| Research Article | ||
Open Vet. J.. 2025; 15(9): 4441-4453 Open Veterinary Journal, (2025), Vol. 15(9): 4441-4453 Research Article Teratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acidIbrahim A. Hamed1, Refat M. Sherif1, Aly A. Shalaby1, Omran E. Abdoslam2*, Qiyi He3, Dongyang Li3, Ting Xu4 and El-Sayed A. El-Sheikh11Plant Protection Department, Faculty of Agriculture, Zagazig University, Zagazig, Egypt 2Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 3College of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou, China 4Beijing Key Laboratory of Biodiversity and Organic Farming, College of Resources and Environmental Sciences, China Agricultural University, Beijing, China *Corresponding Author: Omran E. Abdoslam. Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: o.bait-almal [at] uot.edu.ly Submitted: 06/02/2025 Revised: 15/07/2025 Accepted: 16/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
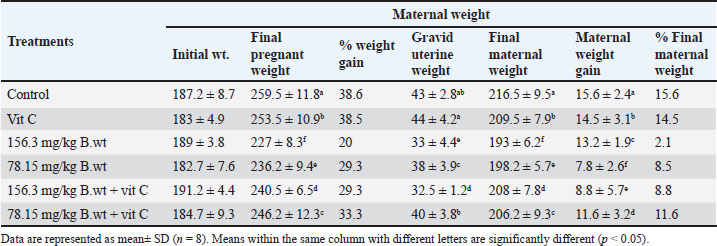
ABSTRACTBackground: Continuous exposure of pregnant females to thiamethoxam (THM) during the organogenesis period causes reproductive toxicity through oxidative stress mechanisms, changes in fetal size and weight, (visceral and skeletal) malformations, histological changes in tissues, and oxidative stress in dams. Aim: This study aimed to investigate the toxicological effects of 1/10 and 1/20 of THM LD50 and the protective effect of vitamin C (vit C) on pregnant females and fetuses. Methods: Forty-eight female rats were divided into six equal groups and were orally treated daily with THM, vit C, and their combinations during the organogenesis period [6th–12th gestation day (GD)]. All pregnant female rats were slaughtered on GD 20. Blood samples, tissues (brain, liver, kidney, and lung), fetuses, and the uterus were examined. Results: Females orally treated with 1/10 and 1/20 of THM LD50 showed the highest weight reduction. Fetal length and weight were significantly decreased in groups treated orally with both doses of THM LD50. A significant increase in the number of resorption sites was recorded in 1/10 of the THM LD50 group compared with the groups treated orally with both doses of THM LD50 combined with vit C. Exposure of dams to high and low doses of THM LD50 resulted in various malformations in the fetuses’ skeletal, palate, cerebral ventricle, cerebrum, liver, kidney, bladder, and ureter. A significant increase in the percentage of changes in the skull, ribs, sternum, and vertebral column was observed in both THM doses. The degree of malondialdehyde was significantly increased in groups treated orally with both doses of THM LD50, while the total antioxidant level was significantly decreased. Conclusion: The results showed the potential of vit C in decreasing teratogenic effects on dams and fetuses exposed to THM, as it reduced the negative effects on different organs of the treated rats. Keywords: Thiamethoxam, Vitamin C, Pregnant rats, Malondialdehyde, Total antioxidant. IntroductionChemical compounds are used in the agricultural sector to control harmful pests to increase agricultural production and meet the needs of the global market (Hamed et al., 2019; El-Sheikh et al., 2023). Pesticide exposure occurs via direct contact during the application or ingestion of contaminated food/water (El-Sheikh and Ashour, 2022). Neonicotinoids are a novel family of agricultural chemicals that account for more than 25% of the global insecticide market. It was used to protect plants against pests and domestic animals from fleas (Costas-Ferreira and Faro, 2021). Imidacloprid and thiamethoxam (THM) are the two main neonicotinoid pesticides that are used in high concentrations, posing risks of environmental and occupational pollution (El-Hak et al., 2022). The doses of THM tested in this study were 1/10 and 1/20 of LD50. THM can generate reactive oxygen species, disrupt antioxidant defense systems, and cross the placenta to affect fetal tissues (Hamed et al., 2023). Repeated exposure to this pesticide group increases the risks of reproductive toxic effects (Costas-Ferreira and Faro, 2021) and causes neurotoxicity (Guney et al., 2007; Calderon-Garciduenas, et al., 2023) effects and abnormalities in animal offspring (Gawade et al., 2013). Studies have demonstrated that 50% of the compound undergoes metabolic processing, with its metabolites being identified in brain tissues of THM-exposed animals (El-Borai et al., 2019). As free radicals, pesticides and their metabolites weaken antioxidant defenses against toxic chemicals and cause redox imbalance (Lobo et al., 2010). Vitamin C (ascorbic acid; vit C) is a water-soluble compound that plays a protective role against xenobiotic intoxications that cause oxidative damage (Djurasevic et al., 2008; Assayed et al., 2010; Di Meo et al., 2016; Kurutas, 2016; Hamed et al., 2023). Vit C protects tissues and (Holzum 1996; Hussein et al., 2014) live cells from oxidative damage by decreasing cell apoptosis (Chang et al., 2012). Vit C reduces biochemical and hematological effects caused by agricultural chemicals in the blood (Saoudi et al., 2021). This substance is available at a relatively low cost and is classified as a non-toxic antioxidant that plays a significant role in reducing the harmful effects of chemicals (Ibrahim et al., 2019). Despite the intensive exposure of animals and humans to this group of insecticides, little data are available on their teratogenic effects. Therefore, this study aimed to elucidate the teratogenic effects (i.e., morphological, visceral, and skeletal abnormalities) of THM, different biochemical parameters, and the role of vit C as an antioxidant material in reducing the harmful effects of THM (Hamed et al., 2019; OECD, 2001; El-Sheikh and Ashour, 2022; Hamed et al., 2023). Materials and MethodsDoses of insecticide and vitamin CThe LD50 of THM is 1563 mg/kg b.wt Environmental Protection Agency, United States 2011. In this experiment, a high dose (1/10 of LD50, 156.3 mg/kg b.wt) and a low dose (1/20 of LD50, 78.15 mg/kg b.wt) were used. Pregnant animals were orally treated with insecticide during the organogenesis period (6th–12th GD). Throughout the trial, the treated rats were given a fresh aqueous solution of vitamin C at a dose of 200 mg/kg body weight and an insecticide every day using distilled water as a vehicle (Hamed et al., 2023). Vit C was administered 30 minutes before THM exposure. Chemical compoundsA commercial formulation of THM (Actara®, 25% WG) was purchased from a local distributor company of Syngenta Crop Protection Agrochemicals (Dokki, Egypt) and imported from Greenboro (Greenboro, NC, USA). L-ascorbic acid (vitamin C; vit C) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All kits used for the examinations were purchased from the Bio-Diagnostic Company (El Tahrir St., Dokki, Egypt). Experimental designForty-eight virgin female non-pregnant albino rats were used for the teratogenic and embryotoxic investigations. The rats were purchased from the breeding animal house of the Faculty of Veterinary Medicine, Zagazig University, Egypt. Rats were housed under specific conditions (25 ºC ± 2ºC, 12-hour light/dark cycle). They were fed a rodent diet and water ad libitum throughout the experiment (NRC, 1996). The rats were weighed after the accommodation time and divided into six groups of eight females per group. The groups were treated as follows: the control group was treated orally with distilled water only. The vit C group was orally administered 200 mg/kg b.wt of vit C. The 1/10 THM LD50 group was orally administered 156.3 mg/kg b.wt of THM. The 1/20 THM LD50 group was orally administered 78.15 mg/kg b.wt of THM. The 1/10 THM LD50 + vit C group was orally administered 156.3 mg/kg b.wt of THM + 200 mg/kg b.wt of vit C. The 1/20 THM LD50 + vit C group was orally administered 78.15 mg/kg b.wt of THM + 200 mg/kg b.wt of vit C. MatingThe estrous cycle was monitored for all animals before mating. For mating, two females and one male were housed in the same cage overnight from 4.00 pm to 8.00 am of the following day. The male was removed the next morning, and the females were examined for signs of mating by flushing the vagina with 0.9% saline using a pipette and then microscopically examining the obtained solution. Mating was confirmed by the presence of sperm cells and plugs. The presence of spermatozoa is recorded as day zero (GD 0) of pregnancy, and a daily increase in weight further confirms pregnancy (Paccola et al., 2018). The treatment schedule involved administration of the test chemical during organogenesis, which is from day 6 to day 12 of gestation according to the Organization for Economic Co-operation and Development Toxicity up A. O. 2001. OECD guideline for testing of organization for economic co-operation and development Paris France. SamplingOn GD 20, the dams were anesthetized, sacrificed for blood sample collection and serum isolation, and then subjected to laparotomy. The uterine horns were obtained, and the number of uterine implants (resorption sites and number of live/dead fetuses) was counted for each dam. The uterus of each dam was excised and opened using scissors. The fetuses and placentae were then separated and weighed. A randomly selected subset of fetuses (n=5/ L) underwent morphological, visceral, and skeletal examinations. Examination of uterus morphologyThe uterus horns of each dam were extracted from the surrounding fat using fine scissors. Graved uteri were opened, and all fetuses (live and dead) were recorded. The uteruses from pregnant rats were weighed, and then the fetuses and placenta were weighed. The number of live and resorbed embryos and implantation sites was also recorded. The uteruses with no evidence of implantation were stained with 2% ammonium sulfide solution to identify resorption sites. Only a resorption site resembling a dark brown clot and with no visible embryonic tissue was classified clearly (Basal et al., 2020). Visceral examination of the fetusesHalf of the fetuses were randomly selected and preserved in Bouin’s solution to preserve visceral teratology (75 ml picric acid, 25 ml formalin 40%, and 5 ml glacial acetic acid) for the assessment of internal soft tissue anomalies using Wilson’s freehand razor blade technique. The fetuses were re-examined for external malformations. The ears and forelimbs were first removed, and then the upper part of the head was cut through the jaws and just above the ears. After examination of the palate, the cut surface of the head was placed on a crock board, and 1-mm slices were made through the head. Then, the slices were placed (cephalic surface upwards) in 70% alcohol in white plastic spot trays. Sectioning was continued from the shoulder joint region through the thoracic and abdominal regions to the kidney region. The sections were then examined for any malformations under a dissecting microscope. According to Wilson (1965), a systematic record was made on a specially designed form of all malformations and anomalies. Skeletal examination of the fetusesTo evaluate the skeletal defects, the remaining fetuses, which were previously kept in 95% ethyl alcohol for a period of 7 days, were eviscerated and stained with alizarin red (60 ml glycerin, 7.5 g potassium hydroxide, 2.5 ml distilled water and completed to 230 ml by distilled water) as described by Staples and Schnell (1964). The muscles of these fetuses were first digested with 2% potassium hydroxide for 6–24 hours according to the size of the fetuses. The digested fetuses were immersed in Mallsch’s solution containing alizarin red for 24 hours. The stained fetuses were kept in Mallsch’s solution alone for 2 days, then rinsed in cold water and cleared by successive passage in glycerol solution at graded concentrations of 50%, 70%, 90%, and 100%. Serum samplesOn the last day of the experiment, the rats were starved overnight and then weighed and sacrificed. Two milliliters of blood from each rat were collected in a centrifuge glass tube and allowed to coagulate under laboratory conditions for 20 minutes, then centrifuged at 3,000 rpm for 10 minutes (Megafuge, Thermo Scientific, Germany). Aliquots of serum samples were transferred and stored in Eppendorf tubes and stored at −20°C until use. Investigation of the malondialdehyde (MDA) and total antioxidant capacity (TAC) levelsA colorimetric method was used for the determination of MDA according to Ohkawa et al. (1979) and total antioxidant TAC according to Koracevic et al. (2001). MDA and TAC were measured using a microplate reader (Infinite M Nano, TECAN, Austria). Histological examinationAt the end of the experiment, samples from rat tissues (brain, liver, kidney, and lung) were examined according to Suvarna et al. (2013). Statistical analysisResults are presented as mean ± SD. Statistical Package for the Social Sciences (version 20.0, Chicago, IL) for Windows software was used to analyze the data using one-way analysis of variance with Duncan’s multiple range test at a significance level of p <0.05 to determine statistical differences among groups. Ethical approvalHousing, care, and all experimental procedures were conducted in compliance with the guidelines of Zagazig University Care and Use of Laboratory Animals (permission number: ZU-IACUC/2/F/121/2022). ResultsGeneral health and mortality observationsNo mortality occurred during the experiment. Generally, signs of toxic effects, including a change in movement and reduced food intake, were observed in rats administered both doses of THM. The groups treated with vit C did not show any visible harmful effects. Effect on the weight parameters of pregnant female ratsThe results in Table 1 show the reduction in the final weight of pregnant females, final maternal weight, gravid uterine weight, and maternal weight gain of pregnant rats compared to control rats. Females treated orally with 1/10 and 1/20 of THM LD50 showed the highest weight reduction compared with the control or vit C groups. Groups treated orally with a combination of low dose (1/20) of THM LD50 and vit C resulted in a significant increase in final pregnant weight, final maternal weight, gravid uterine weight, and maternal weight gain compared with the 1/20 of THM LD50 treated group. However, no significant differences in the same parameters were observed between the control and vit C groups. Table 1. Effect of thiamethoxam, vitamin C, and their combination on final pregnant weight, final maternal weight, gravid uterine weight, and maternal weight gain of pregnant rats (gm) orally administered daily during the organogenesis period.
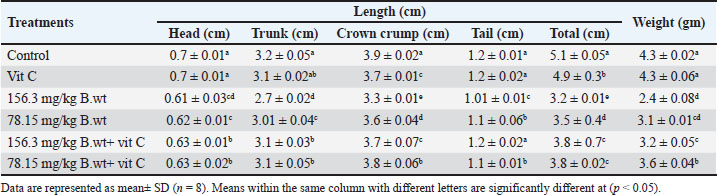
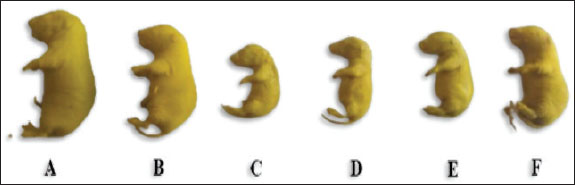
Effect on fetal length and weightTable 2 and Figure 1 show the effects of different doses of THM and their combination with vit C on the weight and length of fetuses. Fetal length and weight of fetuses were significantly decreased in the 1/10 and 1/20 THM LD50 groups compared with those in the vit C and control groups, whereas fetuses in the 1/10 and 1/20 of THM LD50 combined with vit C exhibited low significant differences compared with those in the vit C and control groups. Table 2. Effect of thiamethoxam, vitamin C, and their combinations on fetal length and weight (gm) of pregnant rats treated orally daily during organogenesis.
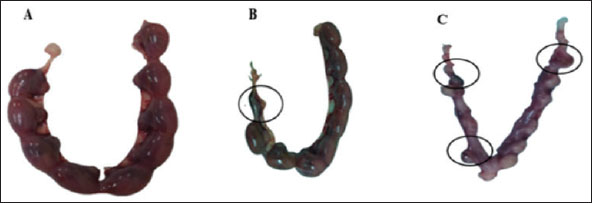
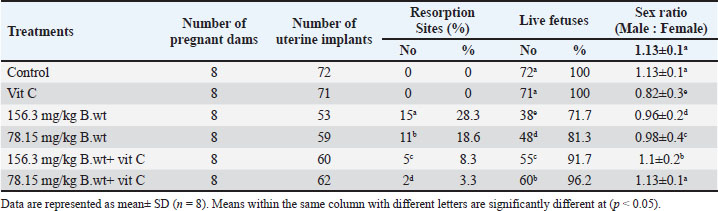
Fig. 1. Lateral view of fetuses obtained from dams treated orally with THM during the organogenesis period showing growth retardation: control (A), vit C (B), 1/10 of THM LD50 (C), 1/20 of THM LD50 (D), 1/10 of THM LD50 + vit C (E), and 1/20 of THM LD50 + vit C (F). Effects on fetal indicesThe uterus of pregnant dams in the control and vit C groups did not show any resorption sites, whereas resorption sites increased by 3.2-fold (p=0.003) in 1/10 of the THM LD50 group compared with the vit C and control groups. The percentages of resorption sites reduced in groups treated with 1/10 of THM LD50 + vit C and 1/20 of THM LD50+ vit C. The sex ratio in animals given orally 1/10 of THM LD50 decreased compared with other groups, as shown in Table 3 and Figure 2.
Fig. 2. Resorption sites on uteruses obtained from the dams. (A) Control uterus, (B) uterus of the dam orally treated with 1/10 of THM LD50 and (C) uterus of the dam orally treated with 1/20 of THM LD50, respectively. Table 3. Effect of thiamethoxam, vitamin C, and their combinations on the uterine external morphology in pregnant rats orally administered daily during the organogenesis period.
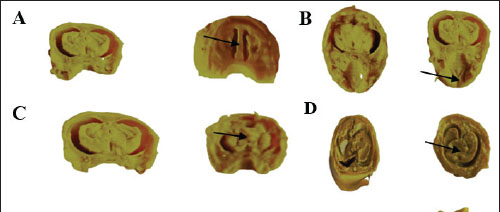
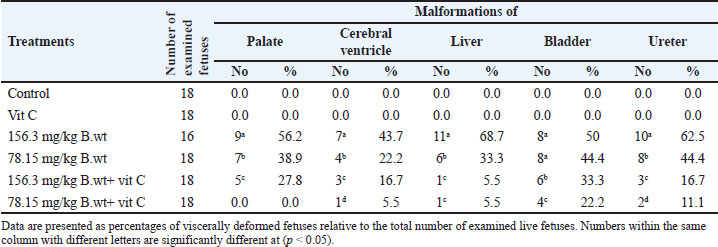
Fetal visceral malformationsThe visceral observations are presented in Table 4 and Figure 3. No visceral malformations were recorded in the fetuses of the control and vit C groups, except for one fetus that exhibited a palate and cerebral ventricle. Exposure of dams to 1/10 and 1/20 of THM LD50 resulted in various malformations in the palate, cerebral ventricle, cerebrum, liver, kidney, bladder, and ureter of the examined fetuses. The recorded visceral malformations were reduced in the groups treated orally with 1/10 and 1/20 of THM LD50 combined with vit C compared with the control and vit C groups.
Fig. 3. Cross and longitudinal sections of the head of a rat fetus. A. Cerebral ventricle, B. Palate. C. The cerebral hemispheres were unequal in size (asymmetric) in the head. D. Hypoplasia of the liver. E. Hypoplasia of the kidney, distends the urinary bladder with urine and dilated ureter (hydro ureter). (Control group at the left). Table 4. Visceral malformations in the feti of pregnant rats orally administered daily with thiamethoxam, vitamin C and their combinations during organogenesis.
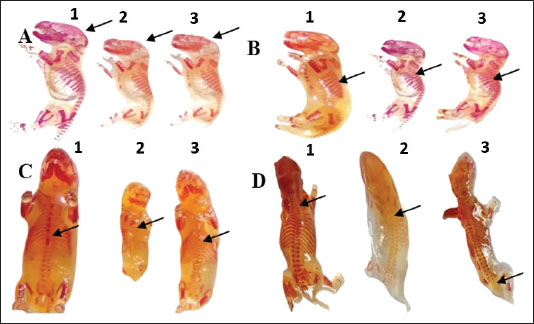
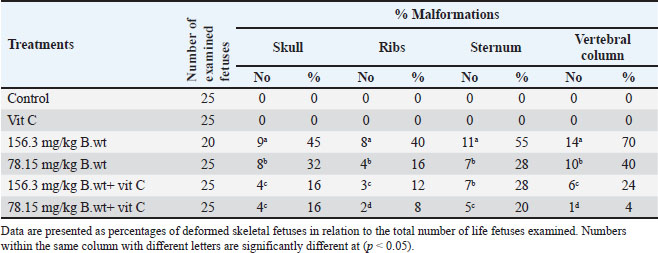
Fetal skeletal malformationsAs shown in Table 5 and Figure 4, the fetuses of the control and vit C groups have normal skeletal structures. Fetuses obtained from dams exposed to 1/10 and 1/20 of THM LD50 showed marked skeletal malformations with a significant increase (p ˂ 0.05) in the percentages of fetuses with skull, ribs, sternum, and vertebral column compared with fetuses of the control and vit C groups. However, several skeletal malformations were recorded in the fetuses of 1/10 of the THM LD50 and 1/20 of the THM LD50 groups of exposed dams. The fetuses of the 1/10 and 1/20 THM LD50 groups showed a significant decrease (p ˂ 0.05) in the percentages of skull, ribs, sternum, and vertebral column compared with the fetuses of the control and vit C groups.
Fig. 4. Skeletal malformations and changes in the fetuses of pregnant rats. A. Incomplete skull bone fusion B. Shortage and zigzag ribs (C) Absence of the sternbrae D. Absence of some coccygeal vertebrae and separation of wings atlas vertebra. (1) Fetuses of the control group, (2) fetuses treated orally with 1/10 of THM LD50, and (3) fetuses treated orally with 1/20 of THM LD50. Table 5. Skeletal malformations in the feti of pregnant rats treated orally daily with thiamethoxam, vitamin C, and their combinations during organogenesis.
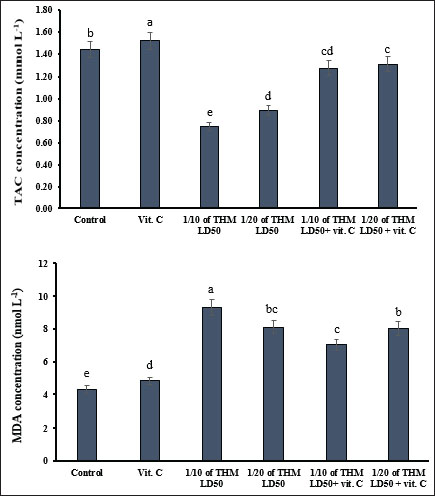
Effect of MDA and TAC as oxidative damage measurementsThe level of MDA was increased significantly (p < 0.05) in dams orally administered with 1/10 and 1/20 of THM LD50 compared with female rats in the control groups. The TAC level was significantly decreased as a result of exposure to the tested doses of THM, while co-administration of vit C to THM-treated rats enhanced the decrease effects of TAC as no significant changes were observed compared with the control group (Fig. 5).
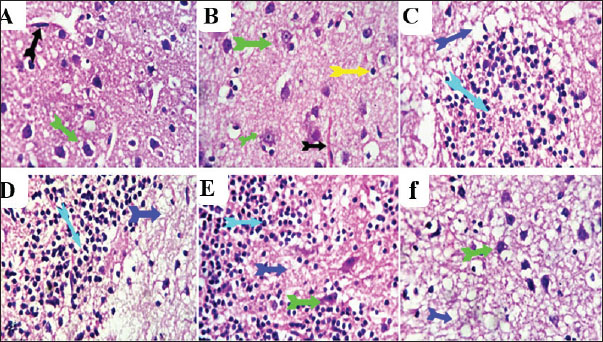
Fig. 5. Effect of thiamethoxam, vitamin C, and their combination on total adipose tissue antigen and malondialdehyde in pregnant female rats (mean± SD). Bars with the same lowercase letter are not significantly different (p < 0.05). Histopathological examinationSections from the brain (Fig. 6) of rats treated orally with 1/10 and 1/20 of THM LD50 showed neurotoxic changes represented by neuronal and axonal degenerative changes with demyelination and occasional focal malacic areas. Meningeal, cerebral, cerebellar, and ventricular vascular congestion and perivascular and perineural edema were observed. Multifocal microgliosis is a highly phagocytic reactive process that engulfs and removes degenerated neuronal and axonal particles. Focal Hippocampal Cellular Degenerative Changes and Ventricular (Lateral and fourth ventricles) Dilatation were recorded in some examined sections. On the other hand, sections from rats treated orally with 1/10 and 1/20 of THM LD50 with vit C revealed a moderate ameliorative effect as represented by normalization of the neurons, axons, and hippocampal cells at different locations. Most of the ventricles and associated vascular and choroid plexus structures were normal. Mild perivascular and perineural edema, in addition to mild focal axonal degeneration and focal gliosis, were observed in some sections compared with the control and vit C groups (Fig. 6).
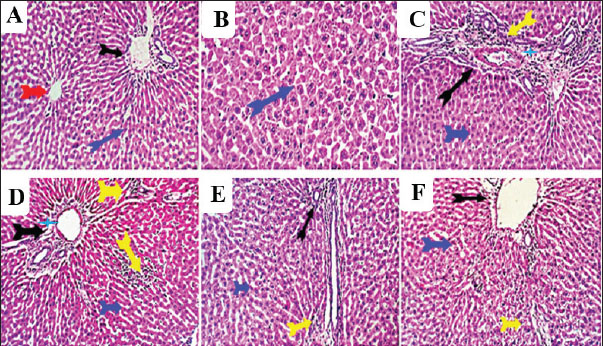
Fig. 6. Photomicrograph of brain sections for histopathological changes: control (A), vit C (B), 1/10 THM LD50 (C), 1/20 THM LD50 (D), 1/10 THM LD50 + vit C (E), and 1/20 THM LD50 + vit C (F). Liver sections (Fig. 7) of the control and vit C groups revealed normal hexagonal plates of hepatocytes. Sections from hepatic tissue of rats treated orally with 1/10 and 1/20 of THM LD50 showed moderate hepatic-toxic changes represented by portal biliary proliferation, lymphocytic cholangitis, and lymphoplasmacytic aggregations, which were also seen in the interstitial tissue partially replacing the hepatic parenchyma. Hepatic sinusoids were focally dilated with mild hypertrophy of Von Kupfer cells. Hepatocytes were focally degenerated, apoptotic, and atrophied. On the other hand, liver sections from rats treated orally with 1/10 and 1/20 THM LD50 and co-administered vit C revealed residual biliary proliferation and mild lymphoplasmacytic infiltration. Mild focal hepatocellular macrosteatosis was observed.
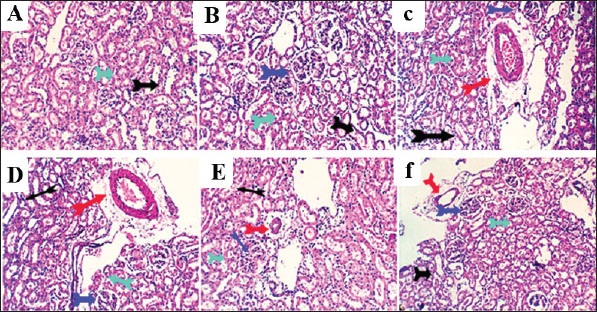
Fig. 7. Photomicrograph of liver sections for histopathological changes: control (A), vit C (B), 1/10 THM LD50 (C), 1/20 THM LD50 (D), 1/10 THM LD50 + vit C (E), and 1/20 THM LD50 + vit C (F). Kidney sections (Fig. 8) of the control and vit C groups revealed normal histomorphology of nephron units, collecting tubules, papillae, and renal pelvis with preserved glomerular tufts lobulation and endothelium, renal vascular structures, and epithelial lining of proximal and distal tubules. Kidney sections from rats orally administered with 1/10 and 1/20 THM LD50 showed moderate nephron-toxic lesions, renal tubular epithelial degenerative changes involving the proximal and distal convoluted tubules, tubular dilatation with hyaline cast formation, particularly affecting the collecting tubules, glomerular lobulation and/or shrinkage, and moderate to marked perivascular edema associated with lymphocytic infiltration. Occasional focal hydronephrotic changes, lymphocytic papillitis, and pyelitis were observed. Kidney sections from rats treated with 1/10 and 1/20 of THM LD50 and co-administered with vit C revealed residual degenerative and ectatic changes in the tubular epithelium in addition to mild perivascular edema and glomerular tuft lobulation.
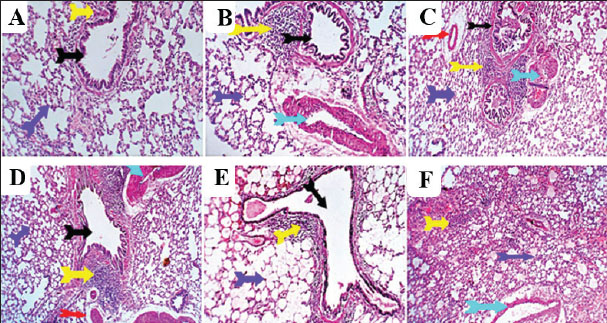
Fig. 8. Photomicrograph of kidney sections for histopathological changes: control (A), vit C (B), 1/10 THM LD50 (C), 1/20 THM LD50 (D), 1/10 THM LD50 + vit C (E), and 1/20 THM LD50 + vit C (F). Lung sections (Fig. 9) of control and vit C-treated orally revealed normal bronchial and bronchiolar micromorphological structures with mucosal and submucosal cellular features. Minimal peribronchiolar lymphoplasmocytic infiltration was observed. The peribronchial and interalveolar blood vessels were normal. The alveolar epithelial lining, comprising pneumocytes type I and II, alveolar macrophages (pneumocyte type III), and perialveolar intersitium, was of normal populations and structures. Mild constrictive hypertrophic changes in some pulmonary blood vessels were recorded in a few sections of the antioxidant-treated rats. Lung sections from rats treated with 1/10 and 1/20 of THM LD50 revealed moderate peribronchial lymphoplasmocytic aggregations, perivascular edema, vascular wall constructive hypertrophic changes with narrow lumina, and FAE. Lung sections from rats treated with 1/10 and 1/20 of THM LD50 co-administered with vit C revealed mild peri-bronchial lympho-plasmocytic aggregates and focal vascular constrictive hypertrophic changes.
Fig. 9. Photomicrograph of lung sections for histopathological changes: control (A), vit C (B), 1/10 THM LD50 (C), 1/20 THM LD50 (D), 1/10 THM LD50 + vit C (E), and 1/20 THM LD50 + vit C (F). DiscussionExperiments on pesticides have shown adverse effects on reproductive organs, with developing fetuses and neonates being highly susceptible to their toxic effects (Eisa et al., 2018; El-Sheikh and Ashour, 2022 Hamed et al., 2023). This study exhibited harmful effects in female rats induced by THM when they were orally treated with 1/10 or 1/20 of THM LD50 during the organogenesis period. The results showed a marked reduction in the body weight of the groups treated with THM. This reduction in body weight is considered a sensitive indicator of pesticide toxicity and can be used as a viable index of causing damage (Mansour and Mossa 2010; Alkahemal-Balawi et al., 2011; Hassan et al., 2022). The overall increased degradation of lipids and proteins due to exposure to various insecticides can also explain the decrease in body weight (El-Shenawy et al., 2010). The results showed that the reduction in maternal weight gain was consistent with the teratological results because the number of embryos in the treated groups was less than that in the control group. This may explain the reduction in maternal BWG of THM-treated pregnant rats (Au-Qare et al., 2000; Bradman et al., 2003; Scifres et al., 2014). THM caused teratological effects by inducing a reduction in the number and viability of fetuses per mother, reduction in length and weight, and increase in implantation loss (Table 3). The same observations were reported with several different toxic xenobiotics, including pesticides (Yancheva et al., 2022), which were explained by the lack of oval production or basic cell constituents (Tuchmann, 1975). The marked loss in fetal weight may be due to the increased metabolism of lipoproteins and subsequent reduction in food and water intake, as well as stress induced by frequent exposure to THM-doses (El-Shenawy et al., 2010). Compartmentalization and transfer of toxic chemicals from the mother to the fetus through the placenta is an important factors for determining fetal resorption (El Ghareeb et al., 2015; El-Borai et al., 2019). Several reports have discussed the penetration of pesticides through placental barriers (Xue et al., 2011; Dewan et al., 2013; Dakrory et al., 2024). The current data confirmed that THM dosage resulted in a significant increase in the percentage of resorptions, a significant reduction in fetal and placental weights, and external fetal morphological and skeletal abnormalities. This result was confirmed by Gawade et al. (2013), who reported that exposure of pregnant rats to imidacloprid caused a dose-dependent post-implantation loss, fetal abnormalities and skeletal malformations, wavy and fused ribs, and absence of thoracic ribs. The observed fetal malformations in the current study were associated with oxidative changes that were induced after exposure of dams to THM, as evidenced by a dose-dependent increase in MDA level with reductions in TAC activity. These results are consistent with those of other studies that confirmed the ability of imidacloprid to induce oxidative damage in various mammalian tissues (Duzguner and Erdogan, 2012; Ahmed and Nasr, 2015; Chakroun et al., 2017; El-Sabbagh et al., 2018) and in male and female reproduction (Kapoor et al., 2011; Bal et al., 2012; Lonare et al., 2014). Based on these findings, the evaluation of fetotoxicity/teratogenicity hazards represents an initial determination of the approximate safety factor and assessment of the teratogenic effect dose level (Wang, 1988). These results provide evidence that more attention should be paid to the possible effects of various toxic substances found in the environment, particularly those with low toxicity that are present in food and water, as they could induce marked changes at the cellular level (El-Sheikh and Galal, 2015). Histological observations on the brain, liver, kidney, and lung of rats treated with THM and its combination with vit C were comparable with other studies conducted on chlorpyrifos and methyl parathion (Johnson et al., 2009 ; Breeland et al., 2023), which revealed that many insecticides induce several histopathological changes in the liver and kidney. The observations in the previous studies corroborate and support our results. This may be attributed to the antioxidant, anti-inflammatory, and hepatoprotective activities of vit C. Therefore, this study indicates that vit C supplementation may ameliorate toxic effects in individuals at risk of prolonged THM exposure. ConclusionHuman beings are exposed to agrochemicals either directly during application or indirectly through the consumption of pesticide-treated agricultural crops. The purpose of this study was to investigate the side effects of an insecticide commonly used in the agricultural field THM by studying its toxicity on animals and the anti-toxic effects of ascorbic acid. The results showed the great role of vitamin C in decreasing oxidative changes from exposure to THM. Therefore, vitamin C intake is important to reduce the negative effects of THM. In addition, vitamin C can be useful in reducing the toxic effects on various organs in individuals who deal with pesticides on a regular basis through field application. For occupational pesticide exposure, prophylactic vit C (≥500 mg/day) may mitigate oxidative damage, though human trials are needed. AcknowledgmentsWe thank Prof. Dr. E.R. El-Attar for his help in performing the histopathological examination. Conflict of interestThe authors declare no conflict of interest. FundingNo funds were received. Authors contributionsI.A.H.: methodology, data curation, and first draft writing. R.M.S: Supervision, concept development, methodology, writing, and manuscript review. A.A.S.: Supervision, writing, reviewing, and editing of the manuscript. O. E. A.: Writing and editing the manuscript. Q. H.: Writing and editing of the manuscript. D. L.: Writing and editing the manuscript. T. X: Writing and editing the manuscript. E.A.E.: Supervision, methodology, writing, reviewing, and editing of the manuscript. Data availabilityAll data from this study are included in this research, and any other inquiries can be directed to the corresponding author. ReferencesAhmed, M.M. and Nasr, S.A. 2015. Protective effect of broccoli and ferulic acid on imidacloprid-induced rat hepatotoxicity. Egypt. J. Biochem. Mol. Biol. 33, 1–15. Alkahemal-Balawi, H.F., Ahmad, Z., Al-Akel, A.S., Al-Misned, F., Suliman, E.A. and Al-Ghanim, K.A. 2011. Toxicity bioassay of lead acetate and effects of its sub-lethal exposure on growth, hematological parameters and reproduction in Clarias gariepinus. Afr. J. Biotechnic. 10(53), 11039. Assayed, M.E., Khalaf, A.A. and Salem, H.A. 2010. Protective effects of garlic extract and vitamin C against cypermethrin-induced teratogenic effects in rat offspring in vivo. Food Chem. Toxicol. 48(11), 3153–3158. Au-Qare, A.W., Abdel-Rahman, A., Kishk, A.M. and Abou-Donia, M. 2000. Placental transfer and pharmacokinetic of a single dermal dose of 14C methyl parathion rats. Toxicol. Sci. 53, 5–12. Bal, R., Türk, G., Tuzcu, M., Yilmaz, O., Kuloglu, T., Gundogdu, R., Gür, S., Agca, A., Ulas, M., Cambay, Z., Tuzcu, Z., Gencoglu, H., Guvenc, M., Ozsahin, AD., Kocaman, N., Aslan, A. and Etem, E. 2012. Assessment of imidacloprid toxicity on reproductive organ system of adult male rats. Health B. 47(5), 434–444. Basal, W.T., Ahmed, A.R.T., Mahmoud, A.A. and Omar, A.R. 2020. Lufenuron induces reproductive toxicity and genotoxic effects in pregnant albino rats and their fetuses. Scientific Rep. 10(1), 19544. Bradman, A., Barr, D.B., Claus-Henn, B.G., Drumheller, T., Curry, C. and Eskenazi, B. 2003. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ. Health 57(14), 1779–1782. Breeland, G., Sinkler, M.A. and Menezes, R.G. 2023. Embryology and bone ossification. Treasure Island (FL): StatPearls Publishing. Available via https://www.ncbi.nlm.nih.gov/books/NBK539718/ (Accessed 1 May 2023). Calderón-Garcidueñas, A.L., Martínez-Valenzuela, M.D.C. and Waliszewski-Kubiak, S.M. 2023. Pesticide exposure and its effects on intrauterine and postnatal development. Rev. Perinatol. Reprod. Humana. 37(1), 23–30. Chakroun, S., Grissa, I., Ezzi, L., Ammar, O., Neffati, F., Kerkeni, E., Najjar, M.F., Haouas, Z. and Ben Cheikh, H. 2017. Imidacloprid enhances liver damage in Wistar rats: biochemical, oxidative damage and histological assessment. J. Coastal Life Med. 5(12), 540–546. Chang, J., Jang, J., Son, G., Cho, H., Quan, S., Choe, H. and Lee, H. 2012. Ascorbic acid ameliorates oxidative damage induced by maternal low-level lead exposure in the hippocampus of rat pups during gestation and lactation. J. Appl. Nutr. 34(9), 233. Costas-Ferreira, C. and Faro, L.R.F. 2021. Neurotoxic effects of neonicotinoids on mammals: what is there beyond the activation of nicotinic acetylcholine receptors?—a systematic review. Int. J. Mol. Sci. 22(16), 8413. Dakrory, A.I., Elsaed, O.I. and Omar, A.R. 2024. Effect of gestational exposure to neonicotinoids insecticide ‘Thiacloprid’ on Wistar rats and their Egypt. J. Zool. 82(82), 92–108. Dewan, P., Jain, V., Gupta, P. and Banerjee, B.D. 2013. Organochlorine pesticide residues in maternal blood, cord blood, placenta, and breastmilk and their relation to birth size. Chemosphere 90, 1704–1710. Di Meo, T., Reed, P., Venditti, V. and Victor. 2016. Role of ROS and RNS sources in physiology and pathology. Oxid. Med. Cell. Long. 2016, 1245049. Djurasevic, F., Cvijic, G., Djordjevic, J. and Davidovic, V. 2008. Influence of vitamin C supplementation on the oxidative status of interscapular brown adipose tissue in rats. J. Thermal Biol. 33, 238–243. Duzguner, V., S. and Erdogan. 2012. Chronic exposure to imidacloprid induces inflammation and oxidative stress in the liver and CNS of rats. Pesticide Biochem. Physiol. 104(1), 58–64. Eisa, A.A.A., Aboelghar, G.E., Ammar, I.M., Metwally, H.G. and Arafa, S.S. 2018. Teratogenic effects induced by chitosan oligosaccharide in female Wistar rats. Environ. Sci. Pollut. Res. Int. 25(10), 9371–9379. El Borai, N., Hadad, S. and Khalifa, H. 2019. Teratogenic effects of imidacloprid in rats: mechanistic role of oxidative stress. Int. J. Pharmacol. Toxicol. 7(2), 35–43. El Ghareeb, A., Hamdi, H., Taha, E.S.F. and Ali, H. 2015. Evaluation of teratogenic potentials of bronchodilator drug on offsprings of albino rats. Sci. ENT. Eng. Res. 6, 534–542. El-Hak, H.N.G., Al-Eisa, R.A., Ryad, L., Halawa, E. and El-Shenawy, N.S. 2022. Mechanisms and histopathological impacts of acetamiprid and azoxystrobin in male rats. Environ. Sci. Pollut. Res. 29, 43114–43125. El-Sabbagh, H.S., Khamiss, O.A., El-Borai, N.B. and El-Khadrawey, B.A. 2018. The potential therapeutic value of green tea and thyme aqueous extracts on imidacloprid toxicity in rats. IJMPR 2(4), 59–67. El-Sheikh, E., Li, D., Hamed, I., Ashour, M. and Hammock, B. 2023. Residue analysis and risk exposure assessment of multiple pesticides in tomato and strawberry markets and their products. Foods 12(10), 1936. El-Sheikh, E.S. and Ashour, M.B. 2022. Diamide insecticides: efficacy, toxicity and analytical methods for residue monitoring in food samples. Egypt. J. Chem. 65, 165–177 El-Sheikh, S. and Galal, A. 2015. Toxic effects of sub-chronic exposure of male albino rats to emamectin benzoate and possible ameliorative role of F. vulgare essential oil. Environ. Toxicol. Pharmacol. 39(3), 1177–1188. El-Shenawy, N.S., El-Salmy, F., Al-Eisa, R.A. and El-Ahmary, B. 2010. Amelioratory effect of vitamin E on organophosphorus insecticide diazinon-induced oxidative stress in mice liver. Pesticide Biochem. Physiol. 96(2), 101–107. Environmental Protection Agency, United States 2011. Thiamethoxam, pesticide tolerance. Fed Regul Agency 76, 159–160. Gawade, L., Dadarkar, S.S., Husain, R. and Gatne, M. 2013. A detailed study of developmental immunotoxicity of imidacloprid in Wistar rats. Food Chem. Toxicol. 51, 61–70. Hamed, I., Sherif, R., El-Sheikh, E., Aldawek, A.M. and Shalaby, A.A. 2023. Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. Open Vet. J. 13(10), 1334–1345. Hamed, I.A., Sherif, R.M., El-Sheikh, E.S.A. and Shallaby, A.A. 2019. Susceptibility and biochemical determination of house fly (Musca domestica L.) to lambda-cyhalothrin. J. Entomol. 16, 91–97. Hassan, A.A., Bel Hadj Salah, K., Fahmy, E.M., Mansour, D.A., Mohamed, S.A.M., Abdallah, A.A., Ashkan, M.F., Majrashi, K.A., Melebary, S.J., El-Sheikh, E.S.A. and El-Shaer, N. 2022. Olive leaf extract attenuates chlorpyrifos-induced neuro- and reproductive toxicity in male albino rats. Life 12, 1500. Holzum, B. 1996. YRC 2894–Developmental toxicity study in rabbits after oral Unpublished report No. 24709, edition no. M-000780-01-1, from Bayer AG, Wuppertal, Germany, dated January 26, 1996. Submitted to the World Health Organization by Bayer Crop Science AG, Monheim, Germany. Geneva: World Health Organization. Hussein, M., Singh, V., Hassan, M., Singh, A. and Yadav, B. 2014. Malformations and teratogenic effects of imidacloprid on chick embryo. Appl. Med. Sci. 2, 67–72. Ibrahim, R., Elkady, M. and Hassanein, A. 2019. Effect of some antioxidants on rats treated with titanium dioxide nanoparticles. Egypt. J. Food Sci. 47(1), 91–103. Johnson, F.O., Chambers, J.E., Nail, C.A., Givaruangsawat, S. and Carr, R.L. 2009. Developmental chlorpyrifos and methyl parathion exposure alters radial-arm maze performance in juvenile and adult rats. Toxicological Sci. 109(1), 132–142. Kapoor, U., Srivastava, M.K. and Srivastava, L.P. 2011. Toxicological impact of technical imidacloprid on ovarian morphology, hormones, and antioxidant enzymes in female rats: a meta-analysis. Food Chem. Toxicol. 49, 3086–3099. Koracevic, D., Koracevic, G., Djordjevic, V., Andrejevic, . S. and Cosic, V2001. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 54(5), 356–361. Kurutas, E. 2016. The role of antioxidants in the cellular response to oxidative/nitrosative stress: current state. Nutr. J. 25(1), 15–71. Lobo, V., Patil, A., Phatak, A. and Chandra, N. 2010. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 4(8), 118–126. Lonare, M., Kumar, M., Raut, S., Badgujar, P., Doltade, S. and Telang, A. 2014. Evaluation of imidacloprid-induced neurotoxicity in male rats: a protective effect of curcumin. Neurochem. Int. 78, 122–129. Manson, J.M. and Kang, Y.J. 1989. Methods for assessing female reproductive and developmental toxicology.In Prin. Methods Toxicol. Hayes, A.W pp: 72–83. Mansour, S.A. and Mossa, A.H. 2010. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pest. Biochem. Physiol. 96, 14–23. NRC, 1996. Guide for the care and use of laboratory animals. National Research Council Academic Press, Washington DC USA. Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95(2), 351–358. Paccola, C.C., Resende, C.G., Stumpp, T., Miraglia, S.M. and Cipriano, I. 2018. The rat estrous cycle revisited: a quantitative and qualitative analysis. Anim. Reprod. (AR). 10(4), 677–683. Saoudi, M., Badraoui, R., Rahmouni, F., Jamoussi, K. and El Feki, A. 2021. Antioxidant and protective effects of A. campestris essential oil against chlorpyrifos-induced kidney and liver injuries in rats. Front. Physiol. 12, 618582. Scifres, C.M., Catov, J.M. and Simhan, H.N. 2014. The impact of maternal obesity and gestational weight gain on early and mid-pregnancy lipid profiles. Obesity 22(3), 932–938. Staples, R.E. and Schnell, V.L. 1964. Refinements in the rapid clearing technique of the KOH-alizarin red S method for fetal bone analysis. Stain Technol. 39, 61–63. Suvarna, K.S., Christopher, L. and Bancroft, J.D. 2013. Bancroft’s Theory and Practice of Histological Techniques. 7th ed. London, UK: Northern General Hospital Sheffield. Toxicity–Up, A. O. 2001. OECD guideline for testing of chemicals. Organization for Economic Co-Operation and Development: Paris, France, 1–14. Tuchmann, H. 1975. Drug effects on fetus. New York: ADIS Press, 7. Wang, G.M. 1988. Regulatory decision-making and the need for exposure data on teratogenic pesticides in test animals. Carcinogen Mutagen 8, 117–126. Wilson, J.G. 1965. Methods for administering agents and detecting malformations in experimental animals. In Teratology: principles and techniques, Eds., Wilson, J.G. and Warkney J. Chicago, IL: University of Chicago Press, 262–277. Xue, L., Yi, H., Huang, Z., Shi, Y.B. and Li, W.X. 2011. Global gene expression during the human organogenesis: from transcription profiles to function predictions. Int. J. Biol. Sci. 7(7), 1068–1076. Yancheva, V., Georgieva, E., Velcheva, I., Iliev, I., Stoyanova, S., Vasileva, T. and Nyeste, K. 2022. Assessment of the exposure of two pesticides on common carp (Cyprinus carpio Linnaeus, 1758): are the prolonged biomarker responses adaptive or destructive?. Comp. Biochem. Physiol. Part C. Toxicol. Pharmacol. 261, 109446 | ||
| How to Cite this Article |
| Pubmed Style Hamed IA, Sherif RM, Shalaby AA, Abdoslam OE, He Q, Li D, Xu T, El-sheikh EA. TTeratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acid. Open Vet. J.. 2025; 15(9): 4441-4453. doi:10.5455/OVJ.2025.v15.i9.51 Web Style Hamed IA, Sherif RM, Shalaby AA, Abdoslam OE, He Q, Li D, Xu T, El-sheikh EA. TTeratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acid. https://www.openveterinaryjournal.com/?mno=241566 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.51 AMA (American Medical Association) Style Hamed IA, Sherif RM, Shalaby AA, Abdoslam OE, He Q, Li D, Xu T, El-sheikh EA. TTeratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acid. Open Vet. J.. 2025; 15(9): 4441-4453. doi:10.5455/OVJ.2025.v15.i9.51 Vancouver/ICMJE Style Hamed IA, Sherif RM, Shalaby AA, Abdoslam OE, He Q, Li D, Xu T, El-sheikh EA. TTeratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acid. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4441-4453. doi:10.5455/OVJ.2025.v15.i9.51 Harvard Style Hamed, I. A., Sherif, . R. M., Shalaby, . A. A., Abdoslam, . O. E., He, . Q., Li, . D., Xu, . T. & El-sheikh, . E. A. (2025) TTeratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acid. Open Vet. J., 15 (9), 4441-4453. doi:10.5455/OVJ.2025.v15.i9.51 Turabian Style Hamed, Ibrahim A., Refat M. Sherif, Aly A. Shalaby, Omran E. Abdoslam, Qiyi He, Dongyang Li, Ting Xu, and El-sayed A. El-sheikh. 2025. TTeratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acid. Open Veterinary Journal, 15 (9), 4441-4453. doi:10.5455/OVJ.2025.v15.i9.51 Chicago Style Hamed, Ibrahim A., Refat M. Sherif, Aly A. Shalaby, Omran E. Abdoslam, Qiyi He, Dongyang Li, Ting Xu, and El-sayed A. El-sheikh. "TTeratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acid." Open Veterinary Journal 15 (2025), 4441-4453. doi:10.5455/OVJ.2025.v15.i9.51 MLA (The Modern Language Association) Style Hamed, Ibrahim A., Refat M. Sherif, Aly A. Shalaby, Omran E. Abdoslam, Qiyi He, Dongyang Li, Ting Xu, and El-sayed A. El-sheikh. "TTeratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acid." Open Veterinary Journal 15.9 (2025), 4441-4453. Print. doi:10.5455/OVJ.2025.v15.i9.51 APA (American Psychological Association) Style Hamed, I. A., Sherif, . R. M., Shalaby, . A. A., Abdoslam, . O. E., He, . Q., Li, . D., Xu, . T. & El-sheikh, . E. A. (2025) TTeratogenic effects of thiamethoxam on female rats and possible protective function of ascorbic acid. Open Veterinary Journal, 15 (9), 4441-4453. doi:10.5455/OVJ.2025.v15.i9.51 |