| Research Article | ||
Open Vet. J.. 2025; 15(4): 1771-1783 Open Veterinary Journal, (2025), Vol. 15(4): 1771-1783 Research Article Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultryAndri Kusmayadi1*, Richa Mardianingrum2 and Yanti Yanti31Department of Animal Science, Universitas Perjuangan Tasikmalaya, Tasikmalaya, Indonesia 2Department of Pharmacy, Universitas Perjuangan Tasikmalaya, Tasikmalaya, Indonesia 3Department of Mechatronics, Faculty of Engineering, Universitas Mayasari Bakti, Tasikmalaya, Indonesia. *Corresponding Author: Andri Kusmayadi. Department of Animal Science, Universitas Perjuangan Tasikmalaya, Tasikmalaya, Indonesia. Email: andrikusmayadi [at] unper.ac.id Submitted: 07/02/2025 Accepted: 15/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
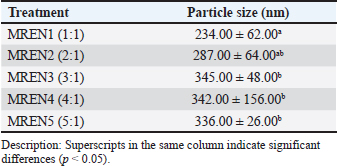
AbstractBackground: The application of nanoencapsulation technology to mangosteen rind aims to increase the stability, bioavailability, and solubility of bioactive compounds. The nanoencapsulation using chitosan and sodium tripolyphosphate (STPP) via ionic gelation yielded the best nanocapsule characteristics compared to other methods. Aim: To investigate the formulation and characterization of mangosteen rind extract nanocapsules (MREN) using chitosan cross-linked with STPP at different ratios as a candidate poultry feed additive. Methods: Ethanol was used as a solvent in the maceration process to extract mangosteen rind. After weighing the mangosteen rind, a solvent was added at a 1:5 (w/v) ratio. At room temperature, maceration lasted for 24 hours. A rotary vacuum evaporator was used to concentrate the filtrate from each extraction process for 20 minutes at a temperature between 40°C and 45°C. Mangosteen rind extract (MRE), chitosan, and STPP were combined to obtain MREN. Chitosan and STPP were used at the following ratios: 1:1, 2:1, 3:1, 4:1, and 5:1 (MREN1–MREN5, respectively). Following mixing, the mixture was homogenized for 5 minutes using a homogenizer, hydrated for 18 hours at 4°C, and then homogenized once more for 30 seconds. With an input temperature of 170°C, feed rate of 15 ml/min, and nozzle atomizer pressure of one bar, spray drying was performed using a spray drier. The final nanocapsule products were then examined for particle size, zeta potential, and morphology. Results: The application of MREN as a candidate poultry feed additive was investigated based on the results of its characterization. The findings demonstrated that the addition of chitosan and STPP to the nanoencapsulation of mangosteen rind extract significantly affected the particle size, zeta potential, and morphology. The particle size increased with increasing chitosan content relative to STPP; the smallest particle size was obtained in the 1:1 treatment at the lowest chitosan dosage. In comparison with the other characteristics, the best and most stable zeta potential was obtained when chitosan was used at 2:1 and 3:1. Compared with the other treatments, the 1:1 treatment resulted in the ideal nanocapsule morphology in terms of morphological features. Conclusion: The mangosteen rind extract nanocapsule product, at a 1:1 ratio, has great potential for application as a feed additive in poultry. Nanoencapsulated mangosteen rind extract products have great potential as feed additives for poultry. Keywords: Characterization, Feed additive, Formulation, Mangosteen rind extract nanocapsules, Poultry. IntroductionMangosteen is a horticultural plant that is widely cultivated in tropical countries, including Indonesia. In Indonesia, West Java Province is the largest producer of mangosteen, and in 2020, its production reached 90,024 tons. The several regions in West Java Province, Tasikmalaya Regency is the largest producer of mangosteen, contributing 45% of national mangosteen production. In 2020, mangosteen production in Tasikmalaya Regency reached 60,468 tons (Noormahesa, 2024). In each part of the mangosteen fruit, the mangosteen rind is the part with the largest composition with a yield percentage reaching 65% (Chaovanalikit et al., 2018), which is reported to contain xanthone (alpha-mangostin) as the highest secondary metabolite compound compared to other parts of the fruit (Dermawan et al., 2019). Xanthones have many pharmacological functions such as antibacterial (Dharasivkar et al., 2017; Gondokesumo et al., 2019; Soliman et al., 2022; Lu et al., 2023; Poetri et al., 2023; Resende et al., 2024), antihyperlipidemic (Abuzaid et al., 2017; Suksamran et al., 2022; Cardozo-Muñoz et al., 2022), and antioxidant (Sriwidodo et al., 2022; Albuquerque et al., 2023; Indiarto et al., 2023; Suhandi et al., 2023). Extraction methods and solvent types are factors that greatly influence the isolation of bioactive components in mangosteen rind (Yuvanatemiya et al., 2022; Cao et al., 2023). Extraction methods and solvent types need to be studied to isolate the best bioactive compounds, especially their antioxidant activity. Mangosteen rind extract contains antioxidant chemicals with unstable properties (Resende et al., 2020), sensitive (Alves et al., 2024), and easily react and oxidize (Kusmayadi et al., 2019a; Yuvanatemiya et al., 2022; Cao et al., 2023; Sefcova et al., 2023). The stability of mangosteen rind extract can be increased by using nanoencapsulation technology (Li et al., 2023; Taouzinet et al., 2023), solubility (Rezaei et al., 2019; Pateiro et al., 2021), bioavailability (Ayala-Fuentes et al., 2021; Prieto et al., 2024), and control drug release by encapsulating active ingredients into nanometer-sized particles (<100 nm) using encapsulant materials (Rohilla et al., 2020; Zhang et al., 2020; Soliman et al., 2022). Encapsulant materials commonly used in the encapsulation process are chitosan (Dharasivkar et al., 2017), lipids (Mirzaeie et al., 2019), proteins (Khosroshahi et al., 2019), polysaccharides (Joshi et al., 2017), and maltodextrin (Dharasivkar et al., 2017; Hwang et al., 2017). Previous research, namely the microencapsulation of MRE using maltodextrin, has several weaknesses, namely that the ability to encapsulate active ingredients is less stable, with sensitive solubility and stability levels (Kusmayadi et al., 2019b). Chitosan and sodium tripolyphosphate (STPP) are used in the ionic gelation method of nanoencapsulation (Soltanzadeh et al., 2021; Kim et al., 2022) and yield the best nanocapsule properties when compared with alternative techniques (Alehosseini et al., 2022; Kim et al., 2022). The anion phosphate group of STPP interacts with the amine group of chitosan by forming cross-links (Susilawati et al., 2022), thereby maintaining the core material’s stability (mangosteen rind extract). Reducing the size of chitosan to nanometers will improve its performance as an encapsulant material. This is because the smaller the particle size, the greater the surface interaction, and mobility, allowing it to become more active. According to Prihastanti et al. (2018), nanoparticles can readily pass through the cell wall pores because their diameter is smaller than the cell wall’s diameter. A polyanion called STPP crosslinks with chitosan to create nanochitosan. STPP is neither mutagenic nor carcinogenic, so it is safe to use as an encapsulant material. The characterization results of the nanoencapsulation formulation of mangosteen rind extract are influenced by the ratio of chitosan to STPP used in the nanoencapsulation process via ionic gelation (Safitri et al., 2022). The size of the nanoparticles decreases as the amount of STPP used increases, and the size of the nanoparticles increases when the amount of STPP is reduced (Al-Nemrawi et al., 2018). Research on the formulation of mangosteen rind extract nanoencapsulation utilizing chitosan and STPP in varying ratios to yield the best properties has not yet been conducted. This research has the potential to investigate the best formulation of the ratio usage between chitosan and STPP as an encapsulant material and crosslinking agent so that it can protect the mangosteen rind extract nanocapsule (MREN) product. The results of this study will be used as a consideration for the formulation of MREN as a feed additive candidate for poultry. One of the main factors influencing the production of effective and efficient nanoencapsulation of herbal ingredients is the ratio of encapsulant material use. Until now, no research has examined the formulation of poultry feed additives based on mangosteen rind extract nanoencapsulation using chitosan and STPP, especially the ratio of its use. The existing research until now is only a study on the microencapsulation of mangosteen rind extract using encapsulant material, namely maltodextrin derived from palm starch (Kusmayadi, 2021). Feed additive in poultry is given as a mixture of feed ingredients in small amounts with the aim of increasing productivity (Kusmayadi et al., 2019c; Hameed et al., 2021), increasing nutritional value and compatibility of feed nutrients that can support poultry growth and reproduction (Tripuratapini et al., 2015), improving health status (Pandit et al., 2023), and increasing poultry production efficiency (Puvaca et al., 2020). Poultry feed additives on the nanoparticle scale aim to increase solubility, improve bioavailability, modify the drug delivery system, increase stability, and increase the absorption of active substances. This study is expected to produce the best formulation and characterization of MREN that are ready to be used as poultry feed additives. Materials and MethodsMaterialsMangosteen rind from Puspahiang District, Tasikmalaya Regency, ethanol 96% (Merck, Germany), chitosan (Merck, Germany), STPP (Merck, Germany), methanol, NaOH, HCl, the standard of α-mangostin, artificial intestinal fluid, aquadest, aquabidest, tween 80, ethyl acetate, anaerobic incubator, analytical scales, vortex, magnetic stirrer (IKA® C-MAG HS7), particle size analyzer “Beckman Coulter LS 13 320” ultrasonicator, scanning electron microscope “JEOL: JSM”6360 LA,” zeta potential analyzer “Beckman Coulter Delsa TM Nano Common Version 2.31/2.03”, auto carbon coater (Joel JEC-560, Japan), thermostatic water bath shaking device “JULABO SW”, rotary evaporator, homogenizer, spray drier, measuring cup, UV spectrophotometer Helios Alpha visible, spectrophotometer (Hitachi U-2810), dissolution tool, micropipette, drop pipette, propipette/rubber suction pump, autoclave, laminar air flow, pH meter, and measuring pipette. MethodsMangosteen rind was extracted using a modified extraction method developed by Ningsih et al. (2017) using ethanol solvent and extraction method maceration. Mangosteen fruit is obtained from Puspahiang District, Tasikmalaya Regency. The mangosteen rind is then cut and sliced to a size of 2 cm. The rind of mangosteen is pounded and sieved to a size of 40 mesh after being dried in an oven set at 40°C–50°C for 24 hours or until the water content is 10%. The flour obtained from the mangosteen rind sieve was weighed to 100 g, and a 1:5 (w/v) solvent ratio was applied. At room temperature, maceration lasted for 24 hours. After filtering each extraction dreg, the filtrate was gathered. The solvent was added to the dregs at a ratio of 1:2. The filtrate from each extraction method was concentrated using a rotary vacuum evaporator (Buchi R-300, Switzerland) at a temperature of 40°C–45°C for up to 20 minutes. The ionic gelation process was used to synthesize mangosteen rind extract nanocapsules (MREN). Mangosteen rind extract (MRE), chitosan solution, and STPP were combined to generate MREN. Chitosan and STPP have the following ratios: 1:1, 2:1, 3:1, 4:1, and 5:1 (MREN1–MREN5). The preparation of a 0.2% chitosan solution was performed by dissolving 0.2 g of chitosan in 100 ml of glacial acetic acid (Merck, Germany), 1% for MRE, and 0.1% glacial acetic acid. Meanwhile, a 0.2% STPP solution was prepared by mixing 0.2 g of STPP into 100 ml of distilled water, as well as the other concentrations. After mixing, it was homogenized with a homogenizer for 5 minutes (T25 digital Ultra Turrax), then hydrated for 18 hours at 4°C, and homogenized again for 30 seconds. With a nozzle atomizer pressure of one bar and an inlet temperature of 170°C, a spray dryer (LabPlant SD-05) was used to perform the process. The feed rate was 15 ml/min. The final nanocapsule products were then examined for shape, zeta potential, and particle size. The mechanism of the ionic gelation interaction between chitosan and STPP occurs through gel formation via ionic bonds between the positive charge of chitosan and the negative charge of STPP. The chitosan used in this study was dissolved in a glacial acetic acid solution (pH < 6) so that it was protonated to form –NH3+, so that it was positively charged. Similarly, STPP was dissolved in glacial acetic acid to undergo ionization and the formation of negatively charged polyphosphate anions. Furthermore, when the STPP solution was added to the chitosan solution, an electrostatic interaction occurred between the –NH3+ group on the chitosan and the –PO4– group on the STPP. This interaction causes crosslinking between the chitosan chains, as indicated by the change of the solution into a gel. In this case, STPP acts as a crosslinking agent that forms the chitosan-STPP gel network. The crosslink strength depends on the ratio of chitosan to STPP. ParametersParticle size, zeta potential, and morphological tests were performed on the resultant mangosteen rind extract nanocapsule products (Hussain et al., 2017; Afifah et al., 2021; Kusmayadi et al., 2019b.). The formulation of mangosteen rind extract nanocapsules with the best characterization will then serve as the foundation for its usage as a poultry feed additive. Data analysisThe parameter testing experiment for each treatment was carried out 5 times. Each formulation’s mangosteen rind extract nanocapsules characterization findings were tabulated and subjected to analysis of variance using Statistical Product and Service Solutions (SPSS) Version 25.0 (IBM, USA). Continue using the Duncan Multiple Range Test if there is a noticeable difference (p < 0.05). Ethical approvalNot needed for this study. ResultsParticle sizeThe particle size of mangosteen rind extract nanoencapsulation needs to be measured to determine the particle size because it affects the stability of the system, bioavailability, release of active substances, and physicochemical characteristics before being given as a feed additive to poultry. Table 1 displays the MREN particle size. The results of the particle size test of mangosteen rind extract nanocapsules showed that the chitosan:STPP ratio had a significant effect (p < 0.05) on particle size. The smallest particle size was observed for the MREN1 treatment, which contained a ratio of 1:1 (234.00), which was significantly different from the other treatments (MREN2–MREN5). The results showed that increasing chitosan to STPP significantly increased the particle size (287.00–345.00). The MREN3 treatment with a ratio of 3:1 (345.00) produced the largest particle size, but with increasing chitosan to STPP ratio, the particle size decreased. The results of the nanoencapsulation study of mangosteen rind extract produced particle sizes ranging from 234.00 to 345.00 nm. This condition indicates that all treatments have nanocapsules particle size values that are still reasonable in the nanoparticle size. This is because the particle size in the nanocapsules product is usually in the range of 1–1,000 nm, but is generally optimal in the range of 100–500 nm in food and pharmaceutical application products. However, smaller particle sizes tend to increase bioavailability and controlled release in the body or environment. This is very important in terms of preparing alternative feed additives for poultry to ensure they are effective and efficient in their use. Table 1. Particle size of mangosteen rind extract nanocapsules.
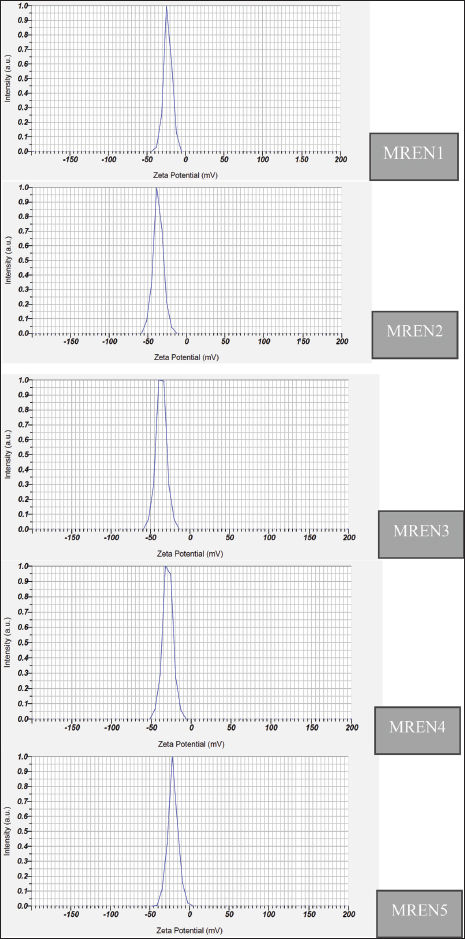
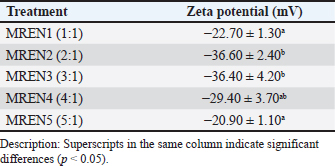
Fig. 1. Zeta potential of mangosteen rind extract nanocapsules. Table 2. Zeta potential of mangosteen rind extract nanocapsules.
Zeta potentialThe zeta potential in nanoencapsulation refers to the electrical charge around the nanoparticles dispersed in the medium. The zeta potential is an important parameter for determining the stability of a nanocapsule system because a high zeta potential value (either positive or negative) indicates a repulsive force between particles, which can prevent aggregation or clumping. Zeta potential testing is used to ascertain a particle’s surface charge type. The zeta potential is a measure of the electrical charge between colloidal particles; the higher the value, the more inhibited the flocculation. Figure 1 and Table 2 show the findings of the study on the zeta potential of a formulation of mangosteen rind extract nanocapsules. The setting of spray drying conditions (inlet temperature, feed rate, nozzle pressure) was based on several previous studies. The optimal inlet temperature was set to 170°C because it can prevent the degradation of the active substance xanthone, but at this temperature, it could still dry the particles well. Likewise, the feed rate used was 15 ml/min because it could balance the production speed and the resulting encapsulation efficiency. Furthermore, the nozzle pressure used is one bar, which is based on this number and is able to produce a uniform droplet size so that the nanoparticles are more stable. This setting has considered avoiding agglomeration, degradation of active substances, and poor encapsulation efficiency. With this optimal setting, the resulting nanoparticles can be more stable, effective, and have a controlled release of active substances in the poultry digestive system. The results of the zeta potential study on mangosteen rind extract nanocapsules were significantly affected by the ratio between chitosan and STPP (p < 0.05). The results ranged from 20.90 to 36.60 mV. The zeta potential values in the MREN1 (1:1) and MREN5 (5:1) treatments containing the lowest and highest chitosan ratios, respectively, produced the lowest zeta potential charge compared with the other treatments with values of –20.90 and –22.70 mV. These results indicate that the zeta potential values in all treatments were negatively charged. Nanocapsules with negatively charged zeta potential are widely used in the pharmaceutical, food, and cosmetic fields because of their more stable nature in biological media and their ability to extend circulation time in the body. Some other advantages of nanocapsules with negative zeta potential are that they can increase dispersion stability, reduce unwanted interactions with cell membranes, extend circulation time in the blood, and play a role in targeted drug delivery applications. This treatment is beneficial to the digestive tract of poultry because the nanoencapsulation product of mangosteen rind extract can work effectively and efficiently.
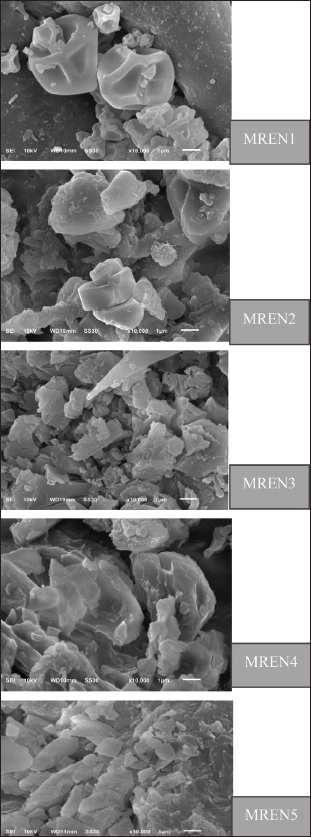
Fig. 2. Morphology of mangosteen rind extract nanocapsules. MorphologyThe morphology of mangosteen rind extract nanocapsules is very important to measure to determine the stability, release of active substances, bioavailability, and interaction with target cells or environments before being given orally to poultry. The morphology of the nanocapsules material produced in this study greatly determines the efficiency of the active substance delivery system in this case α-mangostin in the digestive tract of poultry. The selection of production methods, materials, and processing conditions will greatly affect the shape, size, and stability of nanoencapsulation. Figure 2 displays the findings of the morphological test of the mangosteen rind extract nanocapsules. Morphological characterization of mangosteen rind extract nanocapsules using SEM. The morphology of the nanocapsule product is an indicator to determine the level of stability, release of active substances, and the efficiency of protecting mangosteen rind extract from environmental degradation, such as pH, temperature, and enzymes. The morphology of the nanoparticles used to encapsulate active substances refers to their shape, size, and structure. The shape and morphological structure of the mangosteen rind extract nanoencapsulated are spherical and somewhat oval. The results of this morphology indicate the presence of solid particles with a hollow structure, where the surface of the mangosteen rind extract nanocapsules appears smooth and homogeneous. The results of the mangosteen rind extract nanocapsules show that the mangosteen rind extract is able to be dispersed well in the matrix or in the core with a protective layer on the outside. The morphology of the mangosteen rind extract nanocapsules is greatly determined by the encapsulation method used, the carrier material, and its formulation. DiscussionParticle sizeThe particle size test findings of the mangosteen rind extract nanocapsules, which are shown in Table 1, indicate that the combination of chitosan, STPP, and the mangosteen rind extract formulation significantly (p < 0.05) affects particle size. This demonstrates how variations in the particle size of the resultant nanocapsules are influenced by the comparison of chitosan-STPP as the covering material and mangosteen rind extract as the core material. The particle size increases with increasing proportion of chitosan to STPP, and vice versa. The mangosteen rind extract nanocapsules prepared in this work have particle size test findings ranging from 234.00 to 345.00 nm. According to Rahmawanty et al. (2017), Jeevanandam et al. (2018), and Dewayanti et al. (2023), a particle is considered nanosized if its size falls between 1 and 1,000 nm. These findings are consistent with this range. The particle size of the mangosteen rind extract nanocapsules was in the range of 234.00–345.00 nm, which means that this size is in the range of nanoparticles. However, the large SD value in the MREN4 treatment occurred because the particle size distribution in MREN4 when tested using the particle size analyzer “Beckman Coulter LS 13 320” had a larger difference in the range of values when data collection was carried out on the 1st–5th repetitions compared to other treatments which had lower standard deviation values. The particle size of MREN in all of these treatments is indicated to increase the bioavailability and stability of MREN in the digestive tract of poultry. This result is due to the increased solubility and stability of xanthone bioactive compounds in the mangosteen rind extract nanocapsules. Xanthone compounds have low solubility in water, limiting their absorption in the digestive tract. However, the nanoencapsulation method can increase the dispersion and solubility of xanthones so that they are more easily absorbed in the intestines of poultry. This occurs because mangosteen rind extract nanocapsules can protect xanthone compounds against the digestive environment. Bioactive compounds in mangosteen rind extract can be damaged by acidic stomach pH and digestive enzymes. In addition, nanocapsules at nanoparticle size can maintain the stability of MREN products by protecting bioactive compounds from degradation before they reach the intestines, enabling more compounds to be absorbed. This is because the size of the nanoparticles in MREN has a larger surface area; thus, they are more easily dissolved and absorbed by the intestines as a place for optimal absorption. According to previous studies, the ratio of the coating material used has a significant impact on particle size (Tolve et al., 2016). According to the results presented in Table 1, the greater the particle size produced, the more chitosan coating material was used in comparison with STPP. These findings support the research of Alehosseini et al. (2022), who found that the particle size, zeta potential, and encapsulation effectiveness of nanocapsules are all significantly affected by the ratio of chitosan to STPP used to coat the core material. Larger particle size, higher zeta potential, and higher encapsulation efficiency are the results of using more chitosan than STPP (Soltanzadeh et al., 2021; Alehosseini et al., 2022). Bangun et al. (2018) found that using higher chitosan concentrations led to larger particle sizes. The chitosan:STPP ratios tested were 2.5:1, 5:1, 7.5:1, and 10:1, and the average particle sizes that were increasing were 238.17, 575.20, 706.01, and 1315.37 nm, respectively. These findings support our findings that MREN3 produced the largest particle size (345.00 nm) and MREN1 produced the smallest (234.00 nm). One benefit of using chitosan as a coating material in the right amount is that it creates a good semipermeable membrane with a high degree of flexibility, hardness, and resistance. In addition to improving mechanical properties and providing a highly effective film barrier for chitosan-based goods, the crosslinking process on chitosan can release the medications contained it in a much more controlled manner. One of the most popular crosslinking techniques is the ionic gelation method, in which chitosan and STPP crosslink to form a better film layer that protects the core material and has additional benefits like changing the material’s physicochemical properties and making it much more stable (Atlar et al., 2024). In order to make nanocapsule products more effective and efficient at reaching target cells, the particle size is crucial for controlling their biodistribution and pharmacokinetic characteristics. Reducing the particle size will also change the nanocapsule’s charge and conformation of the nanocapsule, which has a positive effect on the product’s mechanism of action and the nanocapsule’s characteristics in the biological environment (Li et al., 2021). This condition is very helpful as a candidate for a feed additive in poultry, where the application of very small nanocapsules of mangosteen rind extract is expected to have a very good impact on the poultry body’s physiological system, which will be positively correlated with increased poultry productivity. Zeta potentialThe findings demonstrated (Fig. 1; Table 2) that the percentage ratio of chitosan:STPP affected the zeta potential value of mangosteen rind extract nanocapsules. where a comparatively larger zeta potential value was obtained with additional chitosan-STPP additions. The MREN2 and MREN3 treatments in this study had higher zeta potential values (–36.60 to 36.40 mV) than the MREN4 and MREN5 treatments (–29.40 and –20.90 mV). According to these findings, nanocapsule stability in MREN2 and MREN3 therapies was superior to that of other treatments. Nanoparticles with a zeta potential greater than +30 mV and less than 30 mV are more stable, as claimed by Juliantoni et al. (2020). A reasonable level of nanocapsule stability is indicated by a zeta potential value exceeding 30 mV or below –30 mV is generally considered to be physically stable suspensions (Feyzioglu et al., 2016; Zhang et al., 2020). The colloidal compound’s degree of stability is indicated by its zeta potential. The colloidal compound’s degree of stability increases with the zeta potential. A high zeta potential value is anticipated in the electrophoretic process because it can accelerate particle mobility in an electric field while preventing sedimentation or clumping (Midekessa et al., 2020). The zeta potential values in the MREN2 and MREN3 treatments were 30 mV. A negatively charged zeta potential of –30 mV and below (>–30 mV) as occurs in MREN2 and MREN3, will produce strong electrostatic repulsion between particles, preventing aggregation and increasing suspension stability. In addition, nanoparticles with a zeta potential between 30 and 50 mV are more stable in solution so that they do not easily clump when passing through the stomach and intestines of poultry. This has various advantages. In addition to being able to maintain the stability of nanoparticles to prevent clumping, it can also reduce excessive interaction with the intestinal mucosa in preventing irritation, supporting a more controlled and efficient release of active substances, and helping balance intestinal microflora, improving poultry digestive health. In the poultry digestive system, pH and enzymes can affect nanoparticle stability. High negative zeta potential in MREN2 and MREN3 will disperse the particles, resulting in greater distribution of active substances, even in the digestive tract. Nanoparticles with higher negative charges will not be quickly destroyed by digestive enzymes, so active substances such as xanthones from mangosteen rind extract can be available for longer in the intestines. This can prevent the release of active substances too quickly, which can cause the degradation of bioactive compounds before they are optimally absorbed by poultry. A slower release of bioactive substances will help maintain more stable levels of active substances in the blood and increase the bioavailability of xanthones, thereby improving their antioxidant and antimicrobial effects. Other treatments with higher zeta potentials and approaching 0 mV will cause particles to settle and clump together, reducing the effectiveness of the release of active substances. Particle aggregates form more readily in a dispersion system with a low zeta potential to attract one another, and flocculation occurs as a result of the particles’ contact with Van der Waals forces. The repulsion between particles with the same electric charge will stop them from aggregating, but a particularly positive or negative zeta potential value will result in a stronger repulsive force. Because a high zeta potential value, both positive and negative, will be more electrically stable, the zeta value in the zeta potential test should be more than +25 or –25 mV. A low zeta value may cause the particles to flocculate or cluster together, thereby impairing their physical stability (Haidar et al., 2017; Amyliana and Agustini, 2021). The zeta potential is an often-used metric to classify particle interaction and aggregation. Electrophoretic mobility measurements are frequently used to determine this parameter. The mobility and zeta potential can be directly correlated for single particles using a variety of assumptions. Nevertheless, similar approximations are less frequently used for aggregates, which are frequently found in industrial and environmental applications (Engelhardt et al., 2024). The particles will be able to attract one another and flocculate if the zeta potential decreases. While colloids with low zeta potential values tend to thicken or flocculate, colloidal materials with both positive and negative zeta potential values remain stable. To avoid drug delivery aggregation, excellent particles should ideally have a zeta potential charge greater than the dispersing medium. The charge of mangosteen rind extract nanocapsules as a potential feed additive for poultry is summarized by the zeta potential value, which is consistent with other studies that show that the higher the zeta potential value, the greater the electrostatic repulsion between particles and can minimize aggregation or flocculation (Li et al., 2020). In the medication delivery process, the zeta value is a measure of the drug’s capacity to interact with the mucosal layer’s surface absorption barrier. For positively charged submicroparticles to adhere and be deemed mucoadhesive, the mucosal layer should be negatively charged (Ways et al., 2018; Abourehab et al., 2022). The performance of drug therapy can be enhanced by the capacity of submicroparticles with high mucoadhesive qualities to prolong the drug contact period during which the drug is delivered. Similarly, positively charged nanoparticles can boost drug permeability because cell membranes are negatively charged (Desai et al., 2023). Negatively charged zeta potential has the advantage of maintaining the stability of mangosteen rind extract nanocapsules in the fluid in the poultry digestive tract, which is much better than positively charged. This is because the negatively charged zeta potential increases the stability of the suspension, which repels each other, thus preventing agglomeration or sedimentation in the poultry digestive fluid. The poultry intestinal mucosa has a natural negative charge, and the intestinal mucosal layer is composed of negatively charged glycoproteins and mucopolysaccharides. Negatively charged nanoparticles tend to be evenly distributed and not too tightly bound to the intestinal mucosa; thus, absorption is more controlled and does not cause irritation. Conversely, positively charged nanoparticles can cause irritation in the digestive tract due to strong interactions with the mucosa, where positive nanoparticles can stick excessively, causing inflammation or irritation of the intestinal epithelium. This can interfere with normal intestinal function and reduce digestive efficiency. In addition, negatively charged nanoparticles can exert more selective effects on intestinal bacteria. Many beneficial bacteria such as Lactobacillus and Bifidobacterium have negatively charged surfaces. Negatively charged nanoparticles will not disrupt the balance of the microbiota, thus supporting the optimal feed fermentation process (microbiological digestion process) and improving gut health. On the contrary, positive nanoparticles are more toxic to good bacteria. This is because of the strong electrostatic interaction, positively charged nanoparticles can damage the membranes of good bacteria, causing an imbalance in the microflora. This can cause an imbalance in the microbiota, which has the potential to inhibit digestion and increase the risk of pathogen infection. Negatively charged nanoparticles are more efficient in releasing active substances. Negatively charged MREN allows for controlled drug release, so that active substances such as xanthones in mangosteen rind extract or other feed nutrients can be available for a longer period in the intestines of poultry. This treatment can gradually increase nutrient absorption, making it more effective for improving poultry health and growth. On the other hand, positive nanoparticles can cause absorption that is too fast or inefficient because they are hampered by electrostatic interactions, thereby reducing their efficiency. Therefore, selecting negatively charged nanoparticles is a more effective and safe strategy for improving poultry health and performance. MorphologyUsing an SEM, the mangosteen rind extract nanocapsules were examined morphologically. The goal of morphological characteristics utilizing SEM, according to Natalia et al. (2023), is to ascertain the size, texture, and arrangement of constituent particles on the particle layer’s surface. The surface morphology of particles in a sample at both the microscale and nanoscale can be observed using this SEM analysis approach. SEM is a great way to learn about surface structure from a microscopic perspective, including grain boundaries, pores, and layer texture (Sujanto et al., 2015). The mangosteen rind extract nanocapsulated with chitosan and STPP has a very good morphological shape with a round shape, as seen in the morphology of the particles produced in this study (Fig. 2). This is more evident in the MREN1 treatment, in which the mangosteen rind extract is surrounded by chitosan and STPP with a round shape. According to Alves et al. (2016), gallic acid nanoencapsulation with chitosan and STPP resulted in spherical nanoparticles with a reasonably uniform diameter and a spherical nanoencapsulation shape in the presence of self-healing material (Dani et al., 2022). According to Susilawati et al. (2022), nanoencapsulation with chitosan and STPP resulted in oval-shaped nanocapsules. This oval ball shape is the result of an ionic gelation process that creates spherical particles between the active components, specifically, negatively charged mangosteen rind extract, positively charged chitosan, and negatively charged STPP. However, it appears as an uneven MREN morphology in other therapies. The morphological form produced by the mangosteen rind extract nanocapsules in MREN1 treatment has the same percentage between round and oval shapes (50:50). The round and oval shapes produced in this mangosteen rind extract nanocapsules study are indicated to affect stability, release of active substances, and interaction with cells and intestinal mucosa of poultry. The distribution of round particles is more stable in the stomach because it has a lower surface area or volume ratio; thus, it is not quickly degraded by enzymes and gastric acid (diffusion of active substances is slower). The morphology of oval-shaped nanoparticles is easier to attach to the intestinal mucosa, increasing interaction with the intestinal epithelium, but they can be decomposed faster or more easily degraded due to their larger surface area and faster diffusion of active substances. A 50:50 combination of round and oval shapes can be used for more effective control of the release of active substances, with oval shapes for initial release and round shapes for gradual release in the digestive system of poultry. According to the study, nanocapsule goods that were spray-dried with a spray dryer had an uneven morphology, including pockets, fissures, depressions, and a circular or serrated surface. The delay in the film-forming process during the drying of the atomized sample droplets is the cause of the nanocapsule shape imperfection (Alves et al., 2017). The relatively smooth surface layer’s morphology will alter to appear rough as the current density rises; this is shown by fewer fractures and agglomeration on the particle surface layer. Sedimentation or clumping causes agglomeration, which results in a rough surface shape (Natalia et al., 2023). In the meantime, particle shrinkage from the droplets during drying or chilling causes the dented outer surface of the nanocapsule (Amin et al., 2018; Rosenberg et al., 1990). Because the bioactive compounds are shielded by the coating matrix, specifically chitosan-STPP, the resulting nanocapsules have a shape similar to matrix-type nanocapsules. The morphological features of the matrix-shaped nanocapsules demonstrate that the nanoencapsulation procedure was successful. As a low-molecular-weight coating material, chitosan-STPP can function as an effective film layer and help prevent the nanocapsule surface from shrinking. While this morphological test has no effect on the durability of the nanocapsule, it does indicate the activity of its flow within the cell. The morphological features of nanocapsules indicate a propensity for smaller particle aggregation and larger particle size. Increasing the proportion of coating material is one strategy to stop agglomeration (Amin et al., 2018). Chitosan and STPP shield the mangosteen rind extract nanocapsules in the digestive system before the small intestine, ensuring adequate digestion of MREN when it enters the small intestine, according to the results of the morphological test of the mangosteen rind extract nanocapsules. The use of herbal substances in chicken feed additives can be improved due to the benefits of nanoencapsulation technology (Linh et al., 2022). Adil et al. (2024) found that the use of nanoencapsulation technology in poultry farming can improve efficiency, cover off unpleasant tastes and odors, increase bioavailability, increase drug efficacy, and release and target drug delivery systems precisely and in a controlled manner (Kalagatur et al., 2018). Furthermore, chitosan’s outstanding antibacterial properties, cationic charge, safety, biodegradability, and biocompatibility are all enhanced by the encapsulant materials used in this case. To enhance the antibacterial activity of mangosteen rind extract nanocapsules against harmful bacteria, chitosan’s positive charge can promote more adherence to negatively charged microbial membranes. The nanoencapsulation process allows the release of xanthones slowly, thereby prolonging the antibacterial effect. The nanoencapsulation of mangosteen rind extract increases the antibacterial effectiveness of xanthones by increasing their stability, bioavailability, and gradual release, making it more effective in inhibiting the growth of pathogenic bacteria in poultry intestines. These changes contribute to digestive tract health, increased feed efficiency, and better poultry performance. This method is more effective than ordinary extracts that are quickly eliminated from the poultry body. The antibacterial benefits of mangosteen rind extract nanocapsules in poultry can reduce pathogenic bacterial infections in the intestines of poultry by reducing the risk of diarrhea and digestive disorders. Improve the balance of intestinal microbiota by supporting the growth of beneficial bacteria, such as Lactobacillus sp. Reduce dependence on synthetic antibiotics, thereby avoiding the risk of antibiotic resistance. Increase feed efficiency and poultry growth to ensure that poultry are healthier and that weight gain is faster. ConclusionMangosteen rind extract nanocapsule formulations employing different chitosan:STPP ratios resulted in a substantial (p < 0.05) impact on particle size and zeta potential. The particle size increased with increasing chitosan content relative to STPP; the smallest particle size was obtained in the 1:1 treatment at the lowest chitosan dosage. In comparison with the other characteristics, the best and most stable zeta potential was obtained when chitosan was used at 2:1 and 3:1. Compared with the other treatments, the 1:1 treatment resulted in the ideal nanocapsule morphology in terms of morphological features. The mangosteen rind extract nanocapsule product, at a 1:1 ratio, has great potential for application as a feed additive in poultry to boost poultry productivity, according to the characterization data. AcknowledgmentThe authors would like to thank the Directorate of Research, Technology, and Community Service, and the Ministry of Education, Culture, Research, and Technology, Republic of Indonesia for the grant (Grant No. 180/E5/PG.02.00.PL/2023) through the Fundamental Research Scheme. Conflict of interestThe authors declare no conflicts of interest. FundingThis study was funded by the Directorate of Research, Technology, and Community Service, and Ministry of Education, Culture, Research, and Technology, Republic of Indonesia with the grant number (180/E5/PG.02.00.PL/2023) through the Fundamental Research Scheme. Authors’ contributionThe study presented here was carried out in collaboration with all the authors (AK, RM, and YY). AK and RM defined the research theme, methods, and experiments. AK and YY conducted the experiments, organized the data, interpreted the results, and wrote the manuscript. All authors have read, reviewed, and approved the final manuscript. Data availabilityAll data generated or analyzed during this study are included in this manuscript. ReferencesAbourehab, M.A.S., Pramanik, S., Abdelgawad, M.A., Abualsoud, B.M., Kadi, A., Ansari, M.J. and Deepak, A. 2022. Recent advances of chitosan formulations in biomedical applications. Int. J. Mol. Sci. 23(18), 10975; doi:10.3390/ijms231810975. Abuzaid, A.S., Sukandar, E.Y., Kurniati, N.F. and Adnyana, I.K. 2017. Antihyperlidemic effects of mangosteen (Garcinia mangostana L.) pericarp ethanolic extract in high-carbohydrate wistar rats. J. Nat. Remedies. 17(4), 165–173; doi:10.18311/jnr/2017/11051. Adil, S., Banday, M.T., Hussain, S.A., Wani, M.A., Al-Olayan, E., Patra, A.K., Rasool, S., Gani, A., Sheikh, I.U., Khan, A.A. and Muzamil, S. 2024. Impact of nanoencapsulated rosemary essential oil as a novel feed additive on growth performance, nutrient utilization, carcass traits, meat quality and gene expression of broiler chicken. Foods. 13, 1515; doi:10.3390/foods13101515. Afifah, A.N., Dono, N.D. and Zuprizal, Z. 2021. Optimizing antioxidant properties of salam (Syzygium polyanthum) leaf extract through nanoencapsulation technology. IOP Conf. Ser. Earth. Environ. Sci. 686, 012040; doi:10.1088/1755-1315/686/1/012040. Albuquerque, B.R., Dias, M.I., Pinela, J., Calhelha, R.C., Pires, T.C.S.P., Alves, M.J., Corrãa, R.C.G., Ferreira, I.C.F.R., Oliveira, M.B.P.P. and Barros, L. 2023. Insights into the chemical composition and in vitro bioactive properties of mangosteen (Garcinia mangostana L.) Pericarp. Foods 12, 994; doi:10.3390/foods12050994. Alehosseini, E., Shahiri Tabarestani, H., Kharazmi, M.S. and Jafari, S.M. 2022. Physicochemical, thermal, and morphological properties of chitosan nanoparticles produced by ionic gelation. Foods 11(23), 3841; doi:10.3390/foods11233841. Al-Nemrawi, N.K., Alsharif, S.S.M. and Dave, R.H. 2018. Preparation of chitosan-tpp nanoparticles: the influence of chitosan polymeric properties and formulation variables. Int. J. Appl. Pharm. 10(5), 60–65; doi:10.22159/ijap.2018v10i5.263. Alves, A.I., Rodrigues, M.Z., Ribeiro Pinto, M.R., Lago Vanzela, E.S., Stringheta, P.C., Perrone, ã.T. and Ramos, A.M. 2017. Morphological characterization of pequi extract microencapsulated through spray drying. Int. J. Food. Prop. 20, 1298–1305; doi:10.1080/10942912.2017.1343344. Alves, A.D.C.S., Mainardes, R.M. and Khalil, N.M. 2016. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C. 60, 126–134; doi:10.1016/j.msec.2015.11.014. Alves, A., Silva, A.M., Nunes, C., Cravo, S., Reis, S., Pinto, M., Sousa, E., Rodrigues, F., Ferreira, D. and Costa, P.C. 2024. The synthesis and characterization of a delivery system based on polymersomes and a xanthone with inhibitory activity in glioblastoma. Life. 14(1), 132; doi:10.3390/life14010132. Amin, Z.M., Koh, S.P., Syazwani, N., Hamid, A. and Tan, C.P. 2018. Optimization of spray drying parameters for broken rice maltodextrin powder and its microencapsulation efficiency study on VCO microcapsule. J. Nutr. Health. Food. Sci. 6, 1–8; doi:10.15226/jnhfs.2018.001126. Amyliana, N.A. and Agustini, R. 2021. Formulation and characterization of nanoencapsulation yeast black rice by sonication method with Poloxa Mer. UNESA J. Chem. 10(2), 184–191; doi:10.26740/ujc.v10n2.p184-191. Atlar, G.C., Kutlu, G. and Tormuk, F. 2023. Design and characterization of chitosan-based films incorporated with summer savory (Satureja hortensis L.) essential oil for active packaging. Int. J. Biol. Macromol. 254(2), 127732; doi:10.1016/j.ijbiomac.2023.127732. Ayala-Fuentes, J.C. and Chavez-Santoscoy, R.A. 2021. Nanotechnology as a key to enhance the benefits and improve the bioavailability of flavonoids in the food industry. Foods 10(11), 2701; doi:10.3390/foods10112701. Bangun, H., Tandjono, S. and Arianto, A. 2018. Preparation and evaluation of chitosan-tripolyphosphate nanoparticles suspension as an antibacterial agent. J. App. Pharm. Sci. 8(12), 147–156. doi: 10.7324/JAPS.2018.81217. Cao, Y., Jin, Z., Zhu, R. and Liu, K. 2023. The influence of various solvents extraction on chemical properties on Chang 7 shale, Ordos Basin, China. Solid Earth 27(2), 1169–1179; doi:10.5194/egusphere-2023-272. Cardozo-Muñoz, J., Cuca-Suãrez, L.E., Prieto-Rodríguez, J.A., Lopez-Vallejo, F. and Patião-Ladino, O.J. 2022. Multitarget action of xanthones from Garcinia mangostana against α-amylase, α-glucosidase and pancreatic lipase. Molecules 27(10), 3283; doi:10.3390/molecules27103283. Chaovanalikit, A., Mingmuang, A., Kitbunluewit, T., Choldumrongkool, N., Sondee, J. and Chupratum, S. 2018. Anthocyanin and total phenolics content of mangosteen and effect of processing on the quality of Mangosteen products. Int. Food. Res. J. 19(3), 1047–1053. Dani, M., Rusman, R. and Zuprizal, Z. 2022. Nano-encapsulation of Melastoma malabathricum L. fruit extract influence on the growth performance of broiler. J. Ilm. Peternak. Ter. 5(2), 50–56; doi:10.25047/jipt.v5i2.2803. Dermawan, D., Wathoni, N. and Muchtaridi, M. 2019. Host-guest interactions of α– mangostin with (α, β, γ)–cyclodextrins: semi-empirical quantum mechanical methods of PM6 and PM7. J. Young. Pharm. 11(1), 31; doi:10.5530/jyp.2019.11.7. Desai, N., Rana, D., Salave, S., Gupta, R., Patel, P., Karunakaran, B., Sharma, A., Giri, J., Benival, D. and Kommineni, N. 2023. Chitosan: a potential biopolymer in drug delivery and biomedical applications. Pharmaceutics 15(4), 1313; doi:10.3390/pharmaceutics15041313. Dewayanti, A.A. Andriani, D. and Utami, N. 2023. Preparation nanoparticles of ethanol extract of telang flower (Clitoria ternatea L.) with variation concentration of chitosan and tripolyphosphate as antioxidant candidates. Indones. J. Pharm. Nat. Prod. 6(1), 39–44; doi:10.35473/ijpnp.v6i01.2128. Dharashivkar, S.S. and Shirsat, S.B. 2017. Nanoencapsulation: a promising approach for delivery of nutraceuticals. J. Food. Sci. 82(3), 540–551; doi:10.1016/B978-0-12-824408-1.00001-6. Engelhardt, M.B., Sugimoto, T., Papastavrou, G. and Kobayashi, M. 2024. Electrophoretic mobility of nanoparticle aggregates: independence from aggregate size. Colloids and Surf. A.: Physicochem. Eng. Asp. 703(1), 135244; doi:10.1016/j.colsurfa.2024.135244. Feyzioglu, G.C. and Tornuk, F. 2016. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT. 70, 104–110; doi:10.1016/j.lwt.2016.02.037. Gondokesumo, M.E., Pardjianto, B., Sumitro, S.B., Widowati, W. and Handono, K. 2019. Xanthones analysis and antioxidant activity analysis (Applying ESR) of six different maturity levels of mangosteen rind extract (Garcinia mangostana Linn.). Pharmacog. J. 11(2), 369–373; doi:10.5530/pj.2019.11.56. Haidar, G., Clancy, C.J., Shields, R.K., Hao, B., Cheng, S. and Nguyen, M.H. 2017. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum beta-lactamases. Antimicrob. Agents. Chemother. 61:e02534–16; doi:10.1128/AAC.02534-16. Hameed, H.M. 2021. Feed additives in poultry. Assiut. Vet. Med. J. 67(168), 1–14. Hussain, Z., Thu, H.E., Ng, S.F., Khan, S. and Katas, H. 2017. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: a review of new trends and state-of-the-art. Colloids. Surf. B. Biointerfaces. 150(1), 223–241; doi:10.1016/j.colsurfb.2016.11.036. Hwang, J.C. Jeong, D.J. and Jeong, S.S. Nanoencapsulation technology: a review. J. Microbiol. Biotechnol. 27(4), 639–649. Indiarto, R., Reni, R., Utama, G.L., Subroto, E., Pangawikan, A.D. and Djali, M. 2023. The physicochemical, antioxidant, and sensory properties of chocolate biscuits incorporated with encapsulated mangosteen (Garcinia mangostana L.) peel extract. Int. J. Food Prop. 26(1), 122–138, doi: 10.1080/10942912.2022.2159429. Jeevanandam, J., Barhoum, A., Chan, Y.S., Dufresne, A. and Danquah, M.K. 2018. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein. J. Nanotechnol. 3(9), 1050–1074; doi:10.3762/bjnano.9.98. Joshi, K.S., Kumar, S., Yadav, N. and Yadav, A. 2017. Nanoencapsulation: a promising technique for drug delivery. Int. J. Drug Dev. Res. 9(1), 1–7. Juliantoni, Y., Hajrin, W. and Subaidah, W.A. 2020. Nanoparticle formula optimization of juwet seeds extract (Syzygium cumini) using simplex lattice design method. J. Biol. Tropis. 20(3), 416–422; doi:10.29303/jbt.v20i3.2124. Kalagatur, N.K. Nirmal Ghosh, O.S. Sundararaj, N. and Mudili, V. 2018. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 9, 610; doi:10.3389/fphar.2018.00610. Khosroshahi, A.R. and Shahedi, M.M. 2019. Nanoencapsulation: a comprehensive review on its mechanisms and applications. J. Food Sci. Technol. 56(6), 2619–2633. Kim, E.S., Baek, Y., Yoo, H.J., Lee, J.S. and Lee, H.G. 2022. Chitosan-tripolyphosphate nanoparticles prepared by ionic gelation improve the antioxidant activities of astaxanthin in the in vitro and in vivo model. Antioxidants (Basel). 11(3), 479; doi:10.3390/antiox11030479. Kusmayadi, A. 2021. Water content, fungal distribution and specific gravity of mangosteen peel extract microcapsule products at different storage times. Agrointek 15(1), 293–299; doi:10.21107/agrointek.v15i1.7807. Kusmayadi, A., Adriani, L., Abun, A., Muchtaridi, M. and Tanuwiria, U.H. 2019a. Antioxidant activity of mangosteen peel (Garcinia mangostana L.) extracted using different solvents at different times. Drug Invent. Today. 11(1), 44–48. Kusmayadi, A., Adriani, L., Abun, A., Muchtaridi, M. and Tanuwiria, U.H. 2019b. The microencapsulation of mangosteen peel extract with maltodextrin from Arenga starch: formulation and characterization. J. Appl. Pharm. Sci. 9(3), 33–40; doi:10.7324/JAPS.2019.90306. Kusmayadi, A., Bachtiar, K.R. and Prayitno, C.H. 2019c. The effects of mangosteen peel (Garcinia mangostana L.) and turmeric (Curcuma domestica Val) flour dietary supplementation on the growth performance, lipid profile and abdominal fat content in Cihateup ducks. Vet. World. 12(3), 402–408; doi:10.14202/vetworld.2019.402-408. Li, H., Chen, J., Peng, C., Min, F. and Song, S. 2020. Salt coagulation or flocculation? In situ zeta potential study on ion correlation and slime coating with the presence of clay: a case of coal slurry aggregation. Environ. Res. 189, 109875; doi:10.1016/j.envres.2020.109875. Li, J., Guo, M., Wang, Y., Ye, B., Chen, Y. and Yang, X. 2021. Preparation of biological sustained-release nanocapsules and explore on algae-killing properties. J. Adv. Res. 31, 87–96; doi:10.1016/j.jare.2020.12.006. Li, N., Wang, C., Feng, B., Bi, Y., Kong, F., Wang, Z. and Tan, S. 2023. Application of nanoencapsulation technology to improve the stability and bioactivity of tea polyphenols. Food Biosci. 55(1), 103076; doi:10.1016/j.fbio.2023.103076. Linh, N.T., Qui, N.H. and Triatmojo, A. 2022. The effect of nano-encapsulated herbal essential oils on poultry’ s health. Arch. Razi. Inst. 77(6), 2013–2021; doi:10.22092/ARI.2022.358842.2318. Lu, Y., Guan, T., Wang, S., Zhou, C., Wang, M., Wang, X., Zhang, K., Han, X., Lin, J., Tang, Q., Wang, C., and Zhou, W. 2023. Novel xanthone antibacterials: semi-synthesis, biological evaluation, and the action mechanisms. Bioorg. Med. Chem. 83, 117232; doi:10.1016/j.bmc.2023.117232. Midekessa, G., Godakumara, K., Ord, J., Viil, J., Lãttekivi, F., Dissanayake, K., Kopanchuk, S., Rinken, A., Andronowska, A., Bhattacharjee, S., Rinken, T. and Fazeli, A. 2020. Zeta potential of extracellular vesicles: toward understanding the attributes that determine colloidal stability. ACS Omega 5(27), 16701–16710; doi:10.1021/acsomega.0c01582. Mirzaei, F., Zarei, M. and Jafari, M.V. 2019. Nanoencapsulation of food bioactive ingredients: principles and applications critical reviews in food science and nutrition. Academic Press 59(11), 1780–1802. Natalia, G., Budi, E. and Sugihartono, S. Morphological analysis and composition of Ni-Aln composite layers by electrodeposition method using scanning electron microscopy-energy dispersive spectroscopy (Sem-Eds). In Proceedings of the National Physics Seminar (E-Journal). National Physics Seminar 2022 Physics and Physics Education Study Program, Faculty of Mathematics and Natural Sciences, Jakarta State University, Jakarta, Indonesia, 2023, p 11; doi:10.21009/03.1101.FA14. Ningsih, N., Yasni, S. and Yuliani, S. 2017. Synthesis of red mangosteen peel extract nanoparticles and study of functional properties of encapsulated products. J. Teknol. Ind. Pangan. 28(1), 27–35; doi:10.6066/jtip.2017.28.1.27. Noormahesa, R., Nuraini, C. and Unang, U. 2024. Factors affecting mangosteen production (Garcinia mangostana) in Puspahiang Village, Puspahiang District, Tasikmalaya Regency. Paspalum: J. Ilm. Pertan. 12(1), 136–143; doi:10.35138/paspalum.v12i1.698. Pandit, D.L., Yadav, R.B. and Yadav, B. 2023. Assessing the benefits and considerations of functional feed additive administration in broiler chicken. Mal. Anim. Husb. J. 3(1), 46–48; doi:10.26480/mahj.01.2023.46.48. Pateiro, M., Gãmez, B., Munekata, P.E.S., Barba, F.J., Putnik, P., Kovačević,, D.B. and Lorenzo, J.M. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules. 26(6), 1547; doi:10.3390/molecules26061547. Poetri, A.R., Pratiwi, R. and Rismadanti, A. 2023. Effect of mangosteen peel extract (Garcinia mangosteen) to fibroblast number as periodontal disease therapy. Denta. 17(1), 1–7; doi:10.30649/denta.v17i1.1. Prieto, C. and Lagaron, J.M. 2024. Nanoencapsulation and nanocoating of bioactives of application interest in food, nutraceuticals and pharma. Nanomaterials. 14(3), 313; doi: 10.3390/nano14030313. Prihastanti, E., Subagyo, A. and Ngadiwiyana, N. 2018. Effect of combination of NPK and nano silica on the levels of β-carotene and nutritional value of corn (Zea mays L.). IOP Conf. Ser. Mater. Sci. Eng. 434; doi:10.1088/1757-899X/434/1/012117. Puvaca, N., Brkic, I., Jahic, M., Roljevic, S., Nikolic, N., Radovic, G., Ivanisevic, D., Miloradokic, M., Boskovic, D., Ilic, D., Brkanlic, S. and Prodanovic, R. The effect of using natural or biotic dietary supplements in poultry nutrition on the effectiveness of meat production. Sustainability. 12, 4373; doi: 10.3390/su12114373. Rahmawanty, D., Risa, A., Malikhatun, N., Prima, H.R., Nani, K. and Effionora, A. 2017. Nanoparticle preparation and characterization of Haruan fish (Channa Striata) exctract contains albumin from south kalimantan with lonic gelation method. Int. J. Drug Deliv. 9, 47–51; doi:10.5138/09750215.2070. Resende, D.I.S.P., Almeida, M.C., Maciel, B., Carmo, H., Sousa Lobo, J., Dal Pozzo, C., Cravo, S.M., Rosa, G.P., Kane-Pagès, A. and do Carmo Barreto, M. 2020. Efficacy, stability, and safety evaluation of new polyphenolic xanthones towards identification of bioactive compounds to fight skin photoaging. Molecules. 25(12), 2782; doi:10.3390/molecules25122782. Resende, D.I.S.P., Durães, F., Zubarioglu, S., Freitas-Silva, J., Szemerédi, N., Pinto, M., Pinto, E., Martins da Costa, P., Spengler, G. and Sousa, E. 2024. Antibacterial potential of symmetrical twin-drug 3,6-diamino xanthones. Pharmaceuticals. 17(2), 209; doi:10.3390/ph17020209. Rezaei, A., Fathi, M. and Jafari, S.M. 2019. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 88, 146–162; doi:10.1016/j.foodhyd.2018.10.003. Rohilla, P., Bhatt, V., Kumar, N. and Sharma, V. 2020. Nanoencapsulation: a promising technology for food industry. J. Food Sci. Technol. 57(6), 1983–1991. Rosenberg, M., Kopelman, I.J. and Talmon, Y. 1990. Factors affecting retention in spray-drying microencapsulation of volatile materials. J. Agric. Food. Chem. 38, 1288–1294; doi:10.1021/jf00095a030. Safitri, N.L., Puspita, D.W., Junita, J., Sary, L.N.I., Al Adawiyah, R.R., Prihastanti, E. and Suedy, S.W.A. 2022. Effect of nanochitosan coating on the quality of chili pepper (Capsicum frutescens L.) at low temperature storage. Bul. Anat. Fisiol. 7(1), 27–34. Šefcová, M.A., Ortega-Paredes, D., Larrea-ãlvarez, C.M., Mina, I., Guapãs, V., Ayala-Velasteguí, D., Leoro-Garzãn, P., Molina-Cuasapaz, G., Vinueza-Burgos, C. and Revajovã, V. 2023. Effects of lactobacillus fermentum administration on intestinal morphometry and antibody serum levels in salmonella-infantis-challenged chickens. Microorganisms. 11, 256; doi:10.3390/microorganisms11020256. Soliman, N.M., Shakeel, F., Haq, N., Alanazi, F.K., Alshehri, S., Bayomi, M., Alenazi, A.S.M. and Alsarra, I.A. 2022. development and optimization of ciprofloxacin hcl-loaded chitosan nanoparticles using box-behnken experimental design. Molecules 27(14), 44–68; doi:10.3390/molecules27144468. Soltanzadeh, M., Peighambardoust, S.H., Ghanbarzadeh, B., Mohammadi, M. and Lorenzo, J.M. 2021. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum L.) peel extract as a natural source of antioxidants. Nanomaterials (Basel). 11(6), 1439; doi:10.3390/nano11061439. Sriwidodo, S., Pratama, R., Umar, A.K., Chaerunisa, A.Y., Ambarwati, A.T. and Wathoni, N. 2022. Preparation of mangosteen peel extract microcapsules by fluidized bed spray-drying for tableting: improving the solubility and antioxidant stability. Antioxidants 11, 1331; doi:10.3390/antiox11071331. Suhandi, C., Wilar, G., Lesmana, R., Zulhendri, F., Suharyani, I., Hasan, N. and Wathoni, N. 2023. Propolis-based nanostructured lipid carriers for α-mangostin delivery: formulation, characterization, and in vitro antioxidant activity evaluation. Molecules 28(16), 6057; doi:10.3390/molecules28166057. Sujanto, A., Salam, R., Badriyana and Dimyanti. 2015. Scanning electron microscopy (SEM) study for characterization of zirconium alloy oxidation process. BATAN, Yogyakarta: Center for Advanced Materials Science and Technology. Suksamran, N., Anantawat, V., Wattanaarsakit, P., Wei, C., Rahman, M.A., Majima, H.J. and Tangpong, J. Mangosteen vinegar from Garcinia mangostana: quality improvement and antioxidant properties. Heliyon 8, e11943; doi: 10.1016/j.heliyon.2022.e11943. Susilawati, E. and Soewondo, B.P. 2022. Effect of nanoencapsulation on compound activity potential as antioxidants. Jurnal Riset Indonesia 2(1), 1–8; doi:10.29313/jrf.v2i1.692. Taouzinet, L., Djaoudene, O., Fatmi, S., Bouiche, C., Amrane-Abider, M., Bougherra, H., Rezgui, F. and Madani, K. 2023. Trends of nanoencapsulation strategy for natural compounds in the food industry. Processes. 11(5), 1459; doi:10.3390/pr11051459. Tolve, R., Galgano, F., Caruso, M.C., Laure, F., Tchuenbou-Magaia, Condelli, N., Favati, F. and Zhang, Z. 2016. Encapsulation of health-promoting ingredients: applications in foodstuffs. Int. J. Food. Sci. Nutr. 67(8), 888–918; doi:10.1080/09637486.2016.1205552. Tripuratapini, S.I.M, Mudita, D.P. and Candrawati, M.A. 2015. Dry matter and nutrient content of probiotic supplements produced with different levels of rumen waste. Peternak. Trop. 3(1), 105–120. Ways, T.M.M., Lau, W.M. and Khutoryanskiy, V.V. 2018. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers (Basel) 10(3), 267; doi:10.3390/polym10030267. Yuvanatemiya, V., Srean, P., Klangbud, W.K., Venkatachalam, K., Wongsa, J., Parametthanuwat, T. and Charoenphun, N. 2022. A review of the influence of various extraction techniques and the biological effects of the xanthones from mangosteen (Garcinia mangostana L.) pericarps. Molecules 27, 8775; doi:10.3390/molecules27248775. Zhang, H., Lin, J., Chen, S., Xie, J., Chen, L., Wang, Y. and Wang, Z. 2020. Antioxidant and anti-inflammatory activities of chrysanthemum flower extract nanoencapsulated with ascorbic acid. J. Microencapsul. 37(5), 438–447; doi:10.3390/antiox11061068. | ||
| How to Cite this Article |
| Pubmed Style Kusmayadi A, Mardianingrum R, Yanti Y. Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultry. Open Vet. J.. 2025; 15(4): 1771-1783. doi:10.5455/OVJ.2025.v15.i4.29 Web Style Kusmayadi A, Mardianingrum R, Yanti Y. Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultry. https://www.openveterinaryjournal.com/?mno=241594 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.29 AMA (American Medical Association) Style Kusmayadi A, Mardianingrum R, Yanti Y. Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultry. Open Vet. J.. 2025; 15(4): 1771-1783. doi:10.5455/OVJ.2025.v15.i4.29 Vancouver/ICMJE Style Kusmayadi A, Mardianingrum R, Yanti Y. Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultry. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1771-1783. doi:10.5455/OVJ.2025.v15.i4.29 Harvard Style Kusmayadi, A., Mardianingrum, . R. & Yanti, . Y. (2025) Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultry. Open Vet. J., 15 (4), 1771-1783. doi:10.5455/OVJ.2025.v15.i4.29 Turabian Style Kusmayadi, Andri, Richa Mardianingrum, and Yanti Yanti. 2025. Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultry. Open Veterinary Journal, 15 (4), 1771-1783. doi:10.5455/OVJ.2025.v15.i4.29 Chicago Style Kusmayadi, Andri, Richa Mardianingrum, and Yanti Yanti. "Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultry." Open Veterinary Journal 15 (2025), 1771-1783. doi:10.5455/OVJ.2025.v15.i4.29 MLA (The Modern Language Association) Style Kusmayadi, Andri, Richa Mardianingrum, and Yanti Yanti. "Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultry." Open Veterinary Journal 15.4 (2025), 1771-1783. Print. doi:10.5455/OVJ.2025.v15.i4.29 APA (American Psychological Association) Style Kusmayadi, A., Mardianingrum, . R. & Yanti, . Y. (2025) Formulation and characterization of mangosteen rind extract nanocapsules as a feed additive candidate of poultry. Open Veterinary Journal, 15 (4), 1771-1783. doi:10.5455/OVJ.2025.v15.i4.29 |