| Short Communication | ||
Open Vet. J.. 2025; 15(8): 3878-3887 Open Veterinary Journal, (2025), Vol. 15(8): 3878-3887 Short Communication Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancyHafi Nurinasari1,2*, Fitrine Ekawasti3, Sajidan4, Bambang Purwanto1,5, Dono Indarto1,6, Soetrisno1,2, Ariva Syiva’a7, Didik Tulus Subekti3 and Sri Suryatmiati Prihandani31Doctorate Program of Medical Sciences, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 2Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 3Research Organization for Health, National Research and Innovation Agency, Bogor, Indonesia 4Biology Study Program, Faculty of Education, Universitas Sebelas Maret, Surakarta, Indonesia 5Department of Internal Medicine, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 6Department of Physiology and Biomedical Laboratory, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 7Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia *Corresponding Author: Hafi Nurinasari. Doctorate Program of Medical Sciences, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia; Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia. Email: hafinurina [at] staff.uns.ac.id Submitted: 12/02/2025 Revised: 25/06/2025 Accepted: 11/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
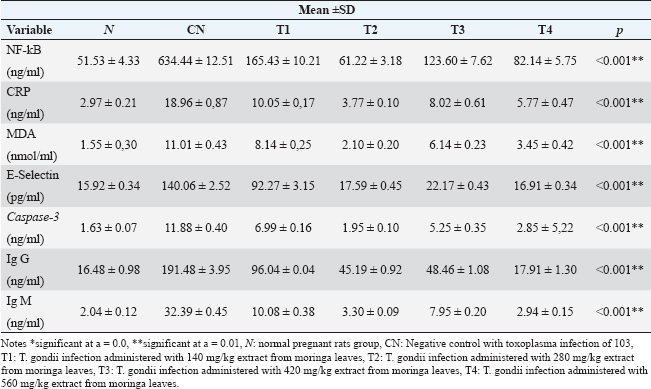
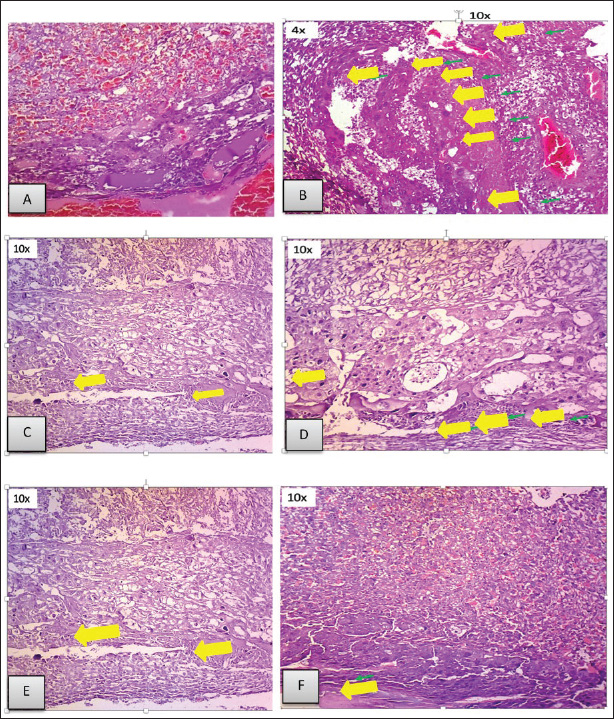
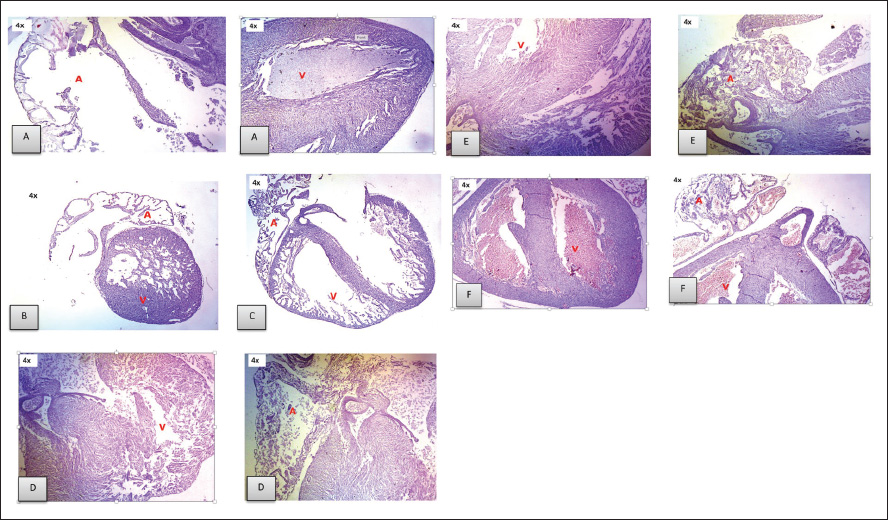
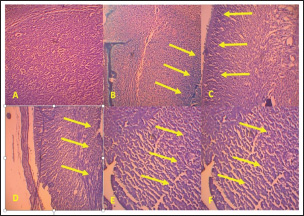
ABSTRACTBackground: Toxoplasmosis is a model for the One Health idea since the parasite that causes Toxoplasma gondii affects almost all animals, including humans. Toxoplasma gondii is among the parasites with the highest success rate in history, able to infect around one-third of all humans globally. Furthermore, among all parasitic infections, T. gondii has one of the highest rates of disease. The expansion of the human and veterinary medical sectors is necessary to address the animal reservoir and decrease the number of toxoplasmosis. Aim: To overcome oxidative stress, intracellular infection mechanisms require therapy using natural substances that are effective, safe, and efficient. Among them, Moringa oleifera contains bioactive chemicals. Methods: In this lab-based investigation, 8-week-old female rats weighing 200–250 g were split into six groups using a randomized control trial with posttest only and control group design. The groups included the normal group, the toxoplasma group, which received a dose of 103 tachyzoite, and the toxoplasma group, which received doses of 140, 280, 420, and 560 mg/kg moringa. Results: On the 16th day of pregnancy, the rats were euthanized, and samples of the mother’s blood were drawn and tested for NF-κB, E-Selectin, C-reactive protein (CRP), MDA, caspase-3, Ig G, and Ig M. Additionally, the placenta, heart, neurons, and fetal neuroglia were assessed histopathologically for rats with toxoplasmosis. NF-κB, MDA, CRP, E-Selectin, caspase-3, Ig M, and Ig G levels differed notably (p < 0.001). This study demonstrated that pregnant rats in the toxoplasmosis model showed improvement in IgG, IgM, E-Selectin, NF-κB, placental necrosis repair, and the fetus’s heart and brain when administered a dosage of 560 mg/kg of M. oleifera leaves. In comparison, M. oleifera leaves at 280 mg/kg doses showed improved CRP, MDA, and caspase-3 levels. Conclusion: Moringa oleifera can be an alternative therapy for toxoplasmosis infection-induced pregnancy in a rat model. It has anti-inflammatory, antiapoptosis, antiparasitic, and antinecrosis effects in pregnant rats with toxoplasmosis models. Keywords: Moringa oleifera, Antiinflammation, Antiapoptosis, Antiparasite, Antinecrosis. IntroductionThe diseases known as toxoplasmosis are caused by the obligatory intracellular Toxoplasma gondii. Toxoplasmosis is a model for the One Health idea since the parasite that causes T. gondii affects humans and animals (Aguirre et al., 2019). An estimated 30%–50% of people worldwide are chronically infected with this parasite, which can infect various warm-blooded animals (Ekawasti et al., 2021). In Indonesia, toxoplasmosis affects between 3% and 70% of the population. Toxoplasmosis infection is more prevalent in populations with certain risk factors, with like to eat raw vegetables, infected milk, and undercooked meat, and those who like to keep cats (Dwinata et al., 2016). There are two ways to spread toxoplasmosis: vertically, through the placenta from the mother to the fetus, and horizontally, through the ingestion of raw vegetables, infected milk, and undercooked meat (Retmanasari et al., 2017). Organ transplants or blood transfusions can infrequently spread the illness (Pleyer et al., 2019). Transmission of toxoplasmosis infection from mother to fetus causes serious problems, such as intracranial disorders, mental retardation, hydrocephalus, chorioretinitis, and even fetal death (Thái et al., 2019). Oxidative stress caused by T. gondii infections leads to an imbalance between the cellular defense system and the system that produces free radicals, either by lowering antioxidant defense activity or by boosting free radical generation (Dincel and Atmaca, 2016). Toxoplasma gondii infection-related oxidative stress can limit trophoblast invasion, decrease angiogenesis, cause endothelial dysfunction, and result in acute atherosclerosis (Dincel and Atmaca, 2016). Upon histopathological analysis, macrophage infiltration revealed necrotic patches in the liver, lungs, heart, Central Nervous System (CNS), and muscles. Other organs affected by toxoplasmosis include the heart, skeletal muscle, fetal membranes, brain, neurons, microglia, liver parenchyma, and leukocytes (Hartati et al., 2007). Acute toxoplasmosis infection is mostly managed with Pyrimethamine, sulfadiazine, and spiramycin. Spiramycin is a safety precaution to prevent infection in the fetus. It is recommended to use pyrimethamine-sulfadiazine for chronic toxoplasmosis (Silva et al., 2017). Both medications directly target tachyzoites and prevent folate metabolism in parasites. However, they also have adverse effects, including elevated creatinine and liver enzyme levels, anemia, and hypersensitivity reactions (Konstantinovic et al., 2017). A new, effective treatment for toxoplasmosis with mild adverse effects that is easy to find is urgently needed. Therapy with the use of natural ingredients and minimal side effects, one of which is M. oleifera (Islam et al., 2021). Additionally, treat chronic toxoplasmosis unadvised to treat chronic toxoplasmosis unless the patient but secondary prevention and supportive therapeutic. Plants of the M. oleifera genus thrive in tropical and subtropical regions with a wide range of soil types and damp to dry weather conditions (Leone et al., 2015; Vergara-Jimenez et al., 2017). Moringa oleifera has anti-infection, immunity, antioxidant, antitumor, and antidiabetic (Razis et al., 2014). Several studies have shown that M. oleifera benefits humans because it contains bioactive compounds. The plant leaves are the most often used and rich in flavonoids, alkaloids, glucosinolate, isothiocyanates, tannins, carotenoids, polyphenols, phenolic acids, and vitamins. Moringa oleifera leaves are used for medicinal purposes because they have many functional bioactive components with pharmacological effects (Razis et al., 2014; Vergara-Jimenez et al., 2017). Moringa oleifera also has neuroprotective and memory-enhancing effects in people with dementia and Alzheimer’s disease (Sutalangka et al., 2013). Because T. gondii is a member of the phylum Apicomplexa, M. oleifera also affects the protozoan plasmodium (Somsak et al., 2016). Based on these findings, scientists are interested in determining how Moringa leaves affect the placenta, neurons, heart, and glia histopathology in a fetal rat model of toxoplasmosis and the improvement of Nuclear Factor Kappa Beta, E-selectin, CRP, malondialdehyde (MDA) Immunoglobulin G, Immunoglobulin M, and caspase-3 in pregnant rats with toxoplasmosis models. Material and MethodsPreparationPowder of M. oleifera leaves was provided by PT. Merapi Farma, Hargobinangun, Pakem, and Sleman Yogyakarta, were extracted using an ethanol solvent for the maceration process. Moringa leaf extraction was conducted at the Research and Development Center for Traditional Medicinal Plants, Tawangmangu, Indonesia. The T. gondii isolates used in this study were provided by the Indonesia Research Centre for Veterinary Science, Ministry of Agriculture. The T. gondii isolate used in this study was a virulent isolate of biotype 1 strain RH (Ekawasti et al., 2021). Experimental animals and groupsWe used the randomized control trial (RCT) with a posttest-only control group design, which recruited 48 female rats (Rattus novergicus) with 200–250 g in body weight and 6–8 weeks old, and split them into six groups using an RCT. In animal studies, anticipating the loss of experimental subjects due to factors like death or unexpected outcomes, it is crucial to increase the initial sample size to ensure sufficient data for meaningful analysis (Charan and Kantharia, 2013). Based on this, this study used eight pregnant rats in each group. All selected rats were first adapted to the Center for Food and Nutrition Studies animal laboratory at Gadjah Mada University, Yogyakarta, for 1 week. Each female rat was mated with one male for 24 hours. Six pregnant rats were randomly assigned: Groups 1 and 2: normal pregnant rats and negative controls with T. gondii infection (N and NC groups). Groups 3–6 were pregnant rats with T. gondii infection treated with 140, 280, 420, and 560 mg/kg moringa leaf extract (T1–T4 groups). Once the female rats had become pregnant, T. gondii induction was carried out on the 12th day of pregnancy (Dubey et al., 1997). The female rats were intraperitoneally injected with 103 tachyzoites throughout pregnancy’s 12th–16th day (injected the parasite on days 12th, 13th, 14th, 15th, and 16th of pregnancy). To evaluate whether or not pregnant rats got T. gondii infection, we measured serum IgG and IgM levels on day 16 of pregnancy. The antibody was measured on the same day the parasite was injected. The administration of M. oleifera leaf extract in the rat group 3–6 was administered orally on 5 days a week in the morning (throughout the 12th–16th day of pregnancy). All control and treatment rats were given the broiler II standard feed and allowed to freely access drinking water. The rats were sacrificed on the last day of pregnancy, and maternal blood samples were taken for and detection of antibody Ig G, Ig M, nuclear factor-κappa B (NF-kB), E-Selectin, C-Reactive Protein, malondialdehyde, and caspase-3 levels. Histopathological analysis of the placenta, neurons, heart, and fetal glia was conducted in the Anatomical Pathology Laboratory, Faculty of Medicine, Universitas Sebelas Maret. Stages of staining of the placenta and fetal heart are using hematoxylin-eosin, while neuron and glial cells use the clover barera staining. Statistical analysisAll serum levels and histopathological results were analyzed statistically using SPSS software. The Shapiro–Wilk normality test was used to determine whether the data followed a normal distribution. One-way ANOVA analysis was used to compare all mean values among the rat groups, followed by the Tukey post hoc test. Significant differences among groups were identified using a 95% confidence level (p < 0.05). Ethical approvalThe National Center for the Replacement, Refinement, and Reduction of Animals in Research (NC3Rs) guidelines for treating animals used as trial subjects in this investigation were followed. The animals were approved by the health research ethics committee of Universitas Sebelas Maret, Surakarta (No. 56/UN27.06.6.1/KEP/EC/2021. Results and DiscussionExamination results of NFkB, CRP, MDA, E-selectin, caspase-3, IgG, and Ig M in toxoplasmosis pregnant rats after administration of M.oleifera (Tables 1 and 2). Figure 1 shows that the N group’s placenta appeared intact, and no necrosis was found. In the placenta of the NT group, necrosis was found in 75% of the area (yellow arrow). In the T1 group, the placenta appeared to have necrosis in approximately 40% of the area. In the T2 group, necrosis was found in approximately 20% of the area. In the T3 group, approximately 20% of the region had necrosis. In the T4 group, we found necrosis in approximately 10% of the areas. Histopathological results of Moringa leaves on the appearance of the fetal heart of the rat model of toxoplasmosis (Fig. 2). Based on Figure 2, normal atria and ventricles were obtained. In the NT group, the ventricles and atria appeared atrophied with thickening of the ventricular walls. In the T1 group, ventricular atrophy and atrial ventricular dilatation were observed. In the T2 group, the ventricles and atria showed only mild dilatation, but otherwise they appeared normal. The atria and ventricles of the toxoplasma-induced fetal heart, which were in the T3 group, appeared to be largely normal. In the T4 group, the atria and ventricles appeared normal. Histopathological results of the brain of the toxoplasmosis fetal rat model (Fig. 3). Table 1. Examination results of NF-kB, CRP, MDA, E-selectin, caspase-3, IgG, and Ig M in toxoplasmosis pregnant rats after administration of M. oleifera.
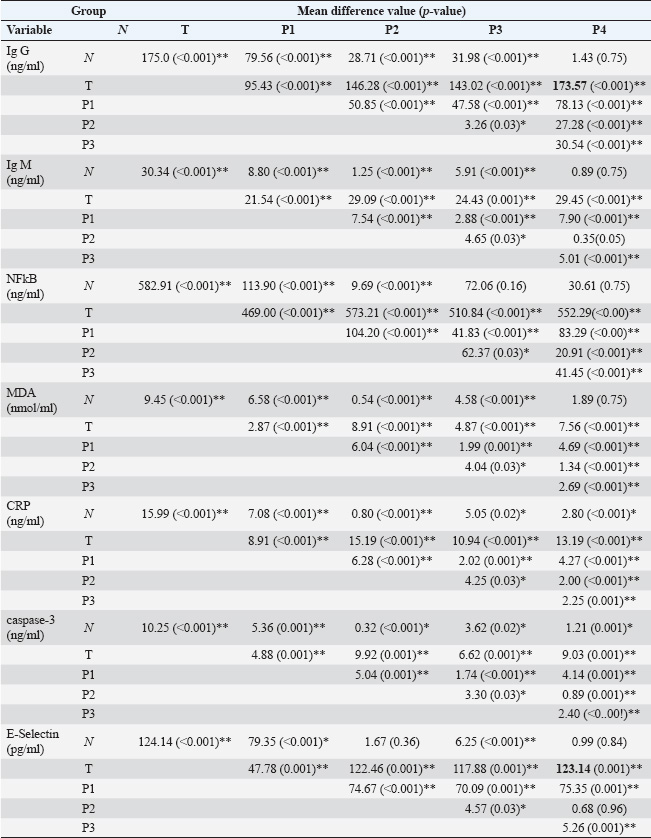
Table 2. Post-hoc results data value of Ig G, Ig M, MDA, CRP, NFkB, E-selectin, and caspase 3 rats after toxoplasma induction and Moringa administration.
Fig. 1. The placenta microanatomy of pregnant rats given toxoplasma and moringa at various doses. A. Normal pregnant rats did not receive any treatment. B. Toxoplasma rats at a dose of 103. C. A 140 mg/kg ethanol extract from Moringa leaves was administered to pregnant rats harboring Toxoplasma. D. A 280 mg/kg ethanol extract from Moringa leaves was administered to pregnant rats harboring Toxoplasma. E. A 420 mg/kg ethanol extract from Moringa leaves was administered to pregnant rats harboring Toxoplasma. F. A dosage of 560 mg/kg of ethanol extract from Moringa leaves was administered to pregnant rats harboring Toxoplasma (yellow arrow indicates areas of necrosis).
Fig. 2. Histopathological examination of the heart of a rat fetus induced by toxoplasmosis treated with various doses of Moringa. A. Histopathological examination of the fetal heart is normal. B. Histopathological examination of the toxoplasma-induced fetal heart. C. Histopathological analysis of the embryonic heart stimulated by toxoplasma using 140 mg/kg of an ethanol-based extract of moringa leaves. D. Histopathological examination of a toxoplasma-induced fetal heart treated with 280 mg/kg ethanol-based Moringa leaf extract. E. Histopathological examination of a toxoplasma-induced fetal heart treated with 420 mg/kg ethanol-based Moringa leaf extract. F. Histopathological analysis of toxoplasma-induced fetal heart tissue treated with 560 mg/kg of an ethanol-based extract of moringa leaves (a: atria; v: ventricle).
Fig. 3. The histological picture, magnified 100 times, reveals a gliosis (increased astrocytes) in the Kluver Barera-stained fetal muscle parenchyma. There is no picture of increased astrocytes (gliosis) in the normal fetal brain exists. B. Gliosis of the toxoplasma fetal brain. C. Gliosis with toxoplasma and 140 mg/kg of Moringa leaf ethanol–based extract. D. Gliosis in toxoplasma in 280 mg/kg Moringa leaf ethanol–based extract. E. Gliosis in toxoplasma and 420 mg/kg of Moringa leaf ethanol extract. F. Gliosis with toxoplasma and 560 mg/kg of Moringa leaf ethanol–based extract (yellow arrow indicates the area of gliosis). The histopathological picture of the normal fetal brain does not reveal an increase in the number of astrocytes (gliosis). Toxoplasma fetal neurons and neuroglia showed severe gliosis in >60% of the total area. Severe gliosis > 60% area was observed in the T1 group. Severe gliosis > 60% area was seen in the T2 group. Severe gliosis > 60% area was observed in the T3 group. The neurons and neuroglia of the T4 group exhibited significant gliosis in 31%–60% of the area. This study demonstrated that in pregnant rats with a toxoplasmosis model, a dose of 560 mg/kg moringa leaf improved Ig G, Ig M, NFkB, and E-selectin levels, repaired placental necrosis, and improved the heart and brain of the fetus. In a pregnant rat model of toxoplasmosis, a dose of 280 mg/kg improved the levels of CRP, MDA, and caspase 3. During infection, T. gondii shows an increase in NFkB, CRP, MDA, Ig G, Ig M, caspase-3 in pregnant rats of the toxoplasmosis model and shows necrosis in the placenta and heart, which is in line with Dincel’s study in which infection with oxidative stress brought on by T. gondii disrupts the balance between the cellular defense system and the system that produces free radicals, namely generating more free radicals, lowering antioxidant defense levels, or doing both (Dincel and Atmaca, 2016). During infection, T. gondii performs autophagy, which plays a role in intracellular survival by maintaining the immune response of the parasite (Lee et al., 2020). When administered to pregnant rats at a dose of 560 mg/kg, an ethanol-based extract of moringa leaves can lower Ig G and M levels and even reach normal levels. This is similar to the results of Nishi et al. (2021), who reported that M.oleifera ethanol-based extract was able to decrease the intracellular form of T. gondii tachyzoites and limit the invasion and intracellular replication of T. gondii, where M. oleifera contains flavonoids and phenolic components, which are important bioactive substances. In a toxoplasma study using ethanol-based extract of Moringa leaves, 560 mg/kg, it can increase Nuclear Factor Kappa β levels. This research is similar to the results of Abdel-Daim et al. (2020), who reported that the effects of cobalt chloride exposure on rats’ kidneys were lessened by moringa leaf extract. Kidney inflammation results from the NFkB inflammatory signaling system, which is elevated in response to cobalt exposure. The administration of moringa considerably increased the expression of NFkB and IL-6. The treatment and control groups were included, with the treatment group exhibiting higher gene expression than the control group (Abdel-Daim et al., 2020). Using the toxoplasma model, this study found that an ethanol-based extract of moringa leaves, at a dose of 560 mg/kg, decreased MDA levels in pregnant rats. MDA levels can be mechanically lowered by administering M. oleifera leaf extract therapy as an antioxidant. Swiss albino rats exposed to radiation-induced oxidative stress were administered M. oleifera leaf extract therapy for 15 days. This treatment efficiently absorbs radiation-induced free radicals. The antioxidants analyzed included ascorbic acid, along with various phenolic compounds such as the phenolic acid ferulic acid; the flavonoids myricetin, epicatechin, and catechin; and polyphenol ellagic acid (Nishi et al., 2021). Moringa at a dose of 560 mg/kg was efficient in lowering CRP levels in pregnant rats in the Toxoplasma model. This research is in line with Abdel-Daim et al. (2020), who reported that giving Moringa oleifera at a dose of 400 mg/kg revealed significant drops in CRP levels in rats exposed to cobalt chloride (CoCl2) (Kou et al., 2018). At high concentrations, M. oleifera extract can directly destroy T. gondii tachyzoites by upsetting the homeostasis of the parasite and releasing NO cellular substances, which are reactive free radicals produced by multiple immune systems and involved in intracellular inactivation processes (Nishi et al., 2021). When administered to pregnant rats in the Toxoplasma model, an ethanol-based extract of moringa leaves (560 mg/kg) can lower E-Selectin levels and even reach close to normal mouse levels. This proves that Moringa leaves have anti-inflammatory effect. This work contradicts a 2019 study by Sumandjar et al. (2019) that found no correlation between the delivery of the Moringa oleifera fraction and the ability of mice given a sepsis model to prevent cell damage by examining E-Selectin expression. Giving the M. oleifera fraction could reduce E-Selectin expression because it is an anti-inflammatory (Sumandjar et al., 2019). The administration of an ethanol-based extract of Moringa leaves at a dose of 560 mg/kg effectively reduced caspase-3 levels. This is similar to a study by Amara et al. (2021) that showed that M. oleifera extract has a protective effect on human neuroblastoma cells (SH-SY5Y) against oxidative damage by controlling the expression of vintage, namely the antioxidant proteins Nrf2 and HO-1, lowering the creation of reactive oxygen species (ROS), and re-establishing the mitochondrial respiratory complex’s activity (Amara et al., 2021). Moringa oleifera at a dose of 560 mg/kg in pregnant rats can improve necrosis in the T. gondii-infected placenta. This research is consistent with Gondo’s (2021) study, in which M. oleifera reduced the inflammatory reaction of plasma cytokines in pregnant rats with diabetes mellitus. Flavonoids in M. oleifera exhibit antioxidant and antiinflammatory properties (Gondo, 2021). This study proved that a single therapy of Moringa leaf ethanol-based extract at a dose of 560 mg/kg yielded valuable outcomes in repairing the heart of the toxoplasmosis rat model. This is consistent with studies conducted by Fenty Alia et al. (2022) that M. oleifera can protect cardiac contractility and cardiac structure from damage and prevent hypertension and endothelial dysfunction. Moringa oleifera has phytochemical compounds as a protective agent against cardiac damage and vascular dysfunction in the cardiovascular disease model, and MO has been suggested as anti-apoptosis, improving cardiac contractility and protecting cardiac structural integrity from damage. Administering methanol extract from Moringa leaves at doses of 200 and 400 mg/kg for 49 days improved high-density lipoprotein (HDL) and reduced body weight, triglycerides, atherogenic index, total cholesterol, and LDL because the leaves contain alkaloids, tannins, flavonoids, terpenoids, and steroids (Nahar et al., 2016). Giving Moringa leaf ethanol extract can prevent intracellular tachyzoites from entering and multiplying, and prevent the release of NO cellular stress chemicals (Dubey et al., 1997). In this study, administration of an ethanol-based extract of Moringa leaves at a dose of 560 mg/kg showed the presence of gliosis but did not result in necrosis of neurons and glia. It is believed that glial cells play a vital part in protecting the host against T. gondii by distributing cytokines, including IL-6, IL-1, IL-10, GM-CSFM, chemotactic cytokines, and IFN gamma (El-Kady et al., 2022). ConclusionThis study showed that Moringa leaves at a dose of 560 mg/kg in pregnant rats of the toxoplasmosis model showed improvement in IgG, IgM, E-Selectin, NFkB, repair of placental necrosis, heart, and brain of the fetus in the toxoplasmosis model rats. In comparison, doses of 280 mg/kg showed improvement in CRP, MDA, and caspase-3, so it can be concluded that M. oleifera can be an alternative therapy and has an antiinflammation, antiapoptosis, antiparasitic, and antinecrosis effect in pregnant rats with toxoplasmosis models. AcknowledgmentsWe hereby extend sincere gratitude to the Ministry of Education, Culture, Research, and Technology, the Central Laboratory of Food and Nutrition Studies (PAU) Gadjah Mada University Yogyakarta, the Centre for Veterinary Research Bogor, the Research and Development Center for Traditional Medicinal Plants Tawangmangu. Conflict of interestThe authors declare that they have no conflicts of interest. FundingScholarship provided by the Anatomical Pathology Laboratory, Faculty of Medicine, Universitas Sebelas Maret for funding and helping complete this work. Authors’ contributionsHN, S, BP, DI, S, AS, DTS, FE: Conceptualized and designed the study: HN, BP, DI, S, SSP: Data collection; HN, FE, DTS: Data analysis and data review for critical intellectual content. All authors have read and approved the final manuscript. Data availabilityAll information supporting the discoveries of this consideration is accessible inside the manuscript. ReferencesAbdel-Daim, M.M., Khalil, S.R., Awad, A. Zeid, E.H.A., El-Aziz, R.A and El-Serehy, H.A. 2020. Ethanolic extract of Moringa oleifera Lam. pp: 1–20. Aguirre, A.A., Longcore, T., Barbieri, M., Dabritz, H., Hill, D., Klein, P.N., Lepczyk, C., Lilly, E.L., McLeod, R., Milcarsky, J., Murphy, C.E., Su, C., VanWormer, E., Yolken, R. and Sizemore, G.C. 2019. The one health approach to toxoplasmosis: epidemiology, control, and prevention strategies. EcoHealth 16(2), 378–390; doi:10.1007/s10393-019-01405-7. Alia, F., Putri, M., Anggraeni, N. and Syamsunarno, M.R.A.A. 2022. The potency of Moringa oleifera Lam. as protective agent in cardiac damage and vascular dysfunction. Front. Pharmacol. 12, 1–18, doi:10.3389/fphar.2021.724439. Amara, I., Ontario, M.L., Scuto, M., Lo Dico, G.M., Sciuto, S., Greco, V., Abid-Essefi, S., Signorile, A., Salinaro, A.T. and Calabrese, V. 2021. Moringa oleifera protects SH-SY5YCells from DEHP-induced endoplasmic reticulum stress and apoptosis. Antioxidants (Basel, Switzerland) 10(4), 532; doi:10.3390/antiox10040532. Charan, J. and Kantharia, N. 2013. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 4(4), 303–306; doi:10.4103/0976-500X.119726. Dincel, G.C., and Atmaca, H.T. 2016. Role of oxidative stress in the pathophysiology of Toxoplasma gondii infection. Int. J. Immunopathol. Pharmacol. 29(2), 226–240; doi:10.1177/0394632016638668. Dubey, J.P., Shen, S.K., Kwok, O.C.H. and Thulliez, P. 1997. Toxoplasmosis in rats (Rattus norvegicus): congenital transmission to first and second generation offspring and isolation of Toxoplasma gondii from seronegative rats. Parasitology 115(1): 9–14; doi:10.1017/S0031182097008950. Dwinata, I.M., Sutarga, I.M. and Damriyasa, I.M. 2016. The potential risk factors for toxoplasmosis in balinese pregnant women-Indonesia. Bali Med. J. 5(1), 130; doi:10.15562/bmj.v5i1.277. Ekawasti, F., Cahyaningsih, U., Dharmayanti, N.L.P.I., Sa’diah, S., Subekti, D.T., Azmi, Z. and Desem, M.I. 2021. Restriction fragment length polymorphism analysis of genes of virulent strain isolate of Toxoplasma gondii using enzyme DdeI. Int. J. One Health 7(2), 196–203. El-Kady, A.M., Al-Megrin, W.A.I., Abdel-Rahman, I.A.M., Sayed, E., Alshehri, E.A., Wakid, M.H., Baakdah, F.M., Mohamed, K., Elshazly, H., Alobaid, H.M., Qahl, S.H., Elshabrawy, H.A., and Younis, S.S. 2022. Ginger is a potential therapeutic for chronic toxoplasmosis. Pathogens (Basel, Switzerland) 11(7), 798; doi:10.3390/pathogens11070798. Gondo, H.K. 2021. Moringa leaf powder (Moringa oleifera) decrease of inflammation plasma cytokine of pregnant rats with diabetes mellitus. Open Access Maced. J. Med. Sci. 9, 1043–1046; doi:10.3889/oamjms.2021.7422. Hartati, S., Raharjo, S. and Widiyono, I. 2007. Studi Gambaran Histopatologis Hepar, Pulmo, Lien dan Otak serta Uji Serologis pada Tikus (Rattus norvegicus) yang diinfeksi Toxoplasma gondii. Stud. Gambaran Histopatologis Hepar, Pulmo, Lien dan Otak serta Uji Serol. pada Tikus (Rattus Nor. yang diinfeksi Toxoplasma gondii) 35(1), 9–15; doi:10.22146/jsv.29283. Islam, Z., Islam, S.M.R., Hossen, F., Mahtab-ul-Islam, K., Hasan, M.R. and Karim, R. 2021. Moringa oleifera is a prominent source of nutrients with potential health benefits. Int. J. Food Sci. 2021, 6627265; doi:10.1155/2021/6627265. Konstantinovic, N., Guegan, H., Stäjner, T., Belaz, S. and Robert-Gangneux, F. 2017. Treatment of toxoplasmosis: current options and future perspectives. Food Waterborne Parasitol. 15, e00036; doi:10.1016/j.fawpar.2019.e00036. Kou, X., Li, B., Olayanju, J.B., Drake, J.M. and Chen, N. 2018. Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients 10(3), 343. doi:10.3390/nu10030343. Lee, J., Choi, J.W., Han, H.Y., Kim, W.S., Song, H.Y., Byun, E.B., Byun, E.H., Lee, Y.H. and Yuk, J.M. 2020. 4-hydroxybenzaldehyde restricts the intracellular growth of Toxoplasma gondii by inducing SIRT1-mediated autophagy in macrophages. Korean J Parasitol 58(1), 7–14; doi:10.3347/kjp.2020.58.1.7. Leone, A., Spada, A., Battezzati, A., Schiraldi, J., Aristil, and Bertoli, S. 2015. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int. J. Mol. Sci. 16(6), 12791–12835; doi:10.3390/ijms160612791. Nahar, S., Faisal, F., Iqbal, J., Rahman, M. and Yusuf, M. 2016. Antiobesity activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Int. J. Basic Clin. Pharmacol. 2014, 1263–1268; doi:10.18203/2319-2003.ijbcp20162427. Nishi, L., Sanfelice, R.A.D.S., da Silva Bortoleti, B.T., Tomiotto-Pellissier, F., Silva, T.F., Evangelista, F.F., Lazarin-Bidóia, D., Costa, I.N., Pavanelli, W.R., Conchon Costa, I., Baptista, A.T.A., Bergamasco, R. and Falavigna-Guilherme, A.L. 2021. Moringa oleifera extract promotes apoptosis-like death in Toxoplasma gondii tachyzoites in vitro. Parasitology 148(12), 1447–1457; doi:10.1017/S0031182021001086. Pleyer, U., Gross, U., Schlüter, D., Wilking, H. and Seeber, F. 2019. Toxoplasmosis in Germany. Dtsch. Arztebl. Int. 116(25), 435–444, doi:10.3238/arztebl.2019.0435. Razis, A.F.A., Ibrahim, M.D. and Kntayya, S.B. 2014. Health benefits of Moringa oleifera. Asian Pacific J. Cancer Prev. 15(20), 8571–8576; doi:10.7314/APJCP.2014.15.20.8571. Retmanasari, A., Widartono, B.S., Wijayanti, M.A. and Artama, W.T. 2017. Prevalence and risk factors for toxoplasmosis in Middle Java, Indonesia. Ecohealth 14(1), 162–170; doi:10.1007/s10393-016-1198-5. Silva, M., Fung, R.K.F., Donnelly, C.B., Videira, P.A. and Sackstein, R. 2017. Cell-specific variation in E-selectin ligand expression among human peripheral blood mononuclear cells: implications for immunosurveillance and pathobiology. J. Immunol. 198(9), 3576–3587; doi:10.4049/jimmunol.1601636. Somsak, V., Borkaew, P., Klubsri, C., Dondee, K., Bootprom, P. and Saiphet, B. 2016. Antimalarial properties of aqueous crude extracts of Gynostemma pentaphyllum and Moringa oleifera Leaves in combination with artesunate in Plasmodium berghei -infected mice. J. Trop. Med. 2016, 8031392; doi:10.1155/2016/8031392. Sumandjar, T., Purwanto, B., Wasita, B., Indarto, D., Cilmiaty, R. and Widyaningsih, V. 2019. The ethyl acetate fraction of Moringa oleifera leaves effects on endothelial stress in rat sepsis model. Bali Med. J. 8(3), 940–943; doi:10.15562/bmj.v8i3.1679. Sutalangka, C., Wattanathorn, J., Muchimapura, S. and Thukham-Mee, W. 2013. Moringa oleifera mitigates memory impairment and neurodegeneration in animal model of age-related dementia. Oxid. Med. Cell. Longev. 2013, 695936; doi: 10.1155/2013/695936. Thái, T.L., Jun, H., Park, S.H., Lê, H.G., Lee, J., Ahn, S.K., Kang, J.M., Myint, M.K., Lin, K., Sohn, W.M., Nam, H.W., Na, B.K., and Kim, T.S. 2019. Seroprevalence of Toxoplasma gondii among school children in Pyin Oo Lwin and Naung Cho, Upper Myanmar. Korean J. Parasitol. 57(3), 303–308; doi:10.3347/kjp.2019.57.3.303. Vergara-Jimenez, M., Almatrafi, M.M. and Fernandez, M.L. 2017. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants, 6(4), 1–13; doi:10.3390/antiox6040091. | ||
| How to Cite this Article |
| Pubmed Style Nurinasari H, Ekawasti F, Sajidan S, Purwanto B, Indarto D, Soetrisno S, Syiva'a A, Subekti DT, Prihandani SS. Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancy. Open Vet. J.. 2025; 15(8): 3878-3887. doi:10.5455/OVJ.2025.v15.i8.54 Web Style Nurinasari H, Ekawasti F, Sajidan S, Purwanto B, Indarto D, Soetrisno S, Syiva'a A, Subekti DT, Prihandani SS. Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancy. https://www.openveterinaryjournal.com/?mno=242529 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.54 AMA (American Medical Association) Style Nurinasari H, Ekawasti F, Sajidan S, Purwanto B, Indarto D, Soetrisno S, Syiva'a A, Subekti DT, Prihandani SS. Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancy. Open Vet. J.. 2025; 15(8): 3878-3887. doi:10.5455/OVJ.2025.v15.i8.54 Vancouver/ICMJE Style Nurinasari H, Ekawasti F, Sajidan S, Purwanto B, Indarto D, Soetrisno S, Syiva'a A, Subekti DT, Prihandani SS. Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancy. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3878-3887. doi:10.5455/OVJ.2025.v15.i8.54 Harvard Style Nurinasari, H., Ekawasti, . F., Sajidan, . S., Purwanto, . B., Indarto, . D., Soetrisno, . S., Syiva'a, . A., Subekti, . D. T. & Prihandani, . S. S. (2025) Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancy. Open Vet. J., 15 (8), 3878-3887. doi:10.5455/OVJ.2025.v15.i8.54 Turabian Style Nurinasari, Hafi, Fitrine Ekawasti, Sajidan Sajidan, Bambang Purwanto, Dono Indarto, Soetrisno Soetrisno, Ariva Syiva'a, Didik Tulus Subekti, and Sri Suryatmiati Prihandani. 2025. Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancy. Open Veterinary Journal, 15 (8), 3878-3887. doi:10.5455/OVJ.2025.v15.i8.54 Chicago Style Nurinasari, Hafi, Fitrine Ekawasti, Sajidan Sajidan, Bambang Purwanto, Dono Indarto, Soetrisno Soetrisno, Ariva Syiva'a, Didik Tulus Subekti, and Sri Suryatmiati Prihandani. "Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancy." Open Veterinary Journal 15 (2025), 3878-3887. doi:10.5455/OVJ.2025.v15.i8.54 MLA (The Modern Language Association) Style Nurinasari, Hafi, Fitrine Ekawasti, Sajidan Sajidan, Bambang Purwanto, Dono Indarto, Soetrisno Soetrisno, Ariva Syiva'a, Didik Tulus Subekti, and Sri Suryatmiati Prihandani. "Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancy." Open Veterinary Journal 15.8 (2025), 3878-3887. Print. doi:10.5455/OVJ.2025.v15.i8.54 APA (American Psychological Association) Style Nurinasari, H., Ekawasti, . F., Sajidan, . S., Purwanto, . B., Indarto, . D., Soetrisno, . S., Syiva'a, . A., Subekti, . D. T. & Prihandani, . S. S. (2025) Anti-toxoplasma effect of Moringa oleifera leaves in a rat model of toxoplasmosis-induced pregnancy. Open Veterinary Journal, 15 (8), 3878-3887. doi:10.5455/OVJ.2025.v15.i8.54 |