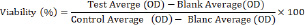| Research Article | ||
Open Vet. J.. 2025; 15(7): 3240-3253 Open Veterinary Journal, (2025), Vol. 15(7): 3240-3253 Research Article Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga ivaSabrine Rebhi1, Kaled Athmouni2, Sabah Dhibi1*, Saida Hidouri3, Hafsia Bouzenna1, Ayda Dhibi4, Nozza Bouzenna1 and Hfaiedh Najla11Laboratory of Biotechnology and Biomonitoring of the Environment and Oasis Ecosystems(LBBEEO), Faculty of Sciences of Gafsa, University of Gafsa, Gafsa, Tunisia 2Department of Life Sciences, Laboratory of Biodiversity and Aquatic Ecosystems, Ecology and Plantonology, Faculty of Sciences Sfax, University of Sfax, Sfax, Tunisia 3Malformative Pathology in Child, University of Monastir, Faculty of Medicine, Monastir, Tunisia 4Computer Science Department, Science College, Northern, Border University, Arar, Kingdom of Saudi Arabia *Corresponding Author: Sabah Dhibi. Laboratory of Biotechnology and Biomonitoring of the Environment and Oasis Ecosystems(LBBEEO), Faculty of Sciences of Gafsa, University of Gafsa, Gafsa, Tunisia. Email: s.dhibi [at] yahoo.fr Submitted: 09/02/2025 Revised: 11/06/2025 Accepted: 17/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Ajuga iva phenolic extract has various biological activities and health benefits. Phenolic compounds extracted from this plant are critical molecules in the pharmacotherapy field. Aim: Our investigation aimed to determine the chemical composition, antioxidant, and anti-inflammatory activities in vitro and in vivo of Ajuga iva ethanolic extract (AIEE). Methods: The diphenylpicrylhydrazyl (DPPH), ABTS, and nitric oxide tests and THP-1 cell model were used to evaluate the ethanolic extract of A. iva’s anti-inflammatory and antioxidant properties in vitro. In addition, the anti-inflammatory activity in vivo was evaluated using the Carageenan method to induce inflammation in 24 Wistar male rats. Results: Our analysis showed that the ethanolic extract AIEE exhibited a high amount of phenolic compounds. In addition, the AIEE extract showed high ability to scavenge DPPH●, ABTS•+, and NO• free radicals in the reaction system, and the scavenging activity values were 79. 96%, 78%, and 77%, respectively. In vitro investigations showed that the AIEE extract exhibited a strong anti-inflammatory potential using TPH-1 as a cell model. The AIEE extract significantly (p < 0.01) decreased 32%, 72.72% and 42.12% the TNα, IL6, and IL8, respectively. In vivo investigation revealed that the AIEE extract exhibited strong anti-inflammatory activity. The histopathological observation confirmed the anti-inflammatory activity of AIEE. Conclusion: These results confirmed that AIEE extracts can be used in the pharmaceutical domain. Keywords: Ajuga iva, Antioxidant activity, Anti-inflammatory activity, Phenolic compounds. IntroductionAn immune system’s intervention is a physiological phenomenon known as inflammation. Inflammation serves as an organism’s primary protection against microbial infections and, in certain cases, as a natural defense system against illnesses such as cancer (Bäck et al., 2019). On the other hand, inflammation can become dangerous in some situations and result in dangerous pathological conditions. Mast cell activation triggers the inflammatory response by releasing a number of vasoactive substances, including bradykinin, histamine, prostaglandins, and serotonin (Dhanisha et al., 2020). Several mediators that promote inflammation, including interleukin-1 beta, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) (An et al., 2020), are involved in orchestrating the intricate inflammatory process. Chronic inflammation increased the chance of developing rheumatoid arthritis, cancer, and skin inflammation, among other diseases. (Greten and Grivennikov, 2019).Therefore, one appealing therapeutic approach to treating diseases associated with inflammation is in order to lessen inflammation. Nevertheless, anti-inflammatory medications, including nonsteroidal anti-inflammatory drugs, have detrimental consequences on human health (Süleyman et al., 2007). In order to meet the demand for potent anti-inflammatory drugs that can mitigate the debilitating side effects of currently prescribed treatments, researchers are searching for safe and effective anti-inflammatory compounds in herbal medicines. A number of studies conducted in the last 10 years have looked for naturally occurring bioactive substances that have analgesic and anti-inflammatory properties. Secondary metabolites of medicinal plants, including polyphenols, flavonoids, terpenoids, and alkaloids, are essential sources for the creation of anti-inflammatory and analgesic drugs (Kazemi et al., 2018; Hermanto et al., 2020). Recent studies have shown that these molecules have anti-inflammatory effects by reducing chemokines, cytokines, pro- and neo-inflammatory mediators, and other inflammatory mediators involved in inflammatory processes (Yaribeygi et al., 2018). The Lamiaceae family, particularly Ajuga iva, which is commonly used in traditional medicine and is referred to as “Chandgoura” in Tunisia, Algeria, and Morocco, is of interest to us in order to enhance the country’s forest resources and produce new goods. Diabetes, fever, toothache, diarrhea, hypertension, hypercholesterolemia, and gastrointestinal diseases are all treated with it (El-Hilaly et al., 2018). Additionally, reports of A. iva’s vasorelaxant, cardiotonic, antibacterial, insecticidal (Aly et al., 2011), and wound-healing qualities have been made (Taleb-Senouci et al., 2009; Bouderbala et al., 2010). The anti-inflammatory potential of Ajuga genus has been reported. Recently, several authors found that the phenolic extract of the Ajuga genus effectively inhibited proinflammatory mediators such as nitric oxide (NO) and TNF-α in vitro (Shin et al., 2020). In addition, another species in the genus, such as Ajuga reptans, has been studied for its anti-inflammatory capacity by decreasing the production of TNF-α, ILs, and COX enzymes (Desalegn and Engidawork, 2023). Ajuga bracteosa is another species within the Ajuga genus that has been evaluated for its anti-inflammatory activity (Gautam et al., 2011). Our hypothesis was that A. iva extracts have strong antioxidant and anti-inflammatory properties due to their rich phytochemical composition, based on their traditional medicinal use and previously identified bioactive constituents. We also expected that these biological effects could be accurately measured using in vitro assays, which would provide scientific confirmation of A. iva’s antiinflammatory and antioxidant properties. Therefore, the aim of our study was to quantify the phenolic compounds of A. iva ethanolic extract and evaluate its antioxidant and anti-inflammatory activities in vitro. Additionally, we examined the possible anti-inflammatory effects of ethanol extract on antagonized carrageenan-induced inflammation in 24 male Wistar rats. Materials and TechniquesPlant materialsAerial parts of A. iva were collected from the Majelbel Abbes region in central Tunisia during the March–April 2023 period. After being cleaned and dried, the pieces were kept for up to 2 weeks in a cold, dry location. Then, using an electric grinder (Blender Moulinex LM2421EG, Tunisia), grind fine powder and store at room temperature until needed. Preparation of the extractThe final powder has been removed by the maceration method, which consists of the dissolution of a solid in a liquid. A 100 ml volume of absolute ethanol was added to 10 g of Ajuga powder and left to stand under agitation (200 rpm) for 24 hours at 32°C. The resulting residue was stored at −20°C for additional analysis after the heterogeneous mixture was filtered and evaporated at ambient temperature under decreased pressure. Characterization of plant extractsTotal phenolic content determinationTotal phenolic compounds were measured using the Folin–Ciocalteu method, as outlined by Singleton and Rossi (Singleton et al., 1965). In particular, 400 μl of Ciocalteu reagent was mixed with 50 μl of ethanolic extract, and the mixture was then left in the dark for 5 minutes. After adding 500 μl of 75% sodium carbonate, the mixture was allowed to sit for an hour. At 765 nm, the solution absorbance was measured at 765 nm. A standard curve plotted with gallic acid was used to determine the total phenolic content, which was then reported as μg of gallic acid equivalent per milliliter extract µg GA/mg. Total amount of flavonoidsThe total flavonoids were quantitatively analyzed by spectrophotometric means using the method described by Zhishen et al. (1999). In summary, 1 ml of distilled water, 75 μl of 5% NaNO2, and 200 μl of Ajuga iva ethanolic extract (AIEE) were mixed together. Then, after a 6-minute incubation time, 75 μl of 10% AlCl3 and a solution of 1 ml of 8% NaOH (1N) combined with 100 μl of distilled water were added. The latter solution was allowed to sit at room temperature for 15 minutes before its absorbance was measured at 510 nm. The outcome was given as μg of quercetin equivalent (µg QE/mg) per milliliter of extract. Determination of condensed tanninsTo determine the total tannin concentration, 20 μl of AIEE (1 mg ml−1) and 600 μl of 4% methanol vanillin solution were mixed together. A volume of 300 μl of concentrated HCl was added after 15 minutes, and the absorbance of the solution at 500 nm was then measured. The total tannin content was calculated by plotting the data against a standard curve using catechin as the standard. The result was expressed as μg of catechin equivalent per milliliter extract (µg CE/mg) (Makkar and Becker 1993). Activity of antioxidantsThe antioxidant capacity of A. iva extract was assessed using two radical scavenging assays: 2,2′-azinobis-(3 ethylbenzothiazoline-6 sulfonic acid radical (ABTS•+) and 2,2 diphenylpicrylhydrazyl radical (DPPH•). According to Öztürk et al. (2011), the AIEE extract’s capacity to scavenge free radicals from DPPH radicals was established. The procedure involved mixing 1 ml of AIEE extract at several concentrations (0.06–1 mg ml−1) with 4 ml of DPPH methanol solution (0.1 mM). The absorbance at 515 nm was used to measure the reaction mixture’s capacity to scavenge free radicals after it had been incubated for 30 minutes in a dark environment. BHT was used as a standard (Öztürk et al., 2011). This activity is given as % DPPH scavenging and calculated according to the following equation:
where Atest represents the absorbance of the extract reaction, and Acontrol represents the absorbance of the control reaction. The antioxidant activities of AIEE extract were also examined by examining their capacity to scavenge the ABTS•+ free radical using the procedure outlined by (Ozgen et al., 2006; Re et al., 1999). When paired with an oxidant (2.45 mM potassium persulfate), ABTS (7 mM in 20 mM sodium acetate buffer, pH 4.5) reacts to produce a stable, dark blue-green radical solution after 13 hours of dark incubation (4°C). The solution was then diluted to an absorbance of 0.7 ± 0.01 at 734 nm to obtain the test reagent. The reaction mixture, which contained 20 μl of sample at several concentrations (0.06–1 mg ml−1) and 3 ml of ABTS reagent, was incubated in a water bath at 30°C for 30 minutes. The test solution becomes colorless as unpaired electrons are sequestered by the antioxidants in the sample. The results were measured at 734 nm. The radical scavenging activity was calculated using the following formula:
where Atest represents the absorbance of the extract reaction, and Acontrol represents the absorbance of the control reaction. Assessment of anti-inflammatory activity using the nitrite testAs previously mentioned, sodium nitroprusside was utilized to produce NO, which was subsequently evaluated using the Griess reagent for the nitric oxide radical scavenging assay (Chera et al., 2022). In conclusion, a sodium nitroprusside solution (2 ml SNP and 0.5 ml PBS, pH 7.4) was combined with 0.5 ml AIEE extract, and the mixture was incubated for 2.5 hours at 25°C. Then, 1 ml of naphthylethylene–diamine–dihydrochloride was added to 0.5 ml of this mixture, together with 1 ml of sulfuric acid. After that, the mixture was vortexed and left to stand in the dark for half an hour. At 546 nm, the absorbance was measured, and the following formula was used to calculate the inhibition percentage:
where Atest represents the absorbance of the extract reaction, and Acontrol represents the absorbance of the control reaction. In vitro antiinflammatory potentialCulture and cell lineThe human monocytic leukemia cell line (THP-1 cells) was obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI 1640/Glutamax-1 medium with 10% fetal bovine serum1% sodium pyruvate, 2% non-essential amino acids, Penicillin 100 U/ml, and streptomycin 100 µg ml−1. The cells were maintained at 37°C in a humidified environment with 5% CO2. MTT test for cell viabilityThe cytotoxicity of the AIEE extract on lipopolysaccharide (LPS)-stimulated and LPS-free THP-1 The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide MTT test was used to determine the number of cells. THP-1 cells were seeded in a 96-well microplate at a suitable density of 5 × 105 cells per well, and after 24 hours, the culture medium was removed and replaced with medium containing AIEE extract at various concentrations (0.1, 0.2, 0.5, 1 and 2 mg ml−1) activated by the presence of lipopolysaccharide (5 mg ml−1) to trigger the inflammatory response in macrophages, as well as macrophage cells primed with AIEE extract only without LPS. The cells were then incubated for 24 hours in a humidified atmosphere at 37°C with 5% CO2 until they were used. The following formula was used to determine cell viability:
Measurement of cytokine production in culture supernatantsHuman cytokine-specific ELISA kits were used to measure the levels of proinflammatory (TNFα, IL-6, and IL-8) and antiinflammatory (IL-10) cytokines. The results were expressed in pg ml−1 and calculated using a standard curve with recombinant cytokines. Briefly, 100 μl of medium diluted in buffer was added to polystyrene wells coated with capture antibody and incubated for 24 hours at 4°C. After washing, 200 μl of PBS assay diluent supplemented with 10% FBS in each well was incubated for 1 hour. Then, each standard, sample, and control should have 100 μl in the corresponding wells. Close the dish and leave it at room temperature for 2 hours. After washing, put 100 μl of detector (detection antibody + SAV-hrp (enzyme) reagent) in each well, plate, and incubate for 1 hour at room temperature. The plates were washed with a wash buffer containing 10% FBS solution supplemented with 0.05% Tween-20 and left in the dark for 30 minutes with 100 μl TMB-ELISA substrate solution. Each well received 50 µl of H2SO4 stop solution, and absorbance was measured at 450 nm with λcorrection at 570 nm. The manufacturer’s instructions were followed when conducting the studies. Three separate experiments, each in triplicate, were conducted to conduct the assays. Animals and treatmentAdult male Wistar rats weighing 120–200 g were used in this study; they were purchased from the Central Pharmacy in Tunisia. All rats were housed in a breeding room under controlled conditions (temperature: 20°C ± 2°C; humidity: 60% ± 5%; 12-hour dark/light cycle), fed standard diets, and allowed free access to tap water. Paw Edema Model induced by carageenanThe anti-inflammatory activity was assessed using the methodology outlined by (Ravi et al., 2009). It received an injection of 0.1 mg l−1 of Carr solution to induce acute inflammation into each group of rats paw’s subplantar area. The carrageenan-induced paw edema model is a well-established assay for evaluating anti-inflammatory compounds (Süleyman et al., 2007). This is the most basic way that requires little equipment but a lot of practice to determine the therapeutic effects of AIEE extract in inflammatory disorders. This method was selected over other methods obtained before any therapy for each group. The rats used in this investigation were randomly divided into four groups of six animals each: Group 1: an untreated group that received sub plantar injection of NCL (0.9%) in a physiological solution. Group 2: inflamed group of rats were injected with carrageenan (Carr). Group 3: was utilized as reference group rats that received carrageenan injections (INDO+Carr) and indomethacin treatment at a dose of 10 mg/kg. Group 4: pretreated with 200 mg/kg p.o. of AIEE extract and then given an injection of carrageenan (AJUGA+Carr). After inducing inflammation, the volume of the injected and control paws was measured at 1, 2, and 4 hours later. The percentage of edema inhibition compared to the paw volume of the saline-treated controls was used to compute the pre-treatment effect, as previously described (Lanhers et al., 1991), according to the following formula:
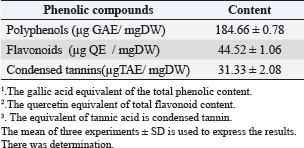
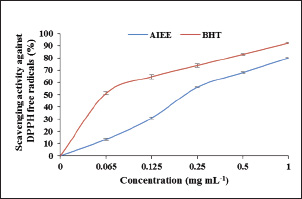
Sample collection and analysisAfter the conclusion of the experiment, the animals were placed in a lying position, and tissue from the right hind paw was removed. The samples were then rinsed in ice-cold normal saline after homogenization in ice-cold phosphate buffer (pH 7.4). The homogenate was then centrifuged at 3,000 × g for 10 minutes. For the Malondialdehyde (MDA) and antioxidant enzyme activity assays [catalase (CAT), superoxide dismutase (SOD), and Glutathione peroxidase (GPx)], the supernatant was removed and stored at −20°C. For the determination of hematological parameters, blood samples were collected and placed into EDTA and heparin tubes. To test oxidative stress, tissues (edema) were dissected from the inflamed skin, homogenized with phosphate-buffered saline (pH=7.4) and centrifuged for 15 minutes at 4,500 rpm. The obtained supernatant was stored at −20°C until analysis. Hematological parameters and inflammatory mediatorsAn automatic SYSMEX SERIE KX-21N hematology analyzer was used at the biochemical laboratory Hospital mdhilaGafsa, Tunisia, to measure the following parameters: hemoglobin (HGB, g/dl), white blood cells (WBC, 109/_l), lymphocyte proportion (Lym, %), and C-reactive protein (CRP) (mg/l). Assessing CRP levelsAfter the inflammatory phase, a specific signal emerged: CRP. According to Morse et al. (2008), it increases in direct proportion to severity. The turbid metric method was used to quantify reactive protein using an automatic COBAS INTEGRA 400 C-reactive analyzer. For CRP, mg l-1 is the unit of measurement. Oxidative stress markersThe total protein carbonyl concentration was measured spectrophotometrically in order to determine the protein oxidation level (Levine et al., 1999). Protein results were expressed as nmol mg−1. According to Niehaus and Samuelsson (Kayali et al., 2006), lipid peroxidation was determined calorimetrically by identifying thio-barbituric acid reactive compounds. Status of enzymatic antioxidantsThe enzymatic antioxidant status of superoxide dismutase activity was assessed using the nitro blue tetrazolium reduction technique (Beauchamp and Fridovich 1971). According to (Aebi et al., 1984), the CAT activity was determined spectrophotometrically by measuring the rate of hydrogen peroxide breakdown at 240 nm. GPx activity was assessed using the methodology described by (Flohe and Günzler 1984). Histological analysisThe paw edema tissues from each experimental group were collected for histological examination. In order to fix the samples, they were first submerged in 10% buffered formalin solution. Then, on slides that were 5 µm thick, tissues were fixed in paraffin and stained with hematoxylin and eosin. A light microscope was used to examine the slides. Statistical analysisSPSS for Windows 20, software (IBM corporation) was used to perform the statistics. One-way analysis of variance followed by least significant difference or Wettest was used to analyze the data, which are presented as the mean ± SD. The significance level was set at 0.05. Ethical approvalThe experimental procedures were carried out in compliance with the guidelines for the care and use of laboratory animals published by the University of Gafsa, Tunisia, and authorized by the Gafsa Committee of Animal Ethics. (reference number: FSG-DLS-21). Dated: 17/06/2025. ResultsTotal phenolic, flavonoid, and tannins contentsPhenol, flavonoids, and tannins were found in the AIEE extract according to a qualitative phytochemical examination. A considerable number of polyphenols (184.66 ± 0.78 µg GAE/mg), flavonoids, and condensed tannins (44.52 ± 1.06 µg QE/mg and 31.33 ± 2.08 µg TAE/mg, respectively) were found in the total phenolic compounds (Table 1). In vitro investigation of the antioxidant potential of the AIEE extractFree radical scavenging using DPPHThe ability of the AIEE extract to scavenge DPPH-free radicals at different concentrations is shown in Figure 1. Our results showed that the AIEE extract free radical-scavenging activity values ranged from 13.41 at 0.065-mg ml−1 to 79.96% at 1-mg ml−1. This extract exhibited a strong capacity to neutralize DPPH free radicals. Table 1. Content of the AIEE extract.
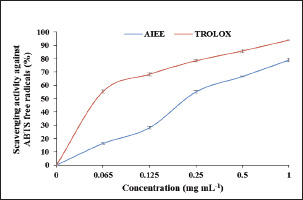
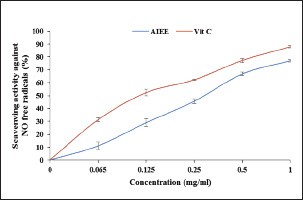
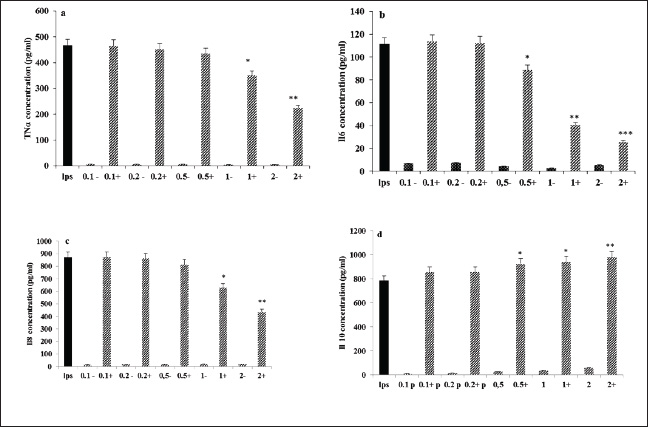
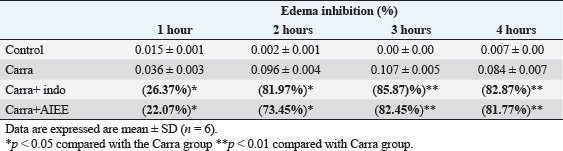
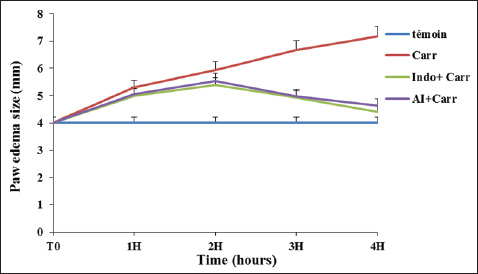
Fig. 1. AIEE extract’s antioxidant properties against DPPH free radicals. The standard was BHT. ABTS or TEAC testThe ABTS-+ free radicals can be neutralized by AIEE extract, as shown in Figure 2. The scavenging activity of AIEE extract ranged from 16.28% at 0.065 mg ml−1 to 78% at 1-mg ml−1. Our results revealed that the AIEE extract has a strong capacity to neutralize ABTS free radicals. Anti-inflammatory activity determined using the nitrite testThe results of the NO radical inhibition test are presented in Figure 3. It appears that the percentage inhibition of this free radical increases with increasing concentrations of vitamin C and AIEE. At a concentration of 1-mg ml−1, AIEE revealed a nitric oxide inhibition percentage of 77%, whereas vitamin C was 87%. Anti-Inflammatory Potential In Vitro Using THP-1 Cell As ModelCytotoxicity assayFigure 4 presents the effects of AIEE and LPS on THP-1 cell line viability. Our findings demonstrated that up to 2-mg ml-1, AIEE extract had no effect on the viability of THP-1 cells. AIEE alone and AIEE plus LPS at 0.1, 0.2, 0.5, 1, and 2-mg ml-1 are the various concentrations. According to our findings, there was no discernible (p < 0.05) impact of AIEE extract concentrations below 2-mg ml-1 on cell viability compared with untreated cells. Impact of AIEE on cytokine production in LPS-stimulated THP-1 cellsTo assess the capacity of AIEE extract to suppress the expression of inflammatory cytokines, including IL-6, TNFα, and IL-8, and to raise IL-10 after 24 hours of exposure, THP-1 cells were first stimulated with LPS (Fig. 5). LPS-stimulated THP-1 cells showed a marked increase in inflammatory cytokine levels. On the other hand, pro-inflammatory cytokines (IL-6, TNFα, and IL-8) were reduced, and IL-10 production was increased when THP-1 cells were treated with AIEE extract at doses of 0.1–2 mg ml−1 (Fig. 5a–c). Effects of AIEE on carrageenan-induced paw edema in ratsThe percentage of inhibition in each group is presented in Table 2. Our findings demonstrated that, at a concentration of 200 mg/kg, the AIEE extract exhibited the strongest anti-inflammatory effect on rat paw edema caused by carrageenan. Four hours after oral treatment, this extract exhibited a substantial inhibitory effect (p < 0.01) (81.77%) in comparison to the INDO group (82.87% at a dose of 10 mg/kg). Furthermore, we are able to verify that the AIEE extract has anti-inflammatory, and can influence acute inflammatory processes. Ajuga iva’s anti-inflammatory effects on the size of edemaFour hours prior to sacrifice, the CARR group received an injection of carrageenan, which caused a significantly greater amount of edema than the control group, which displayed normal paw size (Fig. 6). In the second hour, the edema size was mostly reduced by the combination of the INDO (CARR+INDO) and A. iva extract (CARR+AI) treatments. The results of the trial demonstrated that, in comparison to the reference groups, the Indo and Carr groups, the Ajuga iva extract exhibited a considerable and time-dependent decrease in the size of paw edema. Therefore, it is possible that some of this is due to the phytochemical substances in the extract reducing the release of inflammatory mediators.
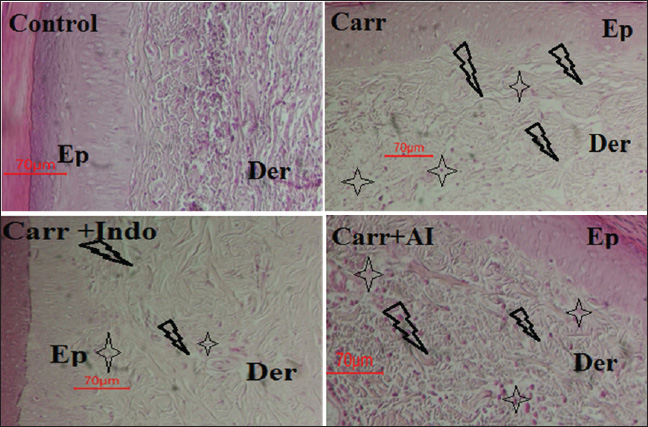
Fig. 2. Antioxidant activity of AIEE extract against the ABTS free radicals. BHT was used as the standard.
Fig. 3. Antioxidant activity of the AIEE extract against the NO free radicals. Vitamin C was used as the standard.
Fig. 4. Cytotoxic effect of AIEE- and AIEE+ extract on THP-1 cells line. Cell viability was assessed using the MTT test. The mean ± SD of the three experiments are represented by each point. Hematological parametersInjections of carageenan cause disruptions in hematologic parameters (Table 3). The CARR group had a significantly higher percentage of lymphocytes and WBCs than the control group. Rats in the CARR group exhibited significantly lower HGB and red blood cell (RBC) levels than those in the healthy group. After AI or INDO treatment, the rats’ values recovered to normal. In this study, we found that the Carr group had significantly altered hematological parameters (WBC and lymphocytes). One important immune system component that contributes to the onset of inflammation is WBC. Nonetheless, hemoglobin and RBCs decrease. Lower hemoglobin levels indicate decreased erythropoietin levels and early RBC breakdown. Effect of A. iva Extract on Oxidative Stress LevelsEffect of AIEE on MDA-level of rats revealed to carrageenanMalondialdehyde levels in carrageenan-exposed rats showed a significant rise in lipid peroxidation in comparison to control rats (Table 4). In contrast, rats pretreated with AIEE had considerably lower MDA levels than the normal group. AIEE treatment brought the MDA level back to a level comparable to that of rats administered carrageenan. Effects of AIEE on the antioxidant status of rats exposed to carrageenanTable 4 presents the impact of carrageenan exposure on the antioxidant status of rats. Rats exposed to carrageenan showed significantly lower SOD and GPx activity (p < 0.001) than controls. Furthermore, rats treated with carrageenan showed a substantial decrease in CAT activity (p < 0.001) in comparison to controls. Additionally, rats that received AIEE before treatment showed a significant improvement in their antioxidant state (p < 0.01). Effect of AIEE Extract on Carageenan-Induced Histological alterationsA histological examination of paw tissue from each group was performed in order to evaluate AIEE’s antiinflammatory effect on acute inflammation (Fig. 4). The Carr, Indo (10 mg/kg/bw) group, and the EtOH extract of A. iva (200 mg/kg/pc) group all showed histological changes according to the analysis (Fig. 7). In contrast to the control group (Fig. 7a), in the Carr group, we saw subcutaneous edema. The epidermis appears spongy, and inflammatory cells, especially polynuclear neutrophils (PN), infiltrate the area of inflammatory tissues (Fig. 7b). Both the reference medication Indo (Fig. 7d) and the EtOH extract group significantly reduced the quantity of cellular penetration and the epidermis’s spongy appearance.
Fig. 5. AIEE- and AIEE+ extract’s impact on TNFα (a), IL-6 (b), IL-8 (c), and Il-10 (d) levels in THP-1 cell lines. Three experiments’ mean ± SD is represented by each point.*p < 0.05 compared with LPS group;**p < 0.01 compared with LPS group;***p < 0.001 compared with the LPS group. Table 2. Acute antiinflammatory effect of the AIEEon carrageenan-induced rat paw edema.
DisscussionIn Tunisia, there has been a recent discussion regarding the role that plants play in traditional medical cures and the potential benefits of phytochemical ingredients in pharmacotherapy. One of these medicinal plants with potential benefits is A. iva. It includes a wide range of chemical substances, including phenolic acids, fatty acids, steroids, terpenoids, tannins, and flavonoids (containing caffeine and chlorogenic acid) (El-Hilaly et al., 2004; Diafat et al., 2016). Thus, both primary and secondary metabolites present in the fruits and foliage of A. iva help explain most of its biological and pharmacological effects (Bennaghmouch et al., 2002). This plant was chosen due to indications of its traditional use, for which biological and chemical research is currently very limited. The current investigation assessed the phytochemical content, antioxidant activity, and anti-inflammatory qualities of A. iva extract in vivo. After phytochemical investigation, the ethanolic extract of A. iva contained notable levels of polyphenols, tannins, and flavonoids. According to numerous research, these phytochemical substances are responsible for the anti-inflammatory and antioxidant qualities of some therapeutic plants (Fawole et al., 2009; Gosta et al., 2012). In vitro, DPPH, ABTS, and NO tests were performed to evaluate the antioxidant activity of A. iva ethanol extract. In this study, the AIEE exhibited the ability to neutralize DPPH-free radicals with scavenging activity values ranging from 13.41% at 0.065 mg/ml to 79.96% at 1 mg/ml, compared to 91% in BHT, which served as a positive control. Our results are in agreement with those of (Saidi et al., 2023), who have proven that the hydroethanolic extract has a very interesting antiradical power against DPPH, which records an IC50 value of (IC50=59.92 ± 0.70 µg/ml). Also, the AIEE extract showed a significant capacity for ABTS•+ radical scavenging activity with values ranging from 16.28% at 0.065 mg/ml to 78% at 1 mg/ml, and reaching 93% in TROLOX, which was employed as a positive control. Similarly, the nitrite test was used to examine the in vitro anti-inflammatory action against the radical NO, yielding a 77% inhibition percentage. This outcome is consistent with research showing that the same plant from a different species (the Ajuga genus) has an effect against the radical NO (Bouderbala et al., 2008). The abundance of polyphenols and flavonoids in a plant extract may contribute to its antioxidant potential. These bioactive substances are identified by their chemical structure and redox characteristics, which are crucial for scavenging free radicals and chelating transition metals (Mohamed et al., 2010). Our study shows that the phenolic, flavonoid, and tannin contents of the High levels of AIEE extract, measuring 184.66 ± 0.78 µg GAE/mg DW (44.52 ± 1.06 µg QE/mg DW and 31.33 ± 2.08 µg TAE/mg DW, respectively). Our findings are consistent with those of Senhaji et al. (2022) also showed that A. iva (L.) contains a lot of catechin tannins, flavonoids, sterols, and saponins (Senhaji et al., 2022). In addition, the results obtained are consistent with the findings of other research (Makni et al., 2013; Salem et al., 2016; Bendif et al., 2017; Fettach et al., 2019; Senhaji et al., 2020; El-Lamey, 2022). They verified that hydroethanoloic extract contains significant amounts of phenolic chemicals.
Fig. 6. The evolution of paw edema size (mm) in control and treated groups: Control (non-treated rats): received injection with isotonic saline solution (NaCl, 0.9%); CARR: rats inflamed by Carrageenan (1%); CARR+AI and CARR+INDO: rats inflamed by Carageenan and treated with AI, or indomethacin, respectively. Table 3. Hemogram parameters of the experimental groups.
Table 4. Effect of AIEE extract on carrageenan-induced oxidative stress in wistar rats. All values are presented as mean ± SD (n=6).
Fig. 7. Histopathological slides of paw edema tissues in rat experimental groups ;control group rats treated with physiological saline (NaCl, 0.9%) showed normal histological structure of both epidermis and dermis; Carr group; Carr-treated rats showed heavy infiltration of polynuclear neutrophils (PN) and a spongy-like appearance and bulla in the epidermis. : Carr + Indo group; Carr-treated rat that received Indomethacin (10 mg/kg/bw) showed a partial protective action. Carr + EtOH extract group; Carr-treated rats that received the EtOH extract (200 mg/kg/bw) showed significantly reduced migration of PN and oedematasis in dermis without any spongy-like feature and bulla Ep: epidermis, Der:dermis: Plus sign :inflammatory cell infiltration; Arrow :edema. Our findings differ greatly from those of Makni et al. (2013) study because the amounts of phenolic compounds and the antioxidant capacity of extracts can change based on the extraction procedures and environmental factors. Using four different solvents (methanol, chloroform, water, and hexane), they examined several Ajuga iva (L.) extracts and discovered that the methanolic and aqueous extracts had the highest phenolic content, with flavonoids exhibiting strong antioxidant ability in all experiments. (DPPH and ABTS) (Makni et al., 2013). Contrary to its aqueous extract, the methanolic extract of A. iva (L.) demonstrated the highest antioxidant capacity, demonstrating the variance in antioxidant activity between the extracts, according to a study by (Bouyahya et al., 2020). The secondary metabolites, such as phenolic chemicals, found in a range of medicinal fruits, vegetables, and plants have been the subject of several investigations (Ouassou et al., 2021; Boutahiri et al., 2022) because these compounds possess antibacterial, anti-inflammatory, and antioxidant properties. In this case, further studies should explore the potential effects of these extracts using animal models. This is the first study to assess the protective impact of AIEE against LPS exposure in THP-1 cells, a human monocytic leukemia cell line. The MTT assay in our study demonstrated that AIEE had no discernible effect on THP-1 cell viability at doses below 2 mgml−1. THP-1 cells were shielded from the pro-inflammatory effects of LPS exposure by pretreatment with AIEE at different doses. This protection is attributed to the strong ability of AIEE to scavenge NO radicals, as confirmed by in vitro studies. Several extracts have been shown to possess anti-inflammatory effects in macrophages, suppressing the production of inflammatory mediators such as NO. Additionally, the presence of phenolic compounds likely contributes to the anti-inflammatory activities, as they have been found to exert beneficial effects on inflammatory processes. After 24 hours of LPS stimulation, the extract decreased the expression of pro-inflammatory cytokines such as IL-6, TNF-α, IL-8, and IL-10, indicating AIEE’s impact on cytokine production in THP-1 cells in response to LPS. As anticipated, THP-1 cells activated by LPS exhibited noticeably higher levels of inflammatory cytokines. However, the release of IL-6, TNF-α, and IL-8 was decreased, and the synthesis of IL-10 was enhanced in a dose-dependent manner when treated with AIEE at concentrations ranging from 0.1 to 2 mgml−1. In contrast, cells treated with AIEE without LPS exposure did not exhibit significant changes in cytokine secretion, particularly IL-10 secretion. These findings are consistent with earlier research that looked into A. iva’s anti-inflammatory qualities. For example, a study discovered that A. iva methanol extract prevented human immune cells from producing inflammatory cytokines such as IL-1β, IL-6, and TNF-α (Bouyahya et al., 2020). Numerous phytochemicals have anti-inflammatory effects on a range of inflammatory illnesses, according to mounting evidence (Yatoo et al., 2018). They are distinguished by elevated reactive oxygen species (ROS) levels, increased immune cell infiltration, and enhanced expression of inflammatory cytokines, all of which are linked to the clinical states of several inflammatory disorders (Liu et al., 2017). Our extract’s high concentration of phenolic components, especially gallic acid and flavonoids, is responsible for its antioxidant effect. Due to their effects on histamine release and arachidonic acid metabolism, which inhibit prostaglandin synthesis and cyclooxygenase expression, these substances are well-known for their anti-inflammatory properties (Akbay et al., 2002). By inhibiting nuclear factor transcription and limiting signal transduction-related kinases, among other ways, flavonoids also affect the production of adhesion molecules and pro-inflammatory cytokines (Derwich et al., 2011). Consequently, studies on medicinal plants used as analgesics should be considered as potential treatments for inflammatory diseases (Bawankule et al., 2014). In an in vivo experiment using a carrageenan (Carr) model, the anti-inflammatory properties of Ajuga iva ethanol extract (EtOH) were compared with those of the nonsteroidal anti-inflammatory drug indomethacin. Indomethacin was used to block prostaglandin biosynthesis in Carr-induced rats, a proven paradigm for evaluating anti-inflammatory drugs (Higgs et al., 1979). This model induces tissue edema, a hallmark of acute inflammation and a key criterion for evaluating anti-inflammatory efficacy (Hemmati et al., 2015). Biphasic inflammation results after Carr injection: in the first phase, pro-inflammatory mediators such as prostaglandin, serotonin, bradykinin, and leukotrienes are synthesized, coupled with an increase in cyclooxygenase-2 (COX-2) mRNA expression (Posadas et al., 2004; Pundarikakshudu et al., 2016). In the second phase, characterized by increased vascular permeability, prostaglandins mediate inflammation and are associated with polynuclear neutrophils PN cell release, infiltration, and migration into the affected tissue (Cuzzocrea et al., 1999). Our results demonstrate that AIEE provides dose-dependent protection against acute inflammation caused by Carr. Compared with the indomethacin group, the EtOH extract considerably reduced paw edema at a dose of 200 mg/kg (81.77% inhibition). Four hours after treatment, the effect peaked. These findings are in line with a prior study showing that A. iva at dosages of 50 and 200 mg/kg reduced paw edema in mice in a dose-dependent manner. (El-Hela et al., 2016). According to our findings, the EtOH extract reduces inflammation by blocking the production and release of inflammatory mediators such as serotonin, histamine, and prostaglandins. A study on the same plant of another species confirmed the anti-inflammatory potential of ajuga; in fact, the 70% ethanol extract of Ajuga bracteosa possesses significant and promising anti-inflammatory activity. The mechanism of anti-inflammatory action is assumed to be mediated by the inhibition of COX-1 and COX-2 (Gautam et al., 2011). Histological examination of the paw tissue from the experimental groups confirmed that the EtOH extract reduced the number of polymorphonuclear cells in the inflamed tissue, further supporting its anti-inflammatory action. Furthermore, inflammatory indicators, including fibrinogen, TNF-α, CRP, and IL-6, are frequently increased during infection or in active illness conditions (Mendall et al., 1997). In our study, compared to the Carr group, AIEE significantly reduced CRP levels from 5.4 mg l−1 to 3.4 mg l−1. Oxidative stress is a major contributor to the pathogenesis of several inflammatory diseases (Yaribeygi et al., 2019). The generation of ROS, a major contributor to inflammation caused by Carr, is essential for the emergence of several inflammatory diseases (Ialenti et al., 1992; Salvemini et al., 1996). High ROS levels can cause protein oxidation and lipid peroxidation (Salem et al., 2015). According to our findings, rats exposed to carrageenan had significantly higher levels of MDA, a marker of lipid peroxidation, than control rats. However, MDA levels were significantly reduced and almost returned to normal following pretreatment with AIEE. Additionally, compared with the control group, rats administered carrageenan exhibited considerably decreased (p < 0.001) GPx and SOD activity. On the other hand, AIEE pretreatment markedly increased antioxidant enzyme activity (p < 0.01). Our results are in line with research showing that A. iva extract lowers lipid peroxidation in rats and raises the levels of enzyme antioxidants such as catalase, GPx, and SOD (Hamden et al., 2008). Similarly, in rats given mercuric chloride, Bahi and Necib (2014) found that Ajuga iva extract decreased lipid peroxidation and raised glutathione, GPx, and GST levels (Bahi and Necib 2014). The antioxidant potential of A. iva leaf aqueous extract was also recently emphasized in a study, which suggested that the protective impact of the EtOH extract may be related to its capacity to promote the generation and activity of antioxidant enzymes during inflammation. ConclusionAccording to this study, the ethanolic extract of Ajuga iva is a valuable substitute for synthetic antioxidants in foods, cosmetics, and pharmaceutical items, as well as an efficient prospective source of polyphenols. The polyphenolic elements are in charge of the antioxidant activity of AIEE, as demonstrated by the effective link between the polyphenolic content and antioxidant capacity. The results of our investigation led to the conclusion that carageenan administration to male rats causes increases in oxidative stress and inflammatory reactive protein (MDA, SOD, CAT, GPx). Additionally, an in vivo study showed that the ethanolic extract of Ajuga iva had stronger anti-inflammatory activity. This possibility may be linked to the presence of natural phytochemicals such as phenols, flavonoids, and tannins. The creation of novel natural pharmaceutical medications with anti-inflammatory, anti-edematous, and antioxidant properties and fewer adverse effects may thus take advantage of these findings. AcknowledgmentsThe authors greatly appreciate the technical support provided Laboratory of Biotechnology and Biomonitoring of the Environment and Oasis Ecosystems(LBBEEO), Faculty of Sciences Gafsa, Tunisia. Conflict of interestThe authors declare that they have no conflict of interest. FundingThis research received no external funding. Authors’ contributionsSabrine Rebhi and Sabah Dhibi designed the study. Sabrine Rebhi, Kaled Athmouni, and Ayda Dhibi performed the in vitro and in vivo experiments. Hafsia Bouzenna and Nozza Bouzenna were responsible for data analysis and interpretation. Sabrine Rebhi and Hfaiedh Najla contributed to manuscript writing and critical revision. All authors read and approved the final version of the manuscript. Data availabilityThe datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request. ReferencesAebi, H. 1984. Catalase in vitro. Methods Enzymol. 105,121–126. Akbay, P., Gertsch, J. and Calis, I. 2002. Novel antileukemic sterol glycosides from Ajugasalicifolia. Helv. Chim. Acta. 85, 1930–1942. Aly, R., Ravid, U., Abu-Nassar, J., Botnick, I., Lebedev, G., Gal, S., Ziadna, H., Achdari, G., Smirov, E. and Meir, A. 2011. Biological activity of natural phytoecdysteroids from Ajuga iva against the Sweet pota to Whitefly Bemisia Tabaci and Persea mite Oligonychus perseae. Pest Manag. Sci. 67, 1493–1498. An, J., Chen, B., Kang, X., Zhang, R., Guo, Y., Zhao, J. and Yang, H. 2020. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am. J. Transl. Res. 12, 2353–2378. Bäck, M., YurdagulJr, A., Tabas, I., Öörni, K. and Kovanen, P.T. 2019. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat. Rev. Cardiol. 16(7), 389–406. Bahi, A. and Necib, Y. 2014. Protective effect of aqueous extract of Ajuga iva (L.) against mercury (II) induced oxidative and renal stress in rats. Int. J. Pharm. Sci. Rev. Res. 27(1), 111–116. Beauchamp, C. and Fridovich, I. 1971. Superoxide dismutase: improved assays and an assay applicable o acrylamide gels. Anal. Biochem. 44, 276–287. Bendif, H., Lazali, M., Mohamed, H., Djamel, M., Boudjeniba, M. and Venskutonis, R. 2017. Biological screening of Ajuga iva extracts obtained using supercritical carbon dioxide and pressurized liquid extraction Citation. J. Med. Bot. 1(0), 33–41. Bennaghmouch, L, Hajjaji, N. and Gmira,2002. North Flavonoïdesd’Ajuga iva (L) Schreb (Labiée). Actes Inst. Agron. Vet, 22, 25–30. Bouderbala, S., Lamri-Senhadji, M., Prost, J., Lacaille-Dubois, M.A. and Bouchenak, M. 2008. Changes in antioxidant defense status in hypercholesterolemic rats treated with Ajuga iva. Phytomedicine 15(6–7), 453–461. Bouderbala, S., Prost, J., Lacaille-Dubois, M.A. and Bouchenak, M. 2010. Iridoid extracts from Ajuga iva increase antioxidant enzyme activities in red blood cells of rats fed a cholesterol-rich diet. Nutr. Res. 30, 358–365. Boutahiri, S., Eto, B., Bouhrim, M., Mechchate, H., Saleh, A., Al kamaly, O., Drioiche, A., Remok, F., Samaillie, J. and Neut, C. 2022. Lavandula pedunculata (Mill.) Cav. aqueous extract antibacterial activity improved by the addition of Salvia rosmarinus Spenn, Salvia lavandulifolia Vahl and Origanum compactum Benth. Life 12, 328. Bouyahya, A., El Omari, N., Elmenyiy, N., Guaouguaou, F.E., Balahbib, A., El-Shazly, M. and Chamkhi, I. 2020. Ethnomedicinal use, phytochemistry, pharmacology, and toxicology of Ajuga iva (L) Schreb. J. Ethnopharmacol. 258, 112875. Chera, E.I., Pop, R.M., Pârvu, M., Sori, O., Uifălean, A., Cătoi, F.A., Cecan, A., Negoescu, A.G., Achimaș, P. and Pârvu, A.E. 2022. Flaxseed ethanol extracts’ antitumor, antioxidant, and anti-inflammatory potential. Antioxidants 11, 892. Cuzzocrea, S., Costantino, G., Mazzon, E., Zingarelli, B., De Sarro, A. and Caputi, A.P. 1999. Protective effects of Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in paw edema induced by carrageenan in the rat. Biochem. Pharmacol. 58(1), 171–176. Derwich, E., Chabir, R. and Taouil, R. 2011. In vitro antioxidant activity and GC/MS studies on the leaves of Menthapiperita (Lamiaceae) from Morocco. Int. J. Pharm. Sci. Drug Res. 3, 130–136. Desalegn, T. and Engidawork, E. 2023. Anti-convulsant activity of soxhlet leaf extracts of Ajuga integrifolia Buch.-Ham. Ex D. Don (Lamiaceae) in Mice. J. Exp. Pharmacol. 5, 241–253. Dhanisha, S.S., Drishya, S. and Guruvayoorappan, C. 2020. Fruit extract of Pithecellobiumdulce (FPD) ameliorates carrageenan-induced acute inflammatory responses via regulating proinflammatory mediators. J. Food Biochem. 44, e13329. Diafat, A., Arrar, L., Derradji, Y. and Bouaziz, F. 2016. Acute and chronic toxicity of the methanolic extract of Ajuga iva in rodents. Nternational. J. Appl. Res. Nat. Prod. 9, 9–16. El-Hela, A.A., Abdel-Hady, N.M., Dawoud, G.T. and Ghoneim, M.M. 2016. HPTLC fingerprint profile of triterpenes of Lamium amplexicaule Benth. and Ajuga iva L.(Lamiaceae) monitored with screening of their anti-inflammatory effect. J. Pharmacog. Phytochem. 5(6), 176. El Hilaly, J., Ennassir, J., Benlyas, M., Alem, C., Amarouch, M. Y. and Filali-Zegzouti, Y. 2018. Anti-inflammatory properties and phenolic profile of six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. King Saud Univ-Sci. 30(4), 519-526. El-Hilaly, J., Lyoussi, B., Wibo, M. and Morel, N. 2004. Vasorelaxant effect of the aqueous extract of Ajuga iva in rat aorta. J. Ethnopharmacol. 93, 69–74. El-Lamey, T.M. and Abd El-Maboud, M.M. 2022. Middle East journal of agriculture research 2022. Middle East J. Agric. Res. 11(1), 41–52. Fawole, O.A., Ndhlala, A.R., Amoo, S.O., Finnie, J.F. and Van Staden, J. 2009. Anti-inflammatory and phytochemical properties of twelve medicinal plants used for treating gastro-intestinal ailments in South Africa. J. Ethnopharmacol.123(2), 237–243. Fettach, S., Mrabti, H.N., Sayah, K., Bouyahya, A., Salhi, N., Cherrah, Y. and El Abbes, F.M. 2019. Phenolic content, acute toxicity of Ajuga iva extracts and assessment of their antioxidant and carbohydrate digestive enzyme inhibitory effects. S. Afr. J. Bot. 125, 381–385. Flohé, L. and Günzler, W.A. 1984. Assays of glutathione peroxidase. Methods Enzymol. 105, 114–121. Gautam, R., Jachak, S.M., and Saklani, A. 2011. Anti-inflammatory effect of Ajuga bracteosa wall Ex Benth. mediated through cyclooxygenase (COX) inhibition. J. Ethnopharmacol. 133(2), 928–930. Gosta, G., Francisco, V., Lopes, M.C., Cruz, M.T. and Batista, M.T. 2012. Intracellular signaling pathways modulated by phenolic compounds: application for new anti-inflammatory drugs discovery. Curr. Med. Chem. 19(18), 2876–2900. Greten, F.R. and Grivennikov, S.I. 2019. Inflammation and cancer: triggers, mechanisms, and Consequences. Immunity 51, 27–41. Hamden, K., Ayadi, F., Jamoussi, K., Masmoudi, H. and Elfeki, A. 2008. Therapeutic effect of phytoecdysteroids rich extract from Ajuga iva on alloxan-induced diabetic rats liver, kidney, and pancreas. Biofactors 33(3), 165–175. Hemmati, A.A., Kalantari, H., Siahpoosh, A., Ghorbanzadeh, B. and Jamali, H. 2015. Anti-inflammatory effect of hydroalcoholic extract of the Washingtoniafilifera seeds in carrageenan-induced paw edema in rats. Jundishapur J. Nat. Pharm. Prod. 10(1), e19887. Hermanto, F.E., Soewondo, A., Tsuboi, H., Ibrahim, M. and Rifa’i, M. 2020. The hepatoprotective effect of cheral as antioxidant and anti-inflammation on mice (Musmusculus) with breast cancer. J. Herbmed. Pharmacol. 9(2), 153–160. Higgs, G.A., Flower, R.J. and Vane, J.R. 1979. A new approach to anti-inflammatory drugs. Biochem. Pharmacol. 28(12), 1959–1961. Ialenti, A., Ianaro, A., Moncada, S. and Di Rosa, M. 1992. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharmacol. 1992;211(2), :177–82. Kazemi, S., Shirzad, H. and Rafieian-Kopaei, M. 2018. Recent findings in the molecular basis of inflammation and anti-inflammatory plants. Curr. Pharm. Des. 24(14), 1551–1562. Lanhers, M.C., Fleurentin, J., Dorfman, P., Mortier, F. and Pelt, J.M. 1991. Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med. 57(3), 225–231. Levine, R. L., Garland, D., Oliver, C. N., Amici, A., Climent, I., Lenz, A. G., Ahn, B.W., Shaltiel, S. and Stadtman, E.R. 1990. [49] Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 186, 464–478. Liu, C.H., Abrams, N.D., Carrick, D.M., Chander, P., Dwyer, J., Hamlet, M.R.J., Macchiarini, F., PrabhuDas, M., Shen, G.L. and Tandon, P. 2017. Biomarkers of chronic inflammation in disease development and prevention: challenges and opportunities. Nat. Immunol. 18, 1175–1180. Makkar, H.P.S. and Becker, K. 1993. Vanillin-HCl method for condensed tannins: effect of organic solvents used for extraction of tannins. J. Chem. Ecol. 19(4), 613–621. Makni, M., Haddar, A., Kriaa,W. and Zeghal, N. 2013. Antioxidant, free radical scavenging, and antimicrobial activities of Ajuga iva leaf extracts. Int. J. Food Prop. 16, 756–765. Mendall, M.A., Patel, P., Asante, M., Ballam, L., Morris, J., Strachan, D.P. and Northfield, T.C. 1997. Relationship of serum cytokine concentrations to cardiovascular risk factors and coronary heart disease. Heart 78(3), 273–277. Mohamed, A.A., Khalil, A.A. and El-Beltagi, H. East .2010. Antioxidant and antimicrobial properties of kaff maryam (Anastatica hierochuntica) and doum palm (Hyphaene thebaica). Grasas y aceites 61(1), 67–75. Morse, L.R., Stolzmann, K., Nguyen, H.P., Jain, N.B., Zayac, C., Gagnon, D.R. and Garshick, E. 2008. Association between mobility mode and C-reactive protein levels in men with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 89(4), 726–731. Ouassou, H., Bouhrim, M., Bencheikh, N., Addi, M., Hano, C., Mekhfi, H., Ziyyat, A., Legssyer, A., Aziz, M. and Bnouham, M. 2021. In vitro antioxidant properties, glucose-diffusion effects,-amylase inhibitory activity, and antidiabetogenic effects of C. Europaea extracts in experimental animals. Antioxidants 10, 1747. Ozgen, M., Reese, R.N., Tulio, A.Z.Jr., Scheerens, J.C. and Miller, A.R. 2006. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2, 2 ‘-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agri. Food Chem. 54(4), 1151–1157. Öztürk, H., Kolak, U. and Meric, C. 2011. Antioxidant, anticholinesterase and antibacterial activities of Jurinea consanguinea DC. Rec. Nat. Prod. 5(1), 43. Posadas, I., Bucci, M., Roviezzo, F., Rossi, A., Parente, L., Sautebin, L. and Cirino, G. 2004. Expression of Concern: Carrageenan-induced mouse paw edema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 142(2), 331–338. Pundarikakshudu, K., Shah, D.H., Panchal, A.H. and Bhavsar, G.C. 2016. Anti-inflammatory activity of fenugreek (Trigonella foenum-graecum Linn) seed petroleum ether extract. Indian J. Pharmacol. 48(4), 441–444. Kayali, R., Cakatay, U., Akçay, T. and Altuğ, T. 2006. Effect of alphalipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of aging rat. Cell Biochem. Funct. 24, 79–85. Ravi, V., Mohamed Saleem, T.S., Patel, S.S., Raamamurthy, J. and Gauthaman, K. 2009. Anti-inflammatory effect of methanolic extract of Solanum nigrum Linn berries. Int. J. Appl. Res. Nat. Prod. 2(2), 33–36. Re, R., Pellegrini, North, Proteggente, A., Pannala, A., Yang, M. and Rice-Evans, C. 1999. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic. Biol. Med. 26(9-10), 1231–1237. Saidi, S., Remok, F., Handaq, N., Drioiche, A., Gourich, A.A., Menyiy, N.E., Amalich, S., Elouardi, M., Touijer, H., Bouhrim, M., Bouissane, L., Nafidi, H.A., Bin Jardan, Y.A., Bourhia, M. and Zair, T. 2023. Phytochemical profile, antioxidant, antimicrobial, and antidiabetic activities of Ajuga iva (L.). Life 13(5), 1165. Salem, M.B., Affes, H., Ksouda, K., Dhouibi, R., Sahnoun, Z., Hammami, S. and Zeghal, K.M. 2015. Pharmacological studies of artichoke leaf extract and their health benefits. Plant Foods Hum. Nutr. 70(4), 441–453. Salem, N., Wissal, W., Dhifi, W., Graya, A., Mnafeg, F., Gharbi, S., Khammassi, S., Brahim, M., Férid, Limam, F., Chekir, R. and Ben Chaouacha-Chekir, R. 2016. Antioxydant activity of Silybum marianum and Ajuga iva natural dyes. Int. J. Control Energy Electr. Eng. 3(2), 6–12. Salvemini, D., Wang, Z.Q., Bourdon, D.M., Stern, M.K., Currie, M.G. and Manning, P.T. 1996. Evidence of peroxynitrite involvement in Carageenan induced rat paw edema. Eur. J. Pharmacol. 303(3), 217–220. Senhaji, S., Lamchouri, F., Bouabid, K., Assem, N., El Haouari, M., Bargach, K. and Toufik, H. 2020. Phenolic contents and antioxidant properties of the aqueous and organic extracts of the Moroccan Ajuga iva Subsp. J. Herbs Spices Med. Plants 26(3), 248–266. Senhaji, S., Lamchouri, F., Boulfia, M., Lachkar, N., Bouabid, K. and Toufik, H. 2022. Mineral c omposition, in vitro inhibitory effects of-amylase,-glucosidase,-galactosidase enzymes, and antibacterial activity of Ajuga iva Subsp. Pseudoiva (DC.) Bric. Biointerface Res. Appl. Chem. 12, 2373–2391. Shin, S.A., Joo, B.J., Lee, J.S., Ryu, G., Han, M., Kim, W.Y., Park, H.H., Lee, J.H. and Lee, C.S. 2020. Phytochemicals as anti-inflammatory agents in animal models of prevalent inflammatory diseases. Molecules 25(24), 5932. Singleton, V.L. and Rossi, J.A. 1965. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic.16, 144–158. Süleyman, H., Demircan, B. and Karagoz, Y. 2007. Anti-inflammatory and side effects of cyclo-oxygenase inhibitors. Am. J. Enol. Vitic. 59(3), 247. Taleb-Senouci, D., Ghomari, H., Krouf, D., Bouderbala, S., Prost, J., Lacaille-Dubois, M.A. and Bouchenak, M 2009. Antioxidant effect of Ajuga iva aqueous extract in streptozotocin-induced diabetic rats. Phytomedicine 16, 623–631. Bawankule, D.U., Trivedi, P., Pal, A., Shanker, K., Singh, M., Sharma, P., Khan, F., Maurya, A.K., Verma, R.K. and Gupta, M.M. 2014. Protective mechanism of lignans from Phyllanthus amarus against galactosamine/lipopolysaccharide-induced hepatitis: an in-vivo and in-silico studies. Curr. Top. Med. Chem. 14(8), 1045–1055. Yaribeygi, H., Atkin, S.L., Pirro, M. and Sahebkar, A. 2019. A review of the anti-inflammatory properties of antidiabetic agents providing protective effects against vascular complications in diabetes. J. Cell Physiol. 234(6), 8286–8294. Yatoo, M.I., Gopalakrishnan, A., Saxena, A., Parray, O.R., Tufani, N.A., Chakraborty, S., Tiwari, R., Dhama, K. and Iqbal, H.M. 2018. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders—a review. Recent Pat. Allergy Drug Discov. 12, 39–58. Yaribeygi, H., Atkin, S.L. and Sahebkar, A. 2018. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 234(2), 1300–1312. Zhishen, J., Mengcheng, T. and Jlanming, West, 1999. The determination of flavonoid contents in mulberry and its scavenging effects on superoxide radicals. Food Chem. 64(4), 555–559. | ||
| How to Cite this Article |
| Pubmed Style Rebhi S, Athmouni K, Dhibi S, Hidouri S, Bouzenna H, Dhibi A, Bouzenna N, Najla H. Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga iva. Open Vet. J.. 2025; 15(7): 3240-3253. doi:10.5455/OVJ.2025.v15.i7.35 Web Style Rebhi S, Athmouni K, Dhibi S, Hidouri S, Bouzenna H, Dhibi A, Bouzenna N, Najla H. Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga iva. https://www.openveterinaryjournal.com/?mno=241953 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i7.35 AMA (American Medical Association) Style Rebhi S, Athmouni K, Dhibi S, Hidouri S, Bouzenna H, Dhibi A, Bouzenna N, Najla H. Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga iva. Open Vet. J.. 2025; 15(7): 3240-3253. doi:10.5455/OVJ.2025.v15.i7.35 Vancouver/ICMJE Style Rebhi S, Athmouni K, Dhibi S, Hidouri S, Bouzenna H, Dhibi A, Bouzenna N, Najla H. Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga iva. Open Vet. J.. (2025), [cited January 25, 2026]; 15(7): 3240-3253. doi:10.5455/OVJ.2025.v15.i7.35 Harvard Style Rebhi, S., Athmouni, . K., Dhibi, . S., Hidouri, . S., Bouzenna, . H., Dhibi, . A., Bouzenna, . N. & Najla, . H. (2025) Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga iva. Open Vet. J., 15 (7), 3240-3253. doi:10.5455/OVJ.2025.v15.i7.35 Turabian Style Rebhi, Sabrine, Kaled Athmouni, Sabah Dhibi, Saida Hidouri, Hafsia Bouzenna, Ayda Dhibi, Nozza Bouzenna, and Hfaiedh Najla. 2025. Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga iva. Open Veterinary Journal, 15 (7), 3240-3253. doi:10.5455/OVJ.2025.v15.i7.35 Chicago Style Rebhi, Sabrine, Kaled Athmouni, Sabah Dhibi, Saida Hidouri, Hafsia Bouzenna, Ayda Dhibi, Nozza Bouzenna, and Hfaiedh Najla. "Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga iva." Open Veterinary Journal 15 (2025), 3240-3253. doi:10.5455/OVJ.2025.v15.i7.35 MLA (The Modern Language Association) Style Rebhi, Sabrine, Kaled Athmouni, Sabah Dhibi, Saida Hidouri, Hafsia Bouzenna, Ayda Dhibi, Nozza Bouzenna, and Hfaiedh Najla. "Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga iva." Open Veterinary Journal 15.7 (2025), 3240-3253. Print. doi:10.5455/OVJ.2025.v15.i7.35 APA (American Psychological Association) Style Rebhi, S., Athmouni, . K., Dhibi, . S., Hidouri, . S., Bouzenna, . H., Dhibi, . A., Bouzenna, . N. & Najla, . H. (2025) Chemical characterization, antioxidant capacity, and anti-inflammatory potentials invitro and in vivo of phenolic compounds extracted from Ajuga iva. Open Veterinary Journal, 15 (7), 3240-3253. doi:10.5455/OVJ.2025.v15.i7.35 |