| Research Article | ||
Open Vet. J.. 2025; 15(7): 3223-3230 Open Veterinary Journal, (2025), Vol. 15(7): 3223-3230 Research Article Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocksElisabetta Mondo1, Raffaele Scarpellini1*, Erika Esposito1, Mariana Roccaro2, Lara Pusinich3, Simone PierMatteo3 and Silvia Piva11Department of Veterinary Medical Sciences, University of Bologna, Bologna, Italy 2Department for Life Quality Studies, University of Bologna, Bologna, Italy 3Practitioner, Bologna, Italy *Corresponding Author: Raffaele Scarpellini. Department of Veterinary Medical Sciences, University of Bologna, Bologna, Italy. Email: raffaele.scarpellini [at] unibo.it Submitted: 10/02/2025 Revised: 30/04/2025 Accepted: 09/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
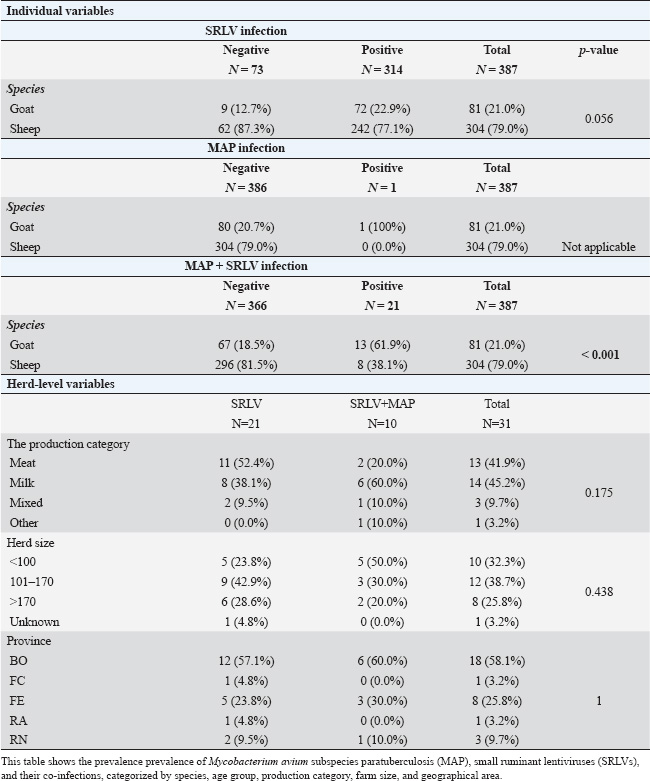
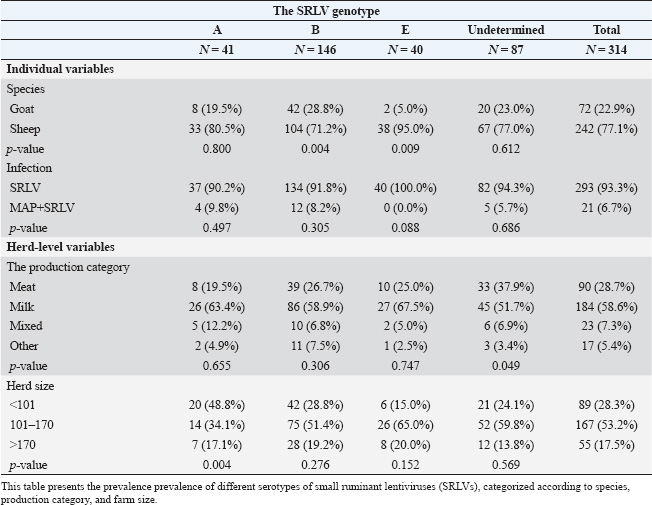
ABSTRACTBackground: Small ruminant lentiviruses (SRLVs) and Mycobacterium avium subsp. paratuberculosis (MAP) are major pathogens affecting sheep and goats, leading to significant economic losses and animal welfare concerns. Aim: To investigate the frequency of SRLV e MAP seropositivity in sheep and goats in the Emilia Romagna region through a comprehensive serological examination. Methods: A total of 387 sera (304 sheep and 81 goats) were subjected to enzyme-linked immunosorbent assay for the detection of antibodies against SRLV serotypes A, B, and E and for the detection of antibodies against MAP. Results: Serological analysis showed that 81.3% of the animals were positive for at least one causative agent, of which 93% were positive for SRLV, 0.3% for MAP, and 6.6% for SRLV-MAP co-infection. Furthermore, in both SRLV-only positive and SRLV-MAP co-infected samples, genotype B was the most representative (45.7%). Conclusion: The results of the study showed the presence of SRLV and paratuberculosis, as well as a prevalence of SRLV-MAP co-infection, for the first time in sheep and goats in the region, as well as the prevalence of SRLV-MAP co-infection, underlying the importance of implementing effective measures to control the spread of pathogens in small ruminants. Keywords: Small ruminant lentivirus (SRLV); Paratuberculosis; Co-infection; Sheep; Goat. IntroductionSmall ruminant lentiviruses (SRLVs) are a group of highly genetically diverse RNA viruses that infect monocytes and macrophages, causing chronic inflammation in multiple tissues (Blacklaws et al., 2012). The transmission of SRLV primarily occurs through the exposure of newborn lambs and kids to virion particles and infected cells present in colostrum and milk, or via respiratory secretions from the mother or nearby adults (Ramírez et al., 2013; Furtado et al., 2020). Additionally, SRLV can be transmitted directly to the fetus in utero or during birth (Stonos et al., 2017). Recent studies have shown that seropositivity to SRLV is a risk factor for Mycobacterium avium subsp. paratuberculosis (MAP) infection due to immunological and physiological interactions. SRLV infection causes chronic inflammation and immunosuppression, increasing susceptibility to MAP and impairing macrophage activity and cytokine dynamics (Cousins et al., 2002; Cecchi et al., 2019). MAP, the etiologic agent of paratuberculosis or Johne’s disease, affects domestic and wild ruminants, causing chronic granulomatous enteritis with tropism for macrophages in the ileum (Whittington et al., 2019). MAP infection develops in several stages, beginning with a subclinical phase in which infected animals shed MAP in feces, contributing to environmental contamination. As the disease progresses, clinical signs include chronic diarrhea, weight loss, and decreased food intake (Manning et al., 2001). SRLV infections, although also chronic and without obvious clinical signs, share with MAP a significant impact on animal health and the small ruminant industry, negatively affecting both animal welfare and economic aspects (Bakker et al., 2010). The economic impact of both pathogens, even in subclinical forms, results in reduced productivity and financial losses for farmers, whereas more severe clinical manifestations can lead to reduced milk and meat production and increased culling due to the synergistic effects of both pathogens (Arsenault et al., 2003; Martínez-Navalón et al., 2013; Windsor et al., 2015). Early diagnosis of MAP and SRLV infections is a major challenge for control and eradication: for SRLV, there are no “gold standard” tests or diagnostic protocols with good sensitivity and specificity; for MAP, diagnosis is based on isolation of the pathogen by fecal culture, a lengthy process due to the slow growth of the microorganism, resulting in difficulties in early detection and timely implementation of control measures, which in small ruminants are mainly based on prevention and biosecurity measures (Robbe-Austerman, 2011; Barquero et al., 2013; Kalogianni et al., 2021; Schaer et al., 2022). Biosecurity measures to control these pathogens are very important and include isolation and quarantine of new animals prior to introduction to the farm, segregation by age group, maintenance of staff and equipment hygiene to prevent cross-contamination, and strict isolation of infected animals to prevent transmission of the pathogen between animals (Reina et al., 2009; Serraino et al., 2013). Therefore, understanding the associated risk factors is crucial for the effective implementation of biosecurity plans. Therefore, understanding the associated risk factors is critical for the effective implementation of biosecurity plans. The aims of this study were: i) to investigate the seroprevalence of MAP and SRLV, with particular reference to its genotypes (A, B, E), among sheep and goats in the Emilia-Romagna region in Italy; ii) to estimate the herd-level prevalence correlated with MAP and SRLV infection. Materials and MethodsSample collectionSerum samples were collected individually from 31 sheep and goat farms located in the Emilia-Romagna region (Italy), randomly selected, and obtained during the internship activities of the Veterinary Medicine Degree Course of the University of Bologna, over the academic years 2016–2017, 2017–2018, and 2018–2019. Samplings were performed in concomitance with the health controls performed by the veterinarian authorized by the Local Health UnitAnimals were selected randomly on the basis of clinical examination characteristics and owner availability. As a result, there was no guarantee of uniformity between the different sampling sites. The individual serum samples were collected through the use of sterile needles with Vacutainer® systems to extract blood from the jugular vein. Subsequently, the collected samples were maintained at room temperature and transported to the laboratory, where they were subjected to centrifugation at 2,000 rpm for 15 minutes to isolate the serum component. The serum was then promptly frozen at −20°C (± 1°C) within Eppendorf® tubes to preserve it for later analysis. For each sampled animal, the following data were recorded: species (sheep/goat), age (<2, 2–4, 5–10, and >10 years). Herd-level variables were also recorded: productive category (milk, meat, mixed, other), herd size (<100 individuals; 101–170; >170), and province (Bologna, Ferrara, Rimini, Ravenna, Forlì-Cesena). Sample analysisTwo different indirect enzyme-linked immunosorbent assays tests were used: one to detect antibodies to SRLV genotypes A, B, and E (Eradikit™ SRLV Genotyping Kit, In3diagnostic, Grugliasco, Italy), and another to detect antibodies to MAP (ID Screen® Paratuberculosis Indirect Kit, Innovative Diagnostics, Grabels, France) (Se: 58.3%; sp: 99.3%). Results were read using an Infinite® F50 spectrophotometer and Magellan™ analysis software (Tecan, Männedorf, Switzerland). Interpretation of results was based on the cut-off values provided by the kit manufacturers. Statistical analysisA descriptive statistical analysis was performed. The apparent prevalence of both MAP and SRLV was calculated by dividing the number of positive sera for the total of sera tested in each farm. At the farm level, the apparent prevalence was calculated by dividing the number of farms with at least one positive serum sample for the total number of included farms. The association between the variables “species”, “age group”, “production category”, “farm size”, “farm geographical area”, and MAP positivity, SRLV positivity (in general and at genotype-level), SRLV-MAP co-infection was also assessed. In the analysis, sheep and goats were considered as one group for all variables except “species,” where they were considered as two separate groups. SRLV genotypes were not considered for the evaluation of the age group, as they represent an unchanging datum over time. Animals of unknown species, age, or farm herd size were not included in the respective analyses. The Chi-squared test or Fisher’s exact test (whenever expected frequencies were less than 5) was used to assess the presence of statistically significant differences in the number of co-infections among SRLV-positive patients, taking into account the variables mentioned above. Analyses were performed using Stata BE (v. 17.0) (StataCorp, Texas, USA). The significance level was set at p < 0.05. Ethical approvalThe farmers’ informed consent and the protocol approved by the Ethics Committee were formally signed by the University of Bologna as part of an agreement with the host companies. This agreement allowed educational traineeships to be conducted at their facilities, guaranteeing compliance with ethical regulations and adherence to the principles of transparency and collaboration with the companies involved. ResultsA total of 387 individual serum samples were collected from 31 farms. Sera comprise 304 (78.6%) sheep and 81 (20.9%) goats. Median age was 5 years (IQR 3–8 years). The median herd size was 152 animals (IQR 89–169). At the farm level, the apparent prevalence of SRLV was 67.7% (21/31) and that of MAP+SRLV was 32.3% (10/31). The majority of farms were sheep farms (21/31, 67.7%), followed by mixed farms (6/31, 19.4%) and goat farms (4/31, 12.9%). Productive category was milk farms (13/31, 41.9%), meat farms (14/31, 45.2%), mixed farms (3/31, 9.7%), and other farms (1/31, 3.2%). Overall, 336 of 387 (86.82%) animals were positive for at least one causative agent. Among these, apparent seroprevalence for MAP was 0.2 % (1/387) and 81.1% (314/387) for SRLV. Apparent cases of co-infection were 5.4% (21/387). The genotypic distribution of the 314 samples positive for SRLVs was as follows: 37 (90.2%) exhibited only the A genotype, 134 (91.8%) carried only the B genotype, and 40 (100.0%) were characterized only by the E genotype. In addition, 82 animals (94.3%) had an indeterminate result and were therefore likely to be infected with more than one genotype. Among the 21 co-infected samples, the distribution of SRLV genotypes was as follows: 4 (9.8%%) possessed the A genotype, 12 (8.2%) possessed the B genotype, and none exhibited the E genotype. Five subjects (5.7%) were positive for more than one genotype. Table 1 presents the percentage of animals positive for MAP, SRLV, and both infections according to the variables considered. Table 2 presents the percentage of animals positive for various SRLV serotypes according to the risk factors considered. Sheep were significantly more frequently infected with genotypes B (p-value=0.004) and E (p-value=0.009). Seropositivity to MAP, and hence co-infection, was significantly more common in goats than in sheep (p-value < 0.001). No significant differences were found in the SRLV genotypes involved in coinfections. At the herd level, no significant differences were found in the prevalence of coinfection according to a productive category and geographical area. However, it was observed that herd sizes <101 and 101–170 were significantly associated with a higher frequency of serotype A (p-value=0.004). DiscussionThis study provides an analysis of the prevalence of MAP, SRLV, and SRLV-MAP coinfection in 31 goat and sheep farms in the Emilia–Romagna region. The collected data indicate a high prevalence of SRLV infection in both sheep and goats, with a positivity rate of 77.1% in sheep and 22.9% in goats, a difference close to statistical significance (p=0.056). This difference between the two species agrees with a study conducted by Jacob-Ferreira et al. (2023) in Portugal, which reported a prevalence of 38.23% in sheep and 51.7% in goat flocks, but in contrast to data from Michiels et al. (2018) in Belgium, which reported a prevalence of 9% in sheep and 6% in goats. The reasons for the different results may depend on the animal management practices, flock densities, and environmental or climatic factors of the respective countries. A wide variety of SRLV prevalence is found in several studies in the literature that considered only one of the two species, ranging from 1.1% to 65% (Karanikolaou et al., 2005; Giangaspero et al., 2011; Agnello et al., 2012; Monika et al., 2017; Thomann et al., 2017; Cirone et al., 2019; Tavella et al., 2021). Differences in prevalence can be attributed to several factors, including geographical area, herd management practices, variability in species and breed susceptibility, and genetic diversity among animals (Leginagoikoa et al., 2006; Gjerset et al, 2009; Jacob-Ferreira et al., 2023). Indeed, Larruskain et al. (2013) suggested that breed genetic characteristics may play a significant role in influencing susceptibility or resistance to SRLV infection and disease progression. Furthermore, the different diagnostic techniques used in the studies, coupled with the existence of different SRLV genotypes (A–E) and their varying geographical distribution, add another layer of complexity when comparing data on a global scale (Pérez et al., 2010; Zhang et al., 2013; Heinrichs et al., 2017). Regarding the geographic distribution of the different genotypes, current information suggests a wide distribution of genotypes A and B in different regions of the world (Gomez-Lucia, 2018; Bazzucchi et al., 2021; Arcangeli et al., 2022). In particular, subtypes A3, A5, A8, A9, A11, A19, A20, A23, A24, B1, B2, and B3 are mainly circulated in Italy (Arcangeli et al., 2022). Genotypes C and D have been identified in more restricted geographical areas: genotype C has so far been identified only in Norway, and genotype D is currently considered limited to Spain and Switzerland (Minguijón et al., 2015; Bazzucchi et al., 2021; Arcangeli et al., 2022). Genotype E (subtypes E1–E2) is closely associated with Italian goats: subtype E1 is mainly associated with goats in northern Italy, whereas subtype E2 has been identified in goats in Sardinia and Umbria (Arcangeli et al., 2022; Carrozza et al., 2023). The serological analysis performed in our study showed that genotype B was the most prevalent genotype in both sheep (71.2%) and goats (28.8%), in line with previous national studies (Agnello et al., 2011; Bazzucchi et al., 2021; Arcangeli et al., 2022). To the best of our knowledge, this is the first time that genotype E has been reported in goats, suggesting potential interspecific transmission (Liapi et al., 2011; Iarussi et al., 2019), given that genotype E was found in two individuals, one of them from mixed farms. The study also assessed the prevalence of MAP in small ruminants. Currently, studies on these species are limited and inconsistent, both in terms of sample size and diagnostic techniques used. Our study showed that 5.7% of the 387 (304 sheep, 81 goats, and 2 not-defined) samples tested were positive for MAP. This result is consistent with previous data from the literature, which indicates that MAP seropositivity generally ranges from 5% to 20% (Attili et al., 2011; Liapi et al., 2012; Salgado et al., 2011; Stau et al., 2012; Kumthekar et al., 2013; Barrero et al., 2017; Galiero et al., 2017; Iarussi et al., 2019). However, it is important to note that the prevalence of seropositivity can vary significantly between geographical regions. For example, studies in Spain reported positivity in 22.5% of goats, whereas in Italy, the reported values ranged from 2.5% to 9.7% in sheep and goats (Stau et al., 2012; Barrero et al., 2017). The notable SRLV-MAP coinfection prevalence is a cause for concern. While sheep had a low coinfection rate (38.1%), goats had a much higher rate (61.9%). This divergence may indicate different susceptibility or transmission dynamics between the two species, but further studies are needed. Table 1. Prevalenceoff MAP, SRL, and coinfections.
Table 2. Prevalence of different SRLV serotypes.
Regarding the prevalence at the herd level, only the “between-herd” prevalence could be calculated because the samples were not fully representative. The observed prevalence was 67.7% for SRLV and 32.3% for coinfection. Although these results are not representative of the entire region, they provide a useful overview for a general assessment of the situation. The patterns observed can guide targeted control measures, inform management practices, and improve disease surveillance. However, further studies are needed to elucidate the mechanisms behind genotype-specific interactions, age-dependent susceptibility, and regional variations. In addition, longitudinal studies can provide insights into disease dynamics over time and the effectiveness of interventions. The major limitation of this study was the lack of a consistent distribution of animals in terms of age, production category, geographical area, and herd size. Additionally, the nature of the samplings (“convenience samplings”) did not allow a systematic selection of the animals. A further limitation concerns the absence of Se and Sp values for Eradikit, which precluded the possibility of performing an accurate analysis of the true prevalence. Despite these limitations, the collected data provide a good basis for understanding MAP, SRLV, and their interactions. ConclusionResearch focusing on SRLV and MAP co-infection in small ruminants remains limited, and even fewer studies have addressed the co-occurrence of infectious diseases of viral and bacterial origin. Although our study was limited by the small sample size and the heterogeneity in the selection of subjects, the preliminary results were remarkable. The epidemiological characteristics of the two pathogens, in agreement with the results of other studies, suggest associations with various factors, including animal-related aspects such as species involvement and management practices specific to Italian small ruminant production in Italy. By comprehensively analyzing these interrelated aspects, we aim to contribute to the development of more targeted and effective measures to safeguard the health and productivity of sheep and goats while addressing the complex challenges posed by these pathogens within the livestock industry. In conclusion, despite the inherent limitations of the study, due to the small sample size and the type of sampling, the results obtained provide a significant contribution to the prevalence of SRLV and MAP, as well as co-infection in small ruminants in the Emilia–Romagna region. Of particular interest is the detection of SRLV genotype E in a goat population, which was documented here for the first time. Although these results are preliminary, they provide a basis for further studies to understand the complex interactions between these pathogens and their impacts on sheep and goat flocks. In addition, the results can be used as a starting point for the development of biosecurity checklists aimed at controlling these diseases. AcknowledgmentsWe acknowledge the support and collaboration of the University of Bologna and the Local Health Unit Company (AUSL) of Bologna in carrying out this study. Special thanks to the participants who provided the serum samples and to the farm owners who allowed us to evaluate their control measures. Conflicts of interestThe authors declare that there are no conflicts of interest regarding the publication of this article. FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Authors’ contributionsConceptualization, S.P., Methodology, E.M., M.R., S.P., and L.P., Data Curation, E.M., M.R., R.S., Writing – Original Draft Preparation, E.M., Writing – Review & Editing, E.M., S.P., and R.S., Visualization, R.S., E.E., S.P., L.P., S.P., M.R., Supervision, S.P., Project Administration, S.P. Data availabilityThe data that support the findings of this study are available from the corresponding author upon request. Informed consent statementAll the information and samples were collected as part of a practice activity of the Veterinary Medicine Degree Course of University of Bologna, for which an agreement with the owner was established, including consent about any research or scientific publication of the data collected. ReferencesAgnello, S., Purpari, G., Lo Giudice, C., Campo, F., Chiaracane, G., Coniglio, A., Stancanelli, A., Feliziani F., Dara, S. and Guercio A. 2012. Artrite – Encefalite Caprina (CAE) in allevamenti da latte nel territorio siciliano. Large An. Rev. XX Congresso nazionale S.I.P.A.O.C. (Società Italiana di Patologia ed Allevamento degli ovini e dei caprini), 5, 68, 2018. Agnello, S., Stancanelli, A., Campo, F., Feliziani, F., Caracappa, S., Purpari, G. and Guercio A. 2011. Artrite-Encefalite Caprina (CAEV) una patologia emergente nel territorio siciliano: programma volontario di eradicazione. In Atti Istisan Congressi 11/C3 del IV Workshop Nazionale di Virologia Veterinaria. Arcangeli, C., Torricelli, M., Sebastiani, C., Lucarelli, D., Ciullo, M., Passamonti, F., Giammarioli, M. and Biagetti M. 2022. Genetic characterization of small ruminant lentiviruses (SRLVs) circulating in naturally infected sheep in Central Italy. Viruses 14(4), 686; doi:10.3390/v14040686. Arsenault, J., Dubreuil, P., Girard, C., Simard, C. and Bélanger, D. 2003. Maedi-visna impact on productivity in Quebec sheep flocks (Canada). Prev. Vet. Med. 59(3), 125–137; doi:10.1016/s0167-5877(03)00086-2. Attili, A.R., Victor, N.N., Silvia, P., Luciana, P., Anastasia, D. and Vincenzo, C. 2011. Ovine paratuberculosis: a seroprevalence study in dairy flocks reared in the marche region, Italy. Vet. Med. Int. 2011, 782875; doi:10.4061/2011/782875. Bakker, D. 2010. Paratuberculosis control measures in Europe. In Paratuberculosis: organism, disease, control. Eds., Bher M.A. and Collins D.M., Preston, UK: CAB Int, pp: 306–315. Barquero, N., Domenech, A., Arjona, A., Fernández-Garayzabal, J.F., Ruiz-Santa-Quiteria, J.A. and Gomez-Lucia, E. 2013. Comparison of two PCR and one ELISA techniques for the detection of small ruminant lentiviruses (SRLVs) in milk of sheep and goats. Res. Vet. Sci. 94(3), 817–819; doi:10.1016/j.rvsc.2013.01.004. Barrero Domínguez, B., Luque, I., Maldonado, A., Huerta, B., Sánchez, M., Gomez Laguna, J. and Astorga, R. 2017. Seroprevalence and risk factors of exposure to caprine arthritis-encephalitis virus in southern Spain. Vet. Rec. 180(9), 226; doi:10.1136/vr.104014. Bazzucchi, M., Pierini, I., Gobbi, P., Pirani, S., Torresi, C., Iscaro, C., Feliziani, F. and Giammarioli, M. 2021. Genomic epidemiology and heterogeneity of SRLV in Italy from 1998 to 2019. Viruses 13(12), 2338; doi:10.3390/v13122338. Blacklaws, B.A. 2012. Small ruminant lentiviruses: immunopathogenesis of visna- maedi and caprine arthritis and encephalitis virus. Comp. Immunol. Microbiol. Infect. Dis. 35(3), 259–269; doi:10.1016/j.cimid.2011.12.003. Carrozza, M.L., Niewiadomska, A.M., Mazzei, M., Abi-Said, M.R., Hué, S., Hughes, J., Gatseva, A. and Gifford, R.J. 2023. Emergence and pandemic spread of small ruminant lentiviruses. Virus Evol. 9(1), vead005; doi:10.1093/ve/vead005. Cecchi, F., Fratini, F., Cerri, D., Bandecchi, P., Cantile, C. and Mazzei, M. 2019. Small ruminant lentivirus and Mycobacterium avium subsp. paratuberculosis: coinfection prevalence and preliminary investigation on genetic resistance to both infections in a Garfagnina goat flock. Large An. Rev. 25, 89–92. Cirone, F., Maggiolino, A., Cirilli, M., Sposato, A., De Palo, P., Ciappetta, G. and Pratelli, A. 2019. Small ruminant lentiviruses in goats in Southern Italy: serological evidence, 138 risk factors and implementation of control programs. Vet. Microbiol. 228, 143–146; doi:10.1016/j.vetmic.2018.11.023. Cousins, D., Condron, R., Eamens, G., Whittington, R. and De Lisle, G. 2002. Paratuberculosis (Johne’s disease). Aust. North Z. Stand. Diagn. Proced. 1, 1–21. Furtado Araújo, J., Andrioli, A., Pinheiro, R.R., Sider, L.H., de Sousa, A.L.M., de Azevedo, D.A.A., Peixoto, R.M., Lima, A.M.C., Damasceno, E.M., Souza, S.C.R. and Teixeira, M.F.D.S. 2020. Vertical transmissibility of small ruminant lentivirus. PLoS One 15(11), e0239916; doi:10.1371/journal.pone.0239916. Galiero, A., Fratini, F., Bravi, P., Turchi, B., Casanovi, E. and Cerri, D. 2017. Serological survey of paratuberculosis in dairy cattle in Garfagnana (Tuscany). J. Hellenic Vet. Med. Soc. 68(4), 641–646. Giammarioli, M., Bazzucchi, M., Puggioni, G., Brajon, G., Dei Giudici, S., Taccori, F., Feliziani, F. and De Mia, G.M. 2011. Phylogenetic analysis of small ruminant lentivirus (SRLV) in Italian flocks reveals the existence of novel genetic subtypes. Virus Genes 43(3), 380–384; doi:10.1007/s11262-011-0653-1. Giangaspero, M., Osawa, T., Orusa, R., Frossard, J.P., Naidu, B., Robetto, S., Tatami, S., Takagi, E., Moriya, H., Okura, N., Kato, K., Kimura, A. and Harasawa, R. 2011. Epidemiological survey of visna-maedi in sheep in northern prefectures of Japan. Vet. Ital. 47(4), 437–451. Gjerset, B., Rimstad, E., Teige, J., Soetaert, K., and Jonassen, C.M. 2009. Impact of natural sheep-goat transmission on the detection and control of small ruminant lentivirus group C infections. Vet. Microbiol. 135(3–4), 231–238; doi:10.1016/j.vetmic.2008.09.069. Gomez-Lucia, E., Barquero, N. and Domenech, A. 2018. Maedi-Visna virus: current perspectives. Vet. Med (Auckl). 9, 11–21; doi:10.2147/VMRR.S136705. Heinrichs, R., Wilkins, W., Schroeder, G. and Campbell, J. 2017. Brief communication communication brève: prevalence of Maedi–visna in Saskatchewan sheep. Can. Vet. J. 8, 183–186. Iarussi F., Paradies P., Sardaro R., Rubino G., Scaltrito D., Pieragostini E. and Petazzi F. 2019. Epidemiology and risk factors of Mycobacterium avium subspecies paratuberculosis in semi-extensive dairy sheep and goat farms of Apulia. Small Ruminant Res. 177, 89–96. Jacob-Ferreira, J., Coelho, A.C., Grau Vila, A., Lacasta, D. and Quintas, H. 2023. Small ruminant lentivirus infection in sheep and goats in North Portugal: seroprevalence and risk factors. Pathogens 12(6), 829; doi:10.3390/pathogens12060829. Kalogianni, A.I., Stavropoulos, I., Chaintoutis, S.C., Bossis, I. and Gelasakis, A.I. 2021. Serological, molecular and culture-based diagnosis of lentiviral infections in small ruminants. Viruses 13(9), 1711; doi:10.3390/v13091711. Karanikolaou, K., Angelopoulou, K., Papanastasopoulou, M., Koumpati-Artopiou, M., Papadopoulos, O. and Koptopoulos, G. 2005. Detection of small ruminant lentiviruses by PCR and serology tests in field samples of animals from Greece. Small Rumin. Res. 58, 181–187; doi:10.1016/j.smallrumres.2005.10.001. Kumthekar, S., Manning, E.J., Ghosh, P., Tiwari, K., Sharma, R.N. and Hariharan, H. 2013. Mycobacterium avium subspecies paratuberculosis was confirmed following serological surveillance of small ruminants in Grenada, West Indies. J. Vet. Diagn. Invest. 25(4), 527–530; doi:10.1177/1040638713490688. Larruskain, A. and Jugo, B.M. 2013. Retroviral infections in sheep and goats: small ruminant lentiviruses and host interaction. Viruses 5(8), 2043–2061; doi:10.3390/v5082043. Leginagoikoa, I., Juste, R.A., Barandika, J., Amorena, B., De Andrés, D., Luján, L., Badiola, J. and Berriatua, E. 2006. Extensive rearing hinders Maedi-Visna Virus (MVV) infection in sheep. Vet. Res. 37(6), 767–778; doi:10.1051/vetres:2006034. Liapi, M., Leontides, L., Kostoulas, P., Botsaris, G., Iacovou, Y., Rees, C., Georgiou, K., Smith, G.C. and Naseby, D.C. 2011. Bayesian estimation of the true prevalence of Mycobacterium avium subsp. paratuberculosis infection in Cypriot dairy sheep and goat flocks. Small Rumin. Res. 95, 174–178. Manning, E.J. and Collins, M.T. 2001. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. 20(1), 133–150; doi:10.20506/rst.20.1.1275. Maresca, C., Giorgi, G., Feliziani, F., Colini, E., Battistacci, L. and Moscati, L. 1999. Indagine epidemiologica sulla paratubercolosi ovina nel territorio di una A.S.L. della regione Umbria. Large An. Rev. 5(4), 71–74. Martínez-Navalón, B., Peris, C., Gómez, E.A., Peris, B., Roche, M.L., Caballero, C., Goyena, E. and Berriatua, E. 2013. Quantitative estimation of the impact of caprine arthritis encephalitis virus infection on milk production by dairy goats. Vet. J. 197(2), 311–317; doi:10.1016/j.tvjl.2012.12.020. Michiels, R., Van Mael, E., Quinet, C., Welby, S., Cay, A.B. and De Regge, N. 2018. Seroprevalence and risk factors related to small ruminant lentivirus infections in Belgian sheep and goats. Prev. Vet. Med. 151, 13–20; doi:10.1016/j.prevetmed.2017.12.014. Minguijón, E., Reina, R., Pérez, M., Polledo, L., Villoria, M., Ramírez, H., Leginagoikoa, I., Badiola, J.J. and García-Marín, J.F., de Andrés, D., Luján, L., Amorena, B., Juste, R.A. 2015. Small ruminant lentivirus infections and diseases. Vet. Microbiol. 181(1–2), 75–89; doi:10.1016/j.vetmic.2015.08.007. Monika, O., Osiński Z. and Kuźmak J. 2017. Bayesian estimation of seroprevalence of small ruminant lentiviruses in sheep from Poland. Prev. Vet. Med. 147, 66–78; doi:10.1016/j.prevetmed.2017.09.001. Pérez, M., Biescas, E., de Andrés, X., Leginagoikoa, I., Salazar, E., Berriatua, E., Reina, R., Bolea, R., de Andrés, D., Juste, R.A., Cancer, J., Gracia, J., Amorena, B., Badiola, J.J. and Luján L. 2010. Visna/maedi virus serology in sheep: survey, risk factors and implementation of a successful control programme in Aragón (Spain). Vet. J. 186(2), 221–225; doi:10.1016/j.tvjl.2009.07.031 Ramírez, H., Reina, R., Amorena, B., de Andrés, D., Martínez, H.A. 2013. Small ruminant lentiviruses: genetic variability, tropism and diagnosis. Viruses. 5(4), 1175–1207; doi:10.3390/v5041175 Reina, R., Berriatua, E., Luján, L., Juste, R., Sánchez, A., de Andrés, D. and Amorena, B. 2009. Prevention strategies against small ruminant lentiviruses: an update. Vet. J. 182(1), 31–37; doi:10.1016/j.tvjl.2008.05.008. Robbe-Austerman, S. 2011. Control of paratuberculosis in small ruminants. Vet. Clin. North. Am. Food. Anim. Pract. 27(3), 609–620, vii; doi:10.1016/j.cvfa.2011.07.007. PMID: 22023839. Salgado, M., Collins, M.T., Salazar, F., Kruze, J., Bölske, G., Söderlund, R., Juste, R., Sevilla, I.A., Biet, F., Troncoso, F. and Alfaro, M. 2011. Fate of Mycobacterium avium subsp. paratuberculosis after application of contaminated dairy cattle manure to agricultural soils. Appl. Environ. Microbiol. 77(6), 2122–2129; doi:10.1128/AEM.02103-10. Schaer, J., Cvetnic, Z., Sukalic, T., Dörig, S., Grisiger, M., Iscaro, C., Feliziani, F., Pfeifer, F., Origgi, F., Zanoni, R.G. and Abril, C.E. 2022. Evaluation of serological methods and a new real-time nested PCR for small ruminant lentiviruses. Pathogens. 11(2), 129; doi:10.3390/pathogens11020129. Sardaro, R., Pieragostini, E., Rubino, G. and Petazzi,F. 2017. Impact of Mycobacterium aviumsubspecies paratuberculosis on profit efficiencyin semi-extensive dairy sheep and goat farms ofApulia, southern Italy. Prev. Vet. Med. 136, 56–64;doi:10.1016/j.prevetmed.2016.11.013. Serraino, A., Ostanello, F., Giacometti, F., Marchetti, G., Arrigoni, N., Ricchi, M., Bonilauri, P. and Zanirato, G. 2013. Paratubercolosi nel Sud Italia: presenza negli allevamenti e valutazione del rischio. Budrio, Bologna: Litografia SAB, pp: 5–48. Stau, A., Seelig, B., Walter, D., Schroeder, C. and Ganter, M. 2012. Seroprevalence of Mycobacterium avium subsp. paratuberculosis in small ruminants in Germany. Small Rumin. Res. 105, 361–365. Stonos, N., Bauman, C., Menzies, P., Wootton, S.K. and Karrow, N.A. 2017. Prevalence of small ruminant lentivirus and Mycobacterium avium subsp. paratuberculosis co-infection in Ontario dairy sheep and dairy goats. Can. J. Vet. Res. 81(2), 155–159. Tavella, A., Capello, K., Bertoni, G. and Bettini, A. 2021. Risk factors associated with the alpine multispecies farming system in the eradication of CAEVs in South Tyrol, Italy. Viruses 13, 1959; doi:10.3390/v13101959. Thomann, B., Falzon, L.C., Bertoni, G., Vogt, H.R., Schüpbach-Regula, G. and Magouras, I. 2017. A census to determine the prevalence and risk factors of caprine arthritis-encephalitis virus and Visna/Maedi virus in the Swiss goat population. Prev. Vet. Med. 137, 52–58; doi:10.1016/j.prevetmed.2016.12.012. Whittington, R., Donat, K., Weber, M.F., Kelton, D., Nielsen, S.S., Eisenberg, S., Arrigoni, N., Juste, R., Sáez, J.L., Dhand, N., Santi, A., Michel, A., Barkema, H., Kralik, P., Kostoulas, P., Citer, L., Griffin, F., Barwell, R., Moreira, M.A.S., Slana, I., Koehler, H., Singh, S.V., Yoo, H.S., Chávez-Gris, G., Goodridge, A., Ocepek, M., Garrido, J., Stevenson, K., Collins, M., Alonso, B., Cirone, K., Paolicchi, F., Gavey, L., Rahman, M.T., de Marchin, E., Van Praet, W., Bauman, C., Fecteau, G., McKenna, S., Salgado, M., Fernández-Silva, J., Dziedzinska, R., Echeverría, G., Seppänen, J., Thibault, V., Fridriksdottir, V., Derakhshandeh, A., Haghkhah, M., Ruocco, L., Kawaji, S., Momotani, E., Heuer, C., Norton, S., Cadmus, S., Agdestein, A., Kampen, A., Szteyn, J., Frössling, J., Schwan, E., Caldow, G., Strain, S., Carter, M., Wells, S., Munyeme, M., Wolf, R., Gurung, R., Verdugo, C., Fourichon, C., Yamamoto, T., Thapaliya, S., Di Labio, E., Ekgatat, M., Gil, A., Alesandre, A.N., Piaggio, J., Suanes, A. and de Waard J.H. 2019. Control of paratuberculosis: who, why and how. A review of 48 countries. BMC Vet. Res. 15(1), 198; doi:10.1186/s12917-019-1943-4. Windsor, P.A. 2015. Paratuberculosis in sheep and goats. Vet. Microbiol. 181(1–2), 161-169; doi:10.1016/j.vetmic.2015.07.019. Zhang, K.S., He, J.J., Liu, Y.J., Shang, Y.J. and Liu X.T. 2013. A seroprevalence survey of maedi-visna among twenty-four ovine flocks from twelve regions of China. J. Integr. Agric. 12(12), 2321–2323. | ||
| How to Cite this Article |
| Pubmed Style Mondo E, Scarpellini R, Esposito E, Roccaro M, Pusinich L, Piermatteo S, Piva S. Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocks. Open Vet. J.. 2025; 15(7): 3223-3230. doi:10.5455/OVJ.2025.v15.i7.33 Web Style Mondo E, Scarpellini R, Esposito E, Roccaro M, Pusinich L, Piermatteo S, Piva S. Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocks. https://www.openveterinaryjournal.com/?mno=242136 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i7.33 AMA (American Medical Association) Style Mondo E, Scarpellini R, Esposito E, Roccaro M, Pusinich L, Piermatteo S, Piva S. Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocks. Open Vet. J.. 2025; 15(7): 3223-3230. doi:10.5455/OVJ.2025.v15.i7.33 Vancouver/ICMJE Style Mondo E, Scarpellini R, Esposito E, Roccaro M, Pusinich L, Piermatteo S, Piva S. Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocks. Open Vet. J.. (2025), [cited January 25, 2026]; 15(7): 3223-3230. doi:10.5455/OVJ.2025.v15.i7.33 Harvard Style Mondo, E., Scarpellini, . R., Esposito, . E., Roccaro, . M., Pusinich, . L., Piermatteo, . S. & Piva, . S. (2025) Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocks. Open Vet. J., 15 (7), 3223-3230. doi:10.5455/OVJ.2025.v15.i7.33 Turabian Style Mondo, Elisabetta, Raffaele Scarpellini, Erika Esposito, Mariana Roccaro, Lara Pusinich, Simone Piermatteo, and Silvia Piva. 2025. Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocks. Open Veterinary Journal, 15 (7), 3223-3230. doi:10.5455/OVJ.2025.v15.i7.33 Chicago Style Mondo, Elisabetta, Raffaele Scarpellini, Erika Esposito, Mariana Roccaro, Lara Pusinich, Simone Piermatteo, and Silvia Piva. "Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocks." Open Veterinary Journal 15 (2025), 3223-3230. doi:10.5455/OVJ.2025.v15.i7.33 MLA (The Modern Language Association) Style Mondo, Elisabetta, Raffaele Scarpellini, Erika Esposito, Mariana Roccaro, Lara Pusinich, Simone Piermatteo, and Silvia Piva. "Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocks." Open Veterinary Journal 15.7 (2025), 3223-3230. Print. doi:10.5455/OVJ.2025.v15.i7.33 APA (American Psychological Association) Style Mondo, E., Scarpellini, . R., Esposito, . E., Roccaro, . M., Pusinich, . L., Piermatteo, . S. & Piva, . S. (2025) Preliminary study on paratuberculosis, small ruminant lentivirus, and co-infection in Emilia Romagna flocks. Open Veterinary Journal, 15 (7), 3223-3230. doi:10.5455/OVJ.2025.v15.i7.33 |