| Research Article | ||
Open Vet. J.. 2025; 15(9): 4454-4469
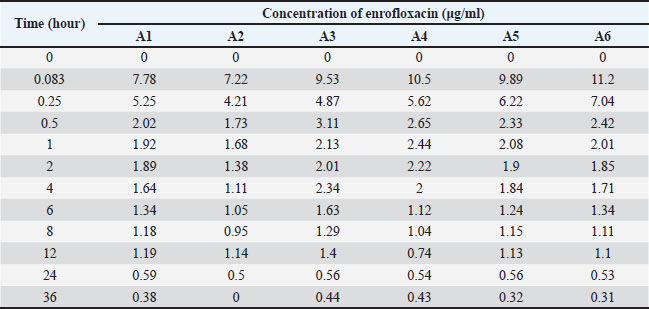
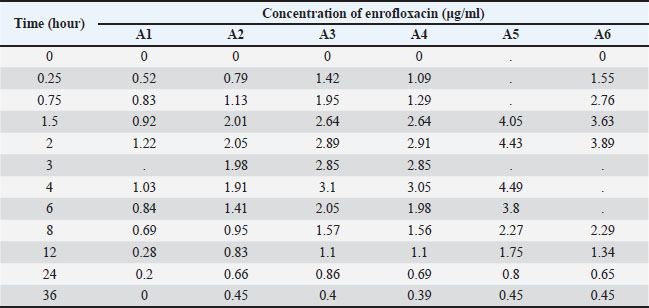
Open Veterinary Journal, (2025), Vol. 15(9): 4454-4469 Research Article Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickensLarissa Alexsandra Felix1*, Beatriz Monte Egito1, Diego Diaz David2, Márcio Gilberto Zangeronimo1, Sheila Rezler Wosiacki3 and Marcos Ferrante1*1Department of Veterinary Medicine, College of Animal Science and Veterinary Medicine, Universidade Federal de Lavras, Minas Gerais, Brazil 2Faculty of Veterinary Science, Universidad Nacional del Litoral, Santa Fe–Argentina 3Department of Veterinary Medicine, College of Animal Science and Veterinary Medicine, Universidade Estadual de Maringá, Umuarama, Paraná, Brazil *Corresponding Author: Marcos Ferrante. Department of Veterinary Medicine, College of Animal Science and Veterinary Medicine, Universidade Federal de Lavras, Minas Gerais, Brazil. Email: marcos.ferrante [at] ufla.br Submitted: 13/02/2025 Revised: 30/07/2025 Accepted: 29/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
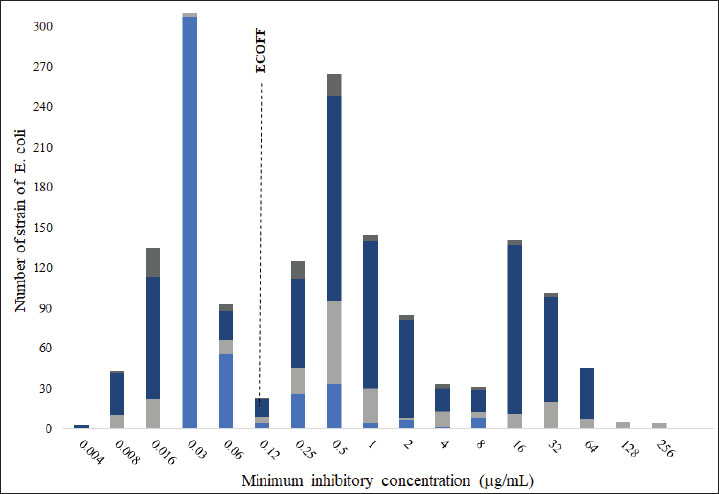
AbstractBackground: Enrofloxacin is one of the most used antibiotics for the treatment of respiratory and gastrointestinal tract diseases in poultry farming worldwide. Pharmacokinetics/pharmacodynamics (PK/PD) modeling is a strategy that allows dose optimization for antibiotic therapy by considering relevant bacteria’s microbiological aspects. Aim: This study aimed to establish a pharmacokinetic model of enrofloxacin in broilers using a nonlinear mixed-effects Model and predict the effectiveness of various oral dosing regimens across a distribution of E. coli minimum inhibitory concentrations (MIC) for two PK/PD targets (fAUC24/MIC ≥ 125 and fAUC24/MIC=28.32). Methods: A PK model was constructed using the Monolix 2024R1 software based on previously published data, which involved 12 clinically healthy male Cobb broilers (38–40 days old, 2.27 ± 0.95 kg). This model served as the basis for Monte Carlo simulations (single oral dose of 10, 20, 30, and 50 mg/kg) and the probability of target attainment (PTA) analysis. The PTA for each protocol was evaluated according to the distribution of MICs considering two PK/PD targets to ensure a comprehensive assessment of treatment efficacy. Results: The best-fit PK model that evaluated the PK of enrofloxacin following intravenous and oral administration was a two-compartment model with first-order absorption and linear elimination. Given the calculated epidemiological cutoff value of 0.125 μg/ml, a dose of 10 mg/kg is adequate for the fAUC24/MIC ≥ 28.32 target, but insufficient for the fAUC24/MIC ≥ 125 target, which requires a higher dose of 20 mg/kg. Conclusion: The PK/PD approach applied in Cobb broiler chickens demonstrates the potential for dose optimization based on MIC distribution, supporting Antimicrobial Stewardship efforts. However, further complementary studies are required to evaluate the predictive capabilities of these indices and to establish the optimal PK/PD targets for in vivo efficacy. Keywords: Antibiotic therapy, Poultry farming, E. coli, Pharmacometrics, PK/PD modeling. IntroductionAvian colibacillosis, a systemic infectious disease caused by avian pathogenic Escherichia coli (APEC), represents one of the most significant bacterial threats to poultry health worldwide. It leads to substantial economic losses due to increased mortality, decreased egg production and hatchability, carcass condemnation, and elevated treatment costs (Dziva and Stevens, 2008; Guabiraba and Schouler, 2015). The etiological agent, E. coli, although typically a commensal inhabitant of the gastrointestinal tract in both animals and humans, it can acquire virulence traits that enable systemic infection in birds. To treat APEC infections, antibiotics such as fluoroquinolones and macrolides, are approved for use in poultry in several countries—except for fluoroquinolones in the United States [Roth et al., 2019; European Medicines Agency (EMA), 2023; Food and Drug Administration (FDA), 2023a; Schmerold et al., 2023; Food and Drug Administration (FDA), 2024]. However, due to the classification of fluoroquinolone as highest priority critically important antimicrobial in human medicine, their use in veterinary medicine should be reserved for cases where first- and second-line treatments fail [European Medicines Agency (EMA), 2020; World Health Organization (WHO), 2024]. Enrofloxacin is a broad-spectrum fluoroquinolone that is developed exclusively for veterinary use and is effective against gram-negative bacteria such as E. coli. It has been widely used to treat respiratory and gastrointestinal tract infections, including colibacillosis, in poultry farming. However, its indiscriminate use in aviculture has likely contributed to the development of bacterial resistance (Roth et al., 2019; Temmerman et al., 2020), potentially compromising the effectiveness of the currently recommended doses. This concern underscores the need to reevaluate these regimens considering emerging resistant E. coli strains. This objective aligns with the Global Action Plan on Antimicrobial Resistance, which highlights the importance of optimizing antimicrobial use to mitigate the escalation of resistance [World Health Organization (WHO), 2015]. In this context, the analysis of the PK/PD relationship of antimicrobials allows integrating the PK and PD properties to estimate a more rational antimicrobial therapy (Landersdorfer and Nation, 2021). PK/PD modeling is widely recommended for optimizing dosage regimens, including by the European Medicines Agency (EMA, 2016a, 2016b), and informs the review of antimicrobial susceptibility breakpoints by the Clinical and Laboratory Standards Institute (CLSI) (Papich et al., 2023). Three PK/PD indices are typically used to predict the efficacy of antimicrobials: time above the MIC (T > MIC), peak concentration divided by MIC (Cmax/MIC), and area under the concentration-time curve divided by MIC (AUC/MIC) (Nielsen and Friberg, 2013). These relationships can be established through in vivo, ex vivo, or in vitro studies using semi-mechanistic PK/PD models, which may yield varying PK/PD targets (Nielsen et al., 2007; Papich, 2014; Rodríguez-Gascón et al., 2021; Toutain et al., 2021). Pharmacokinetic data and established PDT modeling can be used to estimate the efficacy of antimicrobials against a distribution of MICs, supporting updates to therapeutic guidelines. This strategy is similar to that used by Yu et al. (2017) for the determination of dicloxacillin doses against various pathogens and the study by Selig et al. (2022) for piperacillin and tazobactam in critically ill human patients undergoing continuous kidney replacement therapy. Several PK/PD studies have been conducted to evaluate the efficacy of enrofloxacin against bacteria of importance in chickens, and different approaches have been employed across these investigations. For instance, some researchers performed non-compartmental analysis to evaluate the efficacy of fluoroquinolones using standard PK/PD targets (Bonassa et al., 2021; Kang et al., 2019). In contrast, Sang et al. (2016) determined efficacy based on PK/PD targets established in their own study of intestinal contents in E. coli-infected chickens. Given that plasma is the most commonly obtained sample in pharmacokinetic studies and routine farm applications, our study evaluated treatment efficacy based on the PK/PD target proposed by Xiao et al. (2018) which was derived from plasma data. Thus, the present study aimed to establish a pharmacokinetic model of enrofloxacin in broilers using nonlinear mixed-effects modelling (NLMEM) and Monte Carlo simulation to determine the probability of target attainment (PTA) based on two PK/PD targets (fAUC(24)/MIC ≥ 125 and fAUC(24)/MIC=28.32) across a distribution of E. coli minimum inhibitory concentrations (MICs). Materials and MethodsMIC data analysis and epidemiological cutoff (ECOFF) determinationThe range of MICs for enrofloxacin against E. coli was determined based on data from previous studies conducted by Huang et al. (2009); Vanni et al. (2014); Sang et al. (2016); and Temmerman et al. (2020). Furthermore, for PK/PD modeling and, consequently, determination of the PTA, the epidemiological cutoff was established based on the methodology described by Turnidge et al. (2006). ECOFF was calculated in the ECOFFinder provided by CLSI (https://clsi.org/resources/ecoffinder/). The strains were divided into wild-type (WT) and non WT (NWT) strains based on ECOFF (Silley, 2012). DatasetThe pharmacokinetic model of enrofloxacin in broiler chickens was constructed using raw concentration-time data from Kruse (2008). This study involved 12 clinically healthy male Cobb broilers, aged 38–40 days, and weighing on average 2.27 ± 0.95 kg. Birds received a single 10-mg/kg dose of enrofloxacin, either by oral gavage (n=6) or intravenously (n=6). The formulation used was a 5% enrofloxacin solution in physiological saline (purity 98.7%; Bayer) was used, consistent with the product composition described in the original study. The plasma concentrations of enrofloxacin (μg/ml) over time are presented in Supplemental Tables S1 and S2. Pharmacokinetic analysis of enrofloxacin in broilersWe analyzed the plasma concentration-time curves of enrofloxacin using NLMEM in Monolix 2024R1 software (Lixoft SAS, a Simulations Plus company). The PK parameters were estimated by maximum likelihood using the Stochastic Approximation Expectation Maximization algorithm (Mould and Upton, 2013; Traynard et al., 2020). The structural PK models comprised one- and two-compartment systems with first-order elimination. In contrast, the statistical PK models consisted of systems where individual PK parameters were assumed to follow log-normal distributions, and between-subject variability was described using exponential random effects. A base model was established to describe the pharmacokinetic parameters following intravenous administration. Thus, we compared one-, two-, and three-compartment models with linear elimination. Various error models (constant, proportional, or combined) were evaluated to describe the residual variability (ɛ). All individual parameters were considered to have a log-normal distribution. After establishing the intravenous model, an oral pharmacokinetic model was established, following the steps mentioned above, using the values from the intravenous model as initial parameters. Bioavailability (F) was also fixed at 87%, as established in Kruse (2008). The PK parameters were estimated from the final model and reported in the Results section. The most appropriate pharmacostatistical model was selected and evaluated based on the following criteria: goodness-of-fit plots, that is, observations versus individual and population predictions, individual weighted residuals versus individual predictions and time, plots of normalized prediction distribution error (NPDE) versus population predictions and time, and prediction-corrected visual predictive checks (pcVPC); decrease of the objective function (calculated by importance sampling) and Bayesian Information Criterion BIC; and a low relative standard error (RSE < 30%) of PK parameter estimates [Mould and Upton, 2013; Nguyen et al., 2017; Food and Drug Administration (FDA), 2022]. Finally, the robustness of the model was verified using the shrinkage value and a nonparametric bootstrap analysis (1,000 replicates) with a 95% confidence interval. Shrinkage below 20% is acceptable (Savic and Karlsson, 2009; Mould and Upton, 2013; Lavielle and Ribba, 2016). Simulation and PTA analysisMonte Carlo simulations were performed using the final population pharmacokinetic model developed in Monolix (Lixoft SAS, a Simulations Plus company). Simulations included 10,000 virtual individuals (n=10,000), each receiving a single oral dose of enrofloxacin at one of four dosing regimens (10, 20, 30, or 50 mg/kg). These simulations were conducted using the Simulx module of the Monolix Suite. The simulation accounted for the free (unbound) fraction (f) of enrofloxacin in plasma, assuming approximately 22% protein binding in chickens, as reported by Bugyei et al. (1999). For each simulated individual, the unbound area under the plasma concentration-time curve from zero to 24 hours (fAUC24) was calculated, and the fAUC(24) was then divided by the MIC to obtain the fAUC(24)/MIC ratio (Supplemental File 1). This ratio was used as the surrogate PK/PD index. Two PK/PD targets (PDT) were applied to calculate the PTA: (i) fAUC(24)/MIC=28.32, established by Xiao et al. (2018) in an in vivo PK/PD model in broilers for 99.99% bacterial reduction (E=–4), and (ii) fAUC(24)/MIC ≥ 125, a general target associated with fluoroquinolones’ clinical efficacy against Gram-negative pathogens (Wright, 2000; Craig, 2001; Levison and Levison, 2009). PTA was calculated for each dosing regimen across a range of MIC distribution. Doses that reached a PTA ≥ 90% were considered effective, in accordance with the EUCAST Veterinary Subcommittee (VetCAST) (Toutain et al., 2017; Toutain et al., 2019). Ethical approvalEthical approval not needed for this study. ResultsMIC data analysis and ECOFF determinationThe MIC distribution of enrofloxacin against E. coli strains corresponded to a trimodal model, with the first population corresponding to the range of 0.004 to 0.125 µg/ml (Fig. 1). The calculated ECOFF was 0.125 µg/ml. Using the ECOFF as a classification criterion, 43% of the strains were WT, and the remaining 57% was considered NWT (microbiologically resistant).
Fig. 1. Enrofloxacin MIC distribution for the 1734 E. coli strains isolated from broilers. The dotted line represents the ECOFF of 0.125 µg/ml Pharmacokinetic analysis of enrofloxacin in broilersThe final pharmacokinetic model describing enrofloxacin disposition in broiler plasma was identified as a two-compartment model with first-order absorption and linear elimination. The estimated population mean values for the pharmacokinetic parameters were as follows: absorption rate constant (Ka)=0.42 h−¹, clearance (Cl)=209.5 ml/kg/h, central volume of distribution (V1)=785.5 ml/kg, intercompartmental clearance (Q)=1647.5 ml/kg/h, and peripheral volume of distribution (V2)=3234.7 ml/kg. The model included the correlation between Ka, Cl, and V2, with bioavailability fixed at 87% (Supplemental Table S3). The results of the bootstrap analysis (n=1000 replicates) showed narrow 95% confidence intervals and confirmed the parameter estimates’ reliability and robustness (Table 1). Low levels of shrinkage (<20%) indicated sufficient data support for the estimation of random effects. Table 1. Pharmacokinetic model estimates and bootstrap results for enrofloxacin after intravenous and oral administration at a dose of 10 mg/kg body weight.
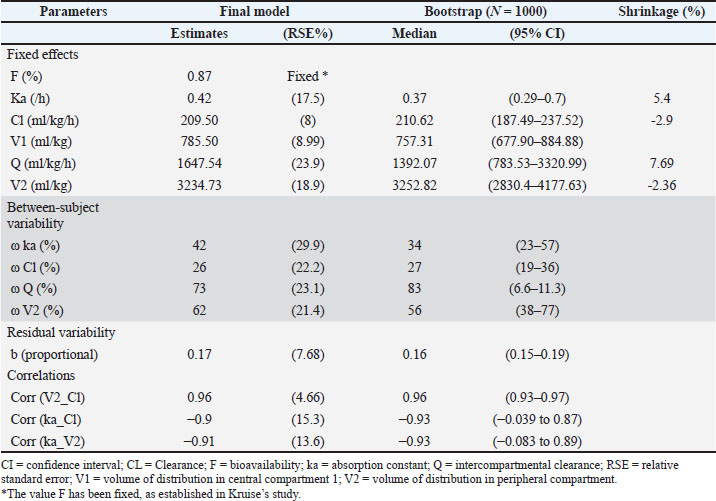
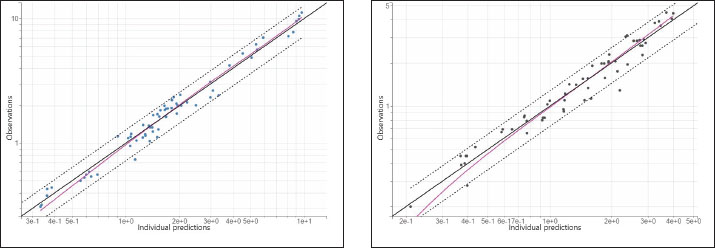
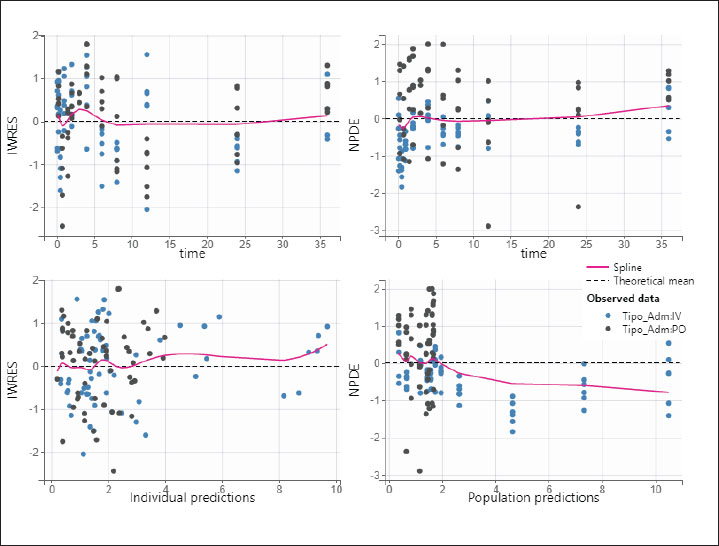
The precision of the PK parameter estimates was less than 30% for the structural parameters, random effects, residual error, and correlations. The observations versus predictions (Fig. 2) do not reveal any misspecification. The observed and predicted enrofloxacin concentrations were matched well by visualizing individual weighted residual versus time after dose and individual predictions. No significant systematic bias was observed for NPDE (Supplemental Fig. S1). The pc-VPC plot (Fig. 3) indicated a good predictive performance of the model. The model satisfactorily predicted the median tendency and dispersion of the observations. Overall, the 2.5th, 50th, and 97.5th percentiles of observed concentrations were within the predicted 95% confidence interval of these percentiles.
Fig. 2. Observed versus individual predictions. The blue dots indicate the intravenous data (left), and the gray dots indicate the oral data (right).
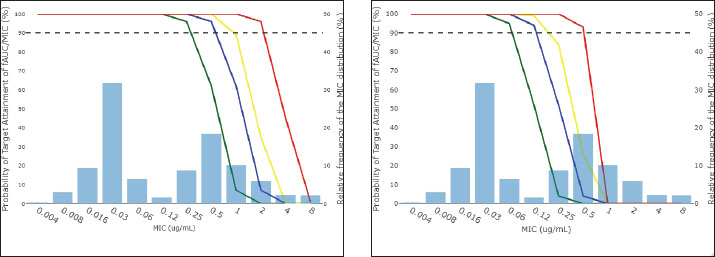
Fig. 3. pcVPC for enrofloxacin concentrations administered intravenously (left) and orally (right). Solid lines represent the 2.5th, 50th, and 97.5th percentiles of the observed values, while shaded areas indicate the 95% prediction intervals derived from simulations. Monte Carlo simulation and PTA analysisThe probabilities of the currently used dose (10 mg/kg) and simulated dose (20, 30, and 50 mg/kg) of enrofloxacin to achieve the fAUC24/MIC target are plotted in Figure 4. For the in vivo defined eradication fAUC24/MIC index (fAUC24/MIC ≥ 28.32), the current dose of 10 mg/kg is predicted to be effective for bacteria up to an MIC of 0.25 µg/ml with a PTA of 96%. The simulated doses of 20 and 30 mg/kg are predicted to be effective for bacteria with MICs of up to 0.5 µg/ml and PTA of 96% and 100%, respectively. The highest dose of 50 mg/kg is predicted to be effective for bacteria with MICs up to 2 µg/ml, with a PTA of 96%.
Fig. 4. PTA fAUC24/MIC ≥ 28.32 (top or left) and fAUC24/MIC ≥ 125 (down or right) for 10 (line green), 20 (line blue), 30 (line yellow), and 50 mg/kg (line red) of enrofloxacin administered via gavage in 10000 simulated broiler chickens. The horizontal black dotted line represents a PTA of 90%. Regarding the generalist index for fluoroquinolones against Gram-negative bacteria (fAUC24/MIC ≥ 125), the current dose of 10 mg/kg is predicted to be effective for bacteria up to a MIC of 0.06 µg/ml with a PTA of 95%. The simulated doses of 20 and 30 mg/kg are predicted to be effective for bacteria with MICs of up to 0.125 µg/ml and PTA of 94% and 99%, respectively. The highest dose of 50 mg/kg is predicted to be effective for bacteria with MICs of up to 0.5 µg/ml, with a PTA of 93%. DiscussionEnrofloxacin is one of the most commonly used antibiotics for treating respiratory and gastrointestinal tract diseases in broilers worldwide. However, fluoroquinolones are deemed critical by the WHO and should be used only in veterinary therapy after exhausting other potentially less critical antimicrobial agents [Food and Drug Administration (FDA), 2018; WHO, 2019]. Therefore, reconsidering the clinical breakpoint is crucial, as clinicians base their treatment protocols on the susceptibility of bacteria to antimicrobial susceptibility testing. Fluoroquinolones are significantly affected by increasing antimicrobial resistance, which could reduce the efficacy of treatments established with the doses recommended in the leaflet (Vanni et al., 2014; Temmerman et al., 2020). Therefore, regimen optimization is necessary to increase therapeutic success and decrease the emergence and spread of antimicrobial resistance (Mouton et al., 2011; Martinez et al., 2012). To characterize the MIC distribution of E. coli strains isolated from chickens, the present study compiled data from four previously published studies: Huang et al. (2009); Vanni et al. (2014); Sang et al. (2016) and Temmerman et al. (2020) comprising a total of 1,734 isolates from birds of different geographic and production backgrounds (Fig. 1). Based on the CLSI clinical breakpoints of enrofloxacin for E. coli strains from poultry, the isolates were classified as susceptible (S, MIC ≤0.25 µg/ml), susceptible with increased exposure (I, 0.5 ≥ MIC ≤ 1 µg/ml), or resistant (R, MIC ≥ 2 µg/ml) [Clinical and Laboratory Standards Institute (CLSI), 2024]. Notably, all four datasets exhibited a tendency toward a trimodal distribuition, with a progressive rightward shift observed in the most recent studies. This trend was particularly evident in the study by Sang et al. (2016) where 38% of isolates presented MICs between 1 and 64 µg/ml In contrast, Huang et al. (2009) reported that 88% of isolates had MIC values ≤0.25 µg/ml, with a prominent peak at 0.003 µg/ml (307/445 strains), and only 3% were classified as resistant (CLSI, 2024). These findings indicate a temporal increase in MIC values among E. coli isolates, indicating a gradual loss of enrofloxacin susceptibility. The resulting ECOFF was 0.125 µg/ml, consistent with the distributions reported by Vanni et al. (2014), Sang et al. (2016), and Temmerman et al. (2020). In contrast, the lower ECOFF of 0.0625 µg/ml observed in the study by Huang et al. (2009) reflects the predominance of highly susceptible strains in that dataset. The pharmacokinetics of enrofloxacin have been studied in various species, particularly in chickens (Abd El-Aziz et al., 1997; Knoll et al., 1999; Gárcia Ovando et al., 1999). Differences in the pharmacokinetics of enrofloxacin formulations are often linked to the excipients and vehicles used. The pharmacokinetic model in our study was based on data from Kruse (2008) which used a 5% enrofloxacin solution in physiological saline (purity 98.7%; Bayer). As demonstrated by Sumano et al. (2001) even 5% oral solutions can show variability in parameters such as Cmax and bioavailability, likely due to formulation-dependent differences. This underscores the importance of specifying vehicle composition, as it may influence drug disposition and efficacy. Additionally, co-administration with other substances (Knoll et al., 1999; Aguilera et al., 2007; Atef et al., 2020) or the presence of an infection (Antonissen et al., 2017) may alter the pharmacokinetics of enrofloxacin. Because enrofloxacin administration typically occurs via drinking water, factors such as water quality, poor tank hygiene, and thermal stress can further influence water intake and the PK/PD behavior of enrofloxacin (Temmerman et al., 2021). The estimated pharmacokinetic parameters are consistent with the established literature. A typical clearance of 209.5 ml/kg/h was estimated, within the range reported in previous studies. Bugyei et al. (1999) reported a clearance of 180 ml/kg/h, whereas Temmerman et al. (2021) and Guo et al. (2010) reported higher values of 485 and 613 ml/kg/h, respectively. Our model presented two compartments (V1=785.5 ml/kg and V2=3234.73 ml/kg), similar to the two-compartment model established by Guo et al. (2010) which obtained values of V1=2,648 ml/kg and V2=2,333 ml/kg. Most studies have established a non-compartmental model, with V values ranging from 1,940 to 5,000 ml/kg (García Ovando et al., 1999; Knoll et al., 1999; Antonissen et al., 2017). These values are comparable when the two compartments are considered. Additionally, the volume in the second compartment is high, which aligns with the drug's behavior, as its lipophilicity results in greater biodistribution (Blokhina et al., 2016). Regarding bioavailability (F), a value of 87% was fixed, as defined by Kruse (2008) which is within the variation range found in published studies, with values ranging from 80% (Bugyei et al., 1999; Temmerman et al., 2021) to 90% (Knoll et al., 1999; Guo et al., 2010). The model adequately predicts enrofloxacin concentrations over time; however, it has the limitation of not considering ciprofloxacin, its active metabolite. Furthermore, no covariate analysis was performed. The PK/PD indices most commonly used to predict fluoroquinolone efficacy are fCmax/MIC=8–10 and fAUC24/MIC ≥ 100 to 125, utilized for gram-negative bacilli (Wright, 2000; Craig, 2001; Levison and Levison, 2009). However, the fAUC24/MIC index is a more universally adopted metric for dose calculation (Toutain et al., 2007). Additionally, AUC is more representative of the pharmacokinetics due to variable drug consumption by animals in in-field use, where medication is administered via water or feed, making it easier to achieve higher AUC values compared to Cmax (Temmerman et al., 2021). The PK/PD index for a specific drug-bacterium combination can be determined using in vivo, in vitro, or ex vivo models. For instance, Xiao et al. (2018) established fAUC0–24h/MIC targets for enrofloxacin at three effect levels (bacteriostatic, bactericidal, and eradication) in chickens infected with E. coli, including a value of ≥ 28.32 for eradication. This target is significantly lower than the general fluoroquinolone reference (≥ 125), influencing dose prediction. Several PKPD studies have been conducted to evaluate the efficacy of enrofloxacin in bacteria of importance in chickens (Sang et al., 2016; Bonassa et al., 2021; Kang et al., 2019). In our study, both values (fAUC24/MIC ≥ 125 and fAUC24/MIC ≥ 28.32) were evaluated, showing that the choice of PK/PD target critically impacts efficacy estimates. Our approach also considers MIC distribution to support dose adjustments tailored to farm-level resistance profiles. Further validation under field conditions is required to confirm the applicability of these targets in poultry production. Based on the results obtained in this study, with the fAUC24/MIC ≥ 125 index, the dose established in the leaflet (10 mg/kg) would be effective only against bacteria with a MIC up to 0.06 µg/ml. The established ECOFF was 0.125 µg/ml; therefore, this dose would not represent the population, requiring a dose adjustment. Temmerman et al. (2021) found a similar result; however, in their study, a dose of 12.5 mg/kg would be effective against bacteria with an MIC of 0.125 µg/ml, whereas our study suggested a dose of 20 mg/kg. However, when observing the results obtained using the index established by Xiao et al. (2018) due to the lower value of the index, the dose of 10 mg/kg becomes effective against bacteria with a MIC up to 0.25 µg/ml, with a PTA of 96%. Thus, this study found a difference in the efficacy of the label dose (10 mg/kg) depending on the PDT used (fAUC24/MIC ≥ 125 or 28.32). Further validation of the proposed doses (10 and 20 mg/kg) requires in vivo pharmacokinetic studies involving E. coli-infected animals and oral administration via water. Therefore, the following in vivo study should evaluate the clinical efficacy of PDT as estimated by Xiao et al. (2018). This study could evaluate and validate the clinical efficacy of the PDT estimated by Xiao et al. (2018). Furthermore, these studies should consider the influence of the metabolite ciprofloxacin. The resistance of E. coli in broiler chickens has been widely studied, and studies have shown that certain mutations in the gnrA, parC, and parE genes, as well as plasmid-mediated resistance, can significantly increase the MIC (Drugdová and Kmeť, 2013; Abdi-Hachesoo et al., 2017; Temmerman et al., 2020). Although our study did not focus on establishing doses based on the bacterial genotype, previous research has demonstrated that some E. coli strains, even with mutations, still have MIC values below 0.5 µg/ml. Based on these findings, the protocols developed in this study may be effective for these strains, providing a more precise and optimized approach to antimicrobial use. The latest update to the main veterinary testing standards document, “Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 7th Edition” (CLSI, 2024), the breakpoint for fluoroquinolones (enrofloxacin and marbofloxacin) against Staphylococcus spp. and E. coli in dogs has been reestablished, with the category "susceptible, dose dependent" being added. This change will significantly impact the breakpoints for the susceptibility of certain bacteria to specific antimicrobials, particularly those for which dose escalation can be safely performed, thereby enabling the treatment of bacteria that would not have been inhibited with standard dosing (Papich et al., 2023) and reinforces the need to establish therapeutic protocols based on the MIC of the infecting agent, ensuring the lowest antibiotic concentration necessary to treat the animal. The present model allows dose calculation based on the MIC to be incorporated into antimicrobial use management plans so that the lowest amount of antibiotic is used in each case, which would be in accordance with Antimicrobial Stewardship practices [Guardabassi and Prescott, 2015; Weese et al., 2015; Lloyd and Page, 2018; Food and Drug Administration (FDA), 2018; Patel et al., 2020; Food and Drug Administration (FDA), 2023b, 2023c]. The PK/PD approach used in this article contributes to antimicrobial stewardship practices because it allows the optimization of antimicrobial use through the optimization/determination of dose regimens for specific agents and species through PK/PD modeling. It can be used to optimize the doses of drugs already used to effectively and rationally prolong the use of the active ingredient. To improve this strategy, it would be interesting if the dose was based on the MIC of the bacterial isolates collected at the poultry farm where the chickens to be treated were located, as this would lead to more precise treatment. Using the lowest dose necessary to effectively treat these animals would reduce the emergence of antimicrobial resistance. ConclusionConsidering the calculated ECOFF of 0.125 µg/ml, a dose of 10 mg/kg would be effective for the fAUC24/MIC ≥ 28.32 targets; however, it would be insufficient for the fAUC24/MIC ≥ 125 targets, requiring a higher dose of 20 mg/kg. This discrepancy highlights the need for further studies to assess the predictive capabilities of these indices in determining antimicrobial efficacy. Moreover, our study demonstrated the potential for dose adjustment based on MIC distribution. Thus, future studies should focus on validating the clinical efficacy of the proposed PDTs before making any changes to the current dosing recommendations. AcknowledgmentsThe authors would like to thank the funding institutions Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES, Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq, Empresa Brasileira de Desenvolvimento Científico e Tecnológico - Embrapa, and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for the scholarships awarded to both undergraduate and postgraduate students and for financing projects of our research group. We thank Dr Diego Dias, who kindly provided us with the raw data as part of an essential partnership to execute this work. Conflict of interestThe authors have no conflicts of interest to declare. FundingThis work was funded by CAPES (88887805525/2023-00), CNPq (448011/2014-0), Embrapa (SEG 20.20.03.002.00.00), and FAPEMIG (APQ-01168-21, APQ-04229-23). This revision reflects the correct grant number for CAPES. Authors’ contributionsLarissa Alexsandra Felix: Methodology, Formal analysis, original draft writing, visualization, and Project administration. Beatriz Monte Egito: Methodology, Writing-Original Draft. Diego Diaz: Data Curation and Resources (PK Dataset). Márcio Gilberto Zangeronim: Writing-Review and Editing.; Sheila Rezler Wosiack: Writing-Review and Editing. Marcos Ferrante: Conceptualization, Writing-Review and Editing and Supervision. Data availabilityAll data supporting the study including Supplementary Material were provided at the end of this manuscript. ReferencesAbd El-Aziz, M.I., Aziz, M.A., Soliman, F.A. and Afify, N.A. 1997. Pharmacokinetic evaluation of enrofloxacin in chickens. Br. Poul. Sci. 38, 164 -168. Abdi-Hachesoo, B., Asasi, K. and Sharifiyazdi, H. 2017. Farm-level evaluation of enrofloxacin resistance in Escherichia coli isolated from broiler chickens during a rearing period. Comp. Clin. Pathol. 26, 471–476. Aguilera, R., Gutiérrez, O.L. and Sumano, L.H. 2007. Enhancement of enrofloxacin serum antibacterial activity by calcium primed broilers. Res. Vet. Sci. 82, 80–84. Antonissen, G., Devreese, M., De Baere, S., Martel, A., Van Immerseel, F. and Croubels, S. 2017. Impact of Fusarium mycotoxins on hepatic and intestinal mRNA expression of cytochrome P450 enzymes and drug transporters, and on the pharmacokinetics of oral enrofloxacin in broiler chickens. Food Chem. Toxicol. 101, 75–83. Atef, M., El-Banna, H.A., Elzorba, H.Y. and Soliman, A.M. 2020. Pharmacokinetics and tissue residue of enrofloxacin in healthy, Eimeria-infected broiler chickens and those pre-treated with amprolium and toltrazuril. Int. J. Vet. Sci. Med. 8, 31–38. Blokhina, S.V., Sharapova, A.V., Ol'Khovich, M.V., Volkova, Т.V. and Perlovich, G.L. 2016. Solubility, lipophilicity, and membrane permeability of some fluoroquinolone antimicrobials. Eur. J. Pharm. Sci. 93, 29–37. Bonassa, K.P.D., Miragliotta, M.Y., Simas, R.C., Eberlin, M.N., Anadón, A., Moreno, R.A. and Reyes, F.G.R. 2021. Pharmacokinetics, pharmacodynamic efficacy prediction indexes and Monte Carlo simulations of enrofloxacin hydrochloride against bacterial strains that induce common clinical diseases in broiler chickens. Front. Vet. Sci. 7, 606872. Bugyei, K., Black, W.D. and McEwen, S. 1999. Pharmacokinetics of enrofloxacin given by the oral, intravenous and intramuscular routes in broiler chickens. Can. J. Vet. Res. 63, 193–200. Clinical and Laboratory Standards Institute (CLSI). 2024. VET01: performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals. Craig, W.A. 2001. Does the dose matter?. Clin. Infect. Dis. 33(Suppl 3), S233–S237. Drugdová, Z. and Kmeť, V. 2013. Prevalence of β-lactam and fluoroquinolone resistance, and virulence factors in Escherichia coli isolated from chickens in Slovakia. Biologia 68, 11–17. Dziva, F. and Stevens, M.P. 2008. Colibacillosis in poultry: unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 37, 355–366. European Medicines Agency (EMA). 2016a. Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products. EMA/CHMP/594085/2015. European Medicines Agency (EMA). 2016b. Guideline for the demonstration of efficacy for veterinary medicinal products containing antimicrobial substances. EMA/CVMP/627/2001-Rev.1. European Medicines Agency (EMA). 2020. Categorization of antibiotics used in animals promotes responsible use to protect public and animal health. EMA/688114/2020. European Medicines Agency (EMA). 2023. Scientific advice under Article 107(6) of Regulation (EU) 2019/6 for the establishment of a list of antimicrobials which shall not be used in accordance with Articles 112, 113 and 114 of the same Regulation or which shall only be used in accordance with these articles subject to certain conditions. Food and Drug Administration (FDA). 2018. Supporting antimicrobial stewardship in veterinary settings: goals for fiscal years 2019–2023. Food and Drug Administration (FDA). 2022. Population pharmacokinetics guidance for industry. FDA-2019-D-2398. Food and Drug Administration (FDA). 2023a. Summary report on antimicrobials sold or distributed for use in food-producing animals. Food and Drug Administration (FDA). 2023b. Supporting antimicrobial stewardship in veterinary settings: goals for fiscal years 2024–2028. Food and Drug Administration (FDA). 2023c. FDA-TRACK: progress on FDA's support of antimicrobial stewardship in veterinary settings. Food and Drug Administration (FDA). 2024. Extralabel use and antimicrobials. García Ovando, H., Gorla, N., Luders, C., Poloni, G., Errecalde, C., Prieto, G. and Puelles, I. 1999. Comparative pharmacokinetics of enrofloxacin and ciprofloxacin in chickens. J. Vet. Pharmacol. Ther. 22, 209–212. Guabiraba, R. and Schouler, C. 2015. Avian colibacillosis: still many black holes. FEMS Microbiol. Lett. 362, 118. Guardabassi, L. and Prescott, J.F. 2015. Antimicrobial stewardship in small animal veterinary practice: from theory to practice. Vet. Clin. N. Am. Small Anim. Pract. 45, 361–376. Guo, Q.J., Huang, L.L., Fang, K., Wang, Y.L., Chen, D.M., Tao, Y.F., Dai, M.H., Liu, Z.L., Peng, D.P. and Yuan, Z.H. 2010. Population pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin in chicken based on retrospective data, incorporating first-pass metabolism. J. Vet. Pharmacol. Ther. 33, 84–94. Huang, T.M., Lin, T.L. and Wu, C.C. 2009. Antimicrobial susceptibility and resistance of chicken Escherichia coli, Salmonella spp., and Pasteurella multocida isolates. Avian Dis. 53, 89–93. Kang, J., Hossain, M.A., Park, H.C., Kim, Y., Lee, K.J. and Park, S.W. 2019. Pharmacokinetic and pharmacodynamic integration of enrofloxacin against Salmonella Enteritidis after administering to broiler chicken by per-oral and intravenous routes. J. Vet. Sci. 20, e15. Knoll, U., Glünder, G. and Kietzmann, M. 1999. Comparative study of the plasma pharmacokinetics and tissue concentrations of danofloxacin and enrofloxacin in broiler chickens. J. Vet. Pharmacol. Ther. 22, 239–246. Kruse, M.C. 2008. Influencia del estrés calórico sobre la farmacocinética y depleción tisular de enrofloxacina en pollos parrilleros [Thesis]. Santa Fe, AR: Universidad Nacional del Litoral, Facultad de Ciencias Veterinarias. Landersdorfer, C.B. and Nation, R.L. 2021. Limitations of antibiotic MIC-based PK–PD metrics: looking back to move forward. Front. Pharmacol. 12, 770518. Lavielle, M. and Ribba, B. 2016. Enhanced method for diagnosing pharmacometric models: random sampling from conditional distributions. Pharm. Res. 33, 2979–2988. Levison, M.E. and Levison, J.H. 2009. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 23, 791–815. Lloyd, D.H. and Page, S.W. 2018. Antimicrobial stewardship in veterinary medicine. Microbiol. Spectr. 6(3), arba-0023-2017. Martinez, M.N., Papich, M.G. and Drusano, G.L. 2012. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 56, 2795–2805. Mould, D.R. and Upton, R.N. 2013. Basic concepts in population modeling, simulation, and model-based drug development – part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometrics Syst. Pharmacol. 2, 1–14. Mouton, J.W., Ambrose, P.G., Canton, R., Drusano, G.L., Harbarth, S., Macgowan, A., Theuretzbacher, U. and Turnidge, J. 2011. Conserving antibiotics for the future: new ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective. Drug Resist. Updat. 14, 107–117. Nguyen, T.H.T., Mouksassi, M.S., Holford, N., Al‐Huniti, N., Freedman, I., Hooker, A.C., John, J., Karlsson, M.O., Mould, D.R., Pérez Ruixo, J.J., Plan, E.L., Savic, R., Van Hasselt, J.G.C., Weber, B., Zhou, C., Comets, E. and Mentré, F. 2017. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst. Pharmacol. 6, 87–109. Nielsen, E.I., Viberg, A., Löwdin, E., Cars, O., Karlsson, M.O. and Sandström, M. 2007. Semimechanistic pharmacokinetic/pharmacodynamic model for assessment of activity of antibacterial agents from time-kill curve experiments. Antimicrob. Agents Chemother. 51, 128 -136. Nielsen, E.I. and Friberg, L.E. 2013. Pharmacokinetic-pharmacodynamic modeling of antibacterial drugs. Pharmacol. Rev. 65, 1053–1090. Papich, M.G. 2014. Pharmacokinetic-pharmacodynamic (PK–PD) modeling and the rational selection of dosage regimes for the prudent use of antimicrobial drugs. Vet. Microbiol. 171, 480–486. Papich, M.G., Gunnett, L.A. and Lubbers, B.V. 2023. Revision of fluoroquinolone breakpoints used for interpretation of antimicrobial susceptibility testing of canine bacterial isolates. Am. J. Vet. Res. 84, ajvr.23.07.0159. Patel, S.J., Wellington, M., Shah, R.M. and Ferreira, M.J. 2020. Antibiotic stewardship in food-producing animals: challenges, progress, and opportunities. Clin. Ther. 42, 1649–1658. Rodríguez-Gascón, A., Solinís, M.A. and Isla, A. 2021. The role of PK/PD analysis in the development and evaluation of antimicrobials. Pharmaceutics 13, 833. Roth, N., Käsbohrer, A., Mayrhofer, S., Zitz, U., Hofacre, C. and Domig, K.J. 2019. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: a global overview. Poult. Sci. 98, 1791–1804. Sang, K., Hao, H., Huang, L., Wang, X. and Yuan, Z. 2016. Pharmacokinetic-pharmacodynamic modeling of enrofloxacin against Escherichia coli in broilers. Front. Vet. Sci. 2, 80. Savic, R.M. and Karlsson, M.O. 2009. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS. J. 11, 558–569. Schmerold, I., Van Geijlswijk, I. and Gehring, R. 2023. European regulations on the use of antibiotics in veterinary medicine. Eur. J. Pharm. Sci. 189, 106473. Selig, D.J., DeLuca, J.P., Chung, K.K., Pruskowski, K.A., Livezey, J.R., Nadeau, R.J. and et al. 2022. Pharmacokinetics of piperacillin and tazobactam in critically ill patients treated with continuous kidney replacement therapy: a mini-review and population pharmacokinetic analysis. J. Clin. Pharm. Ther. 47, 1091–1102. Silley, P. 2012. Susceptibility testing methods, resistance and breakpoints: what do these terms really mean?. Rev. Sci. Tech. 31, 33–41. Sumano, L.H., Gutiérrez, O.L. and Zamora, M.A. 2001. Bioequivalence of four preparations of enrofloxacin in poultry. J. Vet. Pharmacol. Ther. 24, 309–313. Temmerman, R., Garmyn, A., Antonissen, G., Vanantwerpen, G., Vanrobaeys, M., Haesebrouck, F. and Devreese, M. 2020. Evaluation of fluoroquinolone resistance in clinical avian pathogenic Escherichia coli isolates from Flanders (Belgium). Antibiotics 9, 800. Temmerman, R., Pelligand, L., Schelstraete, W., Antonissen, G., Garmyn, A. and Devreese, M. 2021. Enrofloxacin dose optimization for the treatment of colibacillosis in broiler chickens using a drinking behaviour pharmacokinetic model. Antibiotics 10, 604. Toutain, P.L., Bousquet-Melou, A. and Martinez, M. 2007. AUC/MIC: a PK/PD index for antibiotics with a time dimension or simply a dimensionless scoring factor?. J. Antimicrob. Chemother. 60, 1185–1188. Toutain, P.L., Pelligand, L., Lees, P., Bousquet-Mélou, A., Ferran, A.A. and Turnidge, J.D. 2021. The pharmacokinetic/pharmacodynamic paradigm for antimicrobial drugs in veterinary medicine: recent advances and critical appraisal. J. Vet. Pharmacol. Ther. 44, 172–200. Toutain, P.L., Sidhu, P.K., Lees, P., Rassouli, A. and Pelligand, L. 2019. VetCAST method for determination of the pharmacokinetic-pharmacodynamic cutoff values of a long-acting formulation of florfenicol to support clinical breakpoints for florfenicol antimicrobial susceptibility testing in cattle. Front. Microbiol. 10, 465356. Toutain, P.L., Bousquet-Mélou, A., Damborg, P., Ferran, A.A. and Mevius, D., Pelligand, L., Veldman, K.T. and Lees, P. 2017. En route towards european clinical breakpoints for veterinary antimicrobial susceptibility testing: a position paper explaining the VetCAST Approach. Front Microbiol. 15, 2344. Traynard, P., Ayral, G., Twarogowska, M. and Chauvin, J. 2020. Efficient pharmacokinetic modeling workflow with the MonolixSuite: a case study of remifentanil. CPT. Pharmacometrics Syst. Pharmacol. 9, 198–210. Turnidge, J., Kahlmeter, G. and Kronvall, G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cutoff values. Clin. Microbiol. Infect. 12, 418–425. Vanni, M., Meucci, V., Tognetti, R., Cagnardi, P., Montesissa, C., Piccirillo, A., Rossi, A.M., Di Bello, D. and Intorre, L. 2014. Fluoroquinolone resistance and molecular characterization of gyrA and parC quinolone resistance-determining regions in Escherichia coli isolated from poultry. Poult. Sci. 93, 856–863. Waxman, S., San Andres, M.D., Gonzalez, F., De Lucas, J.J., San Andres, M.I. and Rodriguez, C. 2003. Influence of Escherichia coli endotoxin-induced fever on the pharmacokinetic behaviour of marbofloxacin after intravenous administration in goats. J. Vet. Pharmacol. Ther. 26, 65–69. Weese, J.S., Giguère, S., Guardabassi, L., Morley, P.S., Papich, M., Ricciuto, D.R. and Sykes, J.E. 2015. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J. Vet. Intern. Med. 29, 487–498. World Health Organization (WHO). 2015. Global action plan on antimicrobial resistance. Geneva: WHO. World Health Organization (WHO). 2024. WHO list of medically important antimicrobials: a risk management tool for mitigating antimicrobial resistance due to non-human use. Geneva: WHO. Wright, D.H. 2000. Application of fluoroquinolone pharmacodynamics. J. Antimicrob. Chemother. 46, 669–683. Xiao, X., Jiang, L., Lan, W., Jiang, Y. and Wang, Z. 2018. In vivo pharmacokinetic/pharmacodynamic modeling of enrofloxacin against Escherichia coli in broiler chickens. BMC Vet. Res. 14, 1–9. Yu, W., Ji, J., Xiao, T., Ying, C., Fang, J., Shen, P. and Xiao, Y. 2017. Determining optimal dosing regimen of oral administration of dicloxacillin using Monte Carlo simulation. Drug Des. Devel. Ther. 28, 1951 -1956. Supplementary Materials.Pharmacokinetics, pharmacodynamic efficacy prediction indices and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens. Table S1. Plasma concentrations of enrofloxacin (μg/ml) determined in broilers treated intravenously with a single dose of 10 mg/kg.
Table S2. Plasma concentrations of enrofloxacin (μg/ml) determined in broilers treated orally with a single dose of 10 mg/kg.
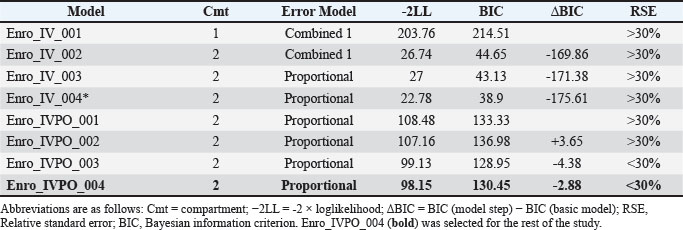
Table S3. Summary model building of enrofloxacin in broilers.
*ωV1 was fixed as the CV was less than 5%, and correlation between Cl and V2 was added. Only ωQ showed an RSE >30%, which was corrected in the IVPO model with the addition of oral route data. Model Enro_IVPO_004 was chosen because it showed better graphical fits and a lower RSE value compared to Enro_IVPO_003. The difference between them is that the value of Cl was no longer fixed in model Enro_IVPO_004.
Fig. S1. Individual weighted residual (IWRES) versus individual predictions (top-left) and time after dose (bottom-left). Normalized prediction errors (NPDE) versus population predictions (top-right) and time after dose (bottom-right). The dark dots represent the intravenous data and the blue dots the oral data. File F1. Method for calculating AUC. First, the following code is added to the model, in the Definition >> Model tab, on additional lines:
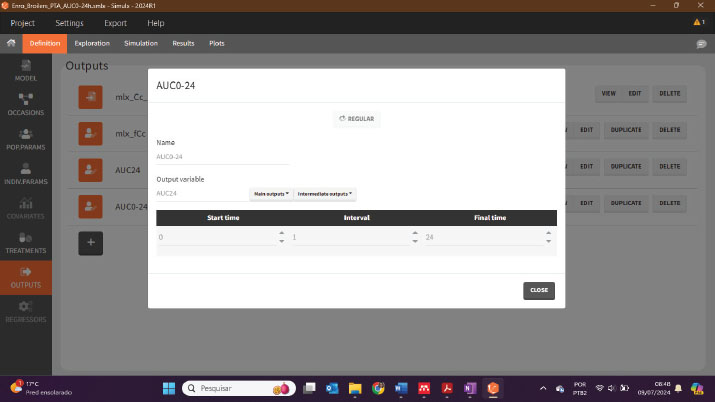
After adding the code to the model, it is necessary to establish an output for AUC 24 hours in the Definitions >> Outputs tab:
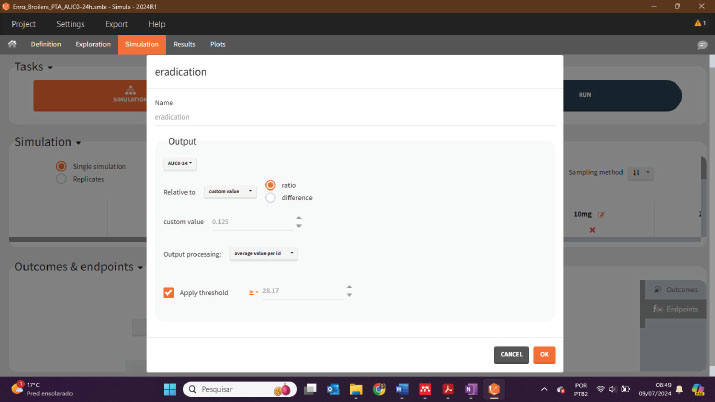
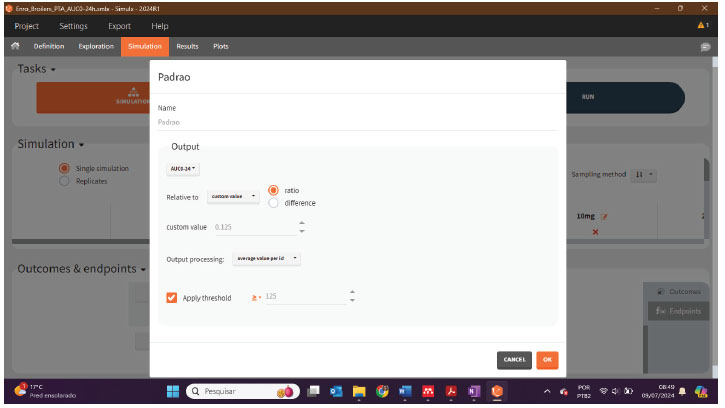
Afterwards, in the Simulation tab, it is necessary to establish the treatments and insert the ACU 24 hours as an output, and then the endpoints can be created, as illustrated below:
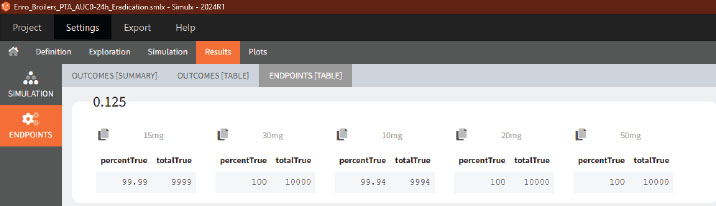
The PTA result will be displayed as a graph in the plots tab and as a percentage, in the Results >> Endopoint tab (Table):
| ||
| How to Cite this Article |
| Pubmed Style Felix LA, Egito BM, David DD, Zangeronimo MG, Wosiacki SR, Ferrante M. Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens. Open Vet. J.. 2025; 15(9): 4454-4469. doi:10.5455/OVJ.2025.v15.i9.52 Web Style Felix LA, Egito BM, David DD, Zangeronimo MG, Wosiacki SR, Ferrante M. Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens. https://www.openveterinaryjournal.com/?mno=242668 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.52 AMA (American Medical Association) Style Felix LA, Egito BM, David DD, Zangeronimo MG, Wosiacki SR, Ferrante M. Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens. Open Vet. J.. 2025; 15(9): 4454-4469. doi:10.5455/OVJ.2025.v15.i9.52 Vancouver/ICMJE Style Felix LA, Egito BM, David DD, Zangeronimo MG, Wosiacki SR, Ferrante M. Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4454-4469. doi:10.5455/OVJ.2025.v15.i9.52 Harvard Style Felix, L. A., Egito, . B. M., David, . D. D., Zangeronimo, . M. G., Wosiacki, . S. R. & Ferrante, . M. (2025) Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens. Open Vet. J., 15 (9), 4454-4469. doi:10.5455/OVJ.2025.v15.i9.52 Turabian Style Felix, Larissa Alexsandra, Beatriz Monte Egito, Diego Diaz David, Márcio Gilberto Zangeronimo, Sheila Rezler Wosiacki, and Marcos Ferrante. 2025. Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens. Open Veterinary Journal, 15 (9), 4454-4469. doi:10.5455/OVJ.2025.v15.i9.52 Chicago Style Felix, Larissa Alexsandra, Beatriz Monte Egito, Diego Diaz David, Márcio Gilberto Zangeronimo, Sheila Rezler Wosiacki, and Marcos Ferrante. "Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens." Open Veterinary Journal 15 (2025), 4454-4469. doi:10.5455/OVJ.2025.v15.i9.52 MLA (The Modern Language Association) Style Felix, Larissa Alexsandra, Beatriz Monte Egito, Diego Diaz David, Márcio Gilberto Zangeronimo, Sheila Rezler Wosiacki, and Marcos Ferrante. "Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens." Open Veterinary Journal 15.9 (2025), 4454-4469. Print. doi:10.5455/OVJ.2025.v15.i9.52 APA (American Psychological Association) Style Felix, L. A., Egito, . B. M., David, . D. D., Zangeronimo, . M. G., Wosiacki, . S. R. & Ferrante, . M. (2025) Pharmacokinetics, pharmacodynamic efficacy prediction indices, and Monte Carlo simulations of enrofloxacin for the treatment of colibacillosis in broiler chickens. Open Veterinary Journal, 15 (9), 4454-4469. doi:10.5455/OVJ.2025.v15.i9.52 |