin was based on its serum concentration, as previously described (Berghoff et al., 2013; Toresson et al., 2019; Kather et al., 2020; Folate Information, 2025). Although all included dogs had already undergone at least two dietary trials without clinical improvement, they were subjected to a new trial including low- and ultra-low-fat formulations for those with PLE. The dietary approach varied among dogs, and it was tailored to individual needs, considering the primary clinician’s recommendations, previous dietary history, owner preferences, palatability, and the presence or absence of PLE. The type of diet was categorized as follows: hydrolyzed protein, restricted antigen, highly digestible, and home-cooked diets (low-fat and ultra-low-fat formulations). Follow-up T1 and T6Follow-up information was obtained from the electronic medical records 1 (T1) and 6 (T6) months after T0. Dogs were considered “short-term responders” if their CCECAI scores at T1 had decreased by more than 25%, whereas they were considered “short-term nonresponders” if their CCECAI scores had decreased by 25% or less, or they had died (Heilmann et al., 2018). Additionally, delta CCECAI (ΔCCECAI) was calculated for each dog as the difference between the CCECAI scores at T6 and at T1. Dogs were considered “medium-term responders” if they were alive at T6 and had a ΔCCECAI < 3. Dogs were considered “medium-term nonresponders” if they did not respond at T1, or had a ΔCCECAI >2, died, or were euthanized due to IRE-related causes. Dogs that died due to other causes or were lost at follow-up were censored from the analyses. Statistical analysisDescriptive statistic was used to present continuous (median and interquartile range (IQR)), and categorical data (absolute and relative frequency). Potential factors associated with the lack of short- and medium-term therapeutic response (predictors) included age, sex, serum albumin concentration, presence of PLE, type of diet, type of anti-inflammatory/immunosuppressive therapy, and cobalamin supplementation. Outcomes included responses at T1 (short-term responders/short-term nonresponders) and T6 (medium-term responders/medium-term nonresponders). The Kolmogorov–Smirnov test for normality was used to evaluate the data distribution of age and serum albumin concentration. After having demonstrated associations between albumin and outcomes, in order to better describe its influence on outcomes, serum albumin concentration was recorded into categories using 0.5 mg/dl and analyzed. Sex, presence of PLE, type of diet, type of immunosuppressive therapy, cobalamin supplementation, and the two outcomes were analyzed as categorical data. Univariate logistic regression was used to evaluate the associations between potential predictors and outcomes. Potential predictors that were significant (p < 0.10) in the univariate analysis were included in the multivariate logistic regression model. The final model variables were tested for collinearity and interactions, and they were removed one by one from the final model either if pairwise interactions were significant (p < 0.05) or if variance inflation factors were higher than 5. All statistics were performed with the IBM SPSS® Statistics package. Ethical approvalNot needed for this study. ResultsA total of 148 dogs with IRE met the inclusion criteria and were retrospectively included in the study. Eighty-five (57.4%) dogs were male (3 neutered), and 63 (42.6%) were female (36 spayed). Several breeds were included: German Shepherd (15 dogs), Yorkshire Terrier (9 dogs), English Setter (7 dogs), Maltese and Jack Russell Terrier (6 dogs each breed), Pinscher and Border Collie (5 dogs each breed), Dachshund (4 dogs), Boxer, Cavalier King Charles Spaniel, Chihuahua, Spanish Levriero and Rottweiler (3 dogs each breed), Australian Sheperd, Bolognese, Cocker Spaniel, Cane Corso, Dobermann, Golden Retriever, Labrador Retriever, American Pitbull Terrier, Poodle, Pug, Siberian Husky and Springer Spaniel (2 dogs each breed), Beagle, Belgian Sheperd, Bernese Mountain dog, Bichon Frisé, Boston Terrier, Cesky Terrier, Dogue de Bordeaux, English Bulldog, French Bulldog, Greyhound, Irish Setter, Parson Russel Terrier, Podenco, Russian Toy, Shiba Inu, Small Italian Levriero, Italian Volpino, Weimaraner, and West Highland White Terrier (1 dog each breed). Thirty-three dogs were mixed-breed. The median age was 5.25 years (IQR 5). At T0, the median CCECAI score was 8 (IQR 4), and the median serum albumin concentration was 2.39 mg/dl (IQR 1.44 mg/dl). Based on serum albumin concentration, 53 dogs (35.8%) were subclassified as IRE-PLE. Lymphoplasmacytic intestinal inflammation was observed in all dogs. Lymphangiectasia was identified in 45 dogs (30.4%). After endoscopy and histological diagnosis (T0), 113 (76.4%) dogs received prednisolone alone, whereas 35 (23.6%) dogs received prednisolone in combination with cyclosporine (21 dogs) or chlorambucil (14 dogs). Of the 53 dogs with IRE-PLE, 38 (71,7%) received only prednisolone, 9 (17%) received prednisolone in combination with chlorambucil, and 6 (11,3%) received prednisolone in combination with cyclosporine. Ninety-five (64.2%) dogs, of which 41 were IRE-PLE, received oral or parenteral cobalamin supplementation. Fifty-six (37.8%) dogs received hydrolyzed protein diets, 45 (30.4%) restricted antigen diets, 29 (19.6%) home-cooked diets (low-fat and ultra-low-fat formulations), and 18 (12.2%) highly digestible diets. Of the 53 dogs with IRE-PLE, 19 (35.8%) received home-cooked diets, 15 (28.3%) received hydrolyzed protein diets, 10 (18.9%) received restricted antigen diets, and 9 (17%) received highly digestible diets. One month after endoscopy and histological diagnosis (T1), 109 dogs (79%) were considered responders and 29 (21%) were considered nonresponders. Among the responders, 27 dogs (24.8%) were IRE-PLE; among the nonresponders, 23 (79.3%) were IRE-PLE. Overall, the median CCECAI score at T1 was 2 (IQR 4). For 10 dogs (6.8%), the CCECAI scores were not available at T1. Between T1 and T6, immunosuppressive therapy remained unchanged in 34 dogs, including 8 dogs with IRE-PLE (23.5%). In contrast, it was switched from monotherapy to multitherapy in 5 dogs, 4 of which had IRE-PLE (80%). In 4 dogs, the immunosuppressive therapy was switched from multitherapy to monotherapy, none of which had IRE-PLE, whereas in 58 dogs, it was tapered and discontinued, with 9 cases (15,5%) exhibiting IRE-PLE. One dog switched from cyclosporine to chlorambucil because of its low effectiveness. During the study period, information on immunosuppressive therapy was not available (death) or incomplete (lost to follow-up) in 46 dogs, of which 32 were IRE-PLE (69.6%). Cobalamin supplementation was discontinued in 42 dogs, of which 7 were IRE-PLE, following normalization of their serum concentration, and then later resumed in 2 of them. Overall, the type of diet was not changed for 66 dogs, of which 14 were IRE-PLE (21.2%); however, it was changed to another category in 35 dogs, of which 6 were IRE-PLE (17.1%). At T6, 128 (86.5%) dogs were evaluable for medium-term response. The medium CCECAI score was 0 (IQR 2); 88 dogs (59.5%) were considered medium-term responders, of which 14 (15.9%) were IRE-PLE, and 40 (27%) were medium-term nonresponders, of which 29 (72.5%) were IRE-PLE. Twenty of the 148 dogs (13.5%) were excluded from the logistic regression analysis. Of the 21 (14.2%) dogs, 19 (90.5%) IRE-PLE died or were euthanized during the study period because of IRE-related causes. Of these dogs, 1 IRE and 14 IRE-PLE died or were euthanized between T0 and T1. Information on dietary, immunosuppressive therapy, and short- and medium-term response is presented in Table 1. Table 1. Information on dietary and immunosuppressive interventions, and short- and medium-term response in 148 dogs diagnosed with immunosuppressive-responsive enteropathy (IRE).
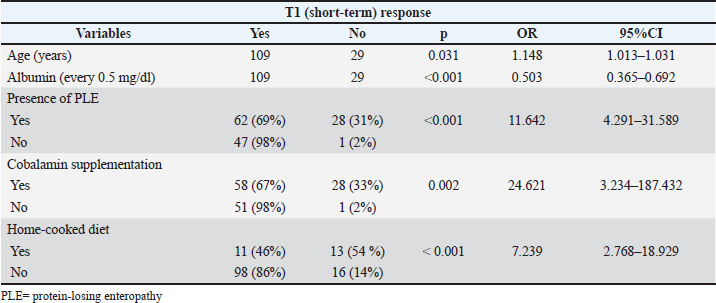
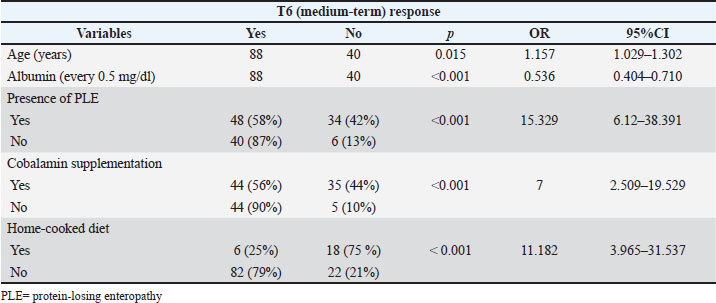
No significant differences in short- and medium-term responses were observed between dogs receiving monotherapy and those receiving multitherapy. Age (years), albumin (every 0.5 mg/dl), presence of PLE, cobalamin supplementation, and home-cooked diet were significantly associated with non-response at both T1 and T6 in the univariate analysis (Tables 2 and 3). Table 2. Univariate analysis. Statistically significant predictors for non-response at T1 in 148 dogs diagnosed with IRE.
Table 3. Univariate analysis. Statistically significant predictors for non-response at T6 in 148 dogs diagnosed with immunosuppressive-responsive enteropathy (IRE).
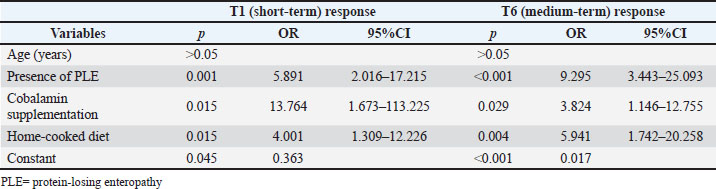
The presence of PLE, cobalamin supplementation, and a home-cooked diet were significantly associated with nonresponse at both T1 and T6 in the multivariate analysis (Table 4). Table 4. Results of multivariate analysis of predictors tested for the association with non-response both at T1 and T6 in 148 dogs diagnosed with immunosuppressive-responsive enteropathy (IRE).
DiscussionThis study aimed to describe short- and medium-term responses to different immunosuppressive and dietary approaches in a population of dogs diagnosed with IRE and explored potential factors associated with the lack of short- and medium-term clinical response. Although IRE is common worldwide, it can be very difficult to manage and predict clinical response to different treatment protocols. The study population consisted of 148 dogs, of which 35.8% were subclassified as IRE-PLE. The median age was 5.25 years, similar to a previous report from Italy, but lower than reports from other countries (Otoni et al., 2018; Guard et al., 2019; Benvenuti et al., 2021). The German Shepherd and Yorkshire Terrier breeds were overrepresented in this study; this is not surprising because both breeds are at increased risk for developing chronic enteropathy and PLE, respectively (Allenspach et al., 2010; Kathrani et al., 2010, 2011; Rudinsky et al., 2017). The median CCECAI score in our study population indicated moderate disease severity. Allenspach et al. observed significantly higher CCECAI scores in dogs with IRE or non-responsive PLE compared with those with FRE (Allenspach et al., 2016). Furthermore, a cut-off CCECAI of 8 was useful to distinguish dogs with FRE-PLE from those with IRE-PLE or non-responsive PLE (Nagata et al., 2020). To the contrary, in our study population, some IRE and IRE-PLE dogs had CCECAI scores < 8 and were consistent with mild disease severity. CCECAI scores were used to evaluate clinical response. An improvement in the median CCECAI score from moderate disease severity to normal was observed within 30 days (T1), and it was maintained in the medium-term period (T6) by the majority of dogs with IRE and some dogs with IRE-PLE. This might suggest that clinical and biochemical (albumin) improvement secondary to the multimodal therapeutic approach might be observed within 30 days in dogs with chronic enteropathies of moderate disease severity, in line with previous observations (Marks et al., 2002; Allenspach et al., 2007, 2016; Benvenuti et al., 2021). Twenty-one dogs died or were euthanized during the study due to IRE-related causes, and 19 of the 21 dogs were IRE-PLE. Of these dogs, 1 was diagnosed with IRE and 14 with IRE-PLE died between T0 and T1. This result suggests that the first month of treatment management is crucial, especially in cases with a more severe disease (PLE), and that the nonresponse to therapy is likely associated with a shorter survival time, which is consistent with previous reports (Nakashima et al., 2015; Gianella et al., 2017). In our study, the majority of dogs received prednisolone as monotherapy, whereas only one-third of dogs received a second-line immunosuppressant (Dandrieux et al., 2013; Allenspach et al., 2016; Dandrieux, 2016; Erdmann and Heilmann, 2017). Interestingly, we observed no significant difference in short- and medium-term response rates between dogs receiving monotherapy and those receiving multitherapy. This could be explained by the multifactorial nature of the pathogenesis of IRE, whose therapeutic management is multimodal and not solely dependent on immunosuppressive protocols. Indeed, a multimodal approach may have a more significant clinical impact than the type of immunosuppressant used. However, this hypothesis needs to be confirmed, and it remains speculative at this time. Moreover, it is difficult to compare our data with those from the veterinary literature. Different types of immunosuppressant agents have been evaluated as monotherapy for the management of dogs with IRE and IRE-PLE, but studies comparing different therapeutic protocols are limited (Allenspach et al., 2007; García-Sancho et al., 2007; Jergens et al., 2010; Dye et al., 2013; Pietra et al., 2013; Simmerson et al., 2014; Nakashima et al., 2015). Cyclosporine and chlorambucil have been described as rescue protocols in dogs not responding to prednisolone, with potentially better outcomes than other immunosuppressants in PLE dogs (Allenspach et al., 2006, 2007; Dandrieux et al., 2013; Green and Kathrani, 2022). To the best of the authors’ knowledge, however, only one study compared two different immunosuppressant protocols in PLE dogs, in which prednisolone and chlorambucil showed greater efficacy compared to prednisolone and azathioprine (Dandrieux et al., 2013). In the medium term, it was possible to taper and discontinue immunosuppressive treatment in almost 40% of the dogs, while the immunosuppressive therapy was switched from multitherapy to monotherapy in 4 dogs, and a second immunosuppressant agent was added in 5 dogs. Finally, one dog switched from one drug to another because of the lack of effectiveness. Indeed, immunosuppressive treatment is usually tapered in responder dogs or intensified through dosage adjustments or drug changes in nonresponder dogs, respectively (Dandrieux, 2016). Overall, a good clinical course in most of our dogs emerged from these results. All IRE-PLE dogs in our study received immunosuppressants in addition to dietary therapy. During the study period, immunosuppressive therapy was discontinued in 9 IRE-PLE dogs. Allenspach and colleagues recently reviewed several reports highlighting the effectiveness of dietary treatment alone in dogs with PLE; this effectiveness was achieved through a variety of dietary approaches (Allenspach and Iennarella-Servantez, 2021). Limited antigen diets were prescribed more often than low-fat diets to Yorkshire terriers with PLE, and many of them experienced improvement in clinical signs and prolonged survival times (Simmerson et al., 2014). Moreover, one study reported that most dogs with PLE and intestinal lymphangiectasia responded positively to a low-fat diet alone (Okanishi et al., 2014). Based on more recent studies, dietary management with low-fat (<30g fat/Mcal ME) or ultra-low-fat (<15g fat/Mcal ME) formulations appears to be the cornerstone of PLE treatment (Tolbert et al., 2022; Myers et al., 2023). Indeed, fat-restricted diets could potentially break the cycle of intestinal protein and lipid leakage, reducing lymphatic flow and lacteal distension (Craven and Washabau, 2019). Currently, it is unclear whether low-fat or ultra-low-fat diets are more effective in PLE cases (Okanishi et al., 2014; Nagata et al., 2020). However, since some dogs with PLE have lacteal dilation due to severe inflammation, a reduced-fat, hydrolyzed diet might be a more suitable therapeutic option (García-Sancho et al., 2007). Although our dogs were all already subjected to at least two subsequent dietary trials without clinical benefit, they were all again subjected to a new dietary trial including low- and ultra-low-fat formulations, with no clinical response. Therefore, all dogs, including those with PLE, received anti-inflammatory/immunosuppressive treatments in addition to dietary interventions. Based on the available literature, immunosuppressants should be carefully considered in dogs with PLE (Craven and Washabau, 2019; Jergens and Heilmann, 2022); however, the administration of corticosteroids was supported by the presence of an intestinal inflammatory infiltrate and the lack of a satisfactory response to dietary trials (Okanishi et al., 2014; Rudinsky et al., 2017). Dogs with PLE that are unresponsive to diet and monotherapy may benefit from a combination of different immunosuppressants (Dandrieux et al., 2013; Salavati Schmitz et al., 2019; Kathrani, 2021). It is the authors’ opinion that the current definition of inflammatory PLE encompasses two groups of patients: dogs with severe lymphangiectasia and mild inflammation who mainly benefit from low-fat formulations, and dogs with severe inflammation and mild lymphangiectasia who benefit from hydrolyzed or low-fat diets combined with anti-inflammatory/immunosuppressive doses of prednisolone. Dietary management alone is likely insufficient to control severe intestinal inflammation, as in the majority of our dogs. The diet plays an important role not only in managing PLE-associated chronic enteropathies but also in managing all types of chronic enteropathies and needs to be integrated into the multimodal treatment plan (Dandrieux, 2016; Marchesi et al., 2017; Kathrani, 2021; Dupouy-Manescau et al., 2024). However, the optimal approach varies significantly among dogs because several factors, including the dog’s genetics, environment, lifestyle, veterinarians’ expertise, the dog’s specific needs, and the owner’s ability to adhere to the feeding plan, influence the dietary choice. To date, both hydrolyzed protein and restricted antigen diets have been successfully employed in managing chronic enteropathy in dogs, and there is no clear evidence favoring one over the other (Kathrani, 2021). Even highly digestible diets, developed for enhanced nutrient absorption, can be effective options (Tolbert et al., 2022). However, digestibility alone may be insufficient for maintaining remission (Mandigers et al., 2010). Moreover, recent research has demonstrated that simply changing the diet, regardless of antigen restriction or supplemental ingredients, can be effective in achieving long-term remission in dogs with IRE (Simpson et al., 2023). In agreement with this information, different types of diets were successfully used in our dogs, in addition to immunomodulatory therapy, while the type of diet was changed only in some dogs to achieve better control of clinical signs. Cobalamin supplementation was administered to approximately 60% of the dogs, as recommended elsewhere (Hall and Day, 2006; Toresson et al., 2018, 2019; Chang et al., 2022; Dor et al., 2024). Hypocobalaminemia frequently occurs in dogs with exocrine pancreatic insufficiency and chronic enteropathy (19%–54%), with and without PLE. Distal small intestinal cobalamin malabsorption, secondary small intestinal dysbiosis, or both might play a role (Batt and Horadagoda, 1989; Allenspach et al., 2007; Berghoff et al., 2013; Heilmann et al., 2016a, 2016b, 2018; Volkmann et al., 2017). Similar to hypoalbuminemia, hypocobalaminemia is a negative prognostic factor in dogs with chronic enteropathy (Allenspach et al., 2007; Volkmann et al., 2017). However, cobalamin supplementation can reverse the risk of hypocobalaminemia (Allenspach et al., 2007), and both oral and parenteral supplementation are effective (Toresson et al., 2018, 2019). The presence of PLE, cobalamin supplementation, and a home-cooked diet were significantly associated with nonresponse at both T1 and T6 in the multivariate analysis. These results are not surprising. First, hypoalbuminemia has previously been reported as a poor prognostic factor, and all dogs with PLE in our study had hypoalbuminemia (Craven et al., 2004; Allenspach et al., 2007). Moreover, other factors already known to negatively impact the prognosis of PLE dogs, such as severity of intestinal and systemic inflammation, bodyweight, altered serum urea, or undernutrition, may have played a role in the lack of therapeutic response (Craven and Washabau, 2019; Jergens and Heilmann, 2022). Second, cobalamin supplementation was administered to dogs with hypocobalaminemia, which is known to be poorly associated with outcome (Allenspach et al., 2007; Volkmann et al., 2017). In addition, hypocobalaminemia is responsible for a wide range of clinical and metabolic effects, including anorexia, lethargy, weight loss, villous atrophy, and malabsorption, that may have negatively impacted the therapeutic response of our dogs (Fordyce et al., 2000; Battersby et al., 2005; Lutz et al., 2013; Ruaux, 2013). Finally, the use of home-cooked diets was significantly associated with nonresponse. A plausible explanation could be found in the type of patient to whom this diet was recommended, rather than in the diet itself. Indeed, all our home-cooked low- and ultra-low-fat formulations were used in selected IRE and IRE-PLE dogs with lymphangiectasia to more precisely set macronutrient profile and prevent further lacteal dilation and lymph leakage (Marks et al., 2002; Okanishi et al., 2014; Rudinsky et al., 2017). It cannot be excluded that the clinical presentation at the time of diet selection played a role in the subsequent failure of treatment response. Overall, the short- and medium-term therapeutic response of our dogs did not appear to be influenced by the type of immunosuppressant, but rather by the presence of hypoalbuminemia (< 2g/dl), hypocobalaminemia, and the need for a restricted fat diet at diagnosis. The first limitation of this study is its retrospective design. Missing information on immunosuppressive therapy and diet may have changed our results. Another limitation is the variability in anti-inflammatory/immunosuppressive treatments and diets used at diagnosis and follow-up, which might have influenced the therapeutic response. Moreover, since histological reassessment of intestinal inflammation following therapeutic interventions is often impracticable, and no single biomarker, whether serologic or mucosal, can be considered as a predictor of disease progression or response to therapy (Jergens and Simpson, 2012), modulation of diet and therapy are often performed according to the clinical response and CCECAI scores (Allenspach et al., 2007; Heilmann et al., 2018; Benvenuti et al., 2021). However, despite the lack of a standardized approach, all dogs received dietary interventions with prednisolone as first-line treatment. Additionally, immunohistochemistry or PCR for antigen receptor rearrangements (PARR) was not performed. Therefore, it was not possible to rule out that some low-grade lymphoma dogs, erroneously classified as IRE or IRE-PLE dogs, might have influenced the therapeutic response (Couto et al., 2018; Lane et al., 2018). Lastly, the influence of other empirical treatments such as gut protectants, antiemetics, and probiotics, as well as the impact of owners in home management and euthanasia request, was not considered in this study, but are certainly worthy of further studies. ConclusionThe clinical course of most dogs with IRE was good. In addition, dietary management alone is likely insufficient for controlling severe intestinal inflammation in some IRE-PLE dogs. Finally, the short- and medium-term therapeutic responses of both IRE and IRE-PLE dogs are not influenced by the type of immunosuppressant, by the presence of hypoalbuminemia, hypocobalaminemia, and the need for a restricted fat diet at diagnosis. AcknowledgmentsNone. FundingThis research received no specific grant. Author contributionsConceptualization and methodology, P.G., A.P., V.M.; formal analysis, A.P.; investigation, F.B., E.B., A.B., F.C., R.Z., V.M., P.G.; data curation, A.P.; writing—original draft preparation, F.B., P.G.; writing—review and editing, F.B., E.B., A.P., A.B., F.C., R.Z., V.M., P.G. All authors have read and agreed to the published version of the manuscript. Conflict of interestThe authors declare that there is no conflict of interest. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAllenspach, K. and Iennarella-Servantez, C. 2021. Canine protein losing enteropathies and systemic complications. Vet. Clin. North Am. Small Anim. Pract. 51, 111–122; doi:10.1016/j.cvsm.2020.09.010 Allenspach, K., Culverwell, C. and Chan, D. 2016. Long-term outcome in dogs with chronic enteropathies: 203 cases. Vet. Rec. 178, 368–368; doi:10.1136/vr.103557 Allenspach, K., House, A., Smith, K., Mcneill, F.M., Hendricks, A., Elson-Riggins, J., Riddle, A., Steiner, J.M., Werling, D., Garden, O.A., Catchpole, B. and Suchodolski, J.S. 2010. Evaluation of mucosal bacteria and histopathology, clinical disease activity and expression of Toll-like receptors in German shepherd dogs with chronic enteropathies. Vet. Microbiol. 146, 326–335; doi:10.1016/j.vetmic.2010.05.025 Allenspach, K., Rüfenacht, S., Sauter, S., Gröne, A., Steffan, J., Strehlau, G. and Gaschen, F. 2006. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid-refractory inflammatory bowel disease. J. Vet. Intern. Med. 20(2006), 239–244. Allenspach, K., Wieland, B., Gröne, A. and Gaschen, F. 2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 21, 700–708. Batt, R.M. and Horadagoda, N.U. 1989. Gastric and pancreatic intrinsic factor-mediated absorption of cobalamin in the dog. Am. J. Physiol. 257, G344–G349; doi:10.1152/ajpgi.1989.257.3.G344 Battersby, I.A., Giger, U. and Hall, E.J. 2005. Hyperammonaemic encephalopathy secondary to selective cobalamin deficiency in a juvenile Border collie. J. Small Anim. Pract. 46, 339–344; doi:10.1111/j.1748-5827.2005.tb00330.x Benvenuti, E., Pierini, A., Bottero, E., Pietra, M., Gori, E., Salvadori, S. and Marchetti, V. 2021. Immunosuppressant-responsive enteropathy and non-responsive enteropathy in dogs: prognostic factors, short- and long-term follow up. Animals 11, 2637; doi:10.3390/ani11092637 Berghoff, N., Parnell, N.K., Hill, S.L., Suchodolski, J.S. and Steiner, J.M. 2013. Serum cobalamin and methylmalonic acid concentrations in dogs with chronic gastrointestinal disease. Am. J. Vet. Res. 74, 84–89; doi:10.2460/ajvr.74.1.84 Cerquetella, M., Rossi, G., Suchodolski, J.S., Schmitz, S.S., Allenspach, K., Rodríguez‐Franco, F., Furlanello, T., Gavazza, A., Marchegiani, A., Unterer, S., Burgener, I.A., Pengo, G. and Jergens, A.E. 2020. Proposal for rational antibacterial use in the diagnosis and treatment of dogs with chronic diarrhoea. J. Small Anim. Pract. 61, 211–215; doi:10.1111/jsap.13122 Chang, C.H., Lidbury, J.A., Suchodolski, J.S. and Steiner, J.M. 2022. Effect of oral or injectable supplementation with cobalamin in dogs with hypocobalaminemia caused by chronic enteropathy or exocrine pancreatic insufficiency. J. Vet. Intern. Med. 36, 1607–1621; doi:10.1111/jvim.16528 Couto, K.M., Moore, P.F., Zwingenberger, A.L., Willcox, J.L. and Skorupski, K.A. 2018. Clinical characteristics and outcome in dogs with small cell T-cell intestinal lymphoma. Vet. Comp. Oncol. 16, 337–343; doi:10.1111/vco.12384 Craven, M., Simpson, J.W., Ridyard, A.E. and Chandler, M.L. 2004. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995–2002). J. Small Anim. Pract. 45, 336–342; doi:10.1111/j.1748-5827.2004.tb00245.x Craven, M.D. and Washabau, R.J. 2019. Comparative pathophysiology and management of protein-losing enteropathy. J. Vet. Intern. Med. 33, 383–402; doi:10.1111/jvim.15406 Dandrieux, J.R.S. 2016. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same?. J. Small Anim. Pract. 57, 589–599; doi:10.1111/jsap.12588 Dandrieux, J.R.S. and Mansfield, C.S. 2019. Chronic enteropathy in canines: prevalence, impact and management strategies. Vet. Med. 10, 203–214; doi:10.2147/VMRR.S162774 Dandrieux, J.R.S., Noble, P.J., Scase, T.J., Cripps, P.J. and German, A.J. 2013. Comparison of a chlorambucil-prednisolone combination with an azathioprine-prednisolone combination for treatment of chronic enteropathy with concurrent protein-losing enteropathy in dogs: 27 cases (2007–2010). J. Am. Vet. Med. Assoc. 242(12), 1705–1714; doi:10.2460/javma.242.12.1705 Dor, C., Nixon, S., Salavati Schmitz, S., Bazelle, J., Černá, P., Kilpatrick, S., Harvey, N.D. and Dunning, M. 2024. Efficacy and tolerance of oral versus parenteral cyanocobalamin supplement in hypocobalaminaemic dogs with chronic enteropathy: a controlled randomised open‐label trial. J. Small Anim. Pract. 65, 317–328; doi:10.1111/jsap.13705 Dossin, O. and Lavoué, R. 2011. Protein-losing enteropathies in dogs. Vet. Clin. North Am. Small Anim. Pract. 41, 399–418; doi:10.1016/j.cvsm.2011.02.002 Dupouy-Manescau, N., Méric, T., Sénécat, O., Drut, A., Valentin, S., Leal, R.O. and Hernandez, J. 2024. Updating the classification of chronic inflammatory enteropathies in dogs. Animals 14, 681; doi:10.3390/ani14050681 Dye, T.L., Diehl, K.J., Wheeler, S.L. and Westfall, D.S. 2013. Randomized, controlled trial of budesonide and prednisone for the treatment of idiopathic inflammatory bowel disease in dogs. J. Vet. Intern. Med. 27, 1385–1391; doi:10.1111/jvim.12195 Folate Information 2025. Gastrointestinal laboratory. Available via https://vetmed.tamu.edu/gilab/research/folate-information/ (Accessed 29 July 2024). Fordyce, H.H., Callan, M.B. and Giger, U. 2000. Persistent cobalamin deficiency causing failure to thrive in a juvenile beagle. J. Small Anim. Pract. 41, 407–410; doi:10.1111/j.1748-5827.2000.tb03233.x García-Sancho, M., Rodríguez-Franco, F., Sainz, A., Mancho, C. and Rodríguez, A. 2007. Evaluation of clinical, macroscopic, and histopathologic response to treatment in nonhypoproteinemic dogs with lymphocytic-plasmacytic enteritis. J. Vet. Intern. Med. 21(11), 11–17. Gianella, P., Lotti, U., Bellino, C., Bresciani, F., Cagnasso, A., Fracassi, F., D’Angelo, A. and Pietra, M. 2017. Clinicopathologic and prognostic factors in short- and long-term surviving dogs with protein-losing enteropathy. Schweiz. Arch. Tierheilkd. 159, 163–169; doi:10.17236/sat00108 Green, J. and Kathrani, A. 2022. Incidence of relapse of inflammatory protein-losing enteropathy in dogs and associated risk factors. J. Vet. Intern. Med. 36, 1981–1988; doi:10.1111/jvim.16561 Guard, B.C., Honneffer, J.B., Jergens, A.E., Jonika, M.M., Toresson, L., Lawrence, Y.A., Webb, C.B., Hill, S., Lidbury, J.A., Steiner, J.M. and Suchodolski, J.S. 2019. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Intern. Med. 33, 1295–1305; doi:10.1111/jvim.15493 Hall, E.J. and Day, M.J. 2016. Diseases of the small intestine. In Textbook of veterinary internal medicine, 8th ed. Eds., Ettinger, S.J., Feldman, E.C. and Cote, E. St Louis, MO: Elsevier Health Sciences, pp. 1516–1533. Heilmann, R. and Erdmann, C. 2017. Chronisch-entzündliche Darmerkrankungen beim Hund – diagnostische und therapeutische Aspekte. Tierärztliche Praxis Ausgabe K. Kleintiere /. Heimtiere 45, 317–327; doi:10.15654/TPK-170366 Heilmann, R.M. and Steiner, J.M. 2018. Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs. J. Vet. Intern. Med. 32, 1495–1508; doi:10.1111/jvim.15247 Heilmann, R.M., Berghoff, N., Mansell, J., Grützner, N., Parnell, N.K., Gurtner, C., Suchodolski, J.S. and Steiner, J.M. 2018. Association of fecal calprotectin concentrations with disease severity, response to treatment, and other biomarkers in dogs with chronic inflammatory enteropathies. J. Vet. Intern. Med. 32, 679–692; doi:10.1111/jvim.15065 Heilmann, R.M., Parnell, N.K., Grützner, N., Mansell, J., Berghoff, N., Schellenberg, S., Reusch, C.E., Suchodolski, J.S. and Steiner, J.M. 2016. Serum and fecal canine α 1 -proteinase inhibitor concentrations reflect the severity of intestinal crypt abscesses and/or lacteal dilation in dogs. Vet. J. 207, 131–139; doi:10.1016/j.tvjl.2015.10.042 Heilmann, R.M., Volkmann, M., Otoni, C.C., Grützner, N., Kohn, B., Jergens, A.E. and Steiner, J.M. 2016. Fecal S100A12 concentration predicts a lack of response to treatment in dogs affected with chronic enteropathy. Vet. J. 215, 96–S100; doi:10.1016/j.tvjl.2016.03.001 Jablonski Wennogle, S.A., Stockman, J. and Webb, C.B. 2021. Prospective evaluation of a change in dietary therapy in dogs with steroid‐resistant protein-losing enteropathy. J. Small Anim. Pract. 62, 756–764; doi:10.1111/jsap.13334 Jergens, A.E. and Heilmann, R.M. 2022. Canine chronic enteropathy-current state-of-the-art and emerging concepts. Front. Vet. Sci. 9, 923013; doi:10.3389/fvets.2022.923013 Jergens, A.E. and Simpson, K.W. 2012. Inflammatory bowel disease in veterinary medicine. Front. Biosci. 4, 1404–1419; doi:10.2741/e470 Jergens, A.E., Crandell, J., Morrison, J.A., Deitz, K., Pressel, M., Ackermann, M., Suchodolski, J.S., Steiner, J.M. and Evans, R. 2010. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: a randomized-controlled trial. J. Vet. Intern. Med. 24, 269–277; doi:10.1111/j.1939-1676.2009.0447.x Kather, S., Grützner, N., Kook, P.H., Dengler, F. and Heilmann, R.M. 2020. Review of cobalamin status and disorders of cobalamin metabolism in dogs. J. Vet. Intern. Med. 34, 13–28; doi:10.1111/jvim.15638 Kathrani, A. 2021. Dietary and nutritional approaches to the management of chronic enteropathy in dogs and cats. Vet. Clinics North Amer. Small Anim. Pract. 51, 123–136; doi:10.1016/j.cvsm.2020.09.005 Kathrani, A., House, A., Catchpole, B., Murphy, A., German, A., Werling, D. and Allenspach, K. 2010. Polymorphisms in the TLR4 and TLR5 gene are significantly associated with inflammatory bowel disease in German shepherd dogs. PLoS. One 5, e15740; doi:10.1371/journal.pone.0015740 Kathrani, A., Werling, D. and Allenspach, K. 2011. Canine breeds at high risk of developing inflammatory bowel disease in the south‐eastern UK. Vet. Rec. 169, 635–635; doi:10.1136/vr.d5380 Lane, J., Price, J., Moore, A., Dandrieux, J.R.S., Clifford, C., Curran, K., Choy, K. and Cannon, C. 2018. Low‐grade gastrointestinal lymphoma in dogs: 20 cases (2010 to 2016). J. Small Anim. Pract. 59, 147–153; doi:10.1111/jsap.12769 Lutz, S., Sewell, A.C., Reusch, C.E. and Kook, P.H. 2013. Clinical and laboratory findings in border collies with presumed hereditary juvenile cobalamin deficiency. J. Am. Anim. Hosp. Assoc. 49, 197–203; doi:10.5326/JAAHA-MS-5867 Mandigers, P.J., Biourge, V., Van Den Ingh, T.S., Ankringa, N. and German, A.J. 2010. A randomized, open-label, positively-controlled field trial of a hydrolyzed protein diet in dogs with chronic small bowel enteropathy. J. Vet. Intern. Med. 24, 1350–1357; doi:10.1111/j.1939-1676.2010.0632.x Marchesi, M.C., Timpano, C.C., Busechian, S., Pieramati, C. and Rueca, F. 2017. The role of diet in managing inflamatory bowel disease affected dogs: a retrospective cohort study on 76 cases. Vet. Ital. 53, 297–302; doi:10.12834/VetIt.566.2700.1 Marks, S.L., Laflamme, D.P. and McAloose, D. 2002. Dietary trial using a commercial hypoallergenic diet containing hydrolyzed protein for dogs with inflammatory bowel disease. Vet. Ther. 3, 109–118. Martínez-López, L.M., Pepper, A., Pilla, R., Woodward, A.P., Suchodolski, J.S. and Mansfield, C. 2021. Effect of sequentially fed high protein, hydrolyzed protein, and high fiber diets on the fecal microbiota of healthy dogs: a cross-over study. Anim. Microbiome 3, 42; doi:10.1186/s42523-021-00101-8 Münster, M., Hörauf, A. and Bilzer, T. 2006. [Assessment of disease severity and outcome of dietary, antibiotic, and immunosuppressive interventions by use of the canine IBD activity index in 21 dogs with chronic inflammatory bowel disease]. Berl. Munch. Tierarztl. Wochenschr. 119, 493–505. Myers, M., Martinez, S.A., Shiroma, J.T., Watson, A.T. and Hostutler, R.A. 2023. Prospective evaluation of low-fat diet monotherapy in dogs with presumptive protein-losing enteropathy. J. Am. Anim. Hosp. Assoc. 59, 74–84; doi:10.5326/JAAHA-MS-7248 Nagata, N., Ohta, H., Yokoyama, N., Teoh, Y.B., Nisa, K., Sasaki, N., Osuga, T., Morishita, K. and Takiguchi, M. 2020. Clinical characteristics of dogs with food-responsive protein-losing enteropathy. J. Vet. Intern. Med. 34, 659–668; doi:10.1111/jvim.15720 Nakashima, K., Hiyoshi, S., Ohno, K., Uchida, K., Goto-Koshino, Y., Maeda, S., Mizutani, N., Takeuchi, A. and Tsujimoto, H. 2015. Prognostic factors in dogs with protein-losing enteropathy. Vet. J. 205, 28–32; doi:10.1016/j.tvjl.2015.05.001 Okanishi, H., Yoshioka, R., Kagawa, Y. and Watari, T. 2014. The clinical efficacy of dietary fat restriction in treatment of dogs with intestinal lymphangiectasia. J. Vet. Intern. Med. 28, 809–817; doi:10.1111/jvim.12327 Otoni, C.C., Heilmann, R.M., García-Sancho, M., Sainz, A., Ackermann, M.R., Suchodolski, J.S., Steiner, J.M. and Jergens, A.E. 2018. Serologic and fecal markers to predict response to induction therapy in dogs with idiopathic inflammatory bowel disease. J. Vet. Intern. Med. 32, 999–1008; doi:10.1111/jvim.15123 Pietra, M., Fracassi, F., Diana, A., Gazzotti, T., Bettini, G., Peli, A., Morini, M., Pagliuca, G. and Roncada, P. 2013. Plasma concentrations and therapeutic effects of budesonide in dogs with inflammatory bowel disease. Am. J. Vet. Res. 74(1), 78–83; doi:10.2460/ajvr.74.1.78 Rhimi, S., Kriaa, A., Mariaule, V., Saidi, A., Drut, A., Jablaoui, A., Akermi, N., Maguin, E., Hernandez, J. and Rhimi, M. 2022. The nexus of diet, gut microbiota and inflammatory bowel diseases in dogs. Metabolites 12, 1176; doi:10.3390/metabo12121176 Ruaux, C.G. 2013. Cobalamin in companion animals: diagnostic marker, deficiency states and therapeutic implications. Vet. J. 196, 145–152; doi:10.1016/j.tvjl.2013.01.025 Rudinsky, A.J., Howard, J.P., Bishop, M.A., Sherding, R.G., Parker, V.J. and Gilor, C. 2017. Dietary management of presumptive protein‐losing enteropathy in Yorkshire terriers. J. Small Anim. Pract. 58, 103–108; doi:10.1111/jsap.12625 Salavati Schmitz, S., Gow, A., Bommer, N., Morrison, L. and Mellanby, R. 2019. Diagnostic features, treatment, and outcome of dogs with inflammatory protein-losing enteropathy. J. Vet. Intern. Med. 33, 2005–2013; doi:10.1111/jvim.15571 Schreiner, N.M., Gaschen, F., Gröne, A., Sauter, S.N. and Allenspach, K. 2008. Clinical signs, histology, and CD3-positive cells before and after treatment of dogs with chronic enteropathies. J. Vet. Intern. Med. 22, 1079–1083; doi:10.1111/j.1939-1676.2008.0153.x Simmerson, S.M., Armstrong, P.J., Wünschmann, A., Jessen, C.R., Crews, L.J. and Washabau, R.J. 2014. Clinical features, intestinal histopathology, and outcome in protein-losing enteropathy in Yorkshire Terrier dogs. J. Vet. Intern. Med. 28, 331–337; doi:10.1111/jvim.12291 Simpson, K.W., Miller, M.L., Loftus, J.P., Rishniw, M., Frederick, C.E. and Wakshlag, J.J. 2023. Randomized controlled trial of hydrolyzed fish diets in dogs with chronic enteropathy. J. Vet. Intern. Med. 37, 2334–2343; doi:10.1111/jvim.16844 Swann, J.W., Garden, O.A., Fellman, C.L., Glanemann, B., Goggs, R., Levine, D.N., Mackin, A.J. and Whitley, N.T. 2019. ACVIM consensus statement on the treatment of immune-mediated hemolytic anemia in dogs. J. Vet. Intern. Med. 33, 1141–1172; doi:10.1111/jvim.15463 Tolbert, M.K., Murphy, M., Gaylord, L. and Witzel-Rollins, A. 2022. Dietary management of chronic enteropathy in dogs. J. Small Anim. Pract. 63, 425–434; doi:10.1111/jsap.13471 Toresson, L., Steiner, J.M., Razdan, P., Spodsberg, E., Olmedal, G., Suchodolski, J.S. and Spillmann, T. 2018. Comparison of efficacy of oral and parenteral cobalamin supplementation in normalising low cobalamin concentrations in dogs: a randomised controlled study. Vet. J. 232, 27–32; doi:10.1016/j.tvjl.2017.12.010 Toresson, L., Steiner, J.M., Spodsberg, E., Olmedal, G., Suchodolski, J.S., Lidbury, J.A. and Spillmann, T. 2019. Effects of oral versus parenteral cobalamin supplementation on methylmalonic acid and homocysteine concentrations in dogs with chronic enteropathies and low cobalamin concentrations. Vet. J. 243(8), 8–14; doi:10.1016/j.tvjl.2018.11.004 Vecchiato, C.G., Pinna, C., Sung, C.H., Borrelli De Andreis, F., Suchodolski, J.S., Pilla, R., Delsante, C., Sportelli, F., Mammi, L.M.E., Pietra, M. and Biagi, G. 2023. Fecal microbiota, bile acids, sterols, and fatty acids in dogs with chronic enteropathy fed a home-cooked diet supplemented with coconut oil. Animals 13, 502; doi:10.3390/ani13030502 Volkmann, M., Steiner, J.M., Fosgate, G.T., Zentek, J., Hartmann, S. and Kohn, B. 2017. Chronic diarrhea in dogs - retrospective study in 136 cases. J. Vet. Intern. Med. 31, 1043–1055; doi:10.1111/jvim.14739 Washabau, R.J., Day, M.J., Willard, M.D., Hall, E.J., Jergens, A.E., Mansell, J., Minami, T. and Bilzer, T.W. 2010. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 24(10), 10–26; doi:10.1111/j.1939-1676.2009.0443.x Willard, M.D., Helman, G., Fradkin, J.M., Becker, T., Brown, R.M., Lewis, B.C. and Weeks, B.R. 2000. Intestinal crypt lesions associated with protein-losing enteropathy in the dog. J. Vet. Intern. Med. 14(2000), 298–307. | ||
| How to Cite this Article |
| Pubmed Style Borella F, Benvenuti E, Pierini A, Borrelli A, Cagnasso F, Zanatta R, Marchetti V, Gianella P. Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathy. Open Vet. J.. 2025; 15(9): 4470-4481. doi:10.5455/OVJ.2025.v15.i9.53 Web Style Borella F, Benvenuti E, Pierini A, Borrelli A, Cagnasso F, Zanatta R, Marchetti V, Gianella P. Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathy. https://www.openveterinaryjournal.com/?mno=244650 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.53 AMA (American Medical Association) Style Borella F, Benvenuti E, Pierini A, Borrelli A, Cagnasso F, Zanatta R, Marchetti V, Gianella P. Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathy. Open Vet. J.. 2025; 15(9): 4470-4481. doi:10.5455/OVJ.2025.v15.i9.53 Vancouver/ICMJE Style Borella F, Benvenuti E, Pierini A, Borrelli A, Cagnasso F, Zanatta R, Marchetti V, Gianella P. Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathy. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4470-4481. doi:10.5455/OVJ.2025.v15.i9.53 Harvard Style Borella, F., Benvenuti, . E., Pierini, . A., Borrelli, . A., Cagnasso, . F., Zanatta, . R., Marchetti, . V. & Gianella, . P. (2025) Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathy. Open Vet. J., 15 (9), 4470-4481. doi:10.5455/OVJ.2025.v15.i9.53 Turabian Style Borella, Franca, Elena Benvenuti, Alessio Pierini, Antonio Borrelli, Federica Cagnasso, Renato Zanatta, Veronica Marchetti, and Paola Gianella. 2025. Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathy. Open Veterinary Journal, 15 (9), 4470-4481. doi:10.5455/OVJ.2025.v15.i9.53 Chicago Style Borella, Franca, Elena Benvenuti, Alessio Pierini, Antonio Borrelli, Federica Cagnasso, Renato Zanatta, Veronica Marchetti, and Paola Gianella. "Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathy." Open Veterinary Journal 15 (2025), 4470-4481. doi:10.5455/OVJ.2025.v15.i9.53 MLA (The Modern Language Association) Style Borella, Franca, Elena Benvenuti, Alessio Pierini, Antonio Borrelli, Federica Cagnasso, Renato Zanatta, Veronica Marchetti, and Paola Gianella. "Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathy." Open Veterinary Journal 15.9 (2025), 4470-4481. Print. doi:10.5455/OVJ.2025.v15.i9.53 APA (American Psychological Association) Style Borella, F., Benvenuti, . E., Pierini, . A., Borrelli, . A., Cagnasso, . F., Zanatta, . R., Marchetti, . V. & Gianella, . P. (2025) Retrospective evaluation of short- and medium-term therapeutic response to different immunosuppressive and dietary approaches in 148 dogs diagnosed with immunosuppressant-responsive enteropathy. Open Veterinary Journal, 15 (9), 4470-4481. doi:10.5455/OVJ.2025.v15.i9.53 |