| Research Article | ||
Open Vet. J.. 2025; 15(8): 3670-3676 Open Veterinary Journal, (2025), Vol. 15(8): 3670-3676 Research Article Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, IraqHella J. Alfatlawy*Faculty of Veterinary Medicine, University of Kufa, Kufa, Iraq *Corresponding Author: Hella Jawad Alfatlawy, Faculty of Veterinary Medicine, University of Kufa, Kufa, Iraq. Email: Halaj.kadhim [at] uokufa.edu.iq Submitted: 18/02/2025 Revised: 15/06/2025 Accepted: 08/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Postbreeding endometritis in Arabian mares poses a significant reproductive challenge, necessitating proper pathogen identification and antimicrobial susceptibility determination. Aim: To investigate the prevalence of intrauterine pathogens and their antimicrobial susceptibility patterns in Arabian mares with postbreeding endometritis. Methods: Seventeen Arabian mares were examined clinically and ultrasonographically. Uterine swabs were collected for bacterial isolation and antimicrobial susceptibility testing. Results: Clinical endometritis was detected in 29.4% of the mares. Ultrasonographic examination revealed varying degrees of uterine wall thickness: slight (58.82%), moderate (11.76%), and severe (29.41%). Escherichia coli was the predominant pathogen (76.47%), followed by Streptococcus spp. (11.76%), with mixed infections occurring in 11.76% of cases. Both pathogens exhibited high resistance to several antimicrobials but significant susceptibility to β-lactam antibiotics and selected fluoroquinolones. Conclusion: Escherichia coli was the primary pathogen associated with postbreeding endometritis in Arabian mares. The high antimicrobial resistance patterns observed emphasize the importance of culture-based treatment selection. β-lactam antibiotics showed promising efficacy against both isolated pathogens. Keywords: Arabian mares, Endometritis, Staphylococcus spp., E. coli, Hira. IntroductionEndometritis is a significant cause of subfertility in mares, resulting in diminished pregnancy and foaling rates. The condition frequently leads to pregnancy failure through mechanisms of early luteolysis and/or embryonic mortality (LeBlanc and Causey, 2009), generating substantial economic and genetic losses within the equine breeding industry. Approximately 25%–60% of barren mares require intensive clinical management and additional breeding cycles to achieve conception, thereby imposing a considerable financial burden on equine owners. Several predisposing factors contribute to endometritis development, including reproductive tract anatomical abnormalities, previous dystocia or retained placenta, and inadequate uterine clearance following intrauterine semen deposition during natural breeding or artificial insemination procedures (Troedsson, 2006). Clinically, endometritis can be classified into three primary categories: acute infection, chronic infection (endometriosis), and persistent post-breeding inflammation (Davis, 2013). Post-mating induced endometritis represents the most prevalent form of non-infectious endometritis (Canisso et al., 2016). In susceptible mares, particularly those with conformational deficiencies, compromised mechanical drainage, or other undetermined factors, bacterial proliferation and fluid accumulation may persist for extended periods (Canisso et al., 2016). Transient endometritis occurs naturally following mating and parturition and represents a physiological response to foreign proteins and bacteria introduced during these events. This normal inflammatory response typically resolves within 24 hours postbreeding and within 6 days postfoaling (Portus et al., 2005; Katila, 2012). The bacterial species most frequently associated with equine endometritis include Streptococcus equi subspecies zooepidemicus, Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae. The predominant bacterial etiologies arise from the vaginal flora, particularly E. coli and hemolytic streptococci (Klein, 2009). Although empirical investigations pertaining to this phenomenon in Iraq are lacking, analogous features of bacterial metritis in Arabian mares have been examined in Egypt (Barbary1 et al., 2016), Saudi Arabia (Hamouda et al., 2012), and the United Arab Emirates (Omar et al., 2022). The present study was conducted to isolate and identify aerobic microbial pathogens from sub-fertile Arabian mares using the Vitek 2 automated identification system, as well as to determine antimicrobial sensitivity profiles and minimal inhibitory concentrations (MICs) for identified isolates through Vitek 2 system analysis. Materials and MethodsStudy areaThe research was conducted from July 2022 to March 2023 at Alselawi Stable for Arabian horse breeding in the Al-Hira district, located 18 km south of Al-Najaf city center in the mid-Euphrates region of Iraq (Fig. 1). The geographical location of the Al-Hira district. Study subjectsSeventeen mature Arabian mares with histories of persistent breeding failure (repeat breeders) were included in this study. The mares ranged in age from 5 to 14 years (mean age 8.3 ± 2.7 years) and varied in parity from nulliparous (n=3) to multiparous (n=14, with 1–5 previous foalings). Among the multiparous mares, 12 had normal foaling histories and two had experienced dystocia during previous deliveries. All animals were maintained on balanced nutrition and housed in semi-closed stables with adequate management practices. Natural insemination was performed exclusively, with all mares bred by a resident stallion kept in the same facility. This stallion occasionally fed on mares from neighboring stables. Selection criteria for repeat breedersMares were classified as repeat breeders based on the following criteria: (1) regular estrous cycles (21 ± 3 days), (2) absence of apparent anatomical abnormalities of the reproductive tract, (3) failure to conceive after at least three properly timed natural breedings during consecutive estrous cycles, and (4) absence of detectable pregnancy 14–16 days postbreeding as confirmed by ultrasonography. Mares with any visible reproductive tract abnormalities or systemic diseases were excluded from the study. Clinical examinationAll mares underwent comprehensive clinical examinations, including vital sign measurement, assessment of vaginal discharge, and evaluation of Caslick’s index. Individual clinical data for each mare were systematically documented in dedicated clinical records. Trans-rectal sonographic monitoringTrans-rectal ultrasonography was used to confirm endometritis cases using an Ultrasound Diagnostic System (Fukuda Co. Ltd., Japan) equipped with a trans-rectal linear transducer (5–7.5 MHz). Diagnostic criteria included measurement of uterine wall thickness and assessment of intraluminal fluid as indicators of endometritis severity. Intrauterine fluid was classified according to Ginther and Pierson (1984) as follows:
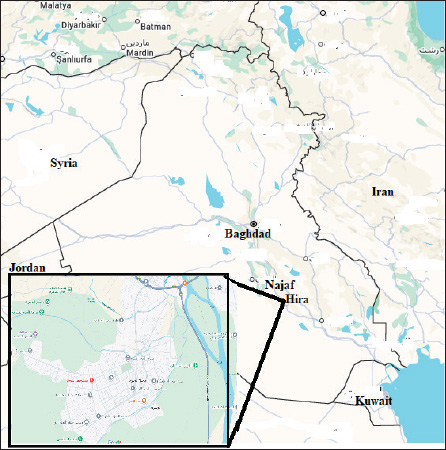
Fig. 1. Reveal the position of Hira at the Najaf governorate /Iraq.
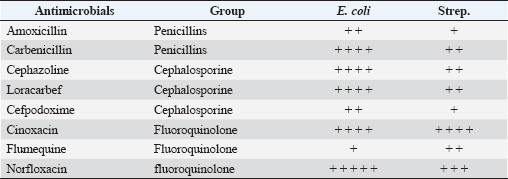
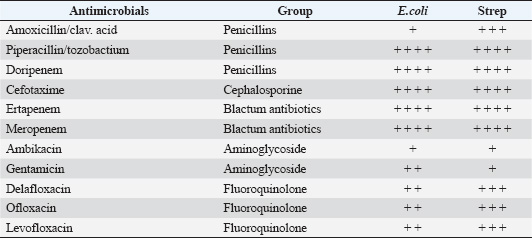
Grade A: Scanty (2 ml). Grade B: Medium (3–10 ml). Grade C: Large (more than 10 ml). Sampling methodologyMicrobiological samples were collected from all study mares following the following protocol: Clitoral fossa and sinuses sampling procedureThe mare was properly restrained in stocks, and the technician used sterile disposable gloves specific to each animal. After wrapping the mare’s tail with a bandage to prevent contamination, the external genitalia were rinsed with sterile distilled water to remove debris. The clitoral fossa was first sampled using a sterile swab, which was immediately placed into Amies transport media with appropriate labeling, followed by sampling of the clitoral sinuses using an identical technique. Cervical swab collectionAfter disinfection of the perineum region of the restrained mare with a wrapped tail, cervical samples were collected by passing a swab by hand while protected with a clean disposable plastic obstetrical sleeve. The swab was then transferred to Amies transport media and labeled. Bacterial identificationIdentification was performed using the automated Vitek® 2 Compact system (Biomérieux, France) in the laboratory of clinical pathology/faculty of veterinary medicine/University of Kufa. Gram-positive and Gram-negative bacteria were identified using Gram-positive ID cards and Gram-negative ID cards, respectively, according to manufacturer protocols. Antimicrobial susceptibility testingMIC values were determined to assess bacterial susceptibility to various antimicrobial agents according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2022). The automated Vitek® 2 Compact system (Biomérieux, France) was used for this analysis, employing AST cards as specified by the manufacturer. Quality control strains, including E. coli ATCC 25922 and Staphylococcus aureus ATCC 29213, were used to validate the testing procedure, as recommended by Wiegand et al. (2008). Ethical approvalThe was approved by the Institutional Animal Care and Use Committee of the University of Kufa. All procedures were performed in accordance with institutional and national guidelines for animal care. Informed consent was obtained from all horse owners. Sample collection was conducted by qualified veterinarians using protocols designed to minimize discomfort. ResultsThe prevalence of clinical endometritis in the present study was 29.4% (5/17 mares), with purulent vaginal discharge being the primary clinical manifestation, with no significant alterations in vital signs were observed. The ultra-sonographic examination revealed varying degrees of uterine wall thickness, which were categorized into three groups based on severity. The measurement of uterine wall thickness of Slight, Moderate, and Severe grades was 58.82% (10/17, mean=2.3 ± 0.2 cm), 11.76% (2/17, mean=3.6 ± 0.3 cm), and 29.41% (5/17, mean=4.3 ± 0.4 cm), respectively. The Intrauterine fluid accumulation was classified into three distinct grades (χ2 test, p < 0.05): Scanty grade (anechoic): 52.94% (9/17), Medium grade (hypoechoic): 23.52% (4/17), and Large grade (hypoechoic with hyperechoic particles): 23.52% (4/17). Microbiological findingsBacterial isolation revealed two predominant pathogens. E. coli was the most prevalent isolate (76.47%, 13/17), followed by Streptococcus spp. (11.76%, 2/17). Mixed infections involving both pathogens were observed in 11.76% (2/17) of cases. The difference in pathogen distribution was statistically significant (Fisher’s exact test, p < 0.001). Antimicrobial susceptibilityBoth isolates exhibited high resistance (>75%) to Carbenicillin, Cephazoline, Iovacarbef, and Cinoxacin. Conversely, significant susceptibility (>90%, p < 0.05) was observed for Piperacillin/tozobactum, Doripenem, Cefotaxime, Ertapenem, and Meropenem. E. coli showed moderate resistance to fluoroquinolones, whereas Streptococcus spp. demonstrated higher susceptibility to this class (Tables 1 and 2). DiscussionPostbreeding endometritis poses a persistent challenge in the reproductive management of mares, particularly within the Arabian breed. This condition not only disrupts fertility in the short term but also has far-reaching effects on breeding efficiency LeBlanc, 2010, foaling rates, and the broader economic stability of equine enterprises. Both the immediate costs associated with diagnosis and treatment and the indirect losses from reduced reproductive output make this disorder a major concern for horse owners and veterinarians alike (Woodward and Troedsson, 2015; Canisso et al., 2020; Schöniger and Schoon, 2020). The diagnosis of endometritis in mares is often complicated by the lack of obvious clinical symptoms. In our study, ultrasonography proved to be a particularly useful tool, enabling the detection of changes in the uterine wall, fluid accumulation, and edema that might otherwise go unnoticed. These findings are in line with recent research advocating the routine use of advanced imaging in equine reproductive assessments (Watson, 2000; Ferris et al., 2019; Khan et al., 2025). The clear association between ultrasound results and clinical signs severity in our cases further supports the value of this approach. Table 1. Resistance of isolates to some antimicrobials.
Table 2. Susceptibility of isolates to certain antimicrobials.
Our microbiological investigations revealed that E. coli was the most frequently isolated pathogen, accounting for 76.47% of the positive cultures. This predominance of E. coli is consistent with recent reports in the veterinary literature, which suggest a shift in the bacterial landscape of equine reproductive infections (Kabir et al., 2024; Christoffersen et al., 2015). The reasons for this trend may be multifactorial, involving changes in breeding management and hygiene practices or the emergence of new resistance patterns Albihn et al., 2003. Considering the regional context, several studies from the Middle East provide useful comparison points. For example, research in Egypt by Barbary et al. (2016) documented a similar transition, with E. coli increasingly replacing Streptococcus species as the leading cause of endometritis in Arabian mares. Their work also highlighted comparable antibiotic resistance profiles, with β-lactam antibiotics generally outperforming other drug classes. In Saudi Arabia, Hamouda et al. (2012) observed a similar pattern, reporting E. coli as the primary pathogen in >60% of mares with reproductive problems, while Streptococcus species accounted for just over a fifth of cases. Their analysis pointed to environmental and management factors unique to the region as possible contributors to these findings. Recent data from the United Arab Emirates further reinforces these observations. Omar et al. (2022) found E. coli to be the main pathogen in nearly 70% of mares with reproductive failure, and their antimicrobial susceptibility testing closely mirrored our own results. Notably, carbapenems and certain fluoroquinolones remained effective, whereas resistance to widely used antibiotics such as aminopenicillin and first-generation cephalosporins was pronounced. Their study also drew attention to breeding management practices in the UAE that may influence infection rates and resistance patterns. The consistency of these findings across Iraq, Egypt, Saudi Arabia, and the UAE Al-Abidy, 2010; Abd-El-Razek et al., 2019) suggests that equine reproductive infections in the Middle East share common features. These may stem from similar environmental conditions, management styles, or even genetic factors within the Arabian horse population. Interestingly, this regional pattern diverges somewhat from what has been reported in Europe and North America, where Streptococcus equi subsp. zooepidemicus has traditionally been the most common cause of endometritis (Troedsson, 2006; LeBlanc and Causey, 2009). Antibiotic resistance remains a significant and growing concern. Our data, which show high rates of resistance among E. coli isolates to several commonly used antibiotics, echo trends reported both regionally and internationally (Kendall et al., 2020; Malaluang et al., 2021). The continued efficacy of β-lactam antibiotics and selected fluoroquinolones offers some reassurance, but the observed variability in susceptibility underscores the importance of culture and sensitivity testing before initiating treatment (Raidal, 2019). The parallels between our findings and those from other parts of the world suggest that local resistance patterns may be shaped by global trends in antimicrobial use, as well as region-specific practices Bohn et al., 2014. From a clinical perspective, these results highlight the need for a more systematic approach to antimicrobial therapy in equine reproductive medicine. Regular monitoring of local resistance trends, adherence to evidence-based treatment protocols, and prudent use of antibiotics are essential steps (Troedsson and Woodward, 2016). There is also a clear need to shift the focus toward prevention and explore alternative treatment options, as well as to deepen our understanding of how resistance develops and spreads (Morris et al., 2020). Finally, expanding regional research efforts across the Middle East will be crucialdevelopinglding a comprehensive picture of pathogen distribution and resistance in Arabian mares. Such studies could pave the way for more targeted and effective strategies to protect the reproductive health and genetic legacy of this important breed. ConclusionThe findings of this study on 17 Arabian mares with histories of persistent breeding failure provide valuable insights into reproductive health management in equine breeding facilities. Our comprehensive clinical, microbiological, and ultrasonographic assessments demonstrated that endometritis was a significant contributing factor to reduced fertility in these mares. The isolation and identification of bacterial pathogens from the clitoral fossa, clitoral sinuses, and cervix using the Vitek® 2 Compact system revealed a predominance of gram-negative bacteria, particularly E. coli and Klebsiella spp., along with some gram-positive organisms. Antimicrobial susceptibility testing revealed varying resistance patterns among isolates, highlighting the importance of culture-directed therapy in treatment selection. Sonographic evaluation of intraluminal fluid accumulation proved to be an effective diagnostic tool, with the majority of repeat breeder mares exhibiting Grade B (medium) fluid accumulation. The correlation between bacterial presence and sonographic findings suggests that both diagnostic methods should be employed for the comprehensive evaluation of reproductive health Zent et al., 1998. These results emphasize the necessity of implementing rigorous hygiene protocols during breeding practices and regular microbiological monitoring of breeding mares, particularly those with reproductive failure histories. Identification of antimicrobial resistance patterns provides valuable guidance for veterinarians managing equine reproductive health. Future studies should evaluate the efficacy of targeted antimicrobial therapies based on susceptibility testing to improve conception rates in mares with persistent breeding failure. AcknowledgmentsThe author expresses sincere gratitude to the Faculty of Veterinary Medicine, University of Kufa, for their institutional support throughout this research. Special appreciation is extended to the horse owners and breeding farms in Al-Najaf province for their cooperation and willingness to participate in this study. Conflict of interestThe authors declare no conflicts of interest regarding the publication of this article. FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The study was conducted without external financial support. Authors’ contributionsHJK was solely responsible for all aspects of this research, including study conception and design, data collection, laboratory analysis, statistical analysis, manuscript preparation, and final approval of the version to be published. Data availabilityThe data that support the findings of this study are available from the corresponding author upon reasonable request. Raw microbial identification data and endometrial sample analyses have been archived and will be maintained for a period of five years following publication. ReferencesAbd-El-Razek, E., Genedy, T. and Elbaz, H. 2019. Ultrasonographic monitoring and treatment of endometritis in mare. J. Curr. Vet. Res. 1, 139–146. Al-Abidy, H.F. 2010. Isolation and identification of pathogenic bacteria from genital tract of Arabian mares affected with genital tract infection and antimicrobial sensitivity. Iraqi J. Vet. Sci. 24, 143–148. Albihn, A., Båverud, V. and Magnusson, U. 2003. Uterine microbiology and antimicrobial susceptibility in isolated bacteria from mares with fertility problems. Acta Vet. Scand. 44, 121–129. Barbary, H.A., Abo-ghonema, I.I., El-Bawab, I.E. and Fadel, M.S. 2016. Diagnosis and treatment of bacterial endometritis in Arabian mares. Alex. J. Vet. Sci. 49, 116–125. Bohn, A.A., Ferris, R.A. and McCue, P.M. 2014. Comparison of equine endometrial cytology samples collected with uterine swab, uterine brush, and low-volume lavage from healthy mares. Vet. Clin. Pathol. 43, 594–600. Canisso, I.F., Segabinazzi, L.G.T.M., Fedorka, C. E. 2020. Persistent breeding-induced endometritis in mares - a multifaceted challenge: from clinical aspects to immunopathogenesis and pathobiology. Int. J. Mol. Sci. 21(4), 1432; doi: 10.3390/ijms21041432 Canisso, I.F., StewBoart, J. and Coutinho da Silva, M.A. 2016. Endometritis: managing persistent postbreeding endometritis. Vet. Clin. N. Am. Equine Pract. 32, 465–480. Christoffersen, M., Woodward, E., Bojesen, A.M., Jacobsen, S., Petersen, M.R., Troedsson, M.H.T. and Lehn-Jensen. 2012. Inflammatory responses to induced infectious endometritis in mares resistant or susceptible to persistent endometritis. BMC Vet. Res. 8, 41–41. CLSI. 2022. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. Davis, H.A., Stanton, M.B., Thungrat, K. and Boothe, D.M. 2013. Uterine bacterial isolates from mares and their resistance to antimicrobials: 8296 cases (2003-2008). J. Am. Vet. Med. Assoc. 242, 977–983. Ferris, R.A., Frisbie, D.D. and McCue, P.M. 2014. Use of mesenchymal stem cells or autologous conditioned serum to modulate the inflammatory response to spermatozoa in mares. Theriogenology 82, 36–42. Ginther, O.J. and Pierson, R.A. 1984. Ultrasonic anatomy and pathology of the equine uterus. Theriogenology 21, 505–516. Gutjahr, S., Paccamonti, D.L., Pycock, J.F., Taverne, M.A., Dieleman, S.J. and van der Weijden, G.C. 2000. Effects of treatment dose and day on uterine response to oxytocin in mares. Theriogenology 54, 447–456. Hamouda, M.A., Al-Hizab, F.A., Ghoneim, I.M., Al-Dughaym, A.M. and Al-Hashim, H.J. 2012. Assessment of endometritis in Arabian mare. Anim. Prod. 14, 99–103. Kabir, A., Lamichhane, B., Habib, T., Adams, A., El-Sheikh Ali, H., Slovis, N.M., Troedsson, M.H.T. and Helmy, Y.A. 2024. Antimicrobial resistance in equines: a growing threat to horse health and beyond-a comprehensive review. Antibiotics 13, 713. Katila, T. 2012. Postmating inflammatory responses of the uterus. Reprod. Domest. Anim. 5, 31–41. Khan, I.U., Khairullah, A.R., Khan, A.Y., Rehman, A.U. and Mustofa, I. 2025. Strategic approaches to improve equine breeding and stud farm outcomes. Vet. World 18, 311–328. Klein, C., Ennen, S., Huchzermeyer, S., Weiss, R. and Wehrend, A. 2009. Analysis of the barrier functions of vulvovaginal fold and cervix to ascending bacterial contamination of the mare’s reproductive tract. Tierarzt. Prax. 80, 113–117. Lambraki, I.A., Cousins, M., Graells, T., Léger, A., Henriksson, P. and Harbarth, S. 2022. Factors influencing antimicrobial resistance in the European food system and potential leverage points for intervention: a participatory, One Health study. PLoS ONE 17, e0263914. LeBlanc, M.M. 2010. Advances in the diagnosis and treatment of chronic infectious and post-mating-induced endometritis in the mare. Reprod. Domest. Anim. 45, 21–27. LeBlanc, M.M. and Causey, R. 2009. Clinical and subclinical endometritis in the mare: both threats to fertility. Reprod. Domest. Anim. 44, 10–22. Malaluang, P., Wilén, E., Lindahl, J., Hansson, I. and Morrell, J.M. 2021. Antimicrobial resistance in equine reproduction. Animals 11, 3035. Morris, H.A., McCue, L.M.P. and Aurich, C. 2020. Equine endometritis: a review of challenges and new approaches. Reproduction 160, R95–R110. Omar, H., Hambidge, M., Firmanes, B., Shabandri, A.M. and Wilsher, S. 2022. Bacteria isolated from equine uteri in the United Arab Emirates: a retrospective study. J. Equine Vet. Sci. 115, 104029. Portus, B.J., Reilas, T. and Katila, T. 2005. Effect of seminal plasma on uterine inflammation, contractility and pregnancy rates in mares. Equine Vet. J. 37, 515–519. Raidal, S.L. 2019. Antimicrobial stewardship in equine practice. Aust. Vet. J. 97, 238–242. Schöniger, S. and Schoon, H.A. 2020. Healthy and diseased equine endometrium: a review of morphological features and molecular analyses. Animals 10, 625. Traub-Dargatz, J.L., Salman, M.D. and Voss, J.L. 1991. Medical problems in adult horses as ranked by equine practitioners. J. Am. Vet. Med. Assoc. 198, 1745–1747. Troedsson, M.H. 2006. Breeding-induced endometritis in mares. Vet. Clin. North Am. Equine Pract. 22, 705–712. Troedsson, M.H.T. and Woodward, E.M. 2016. Our current understanding of the pathophysiology of equine endometritis with an emphasis on breeding-induced endometritis. Reprod. Biol. 16, 8–12. Watson, E.D. 2000. Postbreeding endometritis in the mare. Anim. Reprod. Sci. 60–61, 221–232. Wiegand, I., Hilpert, K. and Hancock, R.E.W. 2008. Agar and broth dilution methods were used to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175. Woodward, E.M. and Troedsson, M.H.T. 2015. Reproductive tract defense mechanisms in mares. Anim. Reprod. Sci. 47(4), 384–389; doi: 10.1111/evj.12403 Zent, W.W., Troedsson, M.H.T. and Xue, J.L. 1998. Post-breeding uterine fluid accumulation in a normal population of thoroughbred mares: a field study. Am. Ass. Equine Pract. 44, 64–65. | ||
| How to Cite this Article |
| Pubmed Style Hella J. Alfatlawy. Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, Iraq. Open Vet. J.. 2025; 15(8): 3670-3676. doi:10.5455/OVJ.2025.v15.i8.30 Web Style Hella J. Alfatlawy. Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, Iraq. https://www.openveterinaryjournal.com/?mno=243460 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.30 AMA (American Medical Association) Style Hella J. Alfatlawy. Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, Iraq. Open Vet. J.. 2025; 15(8): 3670-3676. doi:10.5455/OVJ.2025.v15.i8.30 Vancouver/ICMJE Style Hella J. Alfatlawy. Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, Iraq. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3670-3676. doi:10.5455/OVJ.2025.v15.i8.30 Harvard Style Hella J. Alfatlawy (2025) Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, Iraq. Open Vet. J., 15 (8), 3670-3676. doi:10.5455/OVJ.2025.v15.i8.30 Turabian Style Hella J. Alfatlawy. 2025. Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, Iraq. Open Veterinary Journal, 15 (8), 3670-3676. doi:10.5455/OVJ.2025.v15.i8.30 Chicago Style Hella J. Alfatlawy. "Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, Iraq." Open Veterinary Journal 15 (2025), 3670-3676. doi:10.5455/OVJ.2025.v15.i8.30 MLA (The Modern Language Association) Style Hella J. Alfatlawy. "Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, Iraq." Open Veterinary Journal 15.8 (2025), 3670-3676. Print. doi:10.5455/OVJ.2025.v15.i8.30 APA (American Psychological Association) Style Hella J. Alfatlawy (2025) Microbial profile of post-breeding endometritis in Arabian mares from the Al-Hira District, Iraq. Open Veterinary Journal, 15 (8), 3670-3676. doi:10.5455/OVJ.2025.v15.i8.30 |