| Research Article | ||
Open Vet. J.. 2025; 15(4): 1784-1789 Open Veterinary Journal, (2025), Vol. 15(4): 1784-1789 Research Article Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsiaNutria Widya Purna Anggraini1,2*, Sri Sulistyowati2, Soetrisno Soetrisno1, Bambang Purwanto1,3 and Paramasari Dirgahayu1,41Doctoral Program of Medical Sciences Department, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 2Obstetric and Gynecology Department, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 3Internal Medicine Department, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 4Department of Parasitology and Mycology, Faculty of Medicine, Universitas Sebelas Maret, Surakartan, Indonesia *Corresponding Author: Nutria Widya Purna Anggraini. Doctoral Program of Medical Sciences, Department, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia. Email: nutria_dr [at] staff.uns.ac.id; elis_spog [at] staff.uns.ac.id Submitted: 10/02/2025 Accepted: 22/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
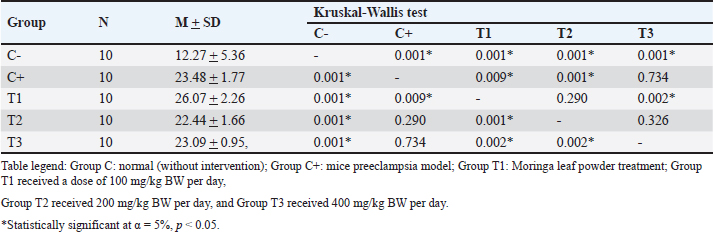
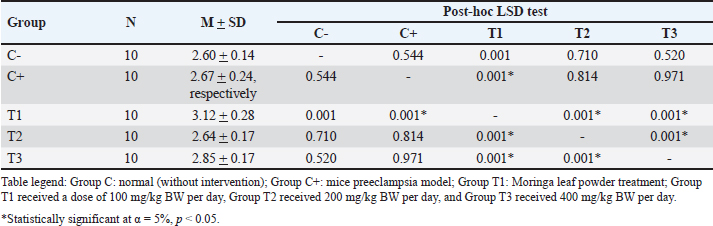
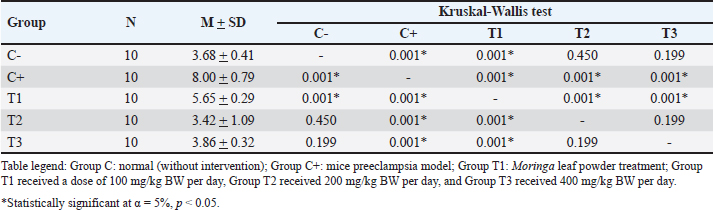
AbstractBackground: Preeclampsia is a serious pregnancy complication characterized by high blood pressure and organ dysfunction, primarily affecting the placenta and kidneys. Reduced placental blood flow can have severe consequences for both the mother and fetus, highlighting the need for effective treatment options. Moringa oleifera leaves, which are known for their potent antioxidant and anti-inflammatory properties, have been explored as a potential intervention to mitigate oxidative stress associated with preeclampsia. Aim: This study aimed to determine the effect of M. oleifera Leaves on C-reactive protein (CRP), Malondialdehyde (MDA), and albuminuria in a mouse model of preeclampsia. Methods: This post controlled experimental study used a preeclampsia-induced mouse model. Female Mus musculus were assigned to five groups: (C0) negative control, (C+) positive control, (T1) treatment group 1 receiving 100 mg/kg BW/day of M. oleifera extract, (T2) treatment group 2 receiving 200 mg/kg BW/day, and (T3) treatment group 3 receiving 400 mg/kg BW/day. The extract was administered orally via gavage for 16 consecutive days, after which the mice were sacrificed. MDA, CRP, and albuminuria levels were measured using ELISA. Results: Moringa oleifera supplementation significantly reduced MDA levels, with the lowest levels observed in the T3 group (22.44 ± 1.66). The T2 group exhibited the lowest CRP levels (2.64 ± 0.17), and albumiuria lowest level was observed in the T2 group (3.42 ± 1.09). Conclusion: Moringa oleifera administration effectively reduced MDA, CRP, and albuminuria levels. As an antioxidant and anti-inflammatory agent, M. oleifera helps suppress inflammation by lowering CRP levels, inhibiting lipid peroxidation to reduce MDA, and improving symptoms of preeclampsia, as evidenced by decreased albuminuria. These findings suggest that incorporating M. oleifera into dietary regimens may have therapeutic effects against preeclampsia-associated symptoms. Keywords: Albuminuria, CRP, MDA, Moringa oleifera, Preeclampsia. IntroductionPreeclampsia is a pregnancy-related disorder characterized by high blood pressure and organ damage, primarily affecting the kidneys. It typically develops after 20 weeks of gestation (Mergiaw, 2010). This condition poses significant risks to both the mother and the developing fetus, requiring effective management strategies to reduce complications and improve health outcomes (Batmomolin, 2020). Preeclampsia, which arises from improper implantation and poor placentation, can result in restricted blood flow to the placenta, leading to placental hypoxia (Torres, 2024). The resulting hypoxic state triggers an inflammatory response, exacerbating the condition by increasing inflammatory markers, such as C-reactive protein (CRP) and malondialdehyde (MDA), which contribute to the progression of albuminuria and kidney damage (Nirupam, 2021). Understanding the underlying mechanisms of preeclampsia is crucial for developing targeted therapies that can prevent or alleviate its adverse effects on maternal and fetal health (Rana, 2019). Until recently, the predominant treatment for preeclampsia has been termination of pregnancy. Despite the development of numerous treatment protocols, the mortality and morbidity associated with preeclampsia remain significant. Consequently, there is an ongoing need for research to identify alternative therapeutic interventions that are readily accessible and can be sourced from the local environment, such as Moringa oleivera leaves, with the aim of ameliorating preeclampsia. Moringa oleifera is a nutrient-dense plant that has garnered attention for its potential therapeutic properties, particularly in managing conditions like preeclampsia due to its anti-inflammatory and antioxidant effects. Moringa contains flavonoids such as Luteolin and Quercetin. Moringa oleifera has been extensively studied for its ability to reduce oxidative stress and inflammation. These compounds may help mitigate the adverse effects associated with preeclampsia by promoting vascular health and improving renal function, thereby offering a promising natural approach to supporting maternal well-being during pregnancy. The role of luteolin in preeclampsia is particularly noteworthy, as it has been found to enhance endothelial function (Ożarowski, 2021) and regulate blood pressure, which are critical factors in managing this condition. Quercetin as an antioxidant plays a role in improving the oxidative effects of stress and also reducing proinflammatory cytokines (Vergara 2017; Herman, 2024). As postulated by various studies (Jones, Smith and Lee, 2020; Lee, Smith and Jones, 2019), Moringa leaves are a rich source of amino acids and minerals. In addition to flavonoids and phenols (Green et al., 2017), Moringa contains Mg (Kang et al., 2018), which has been shown to improve preeclampsia by lowering blood pressure (Zhao et al., 2021). A study by Tinny and Dian (2016) demonstrated that administering the methanol extract of Moringa leaves from the NTT variant influenced serum TNF-α and IL-6 levels, as well as colon tissue MDA levels in DMBA-induced Wistar rats. The effective dose for reducing TNF-α and MDA levels was 20 mg/kg BW/day, whereas IL-6 levels also declined at the same dose, although not significantly (Tinny, 2016). These results align with Prasetio’s study, which highlighted Moringa’s effectiveness in lowering MDA levels in Wistar rats subjected to physical exercise (Prasetio, 2022). Recent findings indicate that ethanolic extracts of M. oleifera, particularly those prepared with 50%–70% ethanol, effectively suppress the secretion of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, CRP, IL-23, IL-12, and IL-10, all of which play key roles in chronic inflammation. A study by Herman-Lara et al. (2024) demonstrated that 700% ethanol extract exhibited strong anti-inflammatory properties, reinforcing its potential as a therapeutic agent for inflammatory conditions. Additionally, research by Subandi et al. (2024) supports these findings, showing that M. oleifera effectively reduces CRP levels, further emphasizing its anti-inflammatory potential (Oliveira, 2024; Subandi 2024) Li et al. (2020) reported the results of research on albuminuria levels in urine specimens in LPS-infused preeclampsia mice that were improved by quercetin treatment significantly (Li q, 2020). Research by Ahmad et al. (2023) reported that M. oleifera exhibits renal protective effects in diabetic rats, potentially reducing albuminuria by inhibiting protein glycation, which is a contributing factor to kidney damage in diabetes (Ahmad, 2023). Studies conducted by Akpan et al. (2018) showed that treatment with M. oleifera can help prevent microalbuminuria, a precursor of diabetic nephropathy, by lowering blood glucose levels and improving kidney function, thereby potentially reducing progression to macroalbuminuria and end-stage renal disease. (Akpan, 2018). Materials and MethodsAnimal model selection The subjects of this study were healthy female Swiss Musculus mice aged 3 months and weighing 20–25 g. The mice were chosen based on the following characteristics: fine fur, clear eyes, no physical defects, or scars, and no previous use in other studies. The exclusion criteria for the study are as follows: the presence of anatomical abnormalities that appear macroscopic. Mice that appear sick, inactive, and/or suffering from diarrhoea., Mice that have died during the study. A total of 50 female mice were divided into 5 treatment groups as follows: C: Normal pregnant mice (negative control) C+: Preeclampsia mouse model injected with 10 ng of anti-Qa-2 on days 1-4 of pregnancy (positive control) (Ortega, et al. 2019; Quiles et al. 2020; Prasetio et al. 2022; Ożarowski et al. 2024; Ramírez-Tortosa et al. 2025). T1: Mice exposed to M. oleifera at 100 mg/kg BW/day. T2: Mice exposed to M. oleifera at 200 mg/kg BW/day. T3: Mice exposed to M. oleifera at 400 mg/kg BW/day. The dosages used in this study were based on previous research. Moringa oleifera ethanol extract was administered orally via gavage for 16 consecutive days, after which the mice were euthanized. MDA, CRP, and albuminuria levels were measured using the ELISA method. MDA, CRP, and Albuminuria ExaminationThe levels of MDA, CRP, and albuminuria were assessed using the Thio Barbituric Acid (TBA) method, and the results were analyzed via spectrophotometry to measure MDA compounds generated from lipid peroxidation and inflammation. The MDA ELISA Kit (MDA Assay Kit competitive ELISA, Abcam - ab238537) and the Urine Protein Assay Kit were used, with absorbance readings recorded at 450 nm using an ELISA Reader. Blood serum was mixed with cold phosphate-buffered saline and centrifuged at 3,000 rpm for 15 minutes. The resulting supernatant was combined with 15% trichloroacetic acid and 0.3% TBA solution in 0.25 N HCl, then incubated in an 80°C water bath for 15 minutes. After cooling, the solution was centrifuged again, and the absorbance was measured at 450 nm. Data analysisThe collected data on MDA, CRP, and albuminuria levels were compiled into distribution and frequency tables for each variable. A one-way ANOVA was conducted under the assumption of normality and homogeneity. In cases in which the data did not follow a normal distribution, an arc-sine transformation was applied. One-way ANOVA was used for normally distributed data, whereas the Kruskal-Wallis test was employed for nonparametric data. The present study incorporated statistical calculations with a significance level of 0.05, consistent with established methodologies (95% confidence interval). Ethical approvalEthical approval from the health research ethics committee of Dr Moewardi General Hospital no: 1.303/V/HREC/2024. ResultsThe study examined MDA, CRP, and albuminuria levels across five experimental groups, each with six replicates: C– (negative control), C+ (positive control), T1 (treatment 1), T2 (treatment 2), and T3 (treatment 3). Effects of treatment on MDA levelsNormality testing indicated a significance value >0.05 (sig. > 0.05), confirming a normal distribution across all groups. Additionally, the variance homogeneity test revealed a significance value above 0.05 (sig > 0.05), indicating equal variance. ELISA revealed an average MDA level of 12.27 ± 5.36 in the normal group (C–) and 23.48 ± 1.77 in the control group (C+). The Shapiro-Wilk normality test confirmed the normal distribution although data homogeneity was not observed. The Kruskal-Wallis test revealed significant differences in MDA levels between the groups (p=0.00, p < 0.05). The descriptive analysis indicated that the highest MDA levels were observed in the T1 group (26.07 ± 2.26), while the lowest was recorded in the T3 group (22.44 ± 1.66). Hypothesis testing confirmed that M. oleifera at a dosage of 400 mg/kg BW/day effectively reduced MDA levels, as described in Table 1. Effects of Treatment on CRP LevelsNormality and homogeneity tests verified that CRP data were normally distributed and homogeneous across groups. ELISA analysis showed an average CRP level of 2.60 ± 0.14 in the normal group (C–) and 2.67 ± 0.24 in the control group (C+). Post-hoc LSD testing revealed significant differences in CRP levels between groups (p=0.00, p < 0.05). Descriptive analysis showed that the T1 group had the highest CRP levels (3.12 ± 0.28), whereas the lowest CRP levels were observed in the T2 group (2.64 ± 0.17). Hypothesis testing confirmed that M. oleifera at a dosage of 200 mg/kg BW/day effectively reduced CRP levels, as described in Table 2. Effects of treatment on Albuminuria levelsThe normality test confirmed that albuminuria data followed a normal distribution, and the homogeneity of variance test indicated equal variance across groups. ELISA analysis showed an average albuminuria level of 3.68 ± 0.41 in the normal group (C–) and 8.00 ± 0.79 in the control group (C+). The Kruskal-Wallis test identified significant differences in albuminuria levels between groups (p=0.00, p < 0.05). The descriptive analysis revealed that the highest albuminuria levels were found in the C+ group (8.00 ± 0.79) and the lowest were observed in the T2 group (3.42 ± 1.09). Hypothesis testing confirmed that M. oleifera at a dosage of 200 mg/kg BW/day effectively reduced albuminuria levels, as described in Table 3. DiscussionMalondialdehydeMalondialdehyde, a byproduct of fatty acid oxidation, is commonly assessed in plasma and tissue samples to evaluate lipid peroxidation and oxidative stress (OS). In pregnancies complicated by intrauterine growth restriction (IUGR), elevated MDA and xanthine oxidase levels in placental tissue, umbilical cord plasma, and maternal blood indicate the significant role of OS in IUGR. A study by Ortega et al. (2019) further demonstrated increased levels of oxidative stress markers, including NOX1, NOX2, iNOS, PARP, and ERK, in the placental tissue of women with cardiovascular disease compared with healthy pregnant women, along with elevated plasma MDA levels (Ortega et al., 2019). Exposure to anti-Qa2 has been shown to increase MDA levels, with the highest levels recorded in the T1 treatment group (26.07 ± 2.26). However, supplementation with M. oleifera effectively reduced MDA levels induced by anti-Qa2 exposure, with the lowest levels observed in the T3 treatment group (22.44 ± 1.66, p < 0.05) (Hikmah, 2019). Similarly, Nadimin et al. (2016) found that M. oleifera extract inhibited the increase in MDA levels in pregnant women, with the intervention group showing lower MDA increases compared to the control group receiving iron and folic acid supplements (Nadimin, 2016). Additionally, García-Montero et al. (2023) demonstrated the antioxidant properties of M. oleifera, which significantly reduced oxidative stress markers, such as superoxide dismutase, catalase, glutathione peroxidase, glutathione, and MDA levels. Notably, this study also reported improvements in neonatal metabolic health, including better blood glucose regulation and reduced Neonatal Intensive Care Unit admissions. (Garcia, 2023). The results of the aforementioned research are in accordance with those of this study, which demonstrated that Moringa leaf ethanol extract significantly reduces MDA levels. Moringa leaves contain flavonoids, which function as antioxidants, and have been shown to reduce MDA levels, which is the primary product of the lipid peroxidation process from polyunsaturated fatty acids, which is a component of the oxidative stress process. Table 1. Analysis of MDA levels.
Table 2. Analysis of CRP levels.
Table 3. Analysis of albuminuria levels.
C-Reactive ProteinC-reactive protein is an acute-phase reactant protein produced by the liver in response to inflammation and is often associated with infections and chronic diseases. The level of ILs serves as an indicator of inflammatory processes that affect overall health (Nehring et al., 2023). Anti-Qa2 exposure elevates CRP levels, with the highest levels recorded in the T1 treatment group (3.12 ± 0.28). Moringa oleifera supplementation significantly reduced these levels, with the lowest CRP concentrations observed in the T3 treatment group (2.64 ± 0.17, p < 0.05) (Hikmah et al., 2019). This study’s findings are consistent with earlier research conducted by Hikmah et al. (2019) thereby substantiating the notion that in instances of preeclampsia, placental hypoxia conditions serve as a catalyst for an augmentation in CRP levels. CRP, in turn, has been identified as a pivotal instigator of the inflammatory response characteristic of preeclampsia. The study results further demonstrate that Moringa leaves have the capacity to attenuate CRP levels in the context of inflammation. Conversely, a study by Nuruhudin et al. (2020) found that M. oleifera leaf extract did not significantly lower high-sensitivity C-reactive protein levels in patients with systemic lupus erythematosus, despite a notable reduction in the MEX SLEDAI score (Nurudhin, 2020). AlbuminuriaUrinary protein levels serve as a clinical marker of endothelial dysfunction, a key factor in the development of preeclampsia. Exposure to anti-Qa2 increased albuminuria, with the highest levels observed in the C+ treatment group (8.00 ± 0.79) (Sakowicz, 2022). Moringa oleifera supplementation significantly reduced albuminuria caused by anti-Qa2 exposure, with the lowest levels found in the T2 treatment group (3.42 ± 1.09, p < 0.05) Sulistyowati (2025). Similarly, Akinrinde et al. (2024) reported that M. oleifera effectively mitigated oxidative stress by reducing MDA, protein carbonyls, and advanced oxidation protein products, while also lowering serum blood urea nitrogen and creatinine levels (Lingam, 2024). The present study demonstrated a substantial reduction in albuminuria levels in mice subjected to a preeclampsia model as a consequence of Moringa leaf administration. This finding aligns with the conclusions of several previous studies. Moringa has antioxidant properties that facilitate the repair of endothelial damage to the glomerulus, resulting in reduced albuminuria levels. A key limitation of the present study was the absence of a rigorous examination of kidney histology to ascertain any enhancements in kidney function. This investigation focused solely on albuminuria levels as a marker of ameliorating preeclampsia. ConclusionThe findings of this study indicate that the ethanol extract of M. oleifera leaves exerts a therapeutic effect against preeclampsia-associated symptoms. It is anticipated that this will emerge as a novel therapeutic option for this condition, with the potential to reduce maternal and fetal morbidity and mortality. The therapeutic effect of the administration of M. oleifera ethanol extract to mice model preeclampsia was demonstrated by a decrease in MDA and CRP levels in blood serum and albuminuria. AcknowledgmentsThe authors extend their sincere gratitude to the Embryology Laboratory, Faculty of Veterinary Medicine, Universitas Airlangga, for their invaluable support during this research. Conflict of interestThe authors declare no conflicts of interest related to this study. FundingThis research received no specific grant. Author’s contributionNutria Widya Purna Anggraini provided the contribution of all authors at submission, designed the analysis, performed the analysis, contributed data, and wrote the paper. Sri Sulistyowati conceived and designed the analysis, Soetrisno designed the analysis, Bambang Purwanto designed the analysis, and Paramasari Dirgahayu performed the analysis. Data availabilityAll data supporting the findings of this study are available in the manuscript. ReferencesAhmad, S., Pandey, A.R., Rai, A.K., Singh, S.P., Kumar, P. and Singh, S. 2023. Moringa oleifera inhibits protein glycation and exerts renoprotective effects in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 305, 116–117. Akpan, H.B., Akande, A.A., Ojewale, A.O., Oladipupo, F.E., Akinpelu, O.F. and Jimoh, S.O. 2018. Moringa oleifera ameliorates nephropathic changes in alloxan-induced diabetic adult wistar rats. Afr. J. Health Sci. 6, 110–118. Batmomolin, A., Ahsan, A., Wiyasa, I.W.A. and Santoso, S. 2020. Ethanolic extract of Moringa oleifera leaves improve inflammation, angiogenesis, and blood pressure in rat model of preeclampsia. J. Appl. Pharm. Sci. 8, 52–57. García-Montero, C., Fraile-Martinez, O., De Leon-Oliva, D., Boaru, D.L., Garcia-Puente, L.M. and De Leãn- Luis, J.A. 2023. Exploring the role of Mediterranean- and Westernized diets and their main nutrients in the modulation of oxidative stress in the placenta: a narrative review. Antioxidants. 11, 1918. Hikmah, E.M.T., Liben, P. and Widjiati. 2019. Anti-Qa2 animal models for preeclampsia preclinical studies: a pathological elevation of blood pressure and proteinuria. Folia Biol. (Poland). 2, 69–78. Hahn, A.B., Tian, H., Wiegand, G. and Soloski, M.J. 2025. Signals delivered via the Qa-2 molecule can synergize with limiting anti-CD3-induced signals to induce T lymphocyte activation. Immunol Investig. 21(3), 203–217. Available via https://pubmed.ncbi.nlm.nih.gov/1350269/ (Accessed 4 February 2025). Herman-Lara, E., Rodríguez-Miranda, J., ãvila-Manrique, S., Dorado-Lãpez, C. and Villalva, M., Jaime, L. 2024. In vitro antioxidant, anti-inflammatory activity and bioaccessibility of ethanolic extracts from Mexican Moringa oleifera leaf. Foods. 13, 2709. Lingam, Y. 2024. A review of role of Moringa oleifera in kidney protection. Int. J. Herb. 12, 29–284 33. Li, Q., Yin, L., Si, Y., Zhang, C., Meng, Y. and Yang West. 2020. Bioflavonoid quercetin improves pathophysiology in a rat model of preeclampsia. Biomed. Pharmacother. 127, 110–122. Mergia, K.K, Mengesh, Y.A.A, Toless, T.T, Makonne, E.E, Gene, S.S and Abeb, A.A, et al 2020. Effects of Thymus schimperi and Moringa stenopetala leaf extracts on lipid peroxidation and total antioxidant status in preeclampsia rat models. J. Complement. Altern. Med. Res. 10, 1–7. Nadimin. 2016. Influence of Moringa leaf exctracy (Moringa oliefera) on the level of MDA (malondialdehyde) in pregnant women. IJSBAR. 27, 48–56. Nehring SM, Goyal A, Patel BC. C reactive protein [Updated 2023 Jul 10]. Treasure Island, FL: StatPearls Publishing; 2025. Available via https://www.ncbi.nlm.nih.gov/books/NBK441843/. Nirupama, R., Divyashree, S., Janhavi, P., Muthukumar, S.P. and Ravindra, P.V. 2021. Preeclampsia: pathophysiology and management. J. Gynecol. Obstet. Hum. Reprod. 50, 101975. Nurudhin, A., Prabowo, N.A., Yulyani, Adnan, Z.A. and Adil. 2020. Effect of Moringa oleifera leaf extract on high sensitivity C-Reactive Protein, ESR And MEX SLEDAI score in lupus patients. Int J. Hum. Health Sci. 20, 291–297. Oliveira Jr, P.C. de, Moraez, D.F. and de, Oliveira C.R. 2024. Exploring the therapeutic effects of Moringa oleifera on inflammation and chronic diseases. Braz. J. Dev. 10, 72477. Ortega, M.A., Romero, B., Asúnsolo, ã., Sola, M., ãlavrez-Rocha, M.J. and Sainz, F. 2019. Patients with incompetent valves in chronic venous insufficiency show increased systematic lipid peroxidation and cellular oxidative stress markers. Oxidative Med Cell Longevity. 2019. Available via https://pubmed.ncbi.nlm.nih.gov/31281583/ (Accessed 4 February 2025). Ożarowski, M., Karpiski, T.M., Szulc, M., Wielgus, K., Kujawski, R. and Wolski, H. 2024. Plant phenolics and extracts in animal models of preeclampsia and clinical trials”review of perspectives for novel therapies. Pharmaceuticals. 14, 269. Prasetio, D.B., Setyaningsih, Y. and Suhartono, Suroto. 2022. Effect of Moringa leaf extract on Malondialdehyde levels in male wistar rats. J. Hunan Univ. Nat. Sci. 49, 424–428. Quiles, J.L., Ramírez-Tortosa, M.C., Gãmez, J.A., Huertas, J.R. and Mataix, J. 2020. Role of vitamin E and phenolic compounds in the antioxidant capacity, measured by ESR, of virgin olive, olive and sunflower oils after frying. Food Chem. 4, 461–468. Ramírez-Tortosa, M.C., Mesa, M.D., Aguilera, M.C., Quiles, J.L., Barã, L. and Ramirez-Tortosa, C.L. 2025. Oral administration of a Turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 147(2), 371–8. Available via https://pubmed.ncbi.nlm.nih.gov/10559523/ (Accessed 4 February 2025). Rana, S., Lemoine, E., Granger, J. and Karumanchi, S.A. Preeclampsia: pathophysiology, challenges, and perspectives. Circ. Res. 7, 1094–10112. Subandi, S., Purwanto, B., Wasita, B. and Soetrisno. 2024. Effects of Quercetin fraction from Moringa oleifera leaf extract on oxidative markers and histological profile of carotid and coronary arteries: an experimental animal study. Indones. J. Pharm. 2, 227–238. Sakowicz, A. 2022. Targeting of nuclear factor Kappa B by drugs adopted for the prevention and treatment of preeclampsia. Int. J. Mol. Sci. 5, 2881. Sulistyowati. 2025. Low-class Ib (HLA-G/Qa-2) MHC protein expression against Hsp-70 and VCAM-1 Profile on Preeclampsia. An experimental animal model of Mus musculus with endothelial dysfunction model. INAJOG. [Internet]. Available via https://www.researchgate.net/publication/277992762_Low_Class_Ib_HLA-GQa-2_MHC_Protein_Expression_against_Hsp-70_and_VCAM-1_Profile_on_Preeclampsia_An_observation_on_experimental_animal_Mus_Musculus_with_Endothelial_Dysfunction_model (Accessed 4 February 2025). Tinny, EE. and South 2016. Methanolic extract of Moringa oleifera reduces serum TNF-α, IL- 6, and Colonic Tissue MDA Levels in DMBA-induced Wistar Rats. J. Ked. Brawijaya. 1, 29. Torres-Torres, J., Espino-y-Sosa, S., Martinez-Portilla, R., Borboa-Olivares, H., Estrada-Gutierrez, G. and Acevedo-Gallegos, S. 2024. A narrative review on the pathophysiology of preeclampsia. Int. J. Mol. Sci. 14, 7569. Vergara-Jimenez, M., Almatrafi, M.M. and Fernandez, M.L. 2017. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants. 4, 9. | ||
| How to Cite this Article |
| Pubmed Style Anggraini NWP, Sulistyowati S, Soetrisno S, Purwanto B, Dirgahayu P. Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsia. Open Vet. J.. 2025; 15(4): 1784-1789. doi:10.5455/OVJ.2025.v15.i4.30 Web Style Anggraini NWP, Sulistyowati S, Soetrisno S, Purwanto B, Dirgahayu P. Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsia. https://www.openveterinaryjournal.com/?mno=242134 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i4.30 AMA (American Medical Association) Style Anggraini NWP, Sulistyowati S, Soetrisno S, Purwanto B, Dirgahayu P. Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsia. Open Vet. J.. 2025; 15(4): 1784-1789. doi:10.5455/OVJ.2025.v15.i4.30 Vancouver/ICMJE Style Anggraini NWP, Sulistyowati S, Soetrisno S, Purwanto B, Dirgahayu P. Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsia. Open Vet. J.. (2025), [cited January 24, 2026]; 15(4): 1784-1789. doi:10.5455/OVJ.2025.v15.i4.30 Harvard Style Anggraini, N. W. P., Sulistyowati, . S., Soetrisno, . S., Purwanto, . B. & Dirgahayu, . P. (2025) Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsia. Open Vet. J., 15 (4), 1784-1789. doi:10.5455/OVJ.2025.v15.i4.30 Turabian Style Anggraini, Nutria Widya Purna, Sri Sulistyowati, Soetrisno Soetrisno, Bambang Purwanto, and Paramasari Dirgahayu. 2025. Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsia. Open Veterinary Journal, 15 (4), 1784-1789. doi:10.5455/OVJ.2025.v15.i4.30 Chicago Style Anggraini, Nutria Widya Purna, Sri Sulistyowati, Soetrisno Soetrisno, Bambang Purwanto, and Paramasari Dirgahayu. "Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsia." Open Veterinary Journal 15 (2025), 1784-1789. doi:10.5455/OVJ.2025.v15.i4.30 MLA (The Modern Language Association) Style Anggraini, Nutria Widya Purna, Sri Sulistyowati, Soetrisno Soetrisno, Bambang Purwanto, and Paramasari Dirgahayu. "Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsia." Open Veterinary Journal 15.4 (2025), 1784-1789. Print. doi:10.5455/OVJ.2025.v15.i4.30 APA (American Psychological Association) Style Anggraini, N. W. P., Sulistyowati, . S., Soetrisno, . S., Purwanto, . B. & Dirgahayu, . P. (2025) Effects of Moringa oleifera leaves on CRP, MDA, and alevels in a mouse model of preeclampsia. Open Veterinary Journal, 15 (4), 1784-1789. doi:10.5455/OVJ.2025.v15.i4.30 |