| Research Article | ||
Open Vet. J.. 2025; 15(4): 1765-1770 Open Veterinary Journal, (2025), Vol. 15(4): 1765-1770 Research Article Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi ArabiaMohammed Ali Al Hejji, Mohammed Ali Al-Sukruwah, Jamal Hussen*Department of Microbiology, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia *Corresponding Author: Jamal Hussen. Department of Microbiology, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia. Email: jhussen [at] kfu.edu.sa Submitted: 25/01/2025 Accepted: 17/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
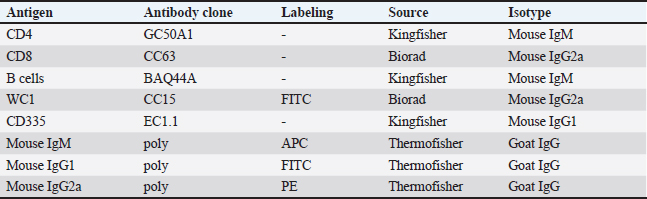
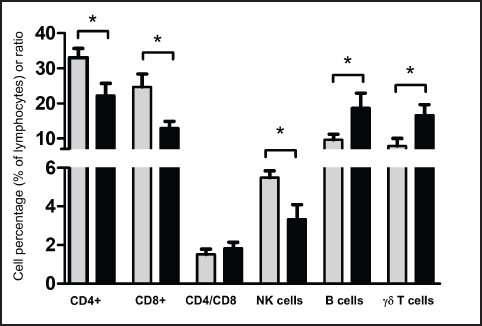
AbstractBackground: Breed-specific differences in humoral and cellular immune parameters have been reported in different species. The Pygmy and Ardi goat populations are two local breeds in Saudi Arabia. Aim: The aim of the present study was to compare the distribution of lymphocyte subsets in the blood of goats of the Ardi and Pygmy breeds. Methods: Monoclonal antibodies against lymphocyte cell surface markers were used in combination with flow cytometry to determine the percentages and absolute numbers of lymphocyte subsets in blood samples collected from the two Saudi Arabian local breeds. Results: The results of the present study show an overall dominance of goat CD4+ T helper cells within blood lymphocytes, followed by comparable percentages of CD8+ cytotoxic T cells, BAQ44A+ B cells, and γδ cells, and a less frequent fraction of NKp46+ NK cells. This indicates lymphocyte subset frequencies that are in agreement with previous reports on the lymphocyte composition in goat blood. The comparison of lymphocyte composition among the goat breeds identified higher percentages as well as absolute numbers of both helper and cytotoxic T cells in Pygmy than in Ardi goats. In contrast, the abundance of γδ cells was higher in Ardi vs. Pygmy goats. Conclusion: The different percentages and absolute numbers of helper and cytotoxic T cells indicate different immune responsiveness of the two goat breeds to extracellular and intracellular pathogens, respectively. The clinical significance of these findings will be investigated in future studies focusing on the comparative immune responses of the two breeds to experimental infections with distinct intracellular and extracellular pathogens. Keywords: Pygmy goats, Ardi goats, Breed, Flow cytometry, Lymphocyte subsets. IntroductionThe most commonly known goat breeds in the world are the Alpine, Boer, Cashmere, Pigmy, Le Mancha, Nubian, Angora, and Saanen breeds (Chiejina and Behnke, 2011). In Saudi Arabia, the Ardi, Hollandi, and Shami goats are among the main goat breeds (Al-Saef, 2013). Pigmy goats, also known as West African dwarf goats, are found in some regions of Saudi Arabia where they are mainly used as zoo animals or kept as companion animals. In addition, they are currently used in beauty competitions and exhibition activities organized in different regions of Saudi Arabia. Recent studies reported an immunologically based higher capacity of Pigmy goats to resist trypanosome and intestinal nematode infections than other goat breeds (Chiejina and Behnke, 2011). The analysis of cellular populations using flow cytometry has been proven to be an accurate methodology for evaluating the competence of the immune system in health and disease (Akanni and Palini, 2006; Davis and Hamilton, 2008; DiGiuseppe and Wood, 2019). The distribution of caprine lymphocyte subsets in the peripheral blood and in several lymphoid and non-lymphoid tissues has been investigated in several studies (Kim et al., 2016; Ismail et al., 1996; Perez-Martinez et al., 2002). In goats, lymphocytes dominate other leukocyte subpopulations in the blood (Kaba et al., 2011). The blood lymphocyte population includes T, B, and natural killer (NK) cells. The T cell population comprises alpha beta T cells, which recognize their antigens in the context of major histocompatibility molecules (MHC), and gamma delta T cells, which dominate the blood of newborn and young ruminants and recognize antigens independently of MHC receptors. CD4+ helper T cells and CD8+ cytotoxic T cells are the major subsets of alpha-beta T cells with an essential role in the response to peptide antigens presented on MHC class II and MHC class I molecules, respectively. As the relative and absolute composition of lymphocytes in the peripheral blood of Pygmy and Ardi goats have not been investigated, the present study used monoclonal antibodies to lymphocyte cell surface markers to determine the percentages and absolute numbers of lymphocyte subsets in blood samples collected from these two local breeds in Saudi Arabia. Materials and MethodsAnimals and blood samplingBlood samples were collected from 13 goats, including six of the Pygmy goat breed (two males and 4 females) and seven of the Ardi goat breed (three males and four females). The goats were aged between 4 and 8 months (mean age was 5.2 for the Pygmy goats and 6.0 months for the Ardi goats). The goats were reared on a private farm in the Al-Ahsa region (Eastern Saudi Arabia). Blood samples (3 ml) were collected from the jugular vein into vacutainer EDTA tubes (Guangzhou Improve Medical Instruments Co., Ltd; Guangzhou, China) and transported to the laboratory within 1 hour for cell separation. Cell separationFor leukocyte isolation via hypotonic lysis of red blood cells, 6 ml of distilled water was added to 2 ml of blood for 20 seconds in a 15 ml sterile falcon tube, followed by the addition of 6 ml 2x PBS to restore tonicity. After centrifugation at 1,000 g for 15 minutes at 4°C, the lysis step was repeated twice for complete removal of the erythrocytes. Finally, the pellet was resuspended in cold PBS (2 x 106 cells/ml). Flow cytometric analysis of leukocyte subsetsIsolated cells (5 x 105 cells/well) were incubated in a 96-well plate for 15 minutes at 4°C with monoclonal antibodies (Table 1) to the lymphocyte markers cluster of differentiation (CD) 4, CD8, CD335 (NKp46), BAQ44A (B cell), and WC1 (γδ T cell). Except for CD4 and CD8 antibodies, which were used in combination, all other antibodies were used for single staining. After incubation, the cells were washed with PBS/BSA buffer (150 μl) and centrifuged for 3 minutes at 300 g and 4°C. To detect primary antibodies, cells were subsequently incubated with fluorochrome-labeled antibodies against mouse immunoglobulin isotypes (Invitrogen). Additional setups were prepared with cells incubated with only isotype control antibodies (goat anti-mouse IgG1-FITC, goat anti-mouse IgG2a-PE, and goat anti-mouse IgM-APC). Staining with propidium iodide (PI) was used to evaluate cell viability with only dead cells stained positive with PI. Finally, the cells were washed (3 minutes at 300 g and 4°C), resuspended in 100 μl buffer, and analyzed by flow cytometry (Becton Dickinson Accuri C6 flow cytometer; Becton Dickinson Biosciences, San Jose, California, USA) by the acquisition of 50.000 cells for each sample. Statistical analysesMeans and standard error of the mean were calculated using the column statistic function of the Prism software (GraphPad). The comparison between means was performed using an unpaired student’s t-test, with p values less than 0.05 indicating significant effects. ResultsRelative composition of blood lymphocytesThe lymphocyte count was calculated by multiplying the percentage of lymphocytes by the total number of leukocytes counted using a Neubauer hemocytometer and light microscopy after the addition of Turk solution. Lymphocyte composition was analyzed by flow cytometry (Fig. 1 A-I). The comparison between the two breeds revealed a significantly (p < 0.05) higher percentage of CD4+ αβ T helper cells in the Pygmy (mean ± SEM=33.0% ± 2.6 % of total lymphocytes) than the Ardi (22.1% ± 3.5 % of total lymphocytes) goat breed (Fig. 2). Similarly, the percentage of CD8+ cytotoxic αβ T cells was significantly higher in the Pygmy (24.7% ± 3.7 % of total lymphocytes) than in the Ardi (13.0% ± 1.8 % of total lymphocytes) goat breed. The higher frequency of both helper and cytotoxic T cells in Pygmy goats resulted in similar (p > 0.05) CD4/CD8 ratios between the two groups. In contrast, the percentage of γδ T cells was significantly (p < 0.05) higher in the Ardi (16.7% ± 2.9 % of lymphocytes) than in the Pygmy (7.8% ± 2.1 % of lymphocytes) goat. The Ardi goats also showed higher (p < 0.05) percentages of B cells (18.7% ± 4.2 % of lymphocytes) than the Pygmy (9.6% ± 1.5 % of lymphocytes) goat. For NK cells, a significantly higher frequency was found in blood from the Pygmy 5.5% ± 0.3 % of lymphocytes than the Ardi (3.3% ± 0.7 % of lymphocytes) goat (Fig. 2). Table 1. Monoclonal antibodies.
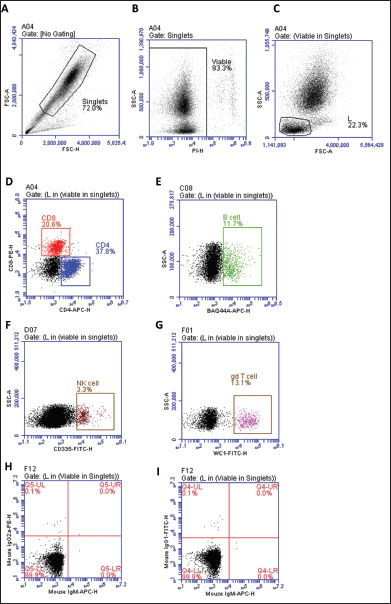
Fig. 1. Separated leukocytes were labeled with fluorescent antibodies and analyzed by flow cytometry. After the exclusion of cell doublets based on forward scatter aria (FSC-A) and forward scatter height (FSC-H) properties (A), viable cells were gated based on negative staining with propidium iodide (PI). (B), Subsequently, a gate was set on lymphocytes based on their FSC and side scatter (SSC) characteristics (C). The populations of CD4+ αβ T cells, CD8+ αβ T cells (D), BAQ44A+ B cells (E), NKp46+ (CD335), natural killer (NK) cells (F), and WC1+ γδ T cells (G) were identified. In addition, isotype control staining with mouse IgG1, IgM, and IgG2a secondary antibodies was performed (H-I).
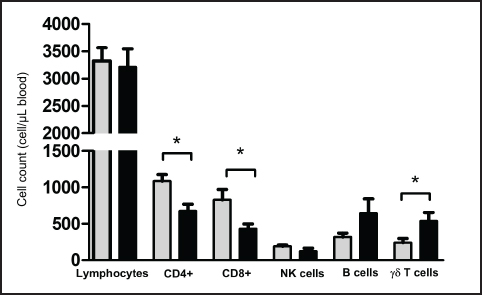
Fig. 2. Frequency of lymphocyte subsets in blood from Pygmy (gray color) and Ardi (black color) goats. Leukocytes were separated from goat blood, labeled with fluorescent antibodies, and analyzed by flow cytometry. The percentages of CD4+ αβ T cells, CD8+ αβ T cells, NKp46+ natural killer (NK) cells, BAQ44A+ B cells, and WC1+ γδ T cells were calculated and presented as a graph. In addition, the CD4 to CD8 ratio (CD4/CD8) was calculated for both goat breeds. Absolute count of blood lymphocyte subsetsThe absolute number of total lymphocytes was calculated after counting leukocytes under the microscope after adding Turk solution to the blood sample (1:10), followed by the multiplication of the lymphocyte percentage with the total white blood cell count. The numbers of total lymphocytes were comparable between the two goat breeds (Fig. 3). Similar to their higher percentages, the absolute numbers of CD4+ αβ T cells and CD8+ αβ T cells were significantly (p < 0.05) higher in the blood from Pygmy goat (1085 ± 92.5 cell/μl for CD4+ and 827.7 ± 142.9 for CD8+ T cells) than those from Ardi goat (678.3 ± 91.3 cell/μl for CD4+ and 430.2 ± 68.8 for CD8+ T cells). In contrast, the number of γδ cells was significantly (p < 0.05) higher in Ardi (540.5 ± 116.2 cell/μl) than in Pygmy (241.5 ± 57.8 cell/μl) goat. The two breeds did not differ (p > 0.05) in the absolute counts of NK and B cells (Fig. 3). DiscussionThe immune response to infection or vaccination is usually associated with changes in immune cell production in the bone marrow and mobilization to tissues, which are reflected by significant changes in the lymphocyte composition of the blood. Therefore, monitoring changes in the distribution of lymphocyte subsets in the blood is one of the most popular methodologies for evaluating the immune system status after infection or vaccination. Species- and breed-specific differences in lymphocyte composition have been reported for several animal species (Faldyna et al., 2001; Macedo et al., 2013; Kim et al., 2016; Yirsaw et al., 2022). In the present study, lymphocyte subsets were identified in blood from two local goat breeds in Saudi Arabia, the Pygmy and the Ardi Saudi goat breeds. In agreement with previous reports on the composition of blood lymphocytes in goats, the results of the present study showed an overall dominance of goat CD4+ T helper cells within blood lymphocytes, followed by comparable percentages of CD8+ cytotoxic T cells, BAQ44A+ B cells, and γδ T cells, and a less frequent fraction of NKp46+ NK cells (Kaba et al., 2011; Totte et al., 2002; Bezos et al., 2012). According to Baliu-Pique et al. (2019), T helper cells are the predominant subset in neonatal and young goat kids, while CD8+ T-cells predominate in the blood of adult animals (Baliu-Pique et al., 2019). The comparison of lymphocyte composition among the goat breeds identified significant differences in the numbers of helper and cytotoxic αβ T cells as well as γδ cells. The capacity of Pygmy goats to resist infectious pathogens such as Trypanosomes has been described in the literature (Chiejina and Behnke, 2011). Higher numbers of both helper and cytotoxic T cells indicate different immune responsiveness of the two goat breeds to extracellular and intracellular pathogens, respectively. To confirm the clinical significance of these results, further studies should focus on the comparative immune responses of the two breeds to experimental infections with distinct intracellular or extracellular pathogens. Several studies have linked breed-specific immunophenotypes to resistance or susceptibility to infectious diseases (Sayers et al., 2008; Corripio-Miyar et al., 2022; Makau et al., 2020). A study by Barbour et al. (2012) identified significant differences between local and imported Saanen goats regarding lymphocyte composition and immune response (Barbour et al., 2012). The authors linked the higher frequency of CD8+ T cells to higher immune responsiveness toward intracellular pathogens. Age-related changes in the maturation status of the goat immune system have been reported in the scientific literature (Baliu-Pique et al., 2019; Koets et al., 2019; Abdelsattar et al., 2021). The present study was limited by the narrow age range of the animals, with all goats aged between 4 and 8 months. An additional limitation of the study is the small number of animals. Therefore, further studies with animals of different ages could be conducted to determine whether the observed differences would still exist among animals of different ages.
Fig. 3. Absolute numbers of lymphocytes and lymphocyte subsets in blood from Pygmy (gray color) and Ardi (black color) goats. Leukocytes were separated from goat blood, counted under a microscope, labeled with fluorescent antibodies, and analyzed by flow cytometry. The absolute numbers of CD4+ αβ T cells, CD8+ αβ T cells, NKp46+ natural killer (NK) cells, BAQ44A+ B cells, and WC1+ γδ T cells were calculated by multiplying their percentages by the absolute number of lymphocytes. Conflict of interestThe authors declare no relevant financial or nonfinancial interests. FundingThis work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [KFU250219]. Author contributionsJamal Hussen conceived the study. Mohammed Ali Al Hejji, Mohammed Ali Al-Sukruwah collected the samples. Jamal Hussen performed flow cytometry and wrote the first draft of the manuscript. All authors have revised and approved the manuscript. Data availabilityThe datasets generated during the current study are available from the corresponding author upon reasonable request. Ethical approvalThis study was conducted in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of King Faisal University, Saudi Arabia (KFU-REC-2024-NOV- ETHICS2861). ReferencesAbdelsattar, M.M., Vargas-Bello-Perez, E., Zhuang, Y., Fu, Y. and Zhang, N. 2021. Effects of age and dietary factors on the blood beta-hydroxybutyric acid, metabolites, immunoglobulins, and hormones of goats. Front. Vet. Sci. 8, 793427. Akanni, E.O. and Palini, A. 2006. Immunophenotyping of peripheral blood and bone marrow cells by flow cytometry. EJIFCC. 17(1), 17–21. Al-Saef, A.M. 2013. Genetic and phenotypic parameters of body weights in saudi aradi goat and their crosses with syrian damascus goat. Small Ruminant Res. 112(1-3), 35–38. Baliu-Pique, M., Kurniawan, H., Ravesloot, L., Verheij, M.W., Drylewicz, J., Lievaart-Peterson, K., Borghans, J.A.M., Koets, A. and Tesselaar, K. 2019. Age-related distribution and dynamics of t-cells in blood and lymphoid tissues of goats. Dev. Comp. Immunol. 93, 1–10. Barbour, E.K., Itani, H.H., Sleiman, F.T., Saade, M.F., Harakeh, S., Nour, A.M. and Shaib, H.A. 2012. Preliminary comparison of different immune and production components in local and imported saanen goats reared under a sub-tropical environment. Trop. Anim. Health Prod. 44(1), 87–93. Bezos, J., Alvarez, J., Moreno, I., de Juan, L., Romero, B., Rodriguez, S., Dominguez, M., Torano, A., Mateos, A., Dominguez, L. and Aranaz, A. 2012. Study of peripheral blood cell populations involved in the immune response of goats naturally infected with mycobacterium caprae. Res. Vet. Sci. 93(1), 163–167. Chiejina, S.N. and Behnke, J.M. 2011. The unique resistance and resilience of the nigerian west african dwarf goat to gastrointestinal nematode infections. Parasit Vectors 4, 12. Corripio-Miyar, Y., Hayward, A., Lemon, H., Sweeny, A.R., Bal, X., Kenyon, F., Pilkington, J.G., Pemberton, J.M., Nussey, D.H. and McNeilly, T.N. 2022. Functionally distinct t-helper cell phenotypes predict resistance to different types of parasites in a wild mammal. Sci. Rep. 12(1), 3197. Davis, W.C. and Hamilton, M.J. 2008. Use of flow cytometry to develop and characterize a set of monoclonal antibodies specific for rabbit leukocyte differentiation molecules. J. Vet. Sci. 9(1), 51–66. DiGiuseppe, J.A. and Wood, B.L. 2019. Applications of flow cytometric immunophenotyping in the diagnosis and posttreatment monitoring of b and t lymphoblastic leukemia/lymphoma. Cytometry B Clin. Cytom. 96(4), 256–265. Faldyna, M., Leva, L., Knotigova, P. and Toman, M. 2001. Lymphocyte subsets in peripheral blood of dogs--a flow cytometric study. Vet. Immunol. Immunopathol. 82(1-2), 23–37. Ismail, H.I., Hashimoto, Y., Kon, Y., Okada, K., Davis, W.C. and Iwanaga, T. 1996. Lymphocyte subpopulations in the mammary gland of the goat. Vet. Immunol. Immunopathol. 52(3). 201–212. Kaba, J., Winnicka, A., Zaleska, M., Nowicki, M. and Bagnicka, E. 2011 Influence of chronic caprine arthritis-encephalitis virus infection on the population of peripheral blood leukocytes. Pol. J. Vet. Sci. 14(4), 585–590. Kim, Y. M., Lee, J. A., Jung, B. G., Kim, T. H., Lee, B. J. adn Suh, G. H. (2016). Reference ranges of hematology and lymphocyte subsets in healthy korean native cattle (hanwoo) and holstein dairy cattle. Anim. Sci. J. 87(6), 796–801. Koets, A., Ravesloot, L., Ruuls, R., Dinkla, A., Eisenberg, S. and Lievaart-Peterson, K. 2019. Effects of age and environment on adaptive immune responses to mycobacterium avium subsp. Paratuberculosis (map) vaccination in dairy goats in relation to paratuberculosis control strategies. Vet. Sci. 6(3), 1–18. Macedo, A.A., Marciano, A.P.V., Rocha, L.M., Alves-Júnior, J.R.F., Faria, A.M.C., Bittar, J.F.F. and Martins-Filho, O.A. 2013. Comparative phenotypic profile of subpopulations of peripheral blood leukocytes in european (Bos taurus taurus) and zebu cattle (Bos taurus indicus). Genet. Mol. Res. 12(4), 6838-–849. Makau, M.C., Powell, J., Prendergast, J., de Laté, P.L., Morrison, L.J., Fisch, A. and Toye, P. 2020. Inverted cd4(+)/cd8(+) t cell ratio in boran (bos indicus) cattle. Vet. Immunol. Immunopathol. 230, 110126. Perez-Martinez, M., Luna, J., Mena, R. and Romano, M.C. 2002. Lymphocytes and t lymphocyte subsets are regionally distributed in the female goat reproductive tract: influence of the stage of the oestrous cycle. Res. Vet. Sci. 72(2), 115–121. Sayers, G., Good, B., Hanrahan, J.P., O’Donovan, J., Mulcahy, G. and Sweeney, T. 2008. Breed differences in mucosal and systemic antibody response to nematode infection in sheep: an important role for ige? Parasitology 135(Pt 1), 71–80. Totte, P., Esteves, I., Gunter, N., Martinez, D. and Bensaida, A. 2002. Evaluation of several flow cytometric assays for the analysis of t-cell responses in goats. Cytometry 49(2), 49–55. Yirsaw, A.W., Gillespie, A., Zhang, F., Smith, T.P.L., Bickhart, D.M., Gunasekaran, K.P., Amir, M., Park, H., Telfer, J.C. and Baldwin, C.L. 2022. Defining the caprine gammadelta t cell wc1 multigenic array and evaluation of its expressed sequences and gene structure conservation among goat breeds and relative to cattle. Immunogenetics 74(3), 347–365. | ||
| How to Cite this Article |
| Pubmed Style Hejji MAA, Al-sukruwah MA, Hussen J. Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi Arabia. Open Vet. J.. 2025; 15(4): 1765-1770. doi:10.5455/OVJ.2025.v15.i4.28 Web Style Hejji MAA, Al-sukruwah MA, Hussen J. Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi Arabia. https://www.openveterinaryjournal.com/?mno=239702 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i4.28 AMA (American Medical Association) Style Hejji MAA, Al-sukruwah MA, Hussen J. Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi Arabia. Open Vet. J.. 2025; 15(4): 1765-1770. doi:10.5455/OVJ.2025.v15.i4.28 Vancouver/ICMJE Style Hejji MAA, Al-sukruwah MA, Hussen J. Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi Arabia. Open Vet. J.. (2025), [cited January 24, 2026]; 15(4): 1765-1770. doi:10.5455/OVJ.2025.v15.i4.28 Harvard Style Hejji, M. A. A., Al-sukruwah, . M. A. & Hussen, . J. (2025) Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi Arabia. Open Vet. J., 15 (4), 1765-1770. doi:10.5455/OVJ.2025.v15.i4.28 Turabian Style Hejji, Mohammed Ali Al, Mohammed Ali Al-sukruwah, and Jamal Hussen. 2025. Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi Arabia. Open Veterinary Journal, 15 (4), 1765-1770. doi:10.5455/OVJ.2025.v15.i4.28 Chicago Style Hejji, Mohammed Ali Al, Mohammed Ali Al-sukruwah, and Jamal Hussen. "Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi Arabia." Open Veterinary Journal 15 (2025), 1765-1770. doi:10.5455/OVJ.2025.v15.i4.28 MLA (The Modern Language Association) Style Hejji, Mohammed Ali Al, Mohammed Ali Al-sukruwah, and Jamal Hussen. "Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi Arabia." Open Veterinary Journal 15.4 (2025), 1765-1770. Print. doi:10.5455/OVJ.2025.v15.i4.28 APA (American Psychological Association) Style Hejji, M. A. A., Al-sukruwah, . M. A. & Hussen, . J. (2025) Flow cytometric analysis of lymphocyte subsets in the peripheral blood of two local goat breeds in Saudi Arabia. Open Veterinary Journal, 15 (4), 1765-1770. doi:10.5455/OVJ.2025.v15.i4.28 |