| Research Article | ||
Open Vet. J.. 2025; 15(5): 2030-2038 Open Veterinary Journal, (2025), Vol. 15(5): 2030-2038 Research Article Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern ThailandNikom Srikacha1 and Pornchai Pornpanom2,3,4*1Department of Animal Sciences, Faculty of Natural Resources, Rajamangala University of Technology Isan, Sakon Nakhon, Thailand 2Akkhraratchakumari Veterinary College, Walailak University, Nakhon Si Thammarat, Thailand 3One Health Research Center, Walailak University, Nakhon Si Thammarat, Thailand 4Informatics Innovation Center of Excellence, Walailak University, Nakhon Si Thammarat, Thailand *Corresponding Author: Pornchai Pornpanom. Akkhraratchakumari Veterinary College, Walailak University, Nakhon Si Thammarat, Thailand. Email: pp.vettech [at] gmail.com Submitted: 16/01/2025 Revised: 13/04/2025 Accepted: 21/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
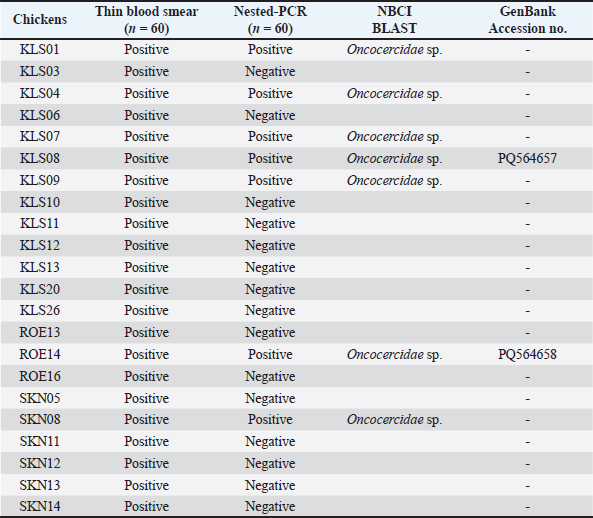
ABSTRACTBackground: Filariasis is a parasitic disease caused by filarial nematodes. The life cycle involves vertebrates host and hematophagous arthropods. Filarial nematodes are viviparous, and their larvae (microfilaria) are produced into the blood circulation. Microfilariae are widely studied in humans and small animals, but are rarely found in domestic chickens. Aim: We aimed to study the prevalence, morphology, and molecular characteristics of microfilariae in village chickens raised in Thailand. Materials and Methods: Sixty thin blood smears were prepared for parasite detection and morphotype observation. A total of 60 genomic DNA were used for nested-PCR amplification of the cytochrome c oxidase 1 (COX1) gene. The amplicons were then sequenced and used for Bayesian phylogenetic analysis. Results: The prevalence of microfilariae in domestic chickens raised in Northeastern Thailand was 36.66%. Three morphotypes of microfilariae were identified. Eleven out of 22 samples exhibited a single morphotype infection. Of these, only two samples were identified as single-strain infections. One sequence isolated from unsheathed microfilaria was phylogenetically grouped with other Eufilaria spp., with 9.95%–10.57 % genetic divergence. Thus, this unsheathed microfilaria was identified as Eufilaria sp. (GenBank accession no: PQ564658). Conclusion: The prevalence of microfilariae in Thai chickens was high. This report highlighted that the sequence of the COX1 gene and morphological characteristics confirmed Eufilaria infection. This study sheds light on the identification and diagnosis of microfilaria and filarial nematode infection in chickens. However, the sensitivity of the available nested-PCR was limited. Further design of primers using our reported sequences may resolve this problem. Thus, the data from this study can be considered as baseline information for further studies. Keywords: Eufilaria, COX1, Morphotypes, Onchocercidae, Thailand. IntroductionFilariasis is a parasitic disease caused by filarial nematodes that parasitize the tissues and tissue spaces of vertebrates (mammals, birds, reptiles, and amphibians) (Bartlett, 2008). There are two families of filarial nematodes, Filariidae and Onchocercidae (Anderson, 2000). However, all avian filarial nematodes are classified under the family Onchocercidae, which includes 16 known genera. Among the eight subfamilies of Onchocercidae, three (Splendidofilariinae, Lemdaninae, Onchocercinae) contain parasites in the avian host (Anderson, 2000). Of the 16 genera of avian filarial nematodes, Pelecitus is the most common genus, occurring across several avian orders. Other frequently observed genera include Chandlerella, Paronchocerca, Splendidofilaria, and Cardiofilaria. Birds in the order Galliformes are specifically affected by eight genera of filarial nematodes (Pelecitus, Paronchocerca, Aproctella, Cardiofilaria, Chandlerella, Splendidofilaria, Lemdana, and Eufilaria) (Bartlett, 2008). Filarial nematodes are viviparous, and adult females reside in tissue spaces. They produce the first-stage larva (microfilaria) in the blood circulation, which then inhabits the blood or skin (Binkienė et al., 2021). Microfilariae develop through the first and second larvae stages, molting before becoming third-stage larvae in arthropod vectors (Manguin et al., 2010). These third-stage larvae can then be transmitted to another avian host during the blood meal (Sehgal et al., 2005). Microfilariae are transmitted by various blood-sucking arthropods, including biting midges, hippoboscid files, black files, mosquitoes, ticks, fleas, and lice (Anderson, 2000; Binkienė et al., 2021; Garrido-Bautista et al., 2023). Some avian filarial nematodes exhibit possible periodicity. Splendidofilaria mavis exhibits the highest rate of parasitemia in the evening and night, whereas parasitemia in the morning and midday is low (Chagas et al., 2021). Filarial nematodes have been extensively studied because they cause diseases such as lymphatic filariasis, onchocerciasis, loaisis, and mansonellosis in humans (Kwarteng et al., 2016). In veterinary medicine, important filarial nematodes include Dirofilaria immitis and Dirofilaria repens, which cause heartworm disease and subcutaneous dirofilariasis, respectively (Genchi et al., 2019). In avian species, filariasis is generally nonpathogenic, but fatal infection has been reported in red-billed blue magpies (Urocissa erythrorhynchus) (Simpson et al., 1996). In chickens (Gallus gallus domesticus), the infection may have negative impacts (Sekiguchi et al., 2018). To our knowledge, there are some reports on the molecular characteristics of Onchocerciade nematodes in chickens (Sekiguchi et al., 2018; Hayashi et al., 2024, Pornpanom and Boonchuay, 2024). Hayashi et al. (2024) reports histopathology and molecular characteristics of the microfilariae of Paronchocerca sp. (subfamily Splendidofilariinae). In Thailand, there are no reports of species or genus of avian filarial nematodes. Only one report on the Onchocercidae family was found among backyard chickens (Pornpanom and Boonchuay, 2024). For the identification of parasite species, it is possible to use the morphological characteristics of microfilariae, but this remains challenging because the morphology of microfilariae among filarial species is similar (Binkienė et al., 2021). The authors assume that using morphology data combined with molecular techniques may enable more accurate identification of parasites. In Thailand, village chickens are raised under a backyard free-ranging system. The rate of infection by blood parasites in Thai chickens is high (Takang et al., 2017; Prasopsom et al., 2020; Piratae et al., 2021; Vaisusuk et al., 2022; Boonchuay et al., 2023). Microfilariae are found in backyard chickens raised in Northern and Southern Thailand (Takang et al., 2017; Boonchuay et al., 2023). However, identification of the species and genera of microfilariae is lacking. Thus, this study aims to describe the genera of microfilariae infecting village chickens in Thailand using their morphological and molecular characteristics. Furthermore, the authors aim to carry out a preliminary investigation of the prevalence of microfilaria in village chickens in Northeastern Thailand. This information is to be considered baseline information for further studies. Material and MethodsEthical approvalThis study was approved by the Walailak University Institutional Animal Care and Use Committee (Approval number: WU-ACUC-66091) and the Institutional Biosafety and Biosecurity (Approval number: WU-IBC-66-066). Blood sample collection and managementFrom March to December 2024, blood samples were collected from 60 village chickens (conventionally sampling, male=41 and female=19) from Northeastern Thailand [Kalasin (16.43′ N, 103.50′ E; 27 samples), Roi Et (16.05′ N, 103.65′ E; 18 samples), and Sakon Nakhon (17.15′ N, 104.13′ E; 15 samples)]. Blood was drawn from the biracial vein, and fresh blood smears were immediately prepared (three smears per chicken). Then, the remaining blood was transferred to an ethylenediaminetetraacetic acid (EDTA) tube and kept in an icebox. The EDTA blood samples were then sent through cold chain transportation for molecular analysis at the Laboratory of Veterinary Clinical Pathology, Akkhraratchakumari Veterinary College, Walailak University (8.64′ N, 99.89′ E). Air-dried blood smears were then fixed with absolute methanol for 1 minute and stained with 10% Giemsa (in phosphate buffer, pH 7.2) for 1 hour. Microscopic examinationSixty blood smears were observed for the existence of microfilariae using a light microscope at 400× magnification (100 fields) and repeated by 1,000× magnification (100 fields) (Pornpanom and Boonchuay, 2024). Pictures of microfilariae were taken using a microscope (Olympus BX43, Olympus, Tokyo, Japan) equipped with a digital camera (OlympusDP27, Olympus) and CellSens imaging software (version 1.18, Olympus). Morphotypes of microfilaria were identified using photographed pictures. The measurement of microfilariae (only length) was performed only in samples infected by single morphotypes using CellSens imaging software (version 1.18, Olympus). Nested-PCR for amplification of cytochrome c oxidase 1 (COX1) in Onchocercidae nematodesSixty EDTA blood samples were extracted for genomic DNA using a Blood Genomic DNA Extraction Mini Kit (FavorPrep, Pingtung, Taiwan). All genomic DNA was then kept at −20ºC until further processing. For amplification of the COX1 gene, the outer primers (OnchoCOI_F1: 5´-TTG TGG AAT GAC TTT TGG YAA T-3´/OnchoCOI_R1: 5´-AAT CTT AAC AGC TCT AGG AAT AGC-3´) and the inner primers (OnchoCOI_F2: 5´-CTG TTA ATC ATA AGA CTA TTG GTA CT-3´/OnchoCOI_R2: 5´-CAG CAC TAA AAT AAG TAC GAG TAT C-3´) were used following the previous report (Garrido-Bautista et al., 2023). The PCR mix was prepared in a total volume of 20 µl, containing 10 µl of master mix (OnePCRTM, Ultra, Bio-Helix, New Taipei City, Taiwan), 1 µl of each primer (10 µm), 6 µl of ultrapure water, and 2 µl of DNA template (concentration < 25 ng/µl in most samples). The thermocycling conditions followed those in a previous report (Pornpanom and Boonchuay, 2024). Both external and internal reactions were as follows: Pre-denaturation (95ºC for 5 minutes), 35 cycles of denaturation (95ºC for 1 minute); annealing (external step: 53ºC for 1 minute and internal step: 50ºC for 1 minute); and extension (72ºC for 1 minute), and finished by the final extension at 72ºC for 10 minutes. Non-template control and positive control [Onchocercidae sp. isolate AVC09 (Pornpanom and Boonchuay, 2024)] were used in all runs. PCR products were detected using 1.5% agarose gel electrophoresis. All positive samples (expected size=850–900 bp) were submitted to U2Bio Thailand (Bangkok, Thailand) for sequencing using Barcode Taq Sequencing technology (Celemics, Seoul, South Korea) in the Illumina MiSeq. Sequences and phylogenetic analysisSeven sequences retrieved from the chickens were available. These seven sequences were compared with known sequences in the National Center for Biotechnology Information (NCBI) database using the Basic Local Alignment Search Tool (BLAST). This is to confirm that they were Onchocercidae nematodes. Two Onchocercidae sequences isolated from a single morphotype infection were deposited in NCBI GenBank. For Bayesian phylogenetic analysis, we used 31 COX1 sequences [following a previous report (Binkienė et al., 2021; Garrido-Bautista et al., 2023)] and our two sequences. The COX1 sequence of Ascaridia galli (KT613888) was used as an outgroup. The phylogeny construction was implemented in MrBayes version 3.2.6 (Ronquist and Huelsenbeck, 2003). The DNA substitution model used in this study was the general time-reversible model. This model was selected based on the hierarchical likelihood ratio test, which was implemented in the mrModeltest 2.3 program (Nylander, 2004). Markov chain Monte Carlo was performed for three million generations with sampling every 100 generations. The first 25% of the three trees were discarded as “burn-in”, and the consensus tree was calculated based on the remaining 22,500 trees. Then, the consensus tree was calculated. The tree was visualized using Figtree version 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree). The sequence homology between different sequences was calculated using the Jukes-Cantor model implemented in MEGA11 (Tamura et al., 2021). Table 1. Detection of microfilaria in village chickens (Gallus gallus domesticus) raised in Northeast Thailand.
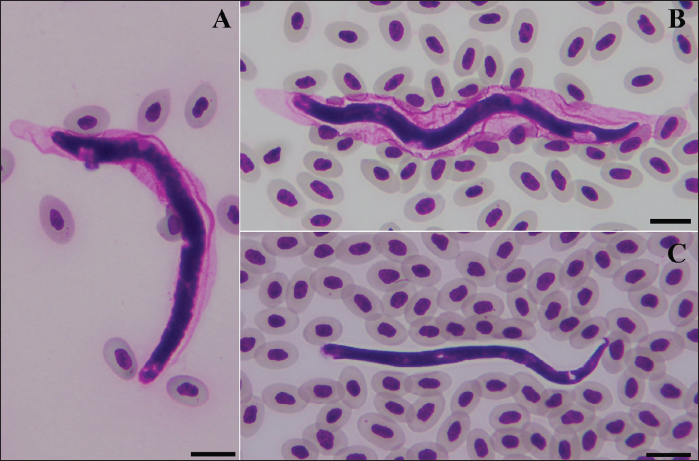
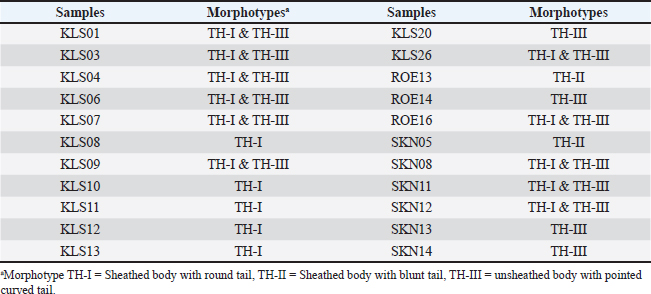
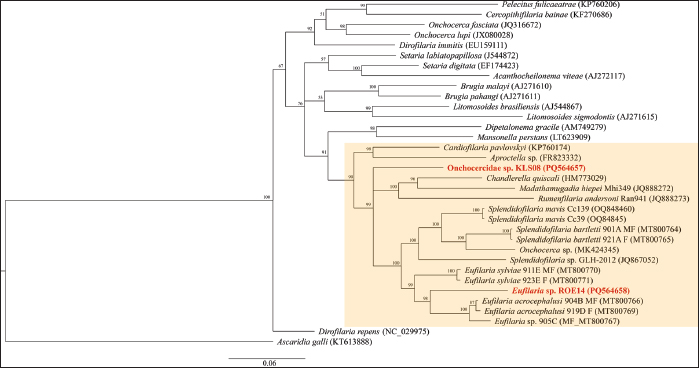
Statistical analysisThe prevalence of microfilaria was calculated based on the results of the thin blood smear examination. The confidence intervals (95% CI) were calculated using the function “binom.approx” in package “epitools”, implemented in R version 4.2.2 (R, 2024). Fisher’s exact test was used to assess differences in the prevalence of microfilariae in each province (Kalasin, Roi Et, and Sakon Nakhon) and between males and females. Fisher’s exact test was implemented in R (R, 2024), and significance was obtained at a p < 0.05. ResultsPrevalence of microfilaria in chickens in ThailandThe infection rate of microfilaria in chickens (G. domesticus) raised in Northeastern Thailand was 36.66% (95% CI: 24.47–48.86). The prevalence of parasites was significantly different among the localities (p < 0.05), with rates of 48.18% in Kalasin, 16.67% in Roi Et, and 40.40% in Sakon Nakhon. Furthermore, the prevalence of microfilaria in males (48.78%) was significantly higher than in females (10.53), p=0.004. The parasite intensity in each chicken was very low, with less than 5 mf found in a whole slide in each sample. Of the 60 samples, only seven were positive for nested-PCR amplification of cytochrome c oxidase 1 (COX1) (Table 1). Morphotypes of microfilariaBased on microscopic analysis of thin blood smears, microfilariae were classified into three morphotypes (Fig. 1). Morphotype TH-I: Sheathed microfilaria with blunt tail (length=83.65 ± 6.78 µm), morphotype TH-II: Sheathed with blunt tail (length=89.40 ± 6.01 µm), and morphotype TH-III: unsheathed with pointed tail (length=74.84 ± 7.39 µm). There were five chickens infected with morphotype TH-I, two were infected with morphotype TH-II, and the other four were infected with morphotype TH-III (Table 2). Furthermore, 11 samples were coinfected with two morphotypes (morphotypes TH-I and TH-III). Molecular characteristics of microfilariaBayesian phylogenetic analysis revealed that all filarial nematodes isolated from avian species belong to the same clade (Fig. 2). However, there were two filarial nematodes isolated from non-avian hosts in this clade, including Madathamugadia hiepei isolated from Turner’s Giant Gecko ( Pachydactylus turneri, GenBank accession no: JQ888272) and Rumenfilaria andersoni isolated from Reindeer ( Rangifer tarandus, GenBank accession no: JQ888273). In this clade, the genetic divergence among the COX1 genes of each avian filarial nematode was 0.00%–18.94% (Fig. 3).
Fig. 1. Morphotypes of microfilaria. Morphotype TH-I: Sheathed body with a round tail (A); morphotype TH-II: Sheathed body with a blunt tail (B); and morphotype TH-III: Unsheathed body with a pointed curved tail (C). Scale bar=10 μm. Table 2. Summary of morphotypes of microfilaria found in village chickens in Northeast Thailand.
Fig. 2. Bayesian phylogenetics of filarial nematodes constructed using the COX1 gene (655 bp). The sequences isolated from this study were given in red-bold. All avian filarial nematodes are grouped together (highlighted). Two filaria nematodes isolated from Pachydactylus turneri (JQ888272) and Rangifer tarandus (JQ888273) were found in this clade. The COX1 sequences of Ascaridia galli (KT613888) were used as an outgroup. Node values (in percentage) indicate posterior clade probabilities. Two Onchocercidae nematodes isolated from chickens raised in Northeastern Thailand exhibited 14.97 % genetic divergence from each other. Onchocercidae sp. KLS08 (GenBank accession no: PQ564657) exhibited 12.07%–13.63 % genetic divergence with Eufilaria spp. and 14.69 % genetic divergence with Splendidofilaria spp. Onchocercidae sp. ROE14 (GenBank accession no: PQ564658) was phylogenetically grouped in the Eufilaria clade, with 9.95%–10.57% genetic divergence. Furthermore, Onchocercidae sp. ROE14 is closely similar to Eufilaria sylviae isolate 923E_F (MT800771), with 9.95% genetic divergence. DiscussionThe infection rate of microfilaria in village chickens in Thailand was relatively high (36.66%). The infection rates at each locality (Kalasin, Roi Et, and Sakon Nakhon) showed a significant difference. Furthermore, the prevalence of microfilaria in males was significantly higher than that in females. Overall, the prevalence of microfilaria in this report (36.66%) was slightly higher than that in a previous report (27.27%) (Pornpanom and Boonchuay, 2024), which might be related to the abundance and species of vectors in Thailand. We assumed that this slight difference in the infection rate might be related to the abundance and species of the vectors in Thailand. Thus, further investigation of the vectors of avian filarial nematodes in this country is needed. Additionally, the relationship between parasite prevalence and environment factors (rainfall, climate, geographic location, and presence of other nearby avian species) may reveal the annual pattern of parasite infection year-round.
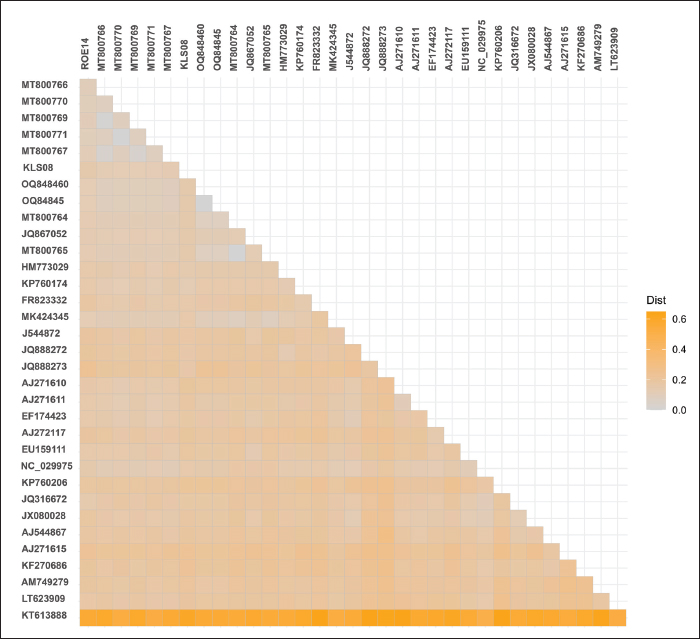
Fig. 3. Heatmap of pairwise genetic distances estimated from nucleotide sequences of the COX1 gene (655 bp) of filarial nematodes using the Jukes-Canter model. Previous studies have successfully identified the vectors of avian filarial nematodes, such as Culicoides arakawae for Paronchocerca (Hayashi et al., 2024), Mansonia crassipes for Cardiofilaria nilesi (Dissanaike and Fernando, 1965), and Simulium uchidai for Splendidofilaria sp. (Fukuda et al., 2005). In Northeastern Thailand, one article reported evidence that Culicoides mahasarakhamense was a potential vector for avian filarial nematodes of the family Onchocercidae (Pramual et al., 2023). Generally, Eufilaria spp. are transmitted by biting midges (Ceratopogonidae) (Anderson, 2000). It is possible that the vector for microfilaria found in this study was C. mahasarakhamense. However, about 100 species of Culicoides have been found in Thailand (Thepparat et al., 2015). Multiple Culicoides species may be possible to transmit Eufilaria and other Onchocercidae nematodes. Several samples were positive for microscopy but negative for nested-PCR. The small number of possible COX1-amplified samples was similar to that reported in a previous report (Pornpanom and Boonchuay, 2024). This limitation is a significant constraint for this study. The available primers designed by Garrido-Bautista et al. (2023) were sufficient for detecting filarial nematodes in passerine birds, but might not be effective for use in chicken parasites. Thus, a molecular diagnosis of microfilaria in chickens is needed. The novel primers may be designed based on our previously reported sequences (PQ564657 and PQ564658). The novel primers with higher sensitivity might shed light on the routine molecular diagnosis of filarial nematodes, especially in countries where the parasites are prevalent. In addition, the novel primers will allow the genetic characteristics of filarial nematodes to be used for parasite identification. On the other hand, the use of another gene (e.g., 18S ribosomal RNA and 25S ribosomal RNA) or the sequencing of a mitogenome (Hayashi et al., 2024) might be applicable. Three morphotypes of microfilaria were identified in this study. The information about the morphotype of microfilaria indicated the diversity of avian filarial nematodes in chickens in Thailand. Thus, further investigations, intensively observing the morphology and morphometry of both microfilaria and adult worms, are needed. The identification of filarial species solely based on the morphology of microfilaria was not recommended because they were highly similar in morphology and morphometry (Sehgal et al., 2005; Hamer et al. 2013). Thus, a combination of microscopic and molecular techniques for studying filarial worms might be useful for further experiments. The feasibility of identification of parasite species would lead to several disciplines of study in avian filarial nematodes, such as pathology, biology, and the development of detection techniques and treatments. Although filarial nematodes are considered to have low pathogenicity, Hayashi et al. (2024) observed some pathological conditions in infected chickens, including blood vessel obstruction and endarteritis in the lung, hyperkeratosis with epithelial metaplasia in the esophagus, infiltration of heterophils and macrophages, and accumulation of cellular debris in the glandular lumen. Furthermore, Sekiguchi et al. (2018) reported the presence of mild depression and open-mouth breathing in chickens infected by filarial nematodes, which was likely attributed to pulmonary collapse. Our chickens exhibited no notable clinical signs and were not euthanized for necropsy. Additionally, no reports of pathological changes in Thai chickens infected with filarial nematodes have been published. Thus, further investigations were necessary. Bayesian phylogenetic analysis of COX1 sequences revealed that our Onchocercidae sp. (ROE14, GenBank accession no: PQ564658) were grouped with E. sylviae, Eufilaria acrocephalusi, and Eufilaria sp., with 89.43%–90.5% similarity. The filarial nematodes found in this study belonged to the genus Eufilaria. The authors highlighted that this is the first report of Eufilaria infection in Thai chickens (G. domesticus). Generally, there were about 17 species of Eufilaria spp., including Eufilaria bartlettae, Eucalyptus delicata, E. kalifai, Eutropis longicaudata, E. alii, Euphydryas asiatica, E. buckleyi, E. capsulate, E. coua, E. hibleri, E. mcintoshi, E. sergenti, E. singhi, E. utae, E. acrocephalusi, and E. sylviae (Granath, 1981; Anderson, 2000; Binkienė et al., 2021). Eufilaria spp. were found in subcutaneous connective tissues, especially the neck region. The microfilaria was short and unsheathed. Eufialria spp. were transmitted by biting midges, such as Culicoides nebeculosus, C. crepuscularis, and Cylindroplegma haematopotus (Anderson, 2000). This suggests that further study on adult nematodes should focus on the subcutaneous connective tissue of chickens, especially the neck region. Furthermore, a study on their vector focused on Culicoides biting midges might reveal information about vectors for microfilaria transmission in chickens. ConclusionThe prevalence of microfilaria in chickens raised in Northeastern Thailand was high (36.66%, 95% CI: 24.47 – 48.86), indicating that the environmental conditions in the region were suitable for the transmission of microfilaria. Morphological characteristics and COX1 gene analysis identified the presence of Eufilaria sp., marking the first documented case of Eufilaria infection in Thai chicken (GenBank accession no: PQ564658). This discovery highlights the need for further investigations into the epidemiology and impact of filarial infections in chickens. Future investigations should focus on developing novel molecular markers for parasite identification. Additionally, further investigation should aim to identify potential vectors responsible for parasite transmission, characterize adult filarial nematodes, and assess the pathological effects of filarial nematodes. Such studies would provide a deeper understanding of filariasis in chickens and its potential impact on animal health and agricultural productivity. AcknowledgmentsThe authors sincerely thank the farmers and livestock authorities for their cooperation in sample collection. This work was supported by Walailak University under the New Researcher Development scheme (Contract Number WU67211). Authors’ contributionsNS: Conceptualization, methodology, sample collection, investigation, data analysis, writing, original draft preparation, and funding acquisition. PP: Conceptualization, methodology, sample collection investigation, data analysis, data curation, writing, review, and editing, and funding acquisition. All authors have read and approved the final manuscript. FundingThis work was supported by Walailak University under the New Researcher Development scheme (Contract Number WU67211). Conflict of interestThe authors declare that there is no conflict of interest. Data availabilityAll data were provided in the manuscript. ReferencesAnderson, R.C. 2000. Book Nematode parasites of vertebrates: their development and transmission, 2nd ed. Wallingford, UK: CAB International. Bartlett, C.M. 2008. Filarioid nematodes. In Parasitic diseases of wild birds. Eds., Atkinson, C.T., Thomas, N.J., Hunter, D.B. Hoboken, NJ: Wiley-Blackwell, pp: 439–462. Binkienė, R., Chagas, C.R.F., Bernotienė, R. and Valkiūnas, G. 2021. Molecular and morphological characterization of three new species of avian Onchocercidae (Nematoda) with emphasis on circulating microfilariae. Parasit Vectors 14, 137. Boonchuay, K., Thomrongsuwannakij, T., Chagas, C.R.F. and Pornpanom, P. 2023. Prevalence and diversity of blood parasites (Plasmodium, Leucocytozoon and Trypanosoma) in backyard chickens (Gallus gallus domesticus) raised in Southern Thailand. Animals 13, 2798. Chagas, C.R.F., Binkienė, R. and Valkiūnas, G. 2021. Description and molecular characterization of two species of avian blood parasites, with remarks on circadian rhythms of avian haematozoa infections. Animals 11(12), 3490. Dissanaike, A.S. and Fernando, M.A. 1965. Cardiofilaria nilesi n.sp., recovered from a chicken experimentally infected with infective larvae from Mansonia crassipes. J. Helminthol. 39(2), 151–158. Fukuda, M., Bain, O., Aoki, C., Otsuka, Y. and Takaoka, H. 2005. Natural infections of Simulium (Nevermαnnia) uchidai (Diptera: Simuliidae) with infective tilariallarvae, probably from a bird, in Oita, Japan. Med. Entomol. Zool. 56(2), 93–98. Garrido-Bautista, J., Harl, J.H., Fuehrer, H.-P., Comas, M., Smith, S., Penn, D.J. and Moreno-Rueda, G. 2023. Prevalence, molecular characterization, and ecological associations of filarioid helminths in a wild population of blue tits (Cyanistes caeruleus). Diversity 15(5), 609. Genchi, M., Rinaldi, L., Venco, L., Cringoli, G., Vismarra, A. and Kramer, L. 2019. Dirofilaria immitis and D. repens in dog and cat: a questionnaire study in Italy. Vet. Parasitol. 267, 26–31. Granath, W.O. 1981. Eufilaria hibleri sp. n. (Nematoda: Filarioidea) from the common grackle (Quiscalus quiscula versicolor). Proc. Helminthol. Soc. Wash. 48, 17–23. Hamer, G.L., Anderson, T.K., Berry, G.E., Makohon-Moore, A.P., Crafton, J.C., Brawn, J.D., Dolinski, A.C., Krebs, B.L., Ruiz, M.O., Muzzall, P.M., Goldberg, T.L. and Walker, E.D. 2013. Prevalence of filarioid nematodes and trypanosomes in American robins and house sparrows, Chicago USA. Int. J. Parasitol. Parasites Wild. 2, 42–49. Hayashi, N., Hosokawa, K., Yamamoto, Y., Kodama, S., Kurokawa, A., Nakao, R. and Nonaka, N. 2024. A filarial parasite potentially associated with the health burden on domestic chickens in Japan. Sci. Rep. 14, 6316. Kwarteng, A., Ahuno, S.T. and Akoto, F.O. 2016. Killing filarial nematode parasites: role of treatment options and host immune response. Infect. Dis. Poverty 5, 86. Manguin, S., Bangs, M.J., Pothikasikorn, J. and Chareonviriyaphap, T. 2010. Review on global co-transmission of human Plasmodium species and Wuchereria bancrofti by Anopheles mosquitoes. Infect. Genet. Evol. 10(2), 159–177. Nylander, J.A.A. 2004. MrModeltest 2.3. Program distributed by the author. Uppsala, Sweden: Evolutionary Biology Centre, Uppsala University. Piratae, S., Vaisusuk, K. and Chatan, W. 2021. Prevalence and molecular identification of Leucocytozoon spp. in fighting cocks (Gallus gallus) in Thailand. Parasitol. Res. 120, 2149–2155. Pornpanom, P. and Boonchuay, K. 2024. Preliminary study on buffy coat smear and molecular detection of microfilaria in domestic chickens (Gallus gallus domesticus) raised in Southern Thailand. Vet. World 17(4), 888–894. Pramual, P., Khamluea, S., Butlun, P. and Promdungdee, A. 2023. Molecular detection of filarial nematode from Culicoides biting midges (Diptera: Ceratopogonidae) in northeastern Thailand. Trop. Biomed. 40(2), 188–193. Prasopsom, P., Salakij, C., Lertwatcharasarakul, P. and Pornpranom, P. 2020. Hematological and phylogenetic studies of Leucocytozoon spp. in backyard chickens and fighting cocks around Kamphaeng Saen, Thailand. Agr. Nat. Resour. 54(6), 595–602. R. 2024. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Ronquist, F. and Huelsenbeck, J.P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. Sehgal, R.N.M., Jones, H.I. and Smith, T.B. 2005. Molecular evidence for host specificity of parasitic nematode microfilariae in some African rainforest birds. Mol. Ecol. 14(13), 3977–3988. Sekiguchi, M., Nonaka, N., Adachi, M., Sekiya, T. and Yamamoto, Y. 2018. Avian filariasis in backyard chickens in Japan. Avian Dis. 62, 326–329. Simpson, V.R., MacKenzie, G. and Harris, E.A. 1996. Fatal microfilarial infection in red billed blue magpies (Urocissa erythrorhynchus). Vet. Rec. 138, 522–523. Takang, P., Pikulkaew, S., Awaiwanont, N. and Numee, S. 2017. Prevalence and risk factors of blood parasites infection in backyard chickens in Chiang Mai. Chiang Mai Vet. J. 15(3), 157–167. Tamura, K., Stecher, G. and Kumar, S. 2021 MEGA11: molecular evolutionary genetics analysis Version 11. Mol. Biol. Evol. 38(7), 3022–3027. Thepparat, A., Bellis, G., Ketavan, C., Ruangsittichai, J., Sumruayphol, S. and Apiwathnasorn, C. 2015. Ten species of Culicoides Latreille (Diptera: Ceratopogonidae) newly recorded from Thailand. Zootaxa 4033(1), 48–56. Vaisusuk, K., Chatan, W., Seerintra, T. and Piratae, S. 2022. High prevalence of Plasmodium infection in fighting cocks in Thailand determined with a molecular method. J. Vet. Res. 66(3), 373–379. | ||
| How to Cite this Article |
| Pubmed Style Srikacha N, Pornpanom P. Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern Thailand. Open Vet. J.. 2025; 15(5): 2030-2038. doi:10.5455/OVJ.2025.v15.i5.19 Web Style Srikacha N, Pornpanom P. Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern Thailand. https://www.openveterinaryjournal.com/?mno=238085 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i5.19 AMA (American Medical Association) Style Srikacha N, Pornpanom P. Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern Thailand. Open Vet. J.. 2025; 15(5): 2030-2038. doi:10.5455/OVJ.2025.v15.i5.19 Vancouver/ICMJE Style Srikacha N, Pornpanom P. Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern Thailand. Open Vet. J.. (2025), [cited January 24, 2026]; 15(5): 2030-2038. doi:10.5455/OVJ.2025.v15.i5.19 Harvard Style Srikacha, N. & Pornpanom, . P. (2025) Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern Thailand. Open Vet. J., 15 (5), 2030-2038. doi:10.5455/OVJ.2025.v15.i5.19 Turabian Style Srikacha, Nikom, and Pornchai Pornpanom. 2025. Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern Thailand. Open Veterinary Journal, 15 (5), 2030-2038. doi:10.5455/OVJ.2025.v15.i5.19 Chicago Style Srikacha, Nikom, and Pornchai Pornpanom. "Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern Thailand." Open Veterinary Journal 15 (2025), 2030-2038. doi:10.5455/OVJ.2025.v15.i5.19 MLA (The Modern Language Association) Style Srikacha, Nikom, and Pornchai Pornpanom. "Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern Thailand." Open Veterinary Journal 15.5 (2025), 2030-2038. Print. doi:10.5455/OVJ.2025.v15.i5.19 APA (American Psychological Association) Style Srikacha, N. & Pornpanom, . P. (2025) Morphological and molecular characterization of microfilariae in chickens (Gallus gallus domesticus) in Northeastern Thailand. Open Veterinary Journal, 15 (5), 2030-2038. doi:10.5455/OVJ.2025.v15.i5.19 |