| Research Article | ||
Open Vet. J.. 2025; 15(5): 2122-2126 Open Veterinary Journal, (2025), Vol. 15(5): 2122-2126 Research Article Ruminoscopy in apparently healthy camel calves (camelus dromedarius): A technique description and ruminoscopic observationsAbdulaziz H. Almuhanna1*, Ayman Elnahas1, Mohamed K. Zabady1, Sayed El-Hawari1, Mohamed Marzok1, Wael Eldeeb1, Isam El Jalii1, Zakriya Al Mohamad1 and Arafat Khalaphallah21Department of Clinical Science, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia 2Division of Internal Medicine, Department of Animal Medicine, Faculty of Veterinary Medicine, Assuit University, Assuit, Egypt *Corresponding Author: Abdulaziz H. Almuhanna. Department of Clinical Science, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia. Email: aalmohana [at] kfu.edu.sa Submitted: 30/01/2025 Revised: 08/04/2025 Accepted: 14/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Accurate diagnosis of digestive disorders in camels requires validation and optimization of new diagnostic techniques to enable clear visualization of the rumen interior. Aim: The aim of this study was to describe the validity of endoscopy for visualization of the rumen in camels. Methods: Ten apparently healthy camel calves were included in this study. Each camel was appropriately restrained, and the endoscope was inserted through the oro-esophageal route to enable visualization of the rumen. Results: Endoscopy via the oral route allowed visualization of the upper digestive organs and rumen. The ruminal mucosa, glandular parts of the rumen, and ruminal contents were also observed. The technique was performed safely, and no clinical complications were observed. Conclusion: The application of ruminoscopy in camels was shown to be a noninvasive and rapid technique that enabled visual access to the appearance and contents. Such research has the potential to guide clinicians toward better diagnosis of ruminal disorders in the future. Keywords: Camel, Ruminoscopy, Rumen, Endoscope, Diagnostics. IntroductionGastrointestinal disorders in camels are of significant importance. These conditions encompass ruminal impaction, ruminal bloat, abomasal ulceration, and swallowed foreign bodies (Fowler, 2011). Furthermore, acute ruminal acidosis is one of the most severe forms of ruminal microbial fermentative disorders, with some cases proving to be fatal within 24 hours (Constable et al. 2016). Endoscopy, which involves the visual examination of bodily cavities using optical devices, is a minimally invasive technique commonly employed for the diagnosis and treatment of numerous digestive issues in both human and veterinary medicine (Sasikala et al. 2019). Gastrointestinal endoscopy in ruminants has been previously implemented either via oral (Franz et al. 2006, McRae et al. 2016, Vijayakumar et al. 2016, Ramos-Zayas et al. 2022), rectal (Van Niekerk et al. 2018), or paralumbar fossa (Franz et al. 2006, Van Niekerk et al. 2018, Dos Santos et al. 2022, Dos Santos et al. 2022) approaches. Gastrointestinal endoscopy of ruminants is a routine, painless, diagnostic, therapeutic, and secure procedure. It has been traditionally utilized for removing esophageal foreign bodies (Vijayakumar et al. 2011, Venkatesakumar et al. 2022, Zabady et al. 2022), diagnosing mega-esophagus (Vijayakumar et al. 2016), and conducting ruminal biopsies (Van Niekerk et al. 2018, Ramos-Zayas et al. 2022). Ruminoscopy is a crucial technique in gastrointestinal endoscopy and plays a vital role in diagnosing ruminoreticular disorders. In cattle, it has been used in the past to examine the ruminal papillae in cases of lactic acidosis (Sasikala et al. 2018). The examination of the rumen and reticular surfaces can help identify pathological conditions. The capability to visualize features naturally and in color is deemed an authentic method, which is superior to other diagnostic tools, such as ultrasonography and radiography (Sasikala et al. 2019). Franz et al. (2006) compared two ruminoscopic techniques (oral and paralumbar fossa approaches) for visualization of the ruminoreticular mucosa and assessment of ruminoreticular contractility in bovine calves. To the best of our knowledge, there is no available literature describing the technique of ruminoscopy in the dromedary camel. In Saudi Arabia, according to our clinical experience at King Faisal University Teaching Hospital and our field visits, there are many cases of gastrointestinal tract diseases, most of which present with non-specific clinical signs such as anorexia, dullness, or retarded growth. Routine clinical examination and laboratory sampling of such cases may provide little diagnostic value. Therefore, the primary objective of this study was to offer a comprehensive description of the ruminoscopy technique in apparently healthy camel calves, with a particular focus on evaluating the normal appearance of the ruminal mucosa, ruminal content, and ruminal motility. Materials and MethodsA total of 10 camel calves aged 1–20 months, of mixed breed (Majaheem, Maghateer, and Waddah), were included in this study. Animals included in this study were assessed as apparently healthy based on normal vital signs, normal appetite, and the absence of visible clinical abnormalities, especially those related to the gastrointestinal tract. Solid food was withheld for 2 days before the procedure to improve the visualization of the rumen. Intravenous glucose (1–2 l of 5% dextrose in normal saline) and fluid therapy were provided for each animal. Each examined camel calf was weighed on a large animal weighing scale to determine the appropriate sedation dosage. Subsequently, the animal was positioned on sternal recumbency and sedated intravenously with xylazine at a dose rate of 0.2 mg/kg (I/V) (Seton 2%; Laboratorios Calier, S.A.C./Barcelonés, Barcelona-Espana). Sedation was administered to ensure better control of animals and to prevent any possible physical damage to the instrument during the clinical procedure. A transparent stomach tube (with fenestrated tip) was used to remove excessive ruminal juice in some animals in which rumen fluid partially impairs appropriate assessment. A customized wooden mouth gag was then placed in the oral cavity. With the aid of two clinicians, the head and neck were manually maintained in a slightly horizontal position to facilitate the passage of the stomach tube. A portable video endoscopy (Fig. 1A) (with insertion tube: 3,300 mm long and 12 mm in diameter) powered by a source of light and an irrigation system and provided with a 3.7 biopsy channel was used (Guangzhou Tueshen Medical Equipment Co., ltd. China). The tip of the insertion tube was then inserted into the central canal of the mouth gag and passed through the oral cavity (Fig. 1B), pharynx, and then advanced and passed through the esophagus. An irrigation system was used to cleanse the camera of saliva or potential food particles that came into contact during the examination. Upon visualizing the opening of the cardia, the tip of the insertion tube is guided toward it to induce swallowing, allowing passage into the rumen. In instances of resistance to swallowing is noted, a 10 ml solution of 2% lidocaine was locally flushed to relax the cardia. The rumen was then visualized for appearance, and its motility was evaluated and scored as strong (obvious motility of all parts of the rumen), moderate (the ruminal motility is of moderate contractility), or weak (complete ruminal stasis). After complete visualization of the rumen, the endoscope was carefully removed, and the animals were allowed to recover from sedation. Ethical approvalThis study was approved by the Research Ethics Committee of King Faisal University (ETHICS2918), (KFU-REC-2024-DEC-ETHICS2918), date 11-12-2024.. ResultsThe ruminoscopy technique was safely performed on the examined animals and did not cause any noticeable pain or discomfort. The oro-esophageal ruminoscopy route facilitates the passage of the insertion tube without injuring the adjacent mucosa. The ruminoscopic examination allowed complete visualization of the esophagus, cardia opening, and rumen content (Fig. 2A, B, and C). In some animals, the esophagus appeared either as a fully opened, cylindrical-like structure or as a star shape, due to contraction of the esophagus in front of the tip of the endoscope. Similarly, the cardia was found as a slit when fully closed (especially in the youngest camel calves) or as a round ring when opened. The length of the inserted endoscopy reaching the cardia in the examined animals ranged from 1.4 to 1.85 m. Ruminal ingesta appeared as green/olive materials, and ruminal juice was also observed in the peripheral regions of the rumen (Fig. 2C). The ruminal wall appeared smooth and pink, with some blood vessels and fine blood capillaries clearly visible (Fig. 2C). The visualization of the rumen also revealed the presence of pouch-like structures known as glandular saccules (Fig. 2D). Regarding the gross appearance of the ruminal mucosa, neither ruminal pillars nor ruminal papillae could be clearly visualized. The motor activity of the ruminal wall was also observed and recorded. We were not able to assess rumen motility according to the number of ruminal cycles per unit time. However, the strength of ruminal wall contraction was used instead as an alternative indicator of rumen motility, which was found to be strong in seven animals and moderate in three of them. In contrast, in no of the animals, we observed complete ruminal stasis.
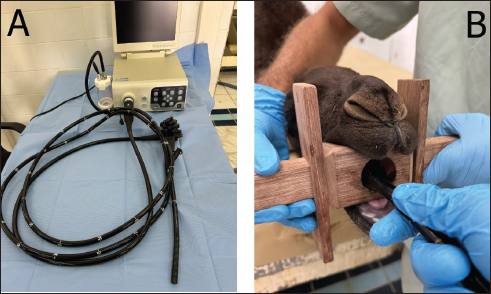
Fig. 1. Endoscopy device (A) used for ruminoscopic examination, which is composed of an insertion tube, video processor with a screen, and irrigation unit. Figure (B) reveals the correct placement of the mouth gag with the tip of the insertion tube inserted through the central canal of the mouth gag.
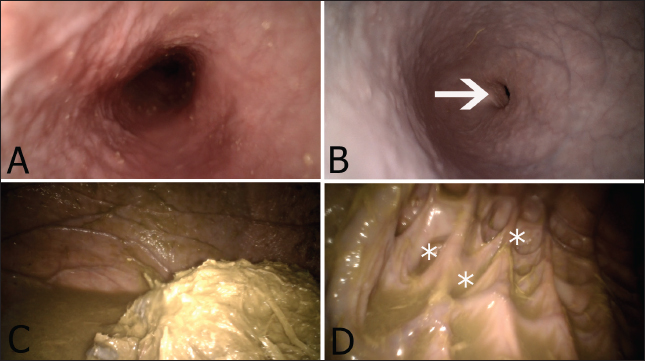
Fig. 2. Appearance of the esophagus as a tube-like structure in the cervical neck area (A). Figure (B) reveals the appearance of the thoracic region of the esophagus, where the cardia opening is clearly visible (arrow). The ruminal content and ruminal juice are seen in figure (C). Figure (D) shows the appearance of the ruminal glandular saccules (asterisks). DiscussionAdvancing camel medicine requires the validation and optimization of new ancillary diagnostic techniques that provide full insights into the digestive system of camels. Therefore, this study was conducted. To the best of our knowledge, this is the first study to provide a full description of ruminoscopy in apparently healthy camel calves. This pilot study has now been shown to be feasible and repeatable and does not pose any potential complications. Although the main aim of this study was to report that ruminoscopy is a useful diagnostic technique in camel medicine, we have also been successful in detecting and diagnosing some unusual ruminal disorders in young and adult camels (unpublished data and which is beyond the remit of this work). Therefore, we envisage that the validation of such a diagnostic technique could be considered as an additional tool for the detection of digestive diseases that necessitate rumen visualization in camels. Ruminoscopy has previously been conducted in bovines through the oro-ruminal (Sasikala et al. 2019) or nasal routes (Sasikala et al. 2022), and both interventions have been shown to result in satisfactory assessments of the rumen. In equine, gastroscopy is typically carried out through the naso-esophageal path (van den Boom 2022); however, the oral approach is commonly adopted in ruminants (Franz et al. 2024), which could be attributed to the anatomical differences in the nasopharyngeal area between both species (Goulden 2002). A study conducted by Sasikala et al. (2018, 2019, 2022) on 20 adult buffalo demonstrated that ruminoscopy is applicable via the nasal route employing an 8 mm diameter endoscope. In our study, in contrast, we voted for the oral approach due to the collapse of the nostrils in camel calves and the narrow-spaced nasal cavity (Shawaf et al. 2021). While oral ruminoscopy poses a risk of tube damage from chewing, we effectively mitigated this risk through sedation and manual restraint. This pilot study demonstrated that ruminoscopy is applicable to camel calves and can be considered a safe and noninvasive clinical procedure. Our results indicated that the esophagus had a bright pink color, with its mucosal surface shining. This finding is similar to what has been reported in other animals, such as bovines (Sasikala et al. 2019). Additionally, the endoscopic appearance of the cardia is also in accordance with previously published work in cattle, in which it appeared as a slit (Sasikala et al. 2019). On the other hand, our results revealed that the morphology of the ruminal wall and the appearance of the mucosal surface are different from that of cattle, with the latter possessing different types of ruminal papillae, such as millet-shaped, petal-shaped, neem leaf-shaped, and finger-like projections papillae (Sasikala et al. 2019). These papillae were not seen in our examined animals since the rumen of camels does not have these anatomical structures, but they do possess specialized small-sized sacs known as glandular saccules (Ibrahim and Almundarij 2023). These saccules were clearly observed in our study, especially in animals with little ruminal content or in those where the ruminal contents were evacuated by a stomach tube. It is possible that the anatomical differences between camels and other species, like ovine and bovine, might be attributed to their different digestive physiology or to overcoming the environmental challenges that camels might encounter. Unlike ruminants and equines, camels are considered pseudo-ruminants due to their unique anatomical characteristics, as they possess three ruminal chambers (Al-Jassim and Hogan 2012). In addition, the pattern of rumen motility differs slightly from that typically reported in other true ruminants (Qureshi and Al-Ani 2008). Our results indicate that the subjective assessment of rumen motility differs between the examined animals. For example, some individuals were assigned with moderate ruminal motility despite having a normal appetite. The explanation for such phenomena can either indicate the presence of undiagnosed subclinical rumen dysfunction or simply the effect of sedation on rumen motility. It was previously reported that the use of xylazine in bovine and ovine resulted in a significant reduction in rumen motility (Braun et al. 2002, Waite et al. 2021). The impact of xylazine on rumen contractility in camels remains unclear; hence, precisely interpreting such results is challenging. Additional research is necessary to explore various sedative agents to identify a suitable option with minimal inhibitory effects on rumen motility. Although we were not able to clearly visualize the reticulum, we envisage that the application of ruminoscopy in camels will facilitate the antemortem diagnosis of some common conditions that would otherwise be detected in postmortem examination, such as ruminal foreign bodies, inflammatory conditions of the rumen, and possibly gastric ulcers. ConclusionThis study revealed that ruminoscopy is a safe, rapid, and noninvasive technique that can provide momentary visualization of the rumen in camel calves. This research has the potential to enhance our future understanding of camel ruminal diseases. In addition, the work described in this paper could pave the way for advancing our understanding of camel digestive medicine. AcknowledgmentsThe authors thank the authorities of the College of Veterinary Medicine at King Faisal University for their unlimited support. Conflict of interestThe authors declare no conflict of interest. FundingNone. Author contributionsAA conceptualized and designed the study. AA, AE, MZ, SE, and MM performed the clinical work. AA and SE wrote the manuscript, and WE, IE, ZA, and AK provided critical assessments of the manuscript. Data availabilityAll data are available from the corresponding author upon reasonable request. ReferencesAl-Jassim, R. and Hogan, J. 2012. The digestive system of the camel, its anatomical, histological and functional characteristics: a comparative approach. In Proceedings 3rd ISOCARD Conference, Oman, pp 75–86. Braun, U., Gansohr, B. and Hässig, M. 2002. Ultrasonographic evaluation of reticular motility in cows after administration of atropine, scopolamine and xylazine. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 49(6), 299–302. Constable, P.D., Hinchcliff, K.W., Done, S.H. and Grünberg, W. 2016. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats, 11th ed. Philadephia, PA: Elsevier Health Sciences. Dos Santos, G.M.A., Borges, L.P.B., de Morais, H.L.M., da Conceição Guilherme, B., dos Santos Albuquerque, R., Rossy, K. C., and Teixeira, P.P.M. 2022. Percutaneous ruminostomy guided by rumenoscopy: study in an experimental model in bovine fetus. BMC Vet. Res. 18(1), 41. Fowler, M.E. 2011. Medicine and surgery of camelids. 3rd ed. Hoboken, NJ: John Wiley & Sons. Franz, S., Gentile, A. and Baumgartner, W. 2006. Comparison of two ruminoscopy techniques in calves. Vet. J. 172(2), 308–314. Franz, S., Hofer, L. and Dadak, A.M. 2024. The role of endoscopy in bovine internal medicine–a review of current indication fields. Vet. J. 106093. Goulden, B. 2002. The equine larynx. N. Z. Vet. J. 50(Suppl. 3), 117. Ibrahim, Z.H. and Almundarij, T.I. 2023. Morphology of the dromedary camel stomach with reference to physiological adaptation. Slov. Vet. Res. 60, 341–352. McRae, K.M., Schultz, M., Mackintosh, C.G. and Shackell, G.H., Martinez, M.F., Knowler, K.J., Williams, M., Ho, C., Elmes, S.N. and McEwan, J.C.. 2016. Ovine rumen papillae biopsy via oral endoscopy; a rapid and repeatable method for serial sampling. N. Z. Vet. J. 64(3), 174–178. Qureshi, A. and Al-Ani, F. 2008. The digestive system. In: Camel Management and Diseases. Amman, Jordan: Dar Ammar Book Publisher, pp: 197–218. Ramos-Zayas, Y., Cantú-Reyes, S.A., Tristán-Casas, I.I. and Kawas, J.R. 2022. A novel oral endoscopic biopsy procedure to obtain rumen epithelial samples. Vet. Sci. 9(5), 230. Sasikala, K.V.G., Sivaraman, S. and Balasubramaniam, G.A. 2018. Ruminoscopy in cattle (Bos taurus) with ruminal lactacidosis—a rapid and novel method to visualize rumen papillary changes. Int. J. Curr. Microbiol. Appl. Sci. 7(5), 3112–3119. Sasikala, K., Vijayakumar, G., Sivaraman, S. and Balasubramaniam, G.A. 2019. Endoscopic evaluation of rumen in cattle (Bos taurus)—a preliminary study. Indian J. Anim. Res. 53(10), 1386–1388. Sasikala, K., Vijayakumar, G., Sivaraman, S. and Balasubramaniam, G.A. 2022. Nasoruminal endoscopy of the rumen and reticulum in buffaloes (Bubalus bubalis)–a preliminary study. Pol. J. Vet. Sci. 25(1), 183–186. Shawaf, T., Almubarak, A., Alhumam, N., Almathen, F. and Hussen, J. 2021. Cytological analysis of tracheal wash and bronchoalveolar lavage fluid in health and respiratory disease in dromedary camels. PeerJ 9, e11723. Van den Boom, R. 2022. Equine gastric ulcer syndrome in adult horses. Vet. J. 283, 105830. Van Niekerk, J.K., Middeldorp, M. and Steele, M.A. 2018. The development of a methodology for ruminal and colon tissue biopsying of young Holstein dairy calves. J. Dairy Sci. 101(8), 7212–7218. Venkatesakumar, E., Vijayakumar, G. and Ponnu Swamy, K.K. 2022. Endoscopic guided retrieval of linear foreign body causing partial oesophageal obstruction in a dairy cow: a case report. Pharma. Innov. J. 11, 2687–2689. Vijayakumar, G., Jegaveera-Pandian, S., Venkatesakumar, E. and Kathirvel, S., Subramanian, M. and Dharmaceelan, S. 2016. Endoscopic diagnosis of megaesophagus in a buffalo. Buffalo Bull. 35(2), 147–150. Vijayakumar, G., Venkateshakumar, E., Sivaraman, S. and Subramanian, M. 2011. Oesophageal obstruction due to trichobezoar in a heifer and its endoscopic removal. Intas. Polivet 12(2), 304–305. Waite, S.J., Cater, J.E., Waghorn, G.C. and Suresh, V. 2021. Effect of sedatives on rumen motility in sheep. Small Rumin. Res. 196, 106284. Zabady, M.K., ElJalii, I., Elnahas, A. and Shawaf, T. 2022. Esophageal obstruction due to trichobezoar in a she-camel (Camelus dromedarius). Open Vet. J. 12(6), 855–858. | ||
| How to Cite this Article |
| Pubmed Style Almuhanna AH, Elnahas A, Zabady MK, El-hawari S, Marzok M, El-deeb W, Jalii IE, Mohamad ZA, Khalphallah A. Ruminoscopy in apparently healthy camel calves (Camelus dromedarius): A technique description and ruminoscopic observations. Open Vet. J.. 2025; 15(5): 2122-2126. doi:10.5455/OVJ.2025.v15.i5.30 Web Style Almuhanna AH, Elnahas A, Zabady MK, El-hawari S, Marzok M, El-deeb W, Jalii IE, Mohamad ZA, Khalphallah A. Ruminoscopy in apparently healthy camel calves (Camelus dromedarius): A technique description and ruminoscopic observations. https://www.openveterinaryjournal.com/?mno=240398 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.30 AMA (American Medical Association) Style Almuhanna AH, Elnahas A, Zabady MK, El-hawari S, Marzok M, El-deeb W, Jalii IE, Mohamad ZA, Khalphallah A. Ruminoscopy in apparently healthy camel calves (Camelus dromedarius): A technique description and ruminoscopic observations. Open Vet. J.. 2025; 15(5): 2122-2126. doi:10.5455/OVJ.2025.v15.i5.30 Vancouver/ICMJE Style Almuhanna AH, Elnahas A, Zabady MK, El-hawari S, Marzok M, El-deeb W, Jalii IE, Mohamad ZA, Khalphallah A. Ruminoscopy in apparently healthy camel calves (Camelus dromedarius): A technique description and ruminoscopic observations. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2122-2126. doi:10.5455/OVJ.2025.v15.i5.30 Harvard Style Almuhanna, A. H., Elnahas, . A., Zabady, . M. K., El-hawari, . S., Marzok, . M., El-deeb, . W., Jalii, . I. E., Mohamad, . Z. A. & Khalphallah, . A. (2025) Ruminoscopy in apparently healthy camel calves (Camelus dromedarius): A technique description and ruminoscopic observations. Open Vet. J., 15 (5), 2122-2126. doi:10.5455/OVJ.2025.v15.i5.30 Turabian Style Almuhanna, Abdulaziz H., Ayman Elnahas, Mohamed K. Zabady, Sayed El-hawari, Mohamed Marzok, Wael El-deeb, Isam El Jalii, Zakriya Al Mohamad, and Arafat Khalphallah. 2025. Ruminoscopy in apparently healthy camel calves (Camelus dromedarius): A technique description and ruminoscopic observations. Open Veterinary Journal, 15 (5), 2122-2126. doi:10.5455/OVJ.2025.v15.i5.30 Chicago Style Almuhanna, Abdulaziz H., Ayman Elnahas, Mohamed K. Zabady, Sayed El-hawari, Mohamed Marzok, Wael El-deeb, Isam El Jalii, Zakriya Al Mohamad, and Arafat Khalphallah. "Ruminoscopy in apparently healthy camel calves (Camelus dromedarius): A technique description and ruminoscopic observations." Open Veterinary Journal 15 (2025), 2122-2126. doi:10.5455/OVJ.2025.v15.i5.30 MLA (The Modern Language Association) Style Almuhanna, Abdulaziz H., Ayman Elnahas, Mohamed K. Zabady, Sayed El-hawari, Mohamed Marzok, Wael El-deeb, Isam El Jalii, Zakriya Al Mohamad, and Arafat Khalphallah. "Ruminoscopy in apparently healthy camel calves (Camelus dromedarius): A technique description and ruminoscopic observations." Open Veterinary Journal 15.5 (2025), 2122-2126. Print. doi:10.5455/OVJ.2025.v15.i5.30 APA (American Psychological Association) Style Almuhanna, A. H., Elnahas, . A., Zabady, . M. K., El-hawari, . S., Marzok, . M., El-deeb, . W., Jalii, . I. E., Mohamad, . Z. A. & Khalphallah, . A. (2025) Ruminoscopy in apparently healthy camel calves (Camelus dromedarius): A technique description and ruminoscopic observations. Open Veterinary Journal, 15 (5), 2122-2126. doi:10.5455/OVJ.2025.v15.i5.30 |