| Research Article | ||
Open Vet. J.. 2025; 15(8): 3787-3793 Open Veterinary Journal, (2025), Vol. 15(8): 3787-3793 Research Article Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational studySimone Della Tommasa1*, Sarah Raspe1, Giacomo Farí2, Annarita Imperante3 and Walter Brehm11Department for Horse, University of Leipzig, Leipzig, Germany 2Department of Biological and Enviromental Science and Technologies (Di. Ste. B.A.), University of Salento, Lecce, Italy 3Field Veterinary Surgeon, Bari, Italy *Corresponding Author: Simone Della Tommasa. Department for Horse, University of Leipzig, Leipzig, Germany. Email: della.tommasa [at] vetmed.uni-leipzig.de Submitted: 14/02/2024 Revised: 30/06/2025 Accepted: 10/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
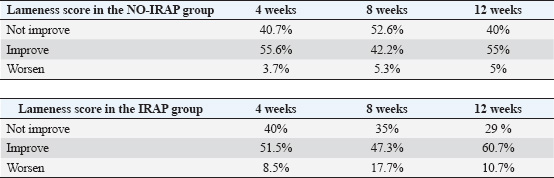
ABSTRACTBackground: Tendon and ligament injuries in equine athletes pose significant challenges, often leading to prolonged recovery, reduced performance, and increased risk of reinjury. Current treatment strategies, including conservative and surgical approaches, have shown limited success in fully restoring tendon integrity. Autologous blood derivatives, such as autologous conditioned serum (ACS), have emerged as potential regenerative therapies. However, the clinical efficacy of ACS in the treatment of equine tendinopathies remains under investigation. Aim: This study aimed to compare the clinical outcomes between intralesional ACS injections and conservative treatment in horses diagnosed with superficial digital flexor tendon (SDFT), deep digital flexor tendon (DDFT), and suspensory ligament (SL) injuries. Methods: This retrospective observational case-control study used clinical data from 100 horses evaluated for lameness between 2017 and 2022. Horses were categorized into two groups: the IRAP group (n=48), which received three intralesional ACS injections at 1-week intervals, and the NO-IRAP group (n=52), which was treated conservatively with NSAIDs and a structured rehabilitation program. Lameness assessments and ultrasonographic evaluations were performed at initial presentation and follow-up at 4, 8, and 12 weeks. The statistical analyses compared changes in lameness scores between the two groups. Results: At 4 weeks, improvement in lameness was observed in 51.5% and 55.6% of the IRAP and NO-IRAP groups, respectively. At 8 weeks, improvement rates were 47% and 42.2%, respectively. By 12 weeks, 60.7% of the IRAP group and 55% of the NO-IRAP group showed improvement. However, a subset of horses in the IRAP group experienced transient worsening of lameness (8.5%–17.7%). Stratification by lesion type revealed better outcomes in the IRAP group for SDFT (58.3% vs. 48%) and SL (51.2% vs. 36%) injuries, whereas the NO-IRAP group had superior results for DDFT injuries (60.9% vs. 46.2%). Conclusion: The study suggests that ACS treatment enhances tendon and ligament healing, particularly in SDFT and SL injuries. However, the transient worsening of lameness observed in some horses receiving ACS warrants further investigation. Although these findings support the potential benefits of ACS, controlled randomized clinical trials are needed to validate its efficacy and optimize treatment protocols for equine tendon and ligament pathologies. Keywords: IRAP, Autologous conditioned serum, Horse, Tendon, Ligament, lameness. IntroductionIn the domain of equine sports and performance, tendon and ligament injuries present formidable challenges, often resulting in lameness, diminished athletic abilities, and premature retirement (Murray et al. 2006; Thorpe et al. 2010; Baxter 2012). The gradual and suboptimal recovery of tendons, marked by the formation of disorganized and collagen-rich scar tissue, increases the susceptibility to reinjury, jeopardizing the prolonged competitiveness of a horse’s racing career (Dyson 2004; Lam et al. 2007; O’meara et al. 2010). The primary component of healthy tendon tissue is predominantly type I collagen, which significantly contributes to its impressive tensile strength and elasticity. In the event of an acute tendon trauma, an inflammatory phase is initiated, marked by the formation of a local hematoma and the induction of an inflammatory reaction at the site of the tendon tear or lesion (James et al. 2008). Approximately 7 days post-injury, the proliferative phase ensues, involving angiogenesis and fibroplasia, accompanied by the haphazard deposition of collagen type III (Spaas et al. 2012). The smaller and less organized fibrils of type III collagen lead to a reduction in the original tensile strength and elasticity (Spaas et al. 2012; Shojaee et al. 2019). Ultimately, 6–8 weeks after the injury, the remodeling phase commences, and it is characterized by a decrease in type III collagen and an increase in the synthesis of collagen type I. However, the repaired tissue does not attain the tensile characteristics of a healthy tendon, resulting in the formation of inflexible scar tissue that carries an elevated risk of tendon reinjury (Maffulli and Kader 2002; Spaas et al. 2012). Despite significant advancements in early tendon injury detection and the introduction of sophisticated therapies, the healing process remains slow, leading to the formation of inferior-quality repaired tendon tissue (O’meara et al. 2010). Similar to other tendinous and ligamentous tissues, the suspensory ligament (SL) exhibits a highly organized, hierarchical architecture predominantly composed of type I collagen fibers. In contrast to most ligamentous structures, the SL is characterized by a substantial content of muscle fibers and non-collagenous matrix components (Smith et al. 2013; Alzola et al. 2018; Smith et al. 2024). This composition demonstrates spatial heterogeneity, varying not only longitudinally along the ligament but also between the forelimb and hindlimb regions, as well as across different equine breeds (Smith et al. 2013; Smith et al. 2024). Epidemiological studies on SL pathology have reported prevalence rates ranging from 3.6% to 10% (Alzola et al. 2018). Approximately 89% of soft tissue injuries involve the superficial digital flexor tendon (SDFT) and deep digital flexor tendon (DDFT), while the remaining 11% affect the SL. In eventing horses, injuries to the SDFT are more prevalent than those to the SL (Alzola et al. 2018; Smith et al. 2024). Conversely, in general-purpose horses, as well as in show jumpers and dressage horses, the SL is the most frequently injured soft tissue structure. Notably, dressage horses tend to sustain SL injuries more commonly in the hindlimb, whereas forelimb SL injuries predominate in other equestrian disciplines (Smith et al. 2024). Despite extensive exploration of conservative and surgical treatments, a definitive solution ensuring complete restoration of the anatomical and functional integrity of injured tissues remains elusive (Patterson-Kane 2009; Textor et al. 2011; Carvalho et al. 2013; Smith et al. 2013). The prolonged healing duration and substandard mechanical properties of tendon scars stem from inherent challenges like poor vascularization and a shortage of progenitor cells within the tendon tissue (Ionita et al. 2008). Acknowledging these hurdles, research efforts have intensified, focusing on regenerative therapies aimed at reinstating normal tissue structure and biomechanics. This has prompted the investigation of autologous blood derivatives, including Autologous conditioned serum (ACS), platelet-rich plasma (PRP), and mesenchymal stem cells, as potential interventions in both human and veterinary medicine (Dahlgren et al. 2002; Molloy et al. 2003; Frisbie et al. 2007; Hraha et al. 2011). Autologous conditioned serum (ACS; synonyms IRAP, Orthokine, Orthogen, Düsseldorf, Germany ACS) is used for intralesional treatment of tendinopathy in horses, but its clinical effect has not been fully documented (Ionita et al. 2008; Weinberger 2008; Textor et al. 2011), and the effects of ACS in tendon lesions remain a subject of inquiry. The rationale for the use of ACS for treating equine tendinopathies is based on several findings. It was shown in an experimental study that the expression of IL-1β (and matrix metalloproteinase-13) is upregulated following overstrain injury of rat tendons, demonstrating that these molecules are important mediators in the pathogenesis of tendinopathy (Molloy et al. 2003; Sun et al. 2008; Ionita et al. 2008; Textor et al. 2011). Rat Achilles tendons exposed to ACS in an experimental study showed enhanced expression of the Col1A1 gene, which led to increased secretion of type I collagen, accelerated recovery of tendon stiffness, and improved histologic maturity of the repair tissue (Jann and Stashak 2008; Majewski et al. 2009; Dakin et al. 2010). The aim of the present observational study was to compare the outcome of the use of intralesional ACS injection with that of conservative treatment in horses with SL, SDFT, and DDFT lesions. Materials and MethodsIn this retrospective observational case-control study, clinical data spanning the years 2017–2022 were collected from horses at an equine clinic for lameness examination. This study focused on horses diagnosed with SDFT, DDFT, and SL lesions. Lameness examinations were conducted by different experienced orthopedic veterinarians during the initial assessment. The lameness examination included: clinical examination of the horse, including detection of swelling and pain at palpation; gait examination at the straight line in trot; longeing in circles in both directions on a hard and soft surface in trot. Lameness was assessed using the AAEP scale. All the horses underwent an ultrasonography examination of the tendon from region IA to IIIB in the fore limb and from IA to IIIC in the hind limbs. Ultrasonography was performed in the transverse and longitudinal planes. The lesions were graded based on 4 grades as described from (Smith et al. 2014). Only horses with acute lameness were included in the study, where the duration of lameness was no longer than 3 weeks, and no previous treatment had been performed. Inclusion criteria required that horses had SDFT, DDFT core lesions, or SL desmitis. The lesions were in the regions indicated above, and they were scaled from 0 to 4 at every ultrasonography examination. All horses underwent follow-up examination at the clinic after 4, 8, or 12 weeks, which was performed by the same veterinarian. Follow-up controls included lameness examinations and ultrasonography of the region of interest. Improvement of the ultrasonography and lameness examination was set with an increase of a grade 1 or more of the scale of the lesion indicated by Smith et al. (2012) and on the AAEP scale for the lameness score (Smith et al. 2013). The horses were categorically divided into two groups: the IRAP group, consisting of horses treated with intralesional injections of IRAP during the follow-up examination and a prescribed training program, and the NO-IRAP group, consisting of horses treated conservatively with NSAIDs and a prescribed training program. In the IRAP group, all horses that received intralesional injections of IRAP three times were included. IRAPs were injected three times, starting from the day after the diagnosis, at a 1-week interval. The training program was customized based on the severity of the lesion and the structure involved, incorporating hand-walking, walking, and trotting. Hand walking for 15 minutes daily was performed on day 0 (day after the first examination in the clinic) and was increased weekly by 5 minutes. At week 10–12, after the first examination, 3-minute trot at the lunge was introduced. Ethical approvalEthical considerations included obtaining approval from relevant institutional review boards and securing informed consent from horse owners or caretakers. Statistical analyses were conducted to compare outcomes between the IRAP and NO-IRAP groups, and the study acknowledged potential sources of bias and limitations. Measures were taken to ensure the confidentiality and security of the collected data, and any funding sources for the study were disclosed. ResultsIn this retrospective, observational case-control study of 100 horses, the average age was 11.7 years (range: 4–23 years). The majority were warmbloods (83%), followed by ponies (16%) and one draft horse (1%). Horses were classified by the level of ridden activity into low (30 horses), medium (53 horses), and high (17 horses). The cohort was divided into two groups: 48 horses received IRAP treatment and 52 did not, NO-IRAP group. Lesions primarily affected the forelimbs (61%) compared to the hindlimbs (39%), with six horses showing lesions in both. The main structures involved were the superficial digital flexor tendon (SDFT, 44.3%), suspensory ligament (SL, 36.1%), and deep digital flexor tendon (DDFT, 19.6%). Multiple injuries were observed in 27% of the horses. At presentation, 38% of the horses had a lameness score of 3/5 or higher, and 62% had a score of 2/5. Swelling and/or heat were noted in 77 horses, and pain on palpation was noted in 28. Follow-up clinical evaluations were performed at 4 weeks (median 30 days), 8 weeks (median 55 days), and 12 weeks (median 85 days). Improvement was defined as a reduction of at least one grade on the AAEP lameness scale, and worsening as an increase of at least one grade. At 4 weeks, improvement in lameness was seen in 55.6% of the NO-IRAP group and 51.5% of the IRAP group, with 40% of the horses in both groups showing no change. At 8 weeks, 47% of the IRAP horses and 42.2% of the NO-IRAP horses improved; 35.3% of the IRAP horses and 52.6% of the NO-IRAP horses showed no change, while 17.7% of the IRAP horses and 5.3% of the NO-IRAP horses worsened. By 12 weeks, 60.7% of IRAP-treated horses and 55% of NO-IRAP horses showed improvement, whereas 28.6%–40%, respectively, remained unchanged. However, worsening lameness was more frequent in the IRAP group (17.7%) than in the NO-IRAP group (≤5%). When analyzed by lesion type, 58.3% of IRAP-treated horses with SDFT lesions showed improvement compared with 48% in the NO-IRAP group. For DDFT lesions, 60.9% of NO-IRAP horses showed improvement compared with 46.2% of IRAP-treated horses, with 23% of the IRAP group showing worsening. Worsening was most often observed in horses with DDFT and SL lesions at 4 and 8 weeks, particularly following injection into the DDFT and SL, which were associated with 70% of worsening cases compared with 30% after SDFT injection. Among the horses with SL injuries, 51.2% in the IRAP group and 36% in the NO-IRAP group improved, while 56% of the NO-IRAP and 39% of the IRAP horses showed no change; worsening occurred in 9.8%–8% of the horses, respectively. Ultrasonographic follow-up in the IRAP group showed lesion grade increases in 32 horses (1 grade), 12 horses (2 grades), and 4 horses with no change at 4 weeks. At 8 weeks, 38 horses worsened by 2 grades, 8 by 3 grades, and 2 remained unchanged. However, by 12 weeks, there was improvement in 42 horses (3 grades) and 6 horses (2 grades). In the NO-IRAP group, lesion grade increases at 4 weeks were observed in 15 horses (1 grade), with 37 showing no change. At 8 weeks, 7 horses worsened by 2 grades, 2 by 3 grades, and 43 remained stable. By 12 weeks, 33 horses had improved by 2 grades, 12 by 2 grades, and 7 by 1 grade. DiscussionThe present retrospective observational case-control study analyzed the intricate landscape of tendon and ligament injuries in equine athletes, emphasizing the challenges posed by the slow and suboptimal recovery of tendons, which often culminate in compromised athletic performance and prolonged healing durations (Lam et al. 2007; James et al. 2008; O’Meara et al. 2010). The study addressed the limitations of current treatments, highlighting the persistent issues of poor vascularization and a shortage of progenitor cells within the tendon tissue, contributing to the formation of inferior scar tissue (James et al. 2008; Spaas et al. 2012; Shojaee et al. 2019). Table 1. Table 1 provides a comparative view of the lameness scores between the “NO-IRAP” and “IRAP” groups over specified time points. In the NO-IRAP group, a higher percentage of horses did not show improvement in lameness compared to the IRAP group. Conversely, the IRAP group exhibited a higher percentage of improvement in lameness, especially at the 8- and 12-week follow-ups. Additionally, the IRAP group showed a higher percentage of worsening of lameness at every follow-up examination, attributed to the intralesional injection of the ACS product. Lameness was considered to have worsened or increased by one or more grades on the AAEP scale.
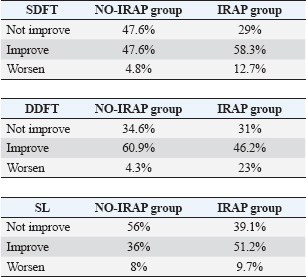
The exploration of regenerative therapies, including autologous blood derivatives such as ACS, brings forth a promising avenue for intervention (Frisbie et al. 2007; Ionita et al. 2008; Hraha et al. 2011; Textor et al. 2011; Carvalho et al. 2013; Smith et al. 2013; Smith et al. 2024). The rationale behind using ACS in equine tendinopathies is rooted in experimental studies demonstrating its ability to modulate key mediators in tendinopathy pathogenesis and enhance the expression of genes associated with collagen synthesis (Ionita et al. 2008; Textor et al. 2011). This, in turn, leads to accelerated recovery and improved histologic maturity of the repaired tissue (Della Tommasa et al. 2023). The core objective of this study was to compare the outcomes of intralesional ACS injection with conservative treatment in horses with tendon and ligament lesions. The results underscore the complexities of equine tendon injuries and challenges in achieving complete restoration of anatomical and functional integrity. While improvements in clinical lameness scores were observed in both groups, the IRAP group showed a notably better outcome on ultrasonographic evaluation at all follow-up intervals—4, 8, and 12 weeks. These findings suggest that although subjective lameness scores provide valuable clinical information, ultrasonography may offer a more sensitive and objective measure of tissue healing, especially in the acute phase. The follow-up period was limited to 12 weeks, as the primary aim was to assess healing during the acute phase of tendon and ligament injury and to determine early responses to treatment. However, it is important to recognize that the relatively short duration of follow-up may not capture the full trajectory of tendon remodeling, particularly in cases in which the underlying pathology includes chronic or degenerative changes. This is especially relevant in warmblood horses, in whom degenerative tendon lesions are relatively common and may not present distinct ultrasonographic differences from acute injuries within a 12-week period. As a result, the interpretation of early healing responses in such cases is challenging. Notably, the study findings revealed variations in lameness scores at different time points, suggesting that the choice between intralesional ACS injections and conservative treatment may impact the trajectory of recovery. The consistent improvement in lameness observed in the IRAP group is encouraging, highlighting the potential benefits of this regenerative therapy in promoting the healing of tendon and ligament injuries. The enhanced expression of the Col1A1 gene, leading to increased secretion of type I collagen, as demonstrated in previous experimental studies (Molloy et al. 2003; Dahlgren et al. 2008; Weinberger et al. 2008), may contribute to the accelerated recovery of tendon stiffness and improved histologic maturity of the repair tissue. Ultrasonographic follow-up over a 12-week period revealed distinct patterns in lesion score progression and recovery between the IRAP-treated and NO-IRAP control groups. At the early stages (4 and 8 weeks), both groups exhibited signs of lesion progression, although the severity appeared more pronounced in the IRAP group. By week 4, 44 horses in the IRAP group showed worsening compared with 15 in the NO-IRAP group, suggesting an initial inflammatory or degenerative response possibly due to disease progression or post-injection inflammatory changes. The trend continued at 8 weeks, with 46 IRAP-treated horses worsening (38 by two grades, 8 by three grades) compared with 9 horses in the NO-IRAP group (7 by two grades, 2 by three grades). However, a notable shift occurred after 12 weeks. The IRAP group showed substantial recovery, with 42 horses improving by three grades and 6 by two grades. This late-phase improvement suggests a delayed but pronounced therapeutic effect of IRAP, likely due to its anti-inflammatory and regenerative properties, including the upregulation of IL-1 receptor antagonist and other growth factors. In contrast, the NO-IRAP group also demonstrated improvement, though to a lesser degree and more gradually, with 33 horses improving by two grades, 12 by two grades, and 7 by one grade. The pattern in the control group may reflect the natural healing process or the effect of standard care without biological modulation. Table 2. Table 1 illustrates the different outcomes observed in the IRAP and NO-IRAP groups based on the structures involved. The IRAP group exhibited a better outcome when the SDFT and the SL were affected, with a higher percentage of horses showing improvement in the lameness score. Conversely, the NO-IRAP group had a better outcome when the DDFT was involved, with a higher percentage of horses showing improvement in the lameness score. Furthermore, the IRAP group demonstrated a higher worsening of lameness when the SDFT and DDFT were involved, whereas the worsening of lameness was comparable when the SL was involved. Lameness was considered to have worsened or increased by one or more grades on the AAEP scale.
Overall, although the IRAP group exhibited a delayed onset of improvement, the magnitude of recovery at 12 weeks was greater, suggesting that IRAP may promote more substantial long-term tissue repair compared with the natural course observed in the control group. These findings support the potential of IRAP therapy for enhancing the resolution of soft tissue lesions, particularly when assessed over a longer posttreatment interval. This difference could reflect the complex biological modulation occurring during IRAP therapy, where short-term inflammation precedes long-term repair. Overall, although the IRAP group exhibited a delayed onset of improvement, the magnitude of recovery at 12 weeks was greater, suggesting that IRAP may promote more substantial long-term tissue repair compared with the natural course observed in the control group. These findings support the potential of IRAP therapy for enhancing the resolution of soft tissue lesions, particularly when assessed over a longer posttreatment interval. Despite these positive trends, it is essential to address the observed tendency of a subset of horses in the IRAP group to experience a temporary worsening of lameness. This finding raises questions about the factors contributing to this outcome and underscores the importance of monitoring and individualized treatment plans. It is plausible that worsening lameness is due to intralesional injection of IRAP, which produces mechanical and physiological stress on the tissue, leading to an acute inflammatory response and transient discomfort. However, this short-term worsening is typically resolved within a few weeks, suggesting that it may be part of a regenerative process. Reactivation of the inflammatory cascade is considered a key step in tendon healing, potentially explaining the initial exacerbation of clinical signs followed by improvement. The structural distribution of the lesions, with the SDFT, DDFT, and SL being the predominant affected areas, adds granularity to the discussion. The study’s consideration of multiple injuries in 27% of cases underscores the multifaceted nature of equine tendon and ligament pathologies. The differential outcomes based on the type of tendon or ligament involved add an additional layer of complexity to the findings. Better outcomes were observed in the IRAP group for SDFT and SL lesions, whereas the NO-IRAP group demonstrated superior results in horses with DDFT injuries. These findings highlight the importance of tailoring treatment approaches according to lesion type, chronicity, and expected regenerative capacity. Despite the advancements in understanding and treating these injuries, this study acknowledges the potential biases and limitations inherent in observational research. The heterogeneity of the study population, including variations in lesion chronicity and the influence of different veterinarians conducting the initial and follow-up examinations, may introduce variability. Moreover, the absence of long-term follow-up data limits conclusions regarding the durability of healing and return to full athletic function. ConclusionIn conclusion, this retrospective observational case-control study provides a comprehensive exploration of the intricate challenges associated with tendon and ligament injuries in equine athletes. The slow and suboptimal recovery of tendons, marked by compromised athletic performance and prolonged healing durations, underscores the pressing need for effective interventions. The investigation into regenerative therapies, particularly ACS, emerges as a promising avenue, with experimental studies demonstrating its potential to modulate key mediators in tendinopathy pathogenesis and enhance collagen synthesis, thereby accelerating recovery. The core objective of comparing intralesional ACS injections with conservative treatment is to reveal variations in lameness scores over time, indicating the potential impact of treatment choice on the trajectory of recovery. However, the study also highlighted complexities, including a subset of horses experiencing temporary worsening of lameness. The favorable outcomes observed in this study contribute to a growing body of evidence supporting the potential efficacy of the IRAP treatment protocol repeated three times at 1 week intervals. However, it is essential to recognize the inherent limitations of observational research, including the heterogeneity of the study population and potential confounding variables. Further controlled studies, including randomized clinical trials, are warranted to validate and refine the protocol, ensuring a comprehensive understanding of its effectiveness in various equine tendon and ligament pathologies. Despite the study’s limitations, the results significantly advance our understanding of potential interventions and considerations for managing these challenging conditions in equine athletes. AcknowledgmentsNone. Conflicts of interestNone. FundingThis research received no external funding. Institutional Review Board StatementThis study is not applicable. Informed consent statementThis form is not applicable. Author contributionsFor research articles with several authors, a short paragraph specifying their individual contributions is required. Conceptualization: SDT, SR, and AI; methodology: SDT, AI, SR, GF; supervision: WB, SDT. All authors have read and agreed to the publication of the finale version of the manuscript. ReferencesAlzola R., Easter C., Riggs C.M., Gardner D.S. and Freeman S.L. 2018. Ultrasonographic-based predictive factors influencing successful return to racing after superficial digital flexor tendon injuries in flat racehorses: a retrospective cohort study in 469 Thoroughbred racehorses in Hong Kong. Equine Vet. J. 50(5), 602–608; doi: 10.1111/evj.12810 Baxter G.M. 2012. Tendon and ligament injuries and disease. In Adams and Stashak’s Lameness in Horses. Ed., Baxter GM. Chichester, FL: Wiley-Blackwell, pp: 927–938. Carvalho, Ade M., Badial, P.R., Álvarez, L.E., Yamada, A.L., Borges, A.S., Deffune, E., Hussni, C.A. and Garcia, Alves A.L. 2013. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrate: a randomized controlled trial. Stem Cell Res. Ther. 4, 85. Dahlgren, L.A. and Harvey, S.C. 2008. Effect of autologous conditioned serum on the metabolism of normal tendon explants. In: 54th Annual Meeting of the Orthopedic Research Society; March 2–5, 2019. Rosemont, IL: Orthopedic Research Society. Dahlgren, L.A., van der Meulen, M.C., Bertram, J.E., Starrak, G.S. and Nixon, A.J. 2002. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J. Orthop. Res. 20, 910–919. Dakin, S.G., Dudhia, J. and Smith, R.K.W. 2010. Modulation of prostaglandin E2 production in equine tendon cells. In: 49th British Equine Veterinary Association Congress; September 8–11. Newmarket, England: Equine Veterinary Journal, Ltd, p: 96. Della Tommasa, S., Brehm W., Farì G., Bernetti, A. and Imperante, A. 2023. Use of autologous conditioned serum (ACS) for osteoarthritis treatment in horses: a systematic review of clinical data. Vet. Sci. 10(12), 707; doi: 10.3390/vetsci10120707 Dyson, S.J. 2004. Medical management of superficial digital flexor tendonitis: a comparative study of 219 horses (1992-2000). Equine Vet J. 36, 415–419; doi: 10.2746/0425164044868422 Frisbie, D.D., Kawcak, C.E., Werpy, N.M., Park, R.D. and McIlwraith, C.W. 2007. Clinical, biochemical, and histologic effects of intraarticular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am. J. Vet. Res. 68, 290–6. Hraha, T.H., Doremus, K.M., McIlwraith, C.W. and Frisbie, D.D. 2011. Autologous conditioned serum: the comparative cytokine profiles of two commercial methods (IRAP and IRAP II) using equine blood. Equine Vet. J. 43(5), 516–521; doi:10.1111/j.2042-3306.2010.00321.x Ionita, J.C. and Brehm, W. 2008. Autologe Blutprodukte in der Regenerativen Therapie: ACS, PRP, ACP, Knochenmark (Autologous blood products for regenerative therapies: ACS, PRP, ACP, bone marrow). In Pferdeheilkunde Forum-Berliner Fortbildungstage. Ed., Lauk, H.D. Berlin, Germany: Hippiatrika Verlag, pp: 588–591. James, R., Kesturu, G., Balian, G. and Chhabra, A.B. 2008. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J. Hand Surg. Am. 33, 102–112; doi: 10.1016/j.jhsa.2007.09.007 Jann H. and Stashak T.S. 2008. Tendon and Paratendon Lacerations. In Equine wound management. Eds., Stashak, T.S. and Theoret, C.L. Ames, Iowa: Wiley-Blackwell, pp: 489–508. Lam, K.H., Parkin, T.D.H., Riggs, C.M. and Morgan, K.L. 2007. Descriptive analysis of the retirement of Thoroughbred racehorses due to tendon injuries at the Hong Kong Jockey Club (1992-2004). Equine Vet. J. 39, 143–148; doi: 10.2746/042516407X159132 Maffulli, N. and Kader, D. 2002. Tendinopathy of tendo achillis. J. Bone Jt Surg-Ser B. 84, 1–8; doi: 10.1302/0301-620X.84B1.0840001 Majewski, M., Ochsner, P.E., Liu, F., Fluckiger, R. and Evans, C.H. 2009. Accelerated healing of the Achilles tendon in response to autologous conditioned serum. Am. J. Sports Med. 37, 2117–2125. Molloy, T., Wang, Y. and Murrell, G. 2003. The roles of growth factors in tendon and ligament healing. Sports Med. 33, 381–394. Murray, R.C., Dyson, S.J., Tranquille, C. and Adams, V. 2006. Association between sport type and performance level and anatomical site of orthopedic injury diagnosis. Equine Vet. J. 36, 411–416; doi: 10.1111/j.2042-3306.2006.tb05578.x O’meara B., Bladon B., Parkin T.D.H., Fraser B. and Lischer C.J. 2010. Investigation of the relationship between race performance and superficial digital flexor tendonitis in the Thoroughbred racehorse. Equine Vet. J. 42, 322–6; doi: 10.1111/j.2042-3306.2009.00021.x Patterson-Kane, J.C. and Firth, E.C. 2009. Pathobiology of exercise-induced superficial digital flexor tendon injury in Thoroughbred racehorses. Vet. J. 181, 79–89. Shojaee, A. and Parham, A. 2019. Strategies of tenogenic differentiation of equine stem cells for tendon repair: current status and challenges. Stem. Cell Res. Ther. 10(1), 181; doi: 10.1186/s13287-019-1291-0 Smith, R.K., Werling, N.J., Dakin, S.G., Alam, R., Goodship, A.E. and Dudhia, J. 2013. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One 8, e75697. Smith R.K. 2024. Treatment of tendinopathies. Equine Vet. Educ. 36(12), 659–672; doi: 10.1111/eve.13987 Spaas, J.H., Guest, D.J. and Van de Walle, G.R. 2012. Tendon regeneration in human and equine athletes. Sport Med. 42, 871–90; doi: 10.1007/BF03262300 Sun, H.B., Li, Y., Fung, D.T., Majeska, R.J., Schaffler, M.B. and Flatow E.L. 2008. Coordinate regulation of IL-1beta and MMP-13 in rat tendons following subculture fatigue damage. Clin. Orthop. Relat. Res. 466, 1555–1561. Textor J. 2011. Autologous biologic treatment of equine musculoskeletal injuries: a platelet-rich plasma and IL-1 receptor antagonist protein. Vet. Clin. North Am. Equine Pract. 27, 275–298. Thorpe, C.T., Clegg, P.D. and Birch, H.L. 2010. A review of tendon injury: why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 42, 174–180; doi: 10.2746/042516409X480395 Weinberger, T. 2008. Regenerative therapiemöglichkeiten beim Pferd-eine Übersicht (Regenerative treatment options in horses-an overview). Pferde Spiegel. 11, 116–119. | ||
| How to Cite this Article |
| Pubmed Style Tommasa SD, Raspe S, Farí G, Imperante A, Brehm W. Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational study. Open Vet. J.. 2025; 15(8): 3787-3793. doi:10.5455/OVJ.2025.v15.i8.43 Web Style Tommasa SD, Raspe S, Farí G, Imperante A, Brehm W. Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational study. https://www.openveterinaryjournal.com/?mno=242768 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.43 AMA (American Medical Association) Style Tommasa SD, Raspe S, Farí G, Imperante A, Brehm W. Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational study. Open Vet. J.. 2025; 15(8): 3787-3793. doi:10.5455/OVJ.2025.v15.i8.43 Vancouver/ICMJE Style Tommasa SD, Raspe S, Farí G, Imperante A, Brehm W. Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational study. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3787-3793. doi:10.5455/OVJ.2025.v15.i8.43 Harvard Style Tommasa, S. D., Raspe, . S., Farí, . G., Imperante, . A. & Brehm, . W. (2025) Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational study. Open Vet. J., 15 (8), 3787-3793. doi:10.5455/OVJ.2025.v15.i8.43 Turabian Style Tommasa, Simone Della, Sarah Raspe, Giacomo Farí, Annarita Imperante, and Walter Brehm. 2025. Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational study. Open Veterinary Journal, 15 (8), 3787-3793. doi:10.5455/OVJ.2025.v15.i8.43 Chicago Style Tommasa, Simone Della, Sarah Raspe, Giacomo Farí, Annarita Imperante, and Walter Brehm. "Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational study." Open Veterinary Journal 15 (2025), 3787-3793. doi:10.5455/OVJ.2025.v15.i8.43 MLA (The Modern Language Association) Style Tommasa, Simone Della, Sarah Raspe, Giacomo Farí, Annarita Imperante, and Walter Brehm. "Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational study." Open Veterinary Journal 15.8 (2025), 3787-3793. Print. doi:10.5455/OVJ.2025.v15.i8.43 APA (American Psychological Association) Style Tommasa, S. D., Raspe, . S., Farí, . G., Imperante, . A. & Brehm, . W. (2025) Autologous conditioned serum IRAP efficacy for tendon and ligament injuries in horses: An observational study. Open Veterinary Journal, 15 (8), 3787-3793. doi:10.5455/OVJ.2025.v15.i8.43 |