| Case Report | ||
Open Vet. J.. 2025; 15(5): 2259-2264 Open Veterinary Journal, (2025), Vol. 15(5): 2259-2264 Case Report Peritoneo pericardial hernioplasty in a 2-month-old Shih TzuRafaela Rodrigues Ribeiro1, Guilherme Pinheiro Santos2,3, Marco Augusto Machado Silva2, Cindy Stefhani dos Santos Silva3, Tayanne Gobbi Mendes3, Rauane Sousa de Moura1 and Iago Martins Oliveira1*1Escola de Ciências Médicas e da Vida, Pontifícia Universidade Católica de Goiás, Goiânia, Brasil 2Departamento de Medicina Veterinária, Escola de Veterinária e Zootecnia, Universidade Federal de Goiás, Rodovia Goiânia - Nova Veneza, Goiânia, Brasil 3Clínica Veterinária HOPE, Goiânia, Brasil *Corresponding Author: Iago Martins Oliveira. Escola de Ciências Médicas e da Vida, Pontifícia Universidade Católica de Goiás, Goiânia, Brasil. Email: iago.vetufg [at] gmail.com Submitted: 23/01/2025 Revised: 13/04/2025 Accepted: 20/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
AbstractBackground: Peritoneopericardial hernias (PPHs) are congenital malformations characterized by continuity defects between the diaphragm and the peritoneum that allow the transposition of abdominal organs into the thoracic cavity. These malformations can lead to clinical, gastroenterological, and respiratory problems, such as vomiting, dyspnea, and tachypnea. They can be congenital, and patients develop clinical signs at a young age or can be diagnosed as incidental when no complications occur. Furthermore, other malformations, such as cryptorchidism and cardiac interventricular communication, can present with congenital HPP. Case Description: This article reports a case of PPH in association with a ventricular septal defect (VSD) in a pediatric canine patient. The dog, a female Shih Tzu, aged 2 months, presented with cyanosis, low weight, decreased body condition score (3/9), and underdevelopment in comparison with other puppies of the same litter. At physical examination, all parameters were within the normal range; however, there was a sound at the base of the heart during auscultation, and echocardiographic examination displayed VSD. Due to the dyspnea, thoracic radiography was performed, and the suspicion of PPH was confirmed. Peritoneopericardial hernioplasty surgery was indicated for a 2-month-old patient. During the surgical procedure, it was not necessary to enter the thoracic cavity in order to close the defect, and suture surgery was performed through the abdominal cavity accessed in the subxiphoidal region. Despite the high risks associated with the procedure, no intraoperative or anesthetic complications occurred. Conclusion: The hernioplasty procedure was considered successful, and the patient’s development and body condition score improvement, with the surgical procedure resolving all signs of respiratory distress. Keywords: Congenital malformation, Cyanosis, Hernia, Surgery, Ventricular septal defect. IntroductionPeritoneopericardial hernias are characterized by continuity defects between the peritoneum and the pericardium due to malformation of the transverse septum during the embryonic phase, which allows abdominal organs to enter the thoracic cavity (Burns et al., 2013). Although this is the most common thoracic malformation in dogs and it has been suggested that genetic factors are involved, its mechanism of development has not been fully elucidated (Morgan et al., 2020). The condition may be an incidental finding in some cases; however, if clinical signs do exist, they tend to be associated with difficulty expanding the thorax or compression of the viscera. The most common signs of PPH are dyspnea, tachypnea, cyanosis, emesis, and regurgitation (Burns et al, 2013; Morgan et al., 2020). Among the other malformations that may be found in conjunction with PPH, the most common are pectus carinatum or excavatum, myofibrillar hypoplasia, umbilical hernia, cryptorchidism, and other congenital heart defects (Morgan et al., 2020). Among congenital heart defects that develop in isolation, ventricular septal defect (VSD) is the fourth most common (Perdoncini and Gusso, 2022). VSD is also more common in toy breeds, such as Yorkshire Terriers and Miniature Poodles (Perdoncini and Gusso, 2022). VSD is characterized by a defect of the interventricular septum that can be classified as perimembranous, muscular, supracrestal, or inlet, depending on the location of the defect. This communication between the ventricles allows regurgitation and mixing of arterial and venous blood, usually with a left-to-right flow that appears as turbulent flow in the B-mode of the echocardiogram (Boon, 1998). Despite cardiac overload, when the flow moves from left to right, clinical signs tend to be minimal, and the main finding is a heart murmur (Perdoncini and Gusso, 2022). However, disease progression can lead to flow reversion to right to left, causing patients to exhibit signs of congestive heart failure (CHF) (Fernandes et al., 2019; Perdoncini and Gusso, 2022; Bruno et al., 2024). Usually, echocardiograms can be used to diagnose and verify the progression of cardiac alterations as well as flow direction (Boon, 1998). Unlike PPH, which can be treated surgically (Morgan et al., 2020; McClaran, 2023), the treatment of VSD tends to be conservative based on pharmacological therapy to control hypertension and CHF (Griffiths, 2010; Perdoncini and Gusso, 2022). This study reports the case of a young patient who presented with PPH concomitant with VSD who underwent hernioplasty in order to correct the continuity defect and control clinical signs related to thoracic structure compression. Case detailsA rescued, female Shih Tzu puppy, approximately 45–50 days of age, was taken for pediatric evaluation along with two of her brothers following the sudden death of another puppy from the same litter. The physical assessment revealed a grade 3 out of 6 heart murmur, although the other parameters were within normal ranges. The patient was the smallest puppy in the litter and present body condition score of 3/9. Subsequently, the patient was referred to an echocardiographic evaluation, which revealed a left-to-right VSD (Fig. 1). Because of the absence of cardiac remodeling or clinical signs, follow-up was indicated, and another echocardiogram associated with a microbubble test was performed to confirm the diagnosis. Upon returning after 15 days, the development of herniation in the diaphragmatic region, pectus carinatum, and posterior myofibrillar hypoplasia was noted. Shortly after the second visit, the patient was referred for emergency care due to cyanosis, expiratory dyspnea, and mild tachycardia. Furosemide (Teuto, Anápolis, GO, Brazil) was administered intravenously (IV) at a dose of 2 mg/kg along with fluid therapy (3 ml/kg/hour). In addition, the patient was hospitalized for monitoring respiratory pattern and vital parameters. Once the condition had stabilized, a new echocardiogram was performed with a microbubble test, which was negative and confirmed the diagnosis of VSD (Fig. 2). During the examination, the patient presented with a second episode of cyanosis and expiratory dyspnea, followed by respiratory arrest while in the lateral decubitus position. Consequently, left lateral, right lateral, and dorsoventral survey X-rays of the thoracic region were obtained, which indicated PPH (Fig. 3). The dog underwent hernioplasty to correct the peritoneopericardial defect. Methadone (Cristalia, Itapira, SP, Brazil) 0.3 mg/kg was administered intramuscularly (IM) as preanesthetic medication. During the preparation, the patient exhibited a heart rate of 180 beats/minute, respiratory rate of 32 breaths/minute, normal-colored mucous membranes, capillary refill time of 2 seconds, and normohydration. Intravenous propofol (Cristalia, Itapira, SP, Brazil) (1 mg/kg) and lidocaine (Bravet, Engenho Novo, RJ, Brazil) (2 mg/kg) were administered to induce anesthesia. During transanesthesia, the patient was maintained on a continuous infusion of fentanyl (ABL, São Paulo, SP, Brazil) (2.5 µg/kg), and the rheumatic anesthetic circuit was maintained with isoflurane at a minimum alveolar concentration of 2%.
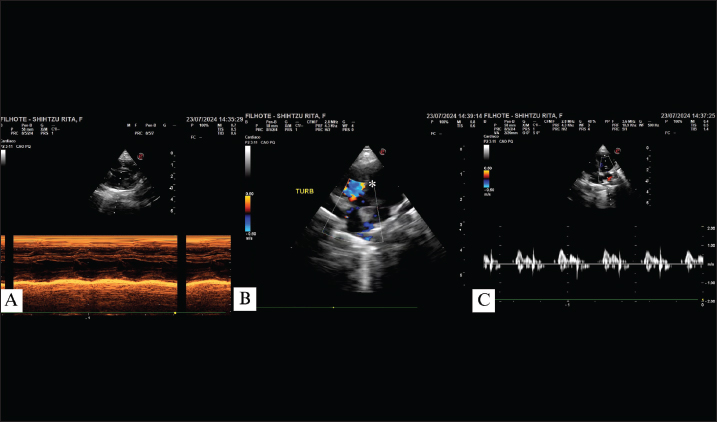
Fig. 1. Echocardiogram of a 45-day-old patient showing right ventricular overload with preserved systolic function in M-mode (A); turbulent flow in the perimembranous ventricular region (*) due to ventricular septal defect (B); E wave surpassing A wave with preserved IVRT values (C). TURB: turbulent flow.
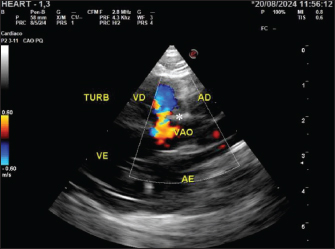
Fig. 2. Echocardiogram of the patient at 60 days, showing turbulent flow (*) in B-mode, in the perimembranous ventricular region in a longitudinal section with a negative microbubble test. TURB, turbulent flow; RV, right ventricle; LV, left ventricle; VSD, ventricular septal defect; RA, right atrium; LA, left atrium. The operative site was scrubbed after shaving in two steps using chlorhexidine 2% and alcohol 70% for definitive antisepsis. The surgical procedure was divided into two stages. In the first stage, umbilical hernioplasty was performed by ablating the hernial sac after an elliptical incision in the skin. Abdominal access was widened by performing preumbilical laparotomy up to the subxiphoid margin, as suggested by McClaran (2023) and Burns et al (2013). In the second stage, the left lateral, left medial, and square hepatic lobes were identified, as well as the gallbladder, inside the pericardial sac. In addition to these organs, adhesions between the liver and pericardial sac were observed during cavity inspection. After releasing the adhesions between the diaphragmatic surface of the liver and the edges of the pericardial sac, the structures were mobilized with a moist gauze pad and repositioned. After inspection, the pericardial-diaphragmatic border was debrided using Metzenbaum scissors. The defect was closed using a 3-0 polydioxanone thread in the Reverdin pattern. Celiorraphy was performed using the same thread in a simple continuous pattern. The subcutaneous space was reduced using 3-0 polyglecaprone thread in an inverted subcuticular pattern. The dermorrhaphy was performed using 4-0 nylon thread in a continuous mattress pattern (Fig. 4).
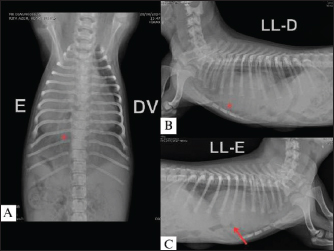
Fig. 3. Dorsoventral radiograph (A); right lateral (B) and left lateral (C) radiographs showing a markedly enlarged cardiac silhouette with a rounded shape and convex projection of the caudal portion (arrow), a sign of the ventral diaphragmatic silhouette, dorsal deviation of the trachea, and the presence of a tubular structure (*), containing gas and extending beyond the diaphragmatic silhouette, most clearly seen in (C), consistent with features suggestive of a peritoneopericardial diaphragmatic hernia. L, left; DV, dorsoventral; LL-D, right laterolateral; LL-E, left laterolateral.
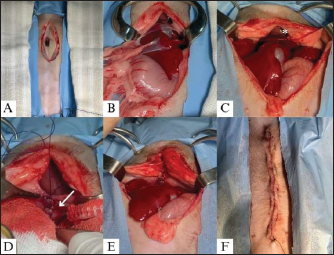
Fig. 4. Midline elliptical incision measuring approximately 5 cm in the preumbilical region (A); Presence of stomach, liver, and gallbladder adhered to the thoracic cavity (B); Repositioning of the viscera and exposure of the pericardioperitoneal defect, enabling visualization of the caudal portion of the heart (*) (C); Creation of the Reverdin pattern (arrow), using Adson forceps, to correct the pericardioperitoneal defect (D); Complete hernioplasty with repositioning of the abdominal organs, with the heart no longer visible (E); Synthesis of the skin tissue using a continuous Wolff suture pattern (F). In the postoperative period, the patient received ceftriaxone (Blau, Cotia, SP, Brazil) (25 mg/kg) IV/BID, meloxicam (Agener União, São Paulo, SP) (0.1 mg/kg) IV/SID, tramadol hydrochloride (Halex Istar, Goiânia, GO, Brazil) (1 mg/kg) IM/TID, and dipyrone (Agener União, São Paulo, SP) (25 mg/kg) IV/TID during hospitalization, where the patient was maintained for 24 hours, with all vital parameters remaining within reference ranges. The patient was discharged with an oral prescription of dipyrone (25 mg/kg) TID for 4 days and meloxicam (0.1 mg/kg) SID for 3 days. The stitches were removed 15 days after the surgical procedure. The patient exhibited significant weight gain and body condition score improvement (to 5/9) and increased levels of exercise and energy, and no further episode of dyspnea was reported 3 months after surgery. DiscussionVSD and PPH are anatomical alterations of embryonic origin that appear to be genetically predisposed (Pages et al., 2018; Morgan et al., 2020), a fact supported by the findings in this case, with a patient having both abnormalities and a history of sudden death of another puppy from the same litter. Although it has not been confirmed, as no necropsy was performed, it is suspected that the other puppy had a cardiothoracic malformation, which corroborates with the current literature (Pages et al., 2018; Morgan et al., 2020). Among dogs, Weimaraners are the primary breed diagnosed with PPH (Burns et al., 2013), although this condition has also been reported in Shih Tzu dogs, as well as VSDs (Morgan et al., 2020). Although less common than PPH, VSD is more prevalent in small-breed dogs, such as the patient, with the Yorkshire Terrier being the breed most predisposed to it (Fernandes et al., 2019; Perdoncini and Gusso, 2022). Although uncommon, PPH in association with VSD has already been reported, and the patient falls within the breeds in which VSD is more common. The turbulent flow in the perimembranous ventricular region led to the diagnosis of VSD with left-to-right flow, which is common in the early stages of heart disease (Fernandes et al., 2019; Coelho et al., 2020; Pacó et al., 2022). Therefore, the prognosis for patients with this condition can still be considered favorable, as it does not have significant systemic repercussions and does not directly affect the quality of life, unlike what occurs when there is a reversal of the flow from right to left (Coelho et al., 2020; Perdoncini and Gusso, 2022). Given this, and the fact that there was no hypertension or cardiac remodeling, despite the overload of the right ventricle, no pharmacological treatment was indicated for the patient. Although VSD was first diagnosed because of the auscultation of a heart murmur and was associated in this case with PPH, these malformations are not always observed together. The clinical signs of respiratory distress present in the patient can be explained by the presence of abdominal organs in the chest cavity, rather than the hemodynamic alterations present in VSD. Hence, there was no reason to believe that the heart condition was cyanotic. The HPP was diagnosed by thoracic radiography, which revealed the presence of abdominal organs in the thoracic region as well as suggestive findings of diaphragmatic discontinuation. Regarding treatment options, VSD treatment is initiated when the patient develops cardiac overload symptoms, shunt flow reversion, or signs of CHF. Diuretics and angiotensin-converting enzyme inhibitors are used, and vasodilators may also be added to control arterial hypertension (Nakamura et al., 2011; Coelho et al., 2020; Perdoncini and Gusso, 2022; Bruno et al., 2024). This treatment aims to control clinical cardiac signs that were not observed by the patient, and only follow-ups were recommended to assess the progression of the condition and clinical signs. However, definitive treatment for VSD is surgical correction, which is generally only performed for muscular and supracrestal defects (Croti et al., 2008; Larsson et al., 2000; Griffiths, 2010; Perdoncini and Gusso, 2022). Therefore, correction with an Amplatzer prosthesis via catheterization, which is the most efficient and least invasive approach, is only performed in cases of muscular VSDs and not perimembranous ones, as in the case reported here, and was therefore not considered a treatment option. Furthermore, surgery to correct perimembranous VSD requires cardiopulmonary bypass equipment, which, to the authors’ knowledge, is not yet available for patients of this size in Brazil, where the case was conducted (Larsson et al., 2000; Croti et al., 2008; Griffiths, 2010; Perdoncini and Gusso, 2022). Considering that clinical signs and respiratory distress were mostly related to HPP, hernioplasty surgery was indicated, even though some articles have indicated that the prognosis is the same for patients undergoing hernioplasty or not (Burns et al., 2013; Morgan et al., 2020). However, it is important to note that this prognosis evaluation is based mostly on the satisfaction of pet owners following diagnosis and clinical monitoring, or after surgical intervention, regarding clinical signs presentation, as well as the average life expectancy of dogs diagnosed with the condition, whether treated surgically or not. Therefore, the consensus on which patients should undergo surgery is ambiguous; however, the literature has suggested that surgery can be recommended for younger dogs and cats showing respiratory or gastrointestinal clinical signs (Burns et al., 2013). Hence, even with the inherently conflicting literature, recommending surgery was the right decision, as it fully resolved the clinical signs and enabled the patient to improve exercise tolerance and exhibit greater enthusiasm for physical activities. The midline incision in the preumbilical region, extending to the subxiphoid portion, allowed the defect to be corrected satisfactorily without the need to enter the thoracic cavity and, consequently, restore negative pressure in the chest (Murphy et al., 2014; Hennik et al., 2021). Although not described in the literature, the elliptical incision allowed for ablation of the hernial sac and a more efficient and esthetically pleasing dermorraphy . In cases in which an enlarged incision and access to the thorax are required, the placement of a chest drain is recommended postoperatively to prevent the development of pneumothorax, which is considered the most concerning surgical complication (Murphy et al., 2014; Morgan et al., 2020). However, in this case, it was possible to perform all surgical maneuvers without enlarging the incision or directly accessing the thoracic cavity, which eliminated the need for a drain to restore negative pressure, minimized the risk of postoperative complications, and contributed to the success of the procedure. In the postoperative period, it is important to observe signs of constrictive pericarditis, pneumopericardium, pericardial effusion, pericardial steatites, and pericardial cyst, as these are other serious possible complications (Murphy et al., 2014; Morgan et al., 2020; Lohinger et al., 2022). There have also been secondary reports of wound dehiscence, surgical site infection, and PPH recurrence (Lohinger et al., 2022). Although none of these complications developed, probably because of the short transsurgical period and the use of appropriate techniques, radiographic examination was recommended for follow-up. However, because the puppy was a rescued dog, the organization did not approve this. Regarding the intraoperative period, the main complications were hypotension, arrhythmia, bleeding, hypoxia, and hypoventilation, which were present in approximately 22% of the cases analyzed (Burns et al., 2013; Morgan et al., 2020). Despite its low incidence, they were particularly concerning in this case because of the concomitant VSD. However, the patient remained stable throughout the intraoperative period, with average blood pressure varying from 60 to 100 mmHg, heart rate varying from 130 to 160 beats/minute, and oxygen saturation maintained at 100% throughout the procedure using mechanical ventilation. In addition, the procedure was performed in a shorter duration than those previously reported, i.e., 41 minutes for the intraoperative period, as opposed to 89 minutes previously reported (Burns et al., 2013; Morgan et al., 2020). The patterns used to synthesize the defect include simple continuous sutures, Reverdin, continuous Wolff, or other appositional patterns, with absorbable or nonabsorbable monofilament thread (Morgan et al., 2020). The pericardioperitoneal defect was repaired using a Reverdin pattern and 3-0 polydioxanone thread, which has a slower absorption, the same method used by Murphy et al. (2014). These implants were chosen to prevent hernia recurrence and allow the defect to heal safely. Continuous Wolff skin suturing was used because of its faster execution than other apposition techniques, with the goal of reducing the surgical duration and minimizing the risk of intraoperative complications. The skin suture was removed after 15 days, and inflammation, wound decay, or other postoperative complications were not observed. Because of the success of the procedure, the patient’s prognosis for PPH was favorable (Burns et al., 2013; Morgan et al., 2020), and there were no signs indicating the need for reintervention six months after surgery. ConclusionRegarding the reported case, it can be concluded that although VSD is a serious heart condition, the patient’s primary clinical signs other than the murmur in the cardiac base region were caused by the presence of abdominal organs in the thoracic cavity due to the peritoneopericardial defect. Considering the above, the importance of imaging tests, radiography, and echocardiographic evaluation in the diagnosis of these malformations is clear. Hernioplasty was effective in resolving the PPH condition and clinical signs, leading to improvement in the patient’s overall condition, including significant weight gain and an increase in exercise tolerance. The postoperative findings corroborate the surgery indication in young patients with respiratory signs. The authors believe that hernioplasty is an excellent and efficient treatment for HPP and should be considered in such cases. For the VSD, periodic monitoring, once every six months, with echocardiograms, was indicated to assess the progression of cardiac alterations and the possible need for pharmacological therapy in the future. AcknowledgmentsThe authors are grateful to the entire veterinary team at the Hope Veterinary Clinic and wish to express their particular regards to M.V. Gabriel Zimmerman, M.V. Roberto Azem, M.V. Luisa Castro, and M.V. Felipe Simeoni. Conflict of interestThe authors declare no conflict of interest. FundingThis research received no specific grant. Authors contributionRRR: Literature revision, manuscript draft, editing, and reviewing; GPS: manuscript review and case conduction; MAMS: manuscript review and case conduction; CSSS: manuscript review; TGM: case diagnosis and conduction, image selection, and manuscript review; IMO: manuscript review and edition; RSM: manuscript draft, revision, edition, image selection, and literature revision. Data availabilityAll data supporting the findings of this study are available in the manuscript. ReferencesBruno, B., Savarino, P., Bussadori, C., Degiovanni, A., Lardone, E., Bertero, A. and Tarducci, A. 2024. Case report: eisenmenger syndrome in a dog with ventricular septal defect: long term management and complications. Front. Vet. Sci. 11, 1393919. Burns, C.G., Bergh, M.S. and Mcloughlin, M.A. 2013. Surgical and nonsurgical treatment of peritoneopericardial diaphragmatic hernia in dogs and cats: 58 cases (1999–2008). J. Am. Vet. Med. Assoc. 242(5), 643–650. Boon, J.A. 1998. Manual of veterinary echocardiography. 2ed. Hoboken, NJ: Willey, Blackweel. Coelho, M.R., Muzzi, R.A.L., Dorneles, E.M.S, Oliveira, L.E.D., Furtado, L.L.A. and Muzzi, L.A.L. 2020. Avaliação da deformação miocárdica pela ecocardiografia feature tracking em gatos com defeito perimembranoso do septo ventricular. Arq. Bras. Med. Vet. 72, 807–813. Croti, U.A., Braile, D.M., de Oliveira, M.A.B and Filho, F.V.G. 2008. Fechamento de comunicação interventricular muscular de via de entrada do ventrículo direito. Braz. J. Cardiovasc. Surg. 23, 589–590. Fernandes, C.G., Frederico, T.C.L., Marques, A.E.G. and Marques, M.G. 2019. Ecocardiografia com contraste à base de microbolhas em um cão com comunicação interventricular perimembranosa e estenose pulmonar—Relato de caso. Griffiths, L.G. 2010. Surgery for cardiac disease in small animals: current techniques. Vet. Clin. North Am. Small Anim. Pract. 40(4), 605–622. Hennink, I., Duver, P., Ulrich, R., Meneses, F., Moioli, M., Adamik, K.N. and Kovacevic, A. 2021 Case report: unusual peritoneopericardial diaphragmatic hernia in an 8-month-old German Shepherd dog, associated with a pericardial pseudocyst and coexisting severe pericardial effusion resulting in right-sided heart failure. Front. Vet. Sci. 8, 673543. Larsson, M.H.M.A., Pereira, L., Jatene, F.B., Freitas, R.F., Barbusci, L.O.D., de Oliveira, S.M. and Abduch, M.C.D. 2000. Clinical diagnosis and alternative surgical treatment of tetralogy of Fallot in a dog. A case report. Arq. Bras. Med. Vet. 52(5), 433–436. Lohinger, C., Gumpenberger, M, Kolm, U.S. and Degasperi, B. 2022. Pneumopericardium with concomitant pericardical effusion following peritoneopericardical diaphragmatic hernia repair in a do. Vet. Record Case Rep. 10(2), e278. McClaran, J.K. 2023. Diaphragmatic and peritoneopericardial diaphragmatic hernias. In: Small animal soft tissue surgery. pp: 308–317. Morgan, K.R.S., Singh, A., Giuffrida, M.A., Balsa, I.M., Hayes, G., Thomson, C.B., Arai, S., Smeak, D.D., Monnet, E., Selmic, L.E., Cray, M., Morris, T., Biskup, J.J., Thieman-Mankin, K., Milovancev, M. and Gatineau, M. 2020. Outcome after surgical and conservative treatments of canine peritoneopericardial diaphragmatic hernia: a multi-institutional study of 128 dogs. Vet. Surg. 49(1), 138–145. Murphy, L.A., Russel, N.J., Dulake, M.I. and Nakamura, R.K. 2014. Constrictive pericarditis following surgical repair of a peritoneopericardial diaphragmatic hernia in a cat. J. Feline Med. Surg. 16(8), 708–712. Nakamura, K., Yamasaki, M., Ohta, H., Sasaki, N., Muramaki, M., Kumara, W.R.B. and Takiguchi, M. 2011. Effects of sildenafil citrate on five dogs with Eisenmenger’s syndrome. J. Small Anim. Pract. 52(11), 595–598. Pacó, T.R., Rocha, C.C., Júnior, Z.J.S. and Antunes, J.M.A.P. 2022. Echocardiographic diagnosis of interventricular septum defect with Eisenmenger syndrome in an adult dog-case report. Acta Vet. Braz. 16, 1. Pages, G., Menaut, P. and Grand, J.G. 2018. Peritoneopericardial diaphragmatic hernia in the dog: a clinical report in a litter of six Dogue de Bordeaux puppies. Rev. Vét. Clin. 53(2), 39–43. Perdoncini, P. and Gusso, A.B.F. 2022. Comunicação interventricular em cães: Revisão. Pubvet 16(06), 1–8. | ||
| How to Cite this Article |
| Pubmed Style Ribeiro RR, Santos GP, Silva MAM, Silva CSDS, Mendes TG, Moura RSD, Oliveira IM. Peritoneopericardial hernioplasty in a two-month-old Shih Tzu. Open Vet. J.. 2025; 15(5): 2259-2264. doi:10.5455/OVJ.2025.v15.i5.43 Web Style Ribeiro RR, Santos GP, Silva MAM, Silva CSDS, Mendes TG, Moura RSD, Oliveira IM. Peritoneopericardial hernioplasty in a two-month-old Shih Tzu. https://www.openveterinaryjournal.com/?mno=239314 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.43 AMA (American Medical Association) Style Ribeiro RR, Santos GP, Silva MAM, Silva CSDS, Mendes TG, Moura RSD, Oliveira IM. Peritoneopericardial hernioplasty in a two-month-old Shih Tzu. Open Vet. J.. 2025; 15(5): 2259-2264. doi:10.5455/OVJ.2025.v15.i5.43 Vancouver/ICMJE Style Ribeiro RR, Santos GP, Silva MAM, Silva CSDS, Mendes TG, Moura RSD, Oliveira IM. Peritoneopericardial hernioplasty in a two-month-old Shih Tzu. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2259-2264. doi:10.5455/OVJ.2025.v15.i5.43 Harvard Style Ribeiro, R. R., Santos, . G. P., Silva, . M. A. M., Silva, . C. S. D. S., Mendes, . T. G., Moura, . R. S. D. & Oliveira, . I. M. (2025) Peritoneopericardial hernioplasty in a two-month-old Shih Tzu. Open Vet. J., 15 (5), 2259-2264. doi:10.5455/OVJ.2025.v15.i5.43 Turabian Style Ribeiro, Rafaela Rodrigues, Guilherme Pinheiro Santos, Marco Augusto Machado Silva, Cindy Stefhani Dos Santos Silva, Tayanne Gobbi Mendes, Rauane Sousa De Moura, and Iago Martins Oliveira. 2025. Peritoneopericardial hernioplasty in a two-month-old Shih Tzu. Open Veterinary Journal, 15 (5), 2259-2264. doi:10.5455/OVJ.2025.v15.i5.43 Chicago Style Ribeiro, Rafaela Rodrigues, Guilherme Pinheiro Santos, Marco Augusto Machado Silva, Cindy Stefhani Dos Santos Silva, Tayanne Gobbi Mendes, Rauane Sousa De Moura, and Iago Martins Oliveira. "Peritoneopericardial hernioplasty in a two-month-old Shih Tzu." Open Veterinary Journal 15 (2025), 2259-2264. doi:10.5455/OVJ.2025.v15.i5.43 MLA (The Modern Language Association) Style Ribeiro, Rafaela Rodrigues, Guilherme Pinheiro Santos, Marco Augusto Machado Silva, Cindy Stefhani Dos Santos Silva, Tayanne Gobbi Mendes, Rauane Sousa De Moura, and Iago Martins Oliveira. "Peritoneopericardial hernioplasty in a two-month-old Shih Tzu." Open Veterinary Journal 15.5 (2025), 2259-2264. Print. doi:10.5455/OVJ.2025.v15.i5.43 APA (American Psychological Association) Style Ribeiro, R. R., Santos, . G. P., Silva, . M. A. M., Silva, . C. S. D. S., Mendes, . T. G., Moura, . R. S. D. & Oliveira, . I. M. (2025) Peritoneopericardial hernioplasty in a two-month-old Shih Tzu. Open Veterinary Journal, 15 (5), 2259-2264. doi:10.5455/OVJ.2025.v15.i5.43 |