| Case Report | ||
Open Vet. J.. 2025; 15(7): 3357-3365 Open Veterinary Journal, (2025), Vol. 15(7): 3357-3365 Case Report Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case seriesAndré Gustavo Alves Holanda1, Arturo Vargas Ruanova2, Enrico Pierluigi Spugnini3, Marla Tereza Frasson4, Carlos Eduardo Fonseca-Alves5,6 and Denner Santos Dos Anjos6*1Department of Surgery, School of Veterinary Medicine and Animal Science, University of São Paulo (FMVZ/USP), São Paulo, Brazil 2Veterinary Practitioner-Electroquimioterapia Veterinaria, Ciudad de México, México 3Biopulse Srl, Rome, Italy 4Veterinary Practitioner, Espírito Santo, Brazil 5Institute of Health Sciences, Universidade Paulista (UNIP), Bauru, Brazil 6Department of Veterinary Surgery and Animal Reproduction, Universidade Estadual Paulista (UNESP), Botucatu, Brazil *Corresponding Author: Denner Santos Dos Anjos, Department of Veterinary Surgery and Animal Reproduction, Universidade Estadual Paulista (UNESP), Botucatu, Brazil. Email: denner.anjosoncology [at] gmail.com Submitted: 29/01/2025 Revised: 15/06/2025 Accepted: 19/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Cutaneous mast cell tumor (MCT) is one of the most common skin neoplasms in cats and typically exhibits clinically benign behavior. Multiple MCTs, recurrent tumors, or infiltrated lymph nodes are associated with a more guarded prognosis. Surgery is the treatment of choice. However, the presence of multiple or large tumors can make the procedure challenging. Electrochemotherapy (ECT) is an effective local treatment, suitable for managing cutaneous or subcutaneous malignant tumors. This case series describes the first report of the use of ECT in the treatment of four cats with MCT. Case Description: Three cats presented with multiple MCTs, ranging from 7 to 50 nodules measuring 0.2 to 1 cm over the head and trunk, and one cat with a large tumor on the head measuring 4.6 cm. None of the patients had macroscopic metastasis detected on complete staging. Based on the anatomic region and limitations of systemic treatment, we proposed the use of ECT with intravenous bleomycin (15,000 IU/m²) as an alternative approach, and complete resolution of the tumors was observed in all patients after treatment. No tumor recurrence was detected during the follow-up period (range: 210–810 days). However, one patient developed new lesions at day 210. Local toxicity induced by ECT was graded as 1 (mild swelling) on a 6-point arbitrary scale (grades 0–5), and alopecia and scar tissue were observed after complete tumor remission. The number of ECT sessions ranged from one to two, with intervals of 14 to 45 days between sessions. Conclusion: The use of ECT may be successfully applied as a viable alternative approach for solitary or multiple MCTs in feline patients as an alternative to surgery or in refractory cases, without significant toxicity, as observed in this case series report. Further research is warranted to support these preliminary findings. Keywords: Bleomycin, Electrochemotherapy, Electric pulses, Electroporation, Mast cell tumor. IntroductionCutaneous mast cell tumor (MCT) is one of the most common skin neoplasms in cats, accounting for up to 20% of feline skin tumors (Miller et al., 1991). They are often solitary and located on the head, neck, and trunk. The vast majority are well-differentiated and exhibit clinically benign behavior. However, multiple MCTs, recurrent tumors, splenic MCTs, or infiltrated lymph nodes are associated with a more guarded prognosis (Litster and Sorenmo, 2006; Oliveira et al., 2020). Surgery is the treatment of choice. However, multiple cutaneous tumors may have a more guarded prognosis than solitary lesions, and surgery may not be feasible, making management more difficult. Taking this into account, other local or systemic treatments may be used to manage these patients (Blackwood et al., 2012; Henry and Herrera, 2013; Berger et al., 2018). The treatments reported in the literature include chemotherapy (Rassnick et al., 2008), tyrosine kinase inhibitors (Berger et al., 2018), and strontium 90 irradiation (Turrel et al., 2006). Electrochemotherapy (ECT) is an effective local treatment that combines the administration of chemotherapeutic drugs with the delivery of permeabilizing electrical pulses. These pulses induce the formation of reversible pores in the plasma membrane of tumor cells, enhancing the uptake of hydrophilic chemotherapy drugs (e.g., bleomycin and cisplatin), thereby potentiating their cytotoxic effects (Spugnini and Baldi, 2019). The secondary mechanisms of action of ECT include antivascular effects and the induction of immunogenic cell death (Hadzialjevic et al., 2024). Tumors of the skin and subcutaneous tissues, regardless of histological type, can be treated with excellent outcomes in cats, dogs, horses, and human patients (Tellado et al., 2022). Furthermore, a favorable response is often achieved in one or two sessions, in most cases (Spugnini et al., 2011; Ferrer-Jorda and Rodríguez-Pizà, 2024). The adverse effects of this treatment are local and well tolerated, including edema and necrosis (Lowe et al., 2017). This therapy quickly became popular among the veterinary community due to its favorable characteristics: ease of administration, effectiveness, low morbidity, and relative inexpensiveness (Spugnini and Baldi, 2019). ECT has been successfully used in cats with cutaneous and subcutaneous tumors, such as squamous cell carcinoma (Dos Anjos et al., 2020) and soft tissue sarcoma (Spugnini et al., 2020). However, to date, the authors are unaware of any studies evaluating the safety and efficacy of ECT for feline MCT. This case series describes the first report of ECT in four cats with cutaneous MCT. Case DetailsThis study was a retrospective and multicentric case series conducted with four owned cats diagnosed with nonmetastatic cutaneous MCTs from three oncology services in Mexico, Brazil, and Italy. ECT was offered to owners after they declined systemic or other local therapies. The procedure was performed in accordance with the guidelines recommended for veterinary medicine (Tellado et al., 2022). Bleomycin at a dose of 15,000 IU/m² was administered as an intravenous bolus. Five minutes later, trains of 8 biphasic pulses with a voltage of 1,000–1,200 V/cm, 1 Hz frequency, lasting 50 + 50 µs each, with 1-ms interpulse intervals, were delivered using a clinical electroporator (Onkodisruptor®, Biopulse Srl, Rome, Italy), certified for veterinary use. The local response to the treatment was evaluated as follows: complete response (CR): disappearance of all target lesions; partial response (PR): at least 30% reduction in the sum of diameters of target lesions; progressive disease (PD): either the appearance of one or more new lesions or at least a 20% increase in the sum of diameters of target lesions; stable disease (SD): less than 30% reduction or 20% increase in the sum of diameters of target lesions (Nguyen et al., 2015). Local toxicity was retrospectively evaluated after the first ECT treatment using a 6-point toxicity score (grades 0–5), where 0—no toxicity, 1—mild swelling, 2—swelling/necrosis <1 cm, 3—severe swelling, 4—deep necrosis, and 5—severe swelling and tissue loss (Lowe et al., 2017). The case details are summarized in Table 1. Case 1A 7-year-old spayed female domestic shorthair cat was referred to a private clinic with a history of multiple nodules over the body refractory to previous treatment with the tyrosine kinase inhibitor toceranib (Palladia, Zoetis, 3.7 mg/kg, administered orally on Monday, Wednesday, and Friday) for 7 months. Upon physical examination, approximately 50 alopecic, non-painful cutaneous lesions were observed, measuring 2–7 millimeters (Fig. 1A). Fine-needle aspiration confirmed nucleated cells with variable granulation, confirming MCT. Surgery was proposed for most of the lesions; however, the owner declined for personal reasons. Therefore, ECT was proposed as an alternative treatment. Complete staging was performed, showing no evidence of abdominal or thoracic metastasis. Hematological and biochemical values were within normal ranges. The cat was sedated with dexmedetomidine, and anesthesia was induced with propofol, as per the manufacturer’s instructions. Propofol is considered an anesthetic as an induction agent, allowing to intubate the animal. Anesthesia was maintained with isoflurane. Before the treatment, incisional biopsies of selected nodules were performed for histopathological confirmation and grading. Table 1. Case characteristics and outcomes of four cats with cutaneous mast cell tumors treated with electrochemotherapy.
The treatment resulted in CR of all lesions at 25 days post-treatment. Alopecia and hypopigmentation were observed after the healing process (Fig. 1B). The cat received post-ECT oral non-steroidal anti-inflammatory drug (meloxicam 0.1 mg/kg, once daily for 3 days) and Gabapentin (10 mg/kg, twice daily, for 15 days) for analgesia. Histopathological examination confirmed both high-grade (partially differentiated MCT) and low-grade (well-differentiated MCT) tumors. Monthly evaluations were performed, and no new or metastatic lesions were observed within 7 months. However, new lesions were observed on the head and forelimbs, resulting in a disease-free interval (DFI) of 210 days. A new session was proposed, but declined by the owner. Case 2A 14-year-old spayed female domestic shorthair cat was referred to a private clinic with a 4.6 x 4.0 cm cutaneous, firm, alopecic, ulcerated mass on the head, with a history of 4 months of progression (Fig. 2A). A complete staging was performed, showing no evidence of abdominal or thoracic metastasis. Hematological and biochemical exams were within normal range. Fine-needle aspiration suggested sarcoma or poorly differentiated MCT. MCT was confirmed through histopathology and immunohistochemistry, with cells positive for vimentin and tryptase. Neoadjuvant chemotherapy and radical surgery were proposed to the owner, who declined treatment for personal reasons. Therefore, we proposed the ECT as an alternative treatment. The cat was premedicated with tiletamine/zolazepam, and anesthesia was induced with propofol, as per the manufacturer’s instructions. The animal was intubated, and anesthesia was maintained with isoflurane for ECT. The patient did not show any side effects and was discharged with prescriptions of oral non-steroidal anti-inflammatory drug (meloxicam 0.1 mg/kg, once daily for three days) and gabapentin (10 mg/kg, twice daily, for 15 days) for analgesia control. One month later, a second ECT session was indicated due to residual macroscopic disease. CR was achieved 42 days after the second ECT session. The patient was discharged with the same prescriptions as in the first ECT session. The toxicity of ECT was classified as grade 1, and alopecia and hypopigmentation were observed following the healing process (Fig. 2B). The patient was followed for 665 days without recurrence or the development of new lesions.
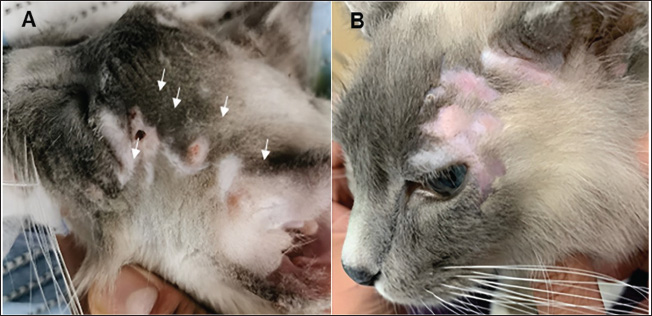
Fig. 1. Feline (Case 1) with mast cell tumors treated with electrochemotherapy. (A): multiple mast cell tumors on the head (white arrows); (B): Complete response 25 days post-ECT.
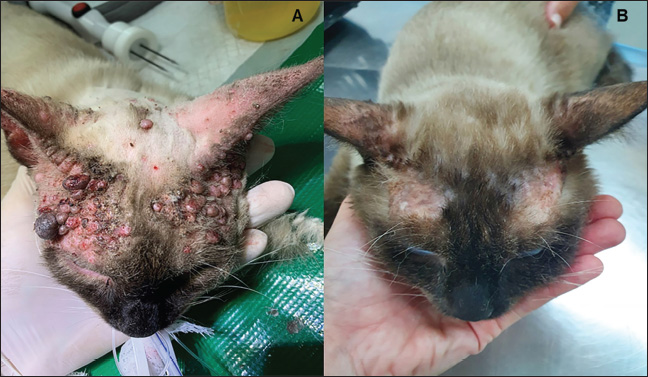
Fig. 2. Feline (Case 2) with mast cell tumor treated with electrochemotherapy. (A): Large mast cell tumor on the left side of the head; (B): Complete response after two ECT sessions. Case 3A 3-year-old neutered male domestic shorthair cat was referred to a private clinic with a history of multiple cutaneous nodules on the head that showed clinical progression over the past year. Physical examination was unremarkable, except for the multiple lesions on the head, ranging from 0.3 to 1.0 cm (Fig. 3A). Fine-needle aspiration confirmed the presence of nucleated cells with variable granulation, confirming MCT (Fig. 4). Furthermore, no abnormalities were found on complete blood count (CBC), biochemical profile, abdominal ultrasound, or thoracic radiography. Based on this clinical presentation and the owner’s decision to decline systemic treatment, ECT was performed. The cat was premedicated with methadone, and anesthesia was induced with propofol, as per the manufacturer’s instructions. The animal was intubated, and anesthesia was maintained with isoflurane. A biopsy was performed before ECT to evaluate the morphological aspects of MCT. The patient showed no side effects and was discharged with prescriptions of oral corticosteroids (prednisolone 1 mg/kg orally q24h) for one week to decrease transitory edema and tramadol (3 mg/kg for three consecutive days q12h) for analgesia. PR was observed 30 days post-treatment, and a second session was performed on day 45. A second biopsy was performed from residual tissue at the time of the second ECT session. The patient was discharged with the same prescriptions as in the first ECT session. CR was achieved after the second session and was observed on day 90 (Fig. 3B). The toxicity of ECT was classified as grade 1, and the healing process was accompanied by alopecia. Histopathological examination of the initial (pre-ECT) biopsy revealed cancer cells with intense pleomorphism, showing large polygonal to spindle-shaped cells with marked anisokaryosis, classified as histiocytic high-grade MCT (seven mitotic figures/10 hpf). The analysis of the second biopsy specimen (collected on day 45 after the first ECT course but before the second ECT session) revealed a low density of apoptotic cells characterized by a pyknotic nucleus with condensed chromatin and eosinophilic cytoplasm (Fig. 5). The patient was rechecked every 3 months, and no new lesions or metastasis were observed for 12 months, after which contact was lost. Case 4A 13-year-old spayed female domestic shorthair cat was referred for the gradual onset of multiple cutaneous nodules localized on the head and neck. Cytology was performed on the nodules (five on the head and two on the neck), resulting in the diagnosis of feline cutaneous MCT. All lesions ranged in size from 3 to 7 mm. The patient was staged with a CBC, biochemical profile, urinalysis, thoracic radiographs, and abdominal ultrasonography. All parameters were within normal limits, except for blood urea nitrogen, which was slightly elevated (33 mg/dl, laboratory range 8–29 mg/dl). One of the largest nodules was excised and confirmed as a well-differentiated feline MCT (Fig. 6). The widespread location of the MCTs prevented wide surgical excision. Among the therapeutic options, strontium 90 plesiotherapy was not available, and the owner declined systemic therapy, such as toceranib, due to difficulties in administering pills to the patient. Thus, ECT was performed as a treatment that might achieve local control.
Fig. 3. Feline (Case 3) with mast cell tumors treated with electrochemotherapy. (A) Multiple mast cell tumors on the head (left image) before the ECT procedure; (B) After two sessions, a complete response was observed on day 90.
Fig. 4. Cytological samples of feline mast cell tumor (Case 3). The analysis revealed a large number of mast cells, characterized by abundant cytoplasm filled with basophilic granules. The central round nucleus exhibits slightly loose chromatin and indistinct nucleoli. Mild anisokaryosis was observed along with a small number of eosinophils. The bottom of the slide shows moderate blood contamination and free basophilic granules.
Fig. 5. Hematoxylin and eosin staining of the feline mast cell tumor (Case 3) observed with an optical optic microscope. (A): lesion prior to treatment (D0), characterized by a dense population of mast cells with undifferentiated morphology (10x); (B): Presence of anisokaryosis and pleomorphism of the cancer cells (arrows), characterized by a polygonal to spindle-shaped cell morphology with the presence of central nucleoli (40x); (C): Tissue biopsy showing mast cell tumor after electrochemotherapy, revealing a low cell density (5x); (D): Cells after ECT (Day 45) were characterized by condensed chromatin and eosinophilic cytoplasm (apoptotic cells—arrows) and more organized collagen fibers, indicating stroma reorganization to a normal pattern (40x). Scale bar 50 µm. The patient was premedicated with a combination of medetomidine and ketamine, as per the manufacturer’s instructions. Anesthesia was induced and maintained with propofol, and the patient was intubated to provide oxygen. After the treatment, the six remaining MCTs decreased by 40% after the first session of ECT, and the patient was discharged with prescriptions of corticosteroids (prednisolone 1 mg/kg orally q24h) for one week to decrease transitory edema and tramadol (3 mg/kg for three consecutive days q12h) for analgesia. A second session was performed after 2 weeks, resulting in the complete resolution of the neoplasms. The patient was discharged with the same prescriptions as in the first ECT session. The toxicity of ECT was classified as grade 1, and alopecia was observed after the healing process. The patient was re-evaluated every 3 months and, at the time of writing this paper, had a DFI of 27 months following the ECT protocol. DiscussionTo the best of the authors’ knowledge, this is the first case series to describe the results of ECT in felines with cutaneous MCT, demonstrating a favorable response without significant adverse effects. ECT combines electric pulses with low-dose chemotherapeutic agents, such as bleomycin, which lead to temporary permeabilization of the tumor cell membrane, thereby enhancing the cytotoxicity of the chemotherapy (Spugnini and Baldi, 2019). Furthermore, ECT is also known to enhance the local immunological response by recruiting antigen-presenting cells and causing the release of damage-associated molecular patterns (Bendix et al., 2022), which may contribute to local control and longer disease-free survival, as shown in the literature for canine MCT (Spugnini et al., 2011; Cemazar et al., 2017; Lowe et al., 2017).
Fig. 6. Hematoxylin and eosin staining of a feline mast cell tumor (Case 4) observed using optical microscopy. (A): Neoplastic cells showing the invasion of the skin by a large number of mast cells (5x). (B): Tissue samples showing a relatively homogeneous population of round cells colonizing the skin of the patient (40x). Scale bar 50 µm. Local treatment via surgery is effective in controlling most feline cutaneous MCTs, which commonly exhibit benign clinical behavior. However, the presence of multiple tumors can limit the surgical approach and negatively impact survival (Litster and Sorenmo, 2006). Furthermore, recurrences can occur in up to 16% of cases, even with complete excision (Molander-McCrary et al., 1998). Strontium 90 irradiation has been reported as an alternative to surgery for single or multiple feline MCTs, with recurrence rates of only 3% (1/35). The study was conducted retrospectively, and the cases may have been selected based on clinical presentation to enhance the results of the technique (Turrel et al., 2006). One of the characteristics of Strontium-90 irradiation is its low penetrability (2–3 mm), making it suitable only for superficial tumors (Russak et al., 2022). In this study, ECT was used in various clinical scenarios of MCT to improve outcomes, particularly in cases where the surgical approach would be challenging due to the presence of multiple nodules or their large size. One of the advantages of using ECT in veterinary oncology is its ease, effectiveness, and safety as observed in this case series and in the literature (Kodre et al., 2009; Lowe et al., 2017; Dos Anjos et al., 2020). Rassnick .et al. (2008) conducted a study on the use of lomustine in 38 cats with cutaneous and visceral MCTs, reporting an overall response rate of 50% and a median response duration of 168 days. Of the 20 cats with primary cutaneous MCTs, 10 (50%) responded to treatment—2 with CR and 8 with PR, resulting in a CR rate of only 10%. Similarly, Berger et al. (2018) evaluated the efficacy of toceranib in 50 cats with MCTs in various locations and observed clinical benefit in 80% of the cases. For cutaneous MCTs, specifically, 86% (19/22) showed clinical benefit, including 7 CR, 10 PR, and 2 cases of SD—yielding a CR rate of 32%. In contrast, all patients in our study achieved CR, following one to two sessions of ECT, with no local recurrence observed. Only one patient developed new lesions after 210 days. Despite the limited number of cases, this finding highlights the potential of ECT as a highly effective local treatment for feline cutaneous MCTs, particularly in cases of multiple tumors, outperforming systemic therapies in terms of CR rates. ECT was associated only with mild and well-tolerated local adverse effects (primarily mild edema, with subsequent alopecia and hypopigmentation during healing) and unlike lomustine (Rassnick et al., 2008) and toceranib (Berger et al., 2018), ECT does not induce systemic side effects—such as anorexia, diarrhea, vomiting, neutropenia, and thrombocytopenia—which can be severe in a subset of patients. Low-grade toxicity with ECT may occur because, in addition to the direct effects of treatment, the electric pulses indirectly affect the tumor vasculature, leading to vasoconstriction, which then helps prevent mast cell degranulation. In this context, primary care involves treatment from the periphery to the center of the lesion, aiming to induce vasoconstriction and prevent the release of inflammatory mediators into the bloodstream from MCTs (De Nardi et al., 2022). In none of our cases were clinical signs of mast cell degranulation observed. Similarly, Kodre et al. (2009) observed no signs of tumor degranulation in 12 canine patients treated with ECT for cutaneous MCTs, with a mean size of 2.9 cm³. Spugnini et al. (2006) observed degranulation (edema and erythema) after ECT in only two of 28 cases of incompletely resected canine MCTs, which subsided within 30 minutes. These findings support the safety of the treatment. The main limitations of this study include its retrospective and multicentric nature and the small number of reported cases. The case information was obtained from medical records, and the follow-up periods were not standardized, varying according to the individual needs of the animals and the preference of the responsible veterinarian. However, these preliminary findings are encouraging and demonstrate the potential applicability of ECT as an alternative treatment for feline MCT. Future prospective studies involving a larger number of animals are essential to better determine remission rates and clarify the clinicopathological factors associated with treatment outcomes. ConclusionThe use of ECT may be successfully applied as a viable alternative approach for solitary or multiple MCTs in feline patients, as an alternative to surgery or in refractory cases, without significant toxicity, as observed in this case series report. Further research is warranted to support these preliminary findings. FundingThis research received no specific grant. Author contributionsAndré Gustavo Alves Holanda, visualization, writing - original draft, writing - review & editing, Arturo Vargas Ruanova, investigation, resources, writing - original draft, Enrico Pierluigi Spugnini, investigation, resources, writing - original draft, Marla Tereza Frasson, investigation, resources, writing - original draft, Carlos Eduardo Fonseca-Alves, visualization, resources, writing - original draft, Denner Santos Dos Anjos, visualization, investigation, resources, writing - original draft, writing - review & editing. Conflict of interestThe authors declare no conflict of interest. Data availabilityAll data supporting the findings of this study are available in the manuscript. ReferencesBendix, M.B., Houston, A., Forde, P.F. and Brint, E. 2022. Electrochemotherapy and immune interactions; a boost to the system? Eur. J. Surg. Oncol. 48, 1895–1900. Berger, E.P., Johannes, C.M., Post, G.S., Rothchild, G., Shiu, K.-B., Wetzel, S. and Fox, L.E. 2018. Retrospective evaluation of toceranib phosphate (Palladia) use in cats with mast cell neoplasia. J. Feline Med. Surg. 20, 95–102. Blackwood, L., Murphy, S., Buracco, P., De Vos, J.P., De Fornel-Thibaud, P., Hirschberger, J., Kessler, M., Pastor, J., Ponce, F., Savary-Bataille, K. and Argyle, D.J. 2012. European consensus document on mast cell tumours in dogs and cats. Vet. Comp. Oncol. 10, e1–e29. Cemazar, M., Ambrozic Avgustin, J., Pavlin, D., Sersa, G., Poli, A., Krhac Levacic, A., Tesic, N., Lampreht Tratar, U., Rak, M. and Tozon, N. 2017. Efficacy and safety of electrochemotherapy combined with peritumoral IL-12 gene electrotransfer of canine mast cell tumours. Vet. Comp. Oncol. 15, 641–654. De Nardi, A.B., dos Santos Horta, R., Fonseca-Alves, C.E., de Paiva, F.N., Linhares, L.C.M., Firmo, B.F., Ruiz Sueiro, F.A., de Oliveira, K.D., Lourenço, S.V., De Francisco Strefezzi, R., Brunner, C.H.M., Rangel, M.M.M., Jark, P.C., Castro, J.L.C., Ubukata, R., Batschinski, K., Sobral, R.A., da Cruz, N.O., Nishiya, A.T. and Dagli, M.L.Z. 2022. Diagnosis, prognosis and treatment of canine cutaneous and subcutaneous mast cell tumors. Cells 11, 618. Dos Anjos, D.S., Sierra, O.R., Spugnini, E.P., De Nardi, A.B. and Fonseca-Alves, C.E. 2020. Comparison of two different doses of bleomycin in electrochemotherapy protocols for feline cutaneous squamous cell carcinoma non-segregated from ultraviolet light exposure. Sci. Rep. 10, 18362. Ferrer-Jorda, E. and Rodríguez-Pizà, I. 2024. Description of outcome and adverse events in 21 cats with locally advanced nasal planum squamous cell carcinoma treated with electrochemotherapy. J. Feline Med. Surg. 26, 1098612X241248043. Hadzialjevic, B., Omerzel, M., Trotovsek, B., Cemazar, M., Jesenko, T., Sersa, G. and Djokic, M. 2024. Electrochemotherapy combined with immunotherapy – a promising potential in the treatment of cancer. Front. Immunol. 14, 1336866. Henry, C. and Herrera, C. 2013. Mast cell tumors in cats. J. Feline Med. Surg. 15, 41–47. Kodre, V., Cemazar, M., Pecar, J., Sersa, G., Cor, A. and Tozon, N. 2009. Electrochemotherapy compared to surgery for treatment of canine mast cell tumours. In Vivo 23, 55–62. Litster, A.L. and Sorenmo, K.U. 2006. Characterisation of the signalment, clinical and survival characteristics of 41 cats with mast cell neoplasia. J. Feline Med. Surg. 8, 177–183. Lowe, R., Gavazza, A., Impellizeri, J.A., Soden, D.M. and Lubas, G. 2017. The treatment of canine mast cell tumours with electrochemotherapy with or without surgical excision. Vet. Comp. Oncol. 15, 775–784. Miller, M.A., Nelson, S.L., Turk, J.R., Pace, L.W., Brown, T.P., Shaw, D.P., Fischer, J.R. and Gosser, H.S. 1991. Cutaneous neoplasia in 340 cats. Vet. Pathol. 28, 389–395. Molander-McCrary, H., Henry, C., Potter, K., Tyler, J. and Buss, M. 1998. Cutaneous mast cell tumors in cats: 32 cases (1991–1994). J. Am. Anim. Hosp. Assoc. 34, 281–284. Nguyen, S.M., Thamm, D.H., Vail, D.M. and London, C.A. 2015. Response evaluation criteria for solid tumours in dogs (v1.0): a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 13, 176–183. Oliveira, M.T., Campos, M., Lamego, L., Magalhães, D., Menezes, R., Oliveira, R., Patanita, F. and Ferreira, D.A. 2020. Canine and feline cutaneous mast cell tumor: a comprehensive review of treatments and outcomes. Top. Comp. Anim. Med. 41, 100472. Rassnick, K.M., Williams, L.E., Kristal, O., Al-Sarraf, R., Baez, J.L., Zwahlen, C.H. and Dank, G. 2008. Lomustine for treatment of mast cell tumors in cats: 38 cases (1999–2005). J. Am. Vet. Med. Assoc. 232, 1200–1205. Russak, O.-M., Verganti, S. and Berlato, D. 2022. Strontium 90 plesiotherapy for the treatment of eyelid squamous cell carcinoma in eight cats. J. Feline Med. Surg. 24, 524–529. Spugnini, E.P. and Baldi, A. 2019. Electrochemotherapy in veterinary oncology. Vet. Clin. North Am. Small Anim. Pract. 49, 967–979. Spugnini, E.P., Vincenzi, B., Baldi, F., Citro, G. and Baldi, A. 2006. Adjuvant electrochemotherapy for the treatment of incompletely resected canine mast cell tumors. Anticancer Res. 26, 4585–4589. Spugnini, E.P., Vincenzi, B., Carocci, F., Bonichi, C., Menicagli, F. and Baldi, A. 2020. Combination of bleomycin and cisplatin as adjuvant electrochemotherapy protocol for the treatment of incompletely excised feline injection-site sarcomas: a retrospective study. Open Vet. J. 10, 267–271. Spugnini, E.P., Vincenzi, B., Citro, G., Dotsinsky, I., Mudrov, T. and Baldi, A. 2011. Evaluation of cisplatin as an electrochemotherapy agent for the treatment of incompletely excised mast cell tumors in dogs. J. Vet. Intern. Med. 25, 407–411. Tellado, M., Michinski, S., Impellizeri, J., Marshall, G., Signori, E. and Maglietti, F. 2022. Electrochemotherapy using thin-needle electrode improves recovery in feline nasal planum squamous cell carcinoma – a translational model. Cancer Drug Resist. 5, 595–611. Tellado, M., Mir, L.M. and Maglietti, F. 2022. Veterinary guidelines for electrochemotherapy of superficial tumors. Front. Vet. Sci. 9, 868989. Turrel, J.M., Farrelly, J., Page, R.L. and McEntee, M.C. 2006. Evaluation of strontium 90 irradiation in treatment of cutaneous mast cell tumors in cats: 35 cases (1992–2002). J. Am. Vet. Med. Assoc. 228, 898–901. | ||
| How to Cite this Article |
| Pubmed Style Holanda AGA, Ruanova AV, Spugnini EP, Frasson MT, Fonseca-alves CE, Anjos DSD. Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case series. Open Vet. J.. 2025; 15(7): 3357-3365. doi:10.5455/OVJ.2025.v15.i7.48 Web Style Holanda AGA, Ruanova AV, Spugnini EP, Frasson MT, Fonseca-alves CE, Anjos DSD. Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case series. https://www.openveterinaryjournal.com/?mno=240165 [Access: November 22, 2025]. doi:10.5455/OVJ.2025.v15.i7.48 AMA (American Medical Association) Style Holanda AGA, Ruanova AV, Spugnini EP, Frasson MT, Fonseca-alves CE, Anjos DSD. Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case series. Open Vet. J.. 2025; 15(7): 3357-3365. doi:10.5455/OVJ.2025.v15.i7.48 Vancouver/ICMJE Style Holanda AGA, Ruanova AV, Spugnini EP, Frasson MT, Fonseca-alves CE, Anjos DSD. Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case series. Open Vet. J.. (2025), [cited November 22, 2025]; 15(7): 3357-3365. doi:10.5455/OVJ.2025.v15.i7.48 Harvard Style Holanda, A. G. A., Ruanova, . A. V., Spugnini, . E. P., Frasson, . M. T., Fonseca-alves, . C. E. & Anjos, . D. S. D. (2025) Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case series. Open Vet. J., 15 (7), 3357-3365. doi:10.5455/OVJ.2025.v15.i7.48 Turabian Style Holanda, André Gustavo Alves, Arturo Vargas Ruanova, Enrico Pierluigi Spugnini, Marla Tereza Frasson, Carlos Eduardo Fonseca-alves, and Denner Santos Dos Anjos. 2025. Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case series. Open Veterinary Journal, 15 (7), 3357-3365. doi:10.5455/OVJ.2025.v15.i7.48 Chicago Style Holanda, André Gustavo Alves, Arturo Vargas Ruanova, Enrico Pierluigi Spugnini, Marla Tereza Frasson, Carlos Eduardo Fonseca-alves, and Denner Santos Dos Anjos. "Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case series." Open Veterinary Journal 15 (2025), 3357-3365. doi:10.5455/OVJ.2025.v15.i7.48 MLA (The Modern Language Association) Style Holanda, André Gustavo Alves, Arturo Vargas Ruanova, Enrico Pierluigi Spugnini, Marla Tereza Frasson, Carlos Eduardo Fonseca-alves, and Denner Santos Dos Anjos. "Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case series." Open Veterinary Journal 15.7 (2025), 3357-3365. Print. doi:10.5455/OVJ.2025.v15.i7.48 APA (American Psychological Association) Style Holanda, A. G. A., Ruanova, . A. V., Spugnini, . E. P., Frasson, . M. T., Fonseca-alves, . C. E. & Anjos, . D. S. D. (2025) Successful treatment of cutaneous mast cell tumors in cats using electrochemotherapy: A case series. Open Veterinary Journal, 15 (7), 3357-3365. doi:10.5455/OVJ.2025.v15.i7.48 |